Abstract
Background:
Ketamine has been reported to exert rapid and sustained antidepressant effects in patients with depression, including patients with treatment-resistant depression. However, ketamine has several drawbacks such as psychotomimetic/dissociative symptoms, abuse potential and neurotoxicity, all of which prevent its routine use in daily clinical practice.
Methods:
Therefore, development of novel agents with fewer safety and usage concerns for the treatment of depression has been actively investigated. From this standpoint, searching for active substances (stereoisomers and metabolites) and agents acting on the N-methyl-D-aspartate (NMDA) receptor have recently gained much attention.
Results:
The first approach includes stereoisomers of ketamine, (R)-ketamine and (S)-ketamine. Although (S)-ketamine has been considered as the active stereoisomer of racemic ketamine, recently, (R)-ketamine has been demonstrated to exert even more prolonged antidepressant effects in animal models than (S)-ketamine. Moreover, ketamine is rapidly metabolized into several metabolites, and some metabolites are speculated as being active substances exerting antidepressant effects. Of such metabolites, one in particular, namely, (2R,6R)-hydroxynorketamine, has been reported to be responsible for the antidepressant effects of ketamine. The second approach includes agents acting on the NMDA receptor, such as glycine site modulators and GluN2B subunit-selective antagonists. These agents have been tested in patients with treatment-resistant depression, and have been found to exhibit rapid antidepressant effects like ketamine.
Conclusion:
The above approaches may be useful to overcome the drawbacks of ketamine. Elucidation of the mechanisms of action of ketamine may pave the way for the development of antidepressant that are safer, but as potent and rapidly acting as ketamine.
Keywords: Ketamine, (S)-ketamine, (R)-ketamine, hydroxynorketamine, GLYX-13, 7-chlorokinurenic acid, GluN2B, mGlu receptor
1. Introduction
Major depressive disorder (MDD) represents a major social problem, with an estimated lifetime prevalence rate in the USA of 16.6% [1]; thus, the economic burden of mood disorders, including MDD, is immense.
Currently, both selective serotonin reuptake inhibitors (SSRIs) and serotonin- and noradrenaline reuptake inhibitors (SNRIs) are widely prescribed for the treatment of MDD. While the large majority of individuals (~70%) with depression exhibit at least some improvement with antidepressant medication, approximately 30% of patients remain resistant to a series of treatments [2, 3]. Moreover, for the currently used antidepressants, it takes about 3-6 weeks before significant therapeutic effect is manifested. Therefore, the focus of drug discovery research has recently shifted to non-monoamine-based agents from the currently prescribed monoamine-based antidepressants. Apart from the biogenic amine system, the glutamatergic system has been identified as a particularly important target to develop new antidepressants [4, 5]. Ketamine, which has been shown to exert robust and rapid antidepressant effects, is an important prototype of agents acting through the glutamatergic system [6]; a number of studies have replicated the rapid and sustained efficacy of ketamine in MDD patients, including patients with treatment-resistant depression (TRD) [7, 8]. However, a number of profound adverse effects, such as psychotomimetic symptoms, abuse potential, neurotoxicity and cognitive impairment, preclude the routine use of ketamine in daily clinical practice.
In order to develop agents that are as potent and rapidly-acting as ketamine against MDD, but with better safety profiles, intensive investigations are under way to understand the mechanisms underlying the antidepressant effects of ketamine. One of the approaches for clarifying the antidepressant effects of ketamine is to identify the active substance(s) that can be detected in the blood following injection of ketamine. Because ketamine is a racemic mixture of (R)-ketamine and (S)-ketamine, and is rapidly and stereoselectively metabolized into several metabolites [9-11], research has been conducted to evaluate the antidepressant potential of each of the stereoisomers and metabolites. Interestingly, recent evidence has suggested that, in addition to (S)-ketamine, which has been considered to be the active component of ketamine exerting anti- depressant effects [12, 13], (R)-ketamine and metabolites of ketamine may also exhibit antidepressant effects without having the unwanted side effects of ketamine and (S)-ketamine [14, 15]. Other approaches to identify agents having a similar efficacy profile to ketamine, but with a better safety profile, are to focus on agents acting at different sites of the N-methyl-D-aspartate (NMDA) receptor from ketamine, based on the assumption that ketamine exerts its antidepressant effects by blocking the NMDA receptor, or to focus on other agents acting within the glutamatergic system [7, 16]. In particular, agents acting on the glycine modulatory site of the NMDA receptor, represented by GLYX-13, have gained much attention recently [17, 18]. These agents have been shown to exert rapid and sustained antidepressant effects, like ketamine, while being devoid of unwanted side effects. Based on these preclinical findings, clinical studies using these agents are on-going, and some encouraging results have been obtained [19, 20].
In this review, recent findings of research using the new approaches mentioned above, aimed at developing safer antidepressants than ketamine, will be reviewed, with particular focus on two emerging approaches, namely, identification of active substance(s) after administration of racemic ketamine, and identification of agents acting on the glycine modulatory site of the NMDA receptor. Moreover, brief mention will also be made of agents acting on metabotropic glutamate (mGlu) receptors besides the NMDA receptor, because some of these agents may share the same pathways as ketamine to exert their antidepressant effects.
2. Antidepressant effects of ketamine (racemic ketamine) and (S)-ketamine
The antidepressant effects of ketamine (0.5 mg/kg administered by intravenous (IV) injection over 40 min) was first demonstrated in patients with MDD in 2000 [6]. The antidepressant effects were confirmed in a subsequent study in which a single injection of ketamine (0.5 mg/kg administered IV over 40 min) exerted rapid (>2 h) and sustained (~1 week) antidepressant effects in patients with TRD [8]. This rapid antidepressant effect of ketamine is in marked contrast to that of conventional antidepressants, which show significant effects only 4–12 weeks postdose [21]. To date, several studies have replicated the rapid and sustained antidepressant effects of ketamine [7]. In addition to the effects of a single injection, the effects of repeated administration of ketamine have also been tested in subjects with TRD [22-24]: while no tolerance occurred at least for a short period, some patients developed relapse after the cessation of ketamine administration. Ketamine has also been reported to rapidly reduce suicidal ideation [25, 26], suggesting the anti-suicide potential of ketamine. Collectively, discovery of the antidepressant effects of ketamine is regarded as the most outstanding finding in depression research in over 60 years.
Ketamine is a racemic mixture containing equal parts of the (S)-ketamine enantiomer and (R)-ketamine enantiomer. Of these, (S)-ketamine has been regarded as the active stereoisomer due to its higher affinity for the NMDA receptor and greater anesthetic potency than (R)-ketamine [12, 13]. Indeed, (S)-ketamine has a three- to fourfold higher affinity for the NMDA receptor than (R)-ketamine [13], and was demonstrated to show antidepressant effects in rodents [27, 28]. As expected, (S)-ketamine was also confirmed in pilot studies to exert antidepressant effects in patients with depression. A comparative study of IV infusion of racemic ketamine (0.5 mg/kg over 50 min) and (S)-ketamine (0.25 mg/kg over 50 min) in two patients with TRD revealed that in one of patients, the antidepressant effects of both became manifest within 1 day, the effects were sustained for more than 3 days, and the depression score (Hamilton Rating Scale for Depression; HAM-D) returned to the pre-treatment level by day 6 [29]. Both patients experienced the psychotomimetic side effects of ketamine following infusion of racemic ketamine, while neither developed these adverse effects following treatment with (S)-ketamine, indicating that treatment with (S)-ketamine may be associated with a lower risk of development of psychotic and dissociative symptoms. Moreover, IV infusion of (S)-ketamine (0.25 mg/kg over 40 min) 6 times over a period of 4 weeks reduced the score on the HAM-D in 5 patients with TRD, the effects appearing within 2 h after each infusion [30]. In contrast to the case in the two patients in the study reported above, in this study, one of the patients developed pronounced dissociative symptoms following treatment with (S)-ketamine, which necessitated discontinuation of the drug. Thus, (S)-ketamine can also elicit psychotomimetic symptoms, similar to racemic ketamine. Recently, a multicenter, randomized, placebo-controlled trial of IV injection of (S)-ketamine (0.2 or 0.4 mg/kg over 40 min) was conducted in 30 patients with TRD [31]. In this study, robust and significant antidepressant effects of (S)-ketamine were observed in patients with TRD at as early as 2 h after the injections. In addition, the 3-day response rates to (S)-ketamine treatment were reported to be 67% in the 0.2 mg/kg arm and 65% in the 0.4 mg/kg arm. In contrast, the reported responses rates to approved oral combinations of antipsychotics and monoamine-based antidepressants for TRD or inadequately responsive MDD are approximately 37%–56% after treatment for 6–12 weeks [21]. It should be noted that (S)-ketamine infusion led to transient dissociative and psychotic symptoms, similar to those occurring in response to administration of racemic ketamine, which subsided within 4 h. Janssen Research & Development, LLC, is currently conducting a clinical trial of intranasal (S)-ketamine (esketamine) administration for the treatment of TRD (ClinicalTrials.gov Identifier: NCT01998958, NCT02782104, etc.).
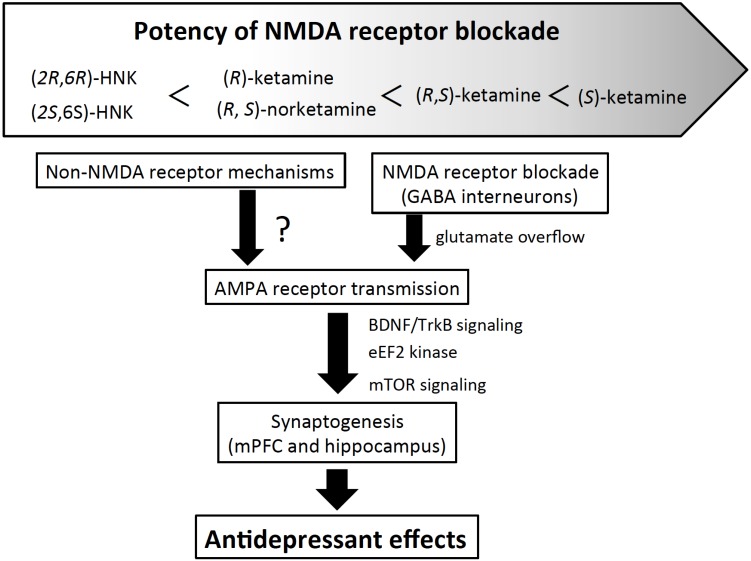
Although the neural mechanisms underlying the antidepressant effects of ketamine still remain to be clearly elucidated, recent studies have indicated that ketamine blocks the NMDA receptor, presumably on GABA interneurons, to disinhibit pyramidal neural activity in discrete brain regions, which leads to an increase in glutamate release. Glutamate overflow triggers a series of signaling pathways, including α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) receptor activation, increase in brain-derived neurotrophic factor (BDNF) secretion and mammalian target of rapamycin (mTOR) signaling. Another hypothesis is that ketamine, by blocking the NMDA receptor, inhibits eukaryotic elongation factor 2 kinase, which increases BDNF production. Either pathway eventually results in an increase in synaptic connectivity (Fig. 1) (for reviews, see [7, 32]).
Fig. (1).
Proposed mechanisms of actions of ketamine, its stereoisomers and their metabolites. Ketamine, its stereoisomers and its metabolites (norketamine and hydroxynorketamine) show different affinity for the NMDA receptor. Both racemic ketamine and (S)-ketamine have been proposed to exert antidepressant effects through blockade of the NMDA receptor, presumably on the GABA interneurons, followed by stimulation of the AMPA receptor. Both (R)-ketamine and HNKs show lower or very weak affinity for the NMDA receptor; therefore, it would appear that they stimulate the AMPA receptor through mechanisms other than NMDA receptor blockade. In any case, AMPA receptor stimulation leads to increase in synaptogenesis via BDNF/TrkB signaling, to exert rapid and sustained antidepressant activity. HNK: hydroxynorketamine.
3. Antidepressant effects of (R)-ketamine
Recently, (R)-ketamine, which had until now been considered as a less potent stereoisomer of racemic ketamine, was demonstrated to exert longer-lasting antidepressant effects than (S)-ketamine [14, 33]. Indeed, in one comparison, (R)-ketamine significantly reversed depressive-like behavior at 7 days after administration in neonatal dexamethasone-treated juvenile mice, while (S)-ketamine no longer showed its antidepressant effects [33]. Moreover, (R)-ketamine exerted more potent antidepressant effects in the chronic social defeat stress model at 6 or 7 days after administration than (S)-ketamine, although both compounds showed still significant effects [14]. In a learned helplessness model, (R)-ketamine, but not (S)-ketamine, showed antidepressant effects at 5 days after administration [14]. In the forced swimming test, learned helplessness test and novelty-suppressed feeding test, (R)-ketamine showed more potent antidepressant effects than (S)-ketamine [15]. Taken together, these results suggest that (R)-ketamine may have more potent and longer-lasting antidepressant effects than (S)-ketamine in some animal models, and that (R)-ketamine may be the more active stereoisomer of racemic ketamine in terms of the antidepressant effects.
Although the neural mechanisms underlying the antidepressant effects of (R)-ketamine are yet to be fully clarified, it is unlikely that NMDA receptor blockade alone is involved. First, (R)-ketamine has been reported to have a three- to fourfold lower affinity for the NMDA receptor than (S)-ketamine [13]. Second, a non-competitive NMDA receptor antagonist, MK-801, showed antidepressant effects at 2 and 4 days, but not at 7 days after administration in the chronic social defeat stress model, while the effect of (R)-ketamine lasted for at least a week in the same model, indicating that the antidepressant effects of MK-801 were shorter-lasting than those of (R)-ketamine [34]. Lastly, in the same model, IV injection of GLYX-13, a partial agonist of the NMDA receptor glycine modulatory site, elicited shorter-lasting antidepressant effects than IV injection of (R)-ketamine [35]: the antidepressant effects of GLYX-13 were observed until 5 days, but not at 7 days after administration [35]. Therefore, although different interactions with NMDA receptor by these agents have to be taken into account, the shorter-lasting antidepressant effects of (S)-ketamine, MK-801 and GLYX-13, might suggest that the longer-lasting antidepressant effects of (R)-ketamine may be mediated by mechanisms other than NMDA receptor blockade alone. Nonetheless, (R)-ketamine, like (S)-ketamine, has been reported to increase the dendritic spine density as well as synthesis of synaptic protein and BDNF in the prefrontal cortex (PFC) and hippocampus, and the antidepressant effects of (R)-ketamine are mediated through AMPA receptor stimulation [14]. Therefore, although the pathway by which (R)-ketamine stimulates AMPA receptor transmission still needs to be elucidated, the actions of both (R)-ketamine and (S)-ketamine may converge at AMPA receptor stimulation, and both change the synaptic plasticity to exert antidepressant effects (Fig. 1).
A recent report has suggested that a metabolite of (R)-ketamine, (2R,6R)-hydroxynorketamine (HNK), is responsible for the antidepressant effects of racemic ketamine, because inhibition of the metabolism of racemic ketamine abolished its antidepressant effects, and (2R,6R)-HNK itself was shown to exert long-lasting antidepressant effects [15]. However, (R)-ketamine has been reported to show long-lasting antidepressant effects in the learned helplessness model after it is locally injected into the medial PFC (mPFC) and hippocampal nuclei [36]. Therefore, (R)-ketamine itself is an active substance, and the roles of its metabolites need to be investigated in further detail.
Interestingly, (R)-ketamine has been reported to be devoid of the drawbacks of racemic ketamine, such as psychotomimetic-like behaviors, abuse potential and neurotoxicity demonstrated in rodents [14]. The reduced abuse potential associated with (R)-ketamine may be corroborated by the recent report that (R)-ketamine administration did not affect [11H]raclopride binding in the monkey striatum [37], which suggests that (R)-ketamine may not affect dopamine release. More importantly, even after repeated intermittent administration (once weekly x 8 weeks), (R)-ketamine did not induce neurotoxicity, such as reduction of the paralbumin (PV)-positive GABA inter- neurons in the mPFC and hippocampus, while (S)-ketamine caused loss of the PV-positive GABA interneurons at a dose that it exerted its antidepressant effects [38]. Therefore, (R)-ketamine may be a safer antidepressant than racemic ketamine and (S)-ketamine over the long-term. In addition, these results also suggest that the mechanisms underlying the psychotomimetic side effects and antidepressant effects of racemic ketamine may differ, and that it may be possible to develop agents with ketamine-like antidepressant potency, but devoid of the unwanted side effects of ketamine.
4. Antidepressant effects of the meta- bolites of ketamine
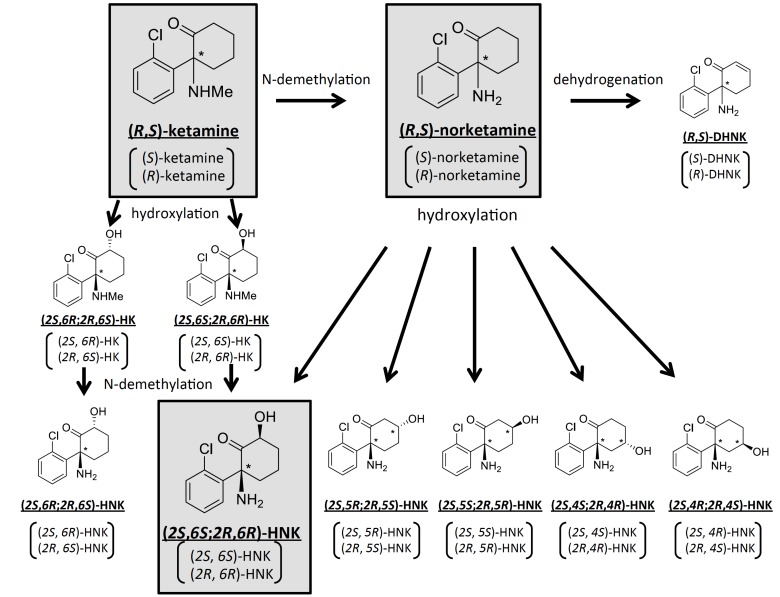
Ketamine is extensively metabolized by multiple human hepatic cytochrome P450 enzyme isoforms, with a resultant plasma elimination half-life of the drug of 1-3 h in humans [39]. Ketamine is stereoselectively biotransformed into a broad array of metabolites [40], including the major N-demethylated metabolite, norketamine, two diastereomeric hydroxyketamines, a series of six diastereomeric HNKs, and dehydronorketamine [9-11] (Fig. 2).
Fig. (2).
Proposed metabolic pathways of racemic ketamine. Racemic ketamine is stereoselectively metabolized into various metabolites by multiple cytochrome P450 enzymes. Among these, ketamine, norketamine, (2S,6S;2R,6R)-HNK and their stereoisomers have been proposed to have antidepressant effects. DHNK: dehydroxynorketamine, HK: hydroxyketamine, HNK: hydroxynorketamine.
It has been suggested that the metabolites of ketamine may also play some roles in the antidepressant effects of ketamine. Among the metabolites of ketamine, both norketamine and HNK have been demonstrated to show antidepressant potential. Indeed, in addition to the major metabolite, norketamine, four of the six HNKs mentioned above were detected in the plasma of MDD patients following ketamine infusion, with (2S,6S;2R,6R)-HNK being the predominantly detected HNK among them [41]. Norketamine has been suggested to be an active metabolite of ketamine, because it has been shown to exert an anesthetic effect and to increase locomotor activity, presumably caused by NMDA receptor blockade (racemic norketamine has been reported to have an approximately 7-fold lower affinity for the NMDA receptor than racemic ketamine) [42]. Indeed, norketamine has been reported to exert acute antidepressant effects in the forced swimming test at a lower potency than ketamine, which is in line with its lower NMDA receptor antagonist potency than that of ketamine [43]. It must be noted that in one reported study, while the antidepressant effects of norketamine were not observed at 3 and 7 days after administration [43], ketamine also did not show sustained antidepressant effects; thus, the sustained antidepressant effects of norketamine need to be investigated in greater detail. Moreover, stereoisomers of norketamine have not been reported to show antidepressant effects.
A recent report has suggested that in addition to norketamine, HNKs may also be involved in the anti- depressant effects of ketamine [15], although the HNKs have not been demonstrated to show any anesthetic effects in rats [44]. First, deuterated ketamine at the C6 position (6,6-dideuteroketamine), to prevent ketamine metabolism, did not elicit antidepressant effects, which could be associated with the decreased level of (2S,6S;2R,6R)-HNK in the brain. It should be noted that 6,6-dideuteroketamine, however, induced locomotor hyperactivity, lending further support to the notion that the neural mechanisms underlying the antidepressant and psychotomimetic symptoms of ketamine may be different. Second, administration of (2R,6R)-HNK or (2S,6S)-HNK (although less potent than (2R,6R)-HNK) elicited antidepressant effects in rodent models, and the effects of (2R,6R)-HNK were sustained. Therefore, (2R,6R)-HNK may be responsible for the antidepressant effects of ketamine. Importantly, (2R,6R)-HNK, unlike ketamine, did not elicit psychotomimetic-like behaviors or show abuse potential in rodents. Although the mechanisms underlying the antidepressant effects of (2R,6R)-HNK remain to be fully investigated, mechanisms other than NMDA receptor blockade may be involved, as (2R,6R)-HNK has little affinity for the NMDA receptor. Nonetheless, as for the case of ketamine, involvement of the AMPA receptor, as the common pathway, has been demonstrated in the antidepressant effects of (2R,6R)-HNK, and (2R,6R)-HNK increased the expressions of GluA1 and GluA2 in the hippocampus, but not in the PFC. Therefore, increase of the AMPA receptor throughput in the hippocampus may be involved in both acute and sustained antidepressant effects of (2R,6R)-HNK, although ketamine and (2R,6R)-HNK may stimulate AMPA receptor signaling via different pathways (Fig. 1). In addition to AMPA receptor stimulation, the roles of blockade of the nicotinic α7 receptor, and subsequent inhibition of serine racemase in the antidepressant actions of HNK have also been proposed [45, 46]. However, this mechanism is unlikely, because dehydroxynorketamine, which exerts more potent inhibition of the nicotinic α7 receptor than (2S,6S)-HNK [45], did not show any antidepressant effects in rodents [43]. Moreover, while the nicotinic α7 receptor inhibition potency of (2R,6R)-HNK is equivalent to that of (2S,6S)-HNK [45], the antidepressant potency differs between the two compounds. Furthermore, systemic administration of norketamine or (2S,6S)-HNK stimulates the phosphorylation of mTOR and its downstream targets in the PFC of rats [47]. However, while norketamine and (2S,6S)-HNK are more potent stimulators of mTOR phosphorylation than ketamine, antidepressant effects of these compounds are much weaker than those of ketamine. Therefore, this mechanism may not have an important role in the antidepressant effects of the metabolites of ketamine. Of note, (2R,6R)-HNK has been reported not to increase mTOR phosphorylation in the hippocampus [15].
5. Antidepressant effects of agents acting on the NMDA receptor
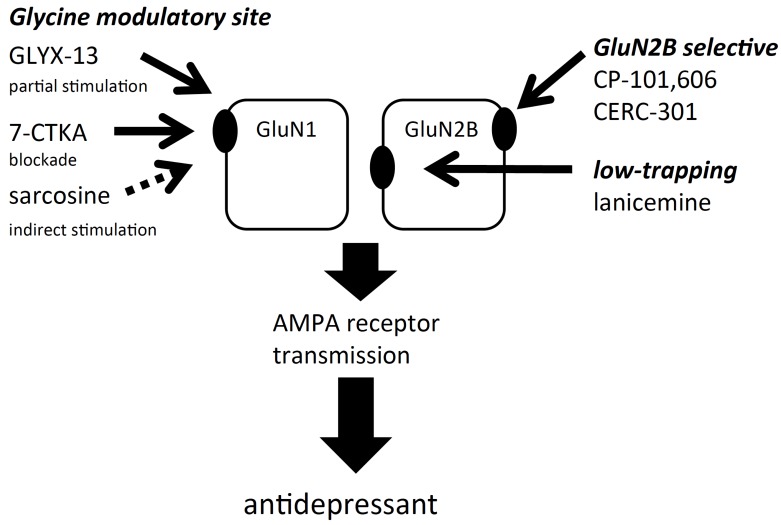
The sites of actions of agents acting on the NMDA receptor are illustrated in Fig. (3).
Fig. (3).
Sites of actions of agents acting on the NMDA receptor. NMDA receptors are tetramers composed of two GluN1 and two GluN2 subunits (GluN2A-GluN2D). Of these, it has been suggested that agents which selectively block the GluN2B subunit-containing NMDA receptor would be devoid of the side effects of ketamine. Similarly, despite acting on the same site of the NMDA receptor channel pore as ketamine, low trapping antagonists such as lanicemine may reduce the side effects of ketamine. Moreover, the NMDA receptor has a glycine modulatory site on GluN1, and agents acting on the glycine modulatory site may also be devoid of the side effects of ketamine. Despite the different sites of actions, these agents have been reported to mediate AMPA receptor stimulation to exert antidepressant effects.
5.1. Agents Acting on the NMDA Receptor
To mitigate the unwanted side effects of ketamine, efforts have been made to develop subtype-selective NMDA receptor antagonists, focusing on the GluN2B subtype. The NMDA receptor is a tetramer composed of two GluN1 and two GluN2 subunits, the latter of which can also be further divided into four subunits (GluN2A-GluN2D) [48]. Of these, the GluN2B subunit-containing NMDA receptor is highly expressed in the brain regions implicated in emotion [48], and may represent the extrasynaptic NMDA receptor involved in the reduction of the BDNF gene expression and surface AMPA receptor expression [49, 50]. In addition, a selective GluN2B antagonist has been reported to exert rapid and sustained antidepressant effects through the same neural mechanisms as ketamine in rodents [51]. A single IV infusion of CP-101,606 (traxoprodil), a selective GluN2B antagonist, elicited antidepressant effects at a dose that was still not sufficient for eliciting dissociative effects in patients with TRD (30 patients), and the antidepressant effects were sustained for a week [19]. MK-0657, another selective GluN2B antagonist was tested in a small, randomized, double-blind, placebo-controlled, crossover pilot study, and oral administration of MK-0657 showed antidepressant actions in patients with TRD (5 patients) as early as on day 5 [52]. A phase 2 trial of MK-0657 (intermittent administration at 12 or 20 mg) as an adjunctive treatment for patients with MDD is currently being conducted by Cercor under the name CERC-301 (ClinicalTrials.gov Identifier: NCT02459236). Based on preclinical pharmacodynamic and pharmacokinetic studies, it has been hypothesized that chronic administration at 8 mg daily (corresponding to approximately 50% predicted receptor occupancy) in humans would elicit rapid onset of antidepressant activity [53].
Another approach is that NMDA receptor channel blockers with lower receptor trapping potential, ability to block the receptor after initial blocks, may be associated with lower rates of psychotomimetic adverse effects as compared to ketamine [54]. In a randomized, double-blind, placebo-controlled, crossover study, the low-trapping NMDA receptor antagonist lanicemine (AZD6765) administered at a single IV dose of 150 mg exerted rapid, but short-lived antidepressant effects (effects were only observed at 80 and 110 min after the infusion) in 22 patients with TRD, without precipitating psychosis or dissociation [55, 56]. In a subsequent larger trial, repeated adjunctive IV infusions (three IV infusions/week for 3 weeks) of lanicemine at two doses (100 and 150 mg) elicited antidepressant effects in patients with TRD at week 2 without any psychotomimetic or dissociative side effects, and the effects lasted for 2 weeks after the cessation of the infusions [55]. In contrast, the clinical efficacy could not be replicated in a recently completed study of adjunctive lanicemine for over 12 weeks (15 infusions at 50 and 100 mg) in the treatment of MDD [57]. It is speculated that the differences in the study outcomes could be attributable to the differences in study design and high response rates to placebo between the two studies.
5.2. Agents Acting on the Glycine Modulatory Site at the NMDA Receptor
NMDA receptors have a glycine modulatory site on the GluN1 subunit, where glycine acts as a co-agonist to regulate NMDA receptor activity. Therefore, agents acting on the glycine modulatory site either positively or negatively regulate NMDA receptor functions. GLYX-13 (rapastinel) is an amidated tetrapeptide (threonine-proline-proline-threonine) with functional partial agonist activity for the NMDA receptor glycine modulatory site, which was derived from a hypervariable region cloned and sequenced from the monoclonal antibody B6B21 [58]. GLYX-13, like ketamine, exerted sustained antidepressant effects, lasting for at least 24 h or 7 days in the forced swimming test [17, 18]. The effects were sustained for much longer than the half-life of GLYX-13 in rats (plasma half-life of GLYX-13 is 7 min); therefore, as for the case of ketamine, enhanced synapto- genesis may be involved in the actions of GLYX-13. Consistent with this assumption, GLYX-13 increased long-term potentiation and NMDA receptor (GluN2B subunit-containing NMDA receptor) current in the hippocampus at 1 week after administration, and increased the number of mature spines in the hippocampus and mPFC at 24 h after administration [18]. GLYX-13 also increased the cell surface expression of the GluN2B receptor and AMPA receptor [18]. These findings may explain the enhanced synaptic plasticity obtained with GLYX-13. Indeed, the antidepressant effects of GLYX-13 at 1 week after administration were blocked by CPP, an NMDA receptor antagonist, indicating that GluN2B receptor upregulation may be involved in the long-lasting antidepressant effects of GLYX-13. Moreover, as in the case of ketamine, the role of mTOR signaling has also been suggested in the sustained antidepressant effects of GLYX-13 [59, 60]. Lending support to this idea, GLYX-13 increased mTOR signaling in the PFC, and local injection of rapamycin, an inhibitor of mTOR signaling, into the mPFC attenuated the sustained antidepressant effects of GLYX-13 [59]. Therefore, enhancement of mTOR signaling and GluN2B receptor-mediated transmission may have important roles in the long-lasting antidepressant effects of GLYX-13, although the relationship between the two signaling still remains unclear. It is noteworthy that the acute anti- depressant effects of GLYX-13 were blocked by an AMPA receptor antagonist [17, 60], although the role of the AMPA receptor in the long-lasting effects of GLYX-13 has not yet been directly studied. In contrast to showing ketamine-like antidepressant effects, GLYX-13 did not show the undesirable side effects of ketamine, such as psychotomimetic-like behaviors and abuse potential, in rodents [17]. Although the precise reason still needs to be determined, a recent finding suggests that GLYX-13 may selectively increase thalamo-cortical synapses without affecting cortico-cortical synapses, unlike ketamine, which enhances both thalamo-cortical and cortico-cortical synapses [59]; this may explain the lack of the psychotomimetic side effects of GLYX-13. In accordance with preclinical results, a randomized, double-blinded, placebo-controlled study in 116 subjects with MDD who had failed to respond to conventional antidepressants revealed that a single IV dose of GLYX-13 reduced the depressive symptoms within 2 h, and that the effects lasted for a week after a single injection [20]. A clinical trial of GLYX-13 for patients with TRD is currently on-going (ClinicalTrials.gov identifier: NCT01684163, NCT02192099, etc.). Of note, very recently, GLYX-13 has been reported to exhibit co-agonist properties at the NMDA receptor independent of the glycine modulatory sites [61], and now they claimed GLYX-13 as an NMDA receptor modulator. Moreover, although GLYX-13 has functional partial agonist activity for the NMDA receptor, it could behave as an antagonist, depending on the NMDA receptor activity, and it is not known which activity is involved in the actions of GLYX-13.
The antidepressant efficacy of agents blocking the glycine modulatory site of the NMDA receptor was demonstrated with 7-chlorokynurenic acid (7-CTKA), an NMDA receptor antagonist acting at the glycine modulatory site of the receptor [62]. 7-CTKA showed acute antidepressant effects in some animal models [63]. Moreover, in a chronic mild stress model in which repeated administration of traditional antidepressants is required to elicit antidepressant effects, 7-CTKA exerted antidepressant effects at 24 h after a single administration, which was sustained for 7 days, similar to ketamine [63]. 7-CTKA reversed reduction of GSK3β phosphorylation, phosphorylation of mTOR signaling molecules and synaptic protein level induced by chronic mild stress in the mPFC, but not in the hippocampus, which was lent further support by the finding that local injection of 7-CTKA into the mPFC elicited antidepressant effects [63]. Moreover, the antidepressant effects of 7-CTKA were abrogated by local injection of LY294002 (a GSK3β activator) or rapamycin (an mTOR inhibitor) into the mPFC [63], indicating that like for the case of ketamine, the GSK3β-mTOR pathway in the mPFC may be involved in the antidepressant effects of 7-CTKA. In addition, phar- macological studies using respective inhibitors and miRNA analysis have revealed that the antidepressant effects of 7-CTKA may also be mediated by BDNF-extracellular signal-regulated kinases/AKT pathways [64]. These effects (reversal of depressive-like behaviors and reduced synaptic plasticity induced by chronic mild stress) following repeated treatment with 7-CTKA were observed without any associated toxicological changes [65]. To enhance brain penetration, 4-chlorokynurenine, which readily enters the brain after systemic administration and is then converted to 7-CTKA within the astrocytes by kynurenine aminotransferase [66], was synthesized. 4-Chlorokynurenine exhibited antidepressant effects in animal models that lasted for 24 h or 7 days after systemic administration [67]. In addition, like for the case of ketamine, AMPA receptor stimulation was also demonstrated to mediate the antidepressant effects of 4-chlorokynurenine [67]. In contrast to ketamine, however, treatment with 7-CTKA or 4-chlorokynurenine was not associated with the manifestation of psychotomimetic-like behaviors or abuse potential in rodents [63, 67]. A clinical trial of 4-chlorokynurenine (AV-101) for patients with MDD is currently on-going (ClinicalTrials.gov identifier: NCT02484456).
In addition to direct regulation of the glycine modulatory site, indirect regulation of the glycine modulatory site by increasing the synaptic glycine levels through inhibition of glycine transporter 1 (GlyT1) may also be an effective approach to regulate the NMDA receptor functions. Sarcosine, a GlyT1 inhibitor, has been reported to exert antidepressant effects in both rodents and humans. Indeed, sarcosine showed antidepressant effects in some conventional animal models of depression after acute treatment as well as reversed depressive-like behaviors after chronic treatment for 3 weeks [68]. Sarcosine increases the synaptic levels of glycine, a co-agonist of the NMDA receptor, by blocking GlyT1, leading to NMDA receptor activation. Although this effect is in contrast to that of ketamine, an NMDA receptor antagonist, sarcosine has also been reported to exert acute antidepressant effects through AMPA receptor-mTOR signaling [69]. However, sarcosine has been assumed to increase AMPA receptor signaling by a pathway different from ketamine, that is, by increasing GluR1 phosphorylation at its protein kinase A site, leading to increase in membrane insertion of the AMPA receptor in the hippocampus [69]. In contrast, the same group reported that AMPA receptor-mTOR signaling in the hippocampus may not be involved in the antidepressant of sarcosine following chronic treatment for 3 weeks, because long-term treatment with sarcosine elicited no antidepressant effects in naive rats despite increased mTOR activation and AMPA receptor membrane insertion [70]. A proof-of-concept clinical trial was performed, which consist of a 6-week, randomized, double-blind flexible dose study in 40 patients with MDD, to test the hypothesis that sarcosine induces a “more rapid and robust response” than citalopram (used as a positive control) [68]; in this study, sarcosine was significantly more effective than citalopram. After 2 weeks, sarcosine was superior to citalopram by approximately 5 points on the HAM-D score. By week 4, 40% of sarcosine-treated patients met remission criteria, and by week 6, 65% of the patients were in remission, while the remission rates in the patients treated with citalopram at week 4 and 6 were 0 and 5%, respectively.
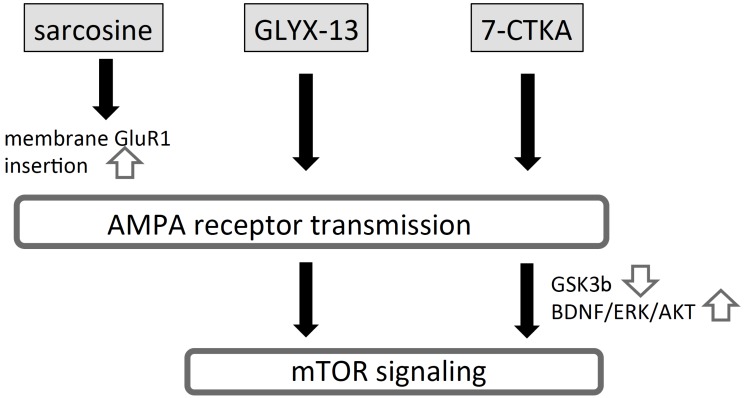
The putative neural mechanisms underlying the anti- depressant effects of the aforementioned agents acting on the glycine modulatory site on the NMDA receptor are illustrated in Fig. (4).
Fig. (4).
Proposed mechanisms of actions of agents acting at the glycine modulatory site on NMDA receptor. Sarcosine, GLYX-13 and 7-CTKA, either directly or indirectly, act at the glycine modulatory site as an agonist, partial agonist and antagonist, respectively. Sarcosine indirectly stimulates the glycine modulatory site by increasing synaptic glycine level via inhibiting glycine transporter 1. Both GLYX-13 and 7-CTKA stimulate the AMPA receptor, although precise mechanisms underlying stimulation of AMPA receptor activity have not been elucidated. While sarcosine indirectly stimulates the glycine modulatory site, it also stimulates transmission at the AMPA receptor, presumably via inducing membrane translocation of GluR1. Therefore, these three agents, despite acting on the glycine modulatory site via different mechanisms, converge at increasing AMPA receptor stimulation to exert antidepressant effects. Of note, very recently, GLYX-13 has been reported to exhibit co-agonist properties at the NMDA receptor independent of the glycine modulatory sites [61], and now they claimed GLYX-13 as an NMDA receptor modulator. 7-CTKA: 7-chlorokynurenic acid.
6. Antidepressant effects of agents acting on the metabotropic glutamate receptors
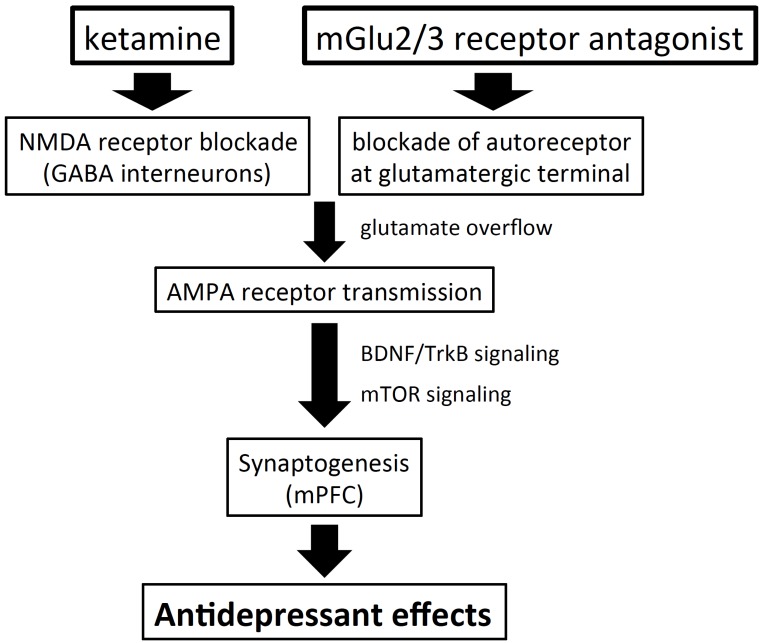
Metabotropic glutamate (mGlu) receptors have an important role in regulating glutamatergic transmission and have been implicated in the expression of moods and emotions [71]. Thus, mGlu receptors, consisting 8 subtypes (mGlu1 – mGlu8), have been investigated as attractive targets for the development of drugs to treat psychiatric disorders. Among the mGlu receptors, mGlu2/3, mGlu5 and mGlu7 receptors are of interest as targets for the development of antidepressants. In particular, mGlu2/3 receptor antagonists have been reported to show similar antidepressant effects, with similar underlying neural mechanisms, to ketamine, which raises the possibility that mGlu2/3 receptor antagonists may be developed as suitable alternatives to ketamine for the treatment of depression. Like ketamine, mGlu2/3 receptor antagonists, such as MGS0039 and LY341495, have been demonstrated to show antidepressant effects in rodents [72, 73] through AMPA receptor stimulation [74, 75] and subsequent increase in BDNF/TrkB signaling [76] and mTOR signaling [77]. Moreover, like ketamine, mGlu2/3 receptor antagonists exerted rapid antidepressant effects lasting for more than a week in both the chronic social defeat stress model [78] and chronic unpredictable stress model [79]. Interestingly, in the chronic social defeat stress model, reversal of depressive-like behaviors by an mGlu2/3 receptor antagonist coincided with reversal of reduction of BDNF/TrkB signaling, synaptic protein synthesis and dendritic spine density in the PFC and hippocampus [78]. In addition, an mGlu2/3 receptor antagonist increased phosphorylation of mTOR signaling and synaptic protein synthesis in the mPFC [80]. Therefore, like the case for ketamine, the rapid and sustained antidepressant effects of mGlu2/3 receptor antagonists may be mediated through stimulation of the processes involved in synaptogenesis, including synaptic protein synthesis and enhanced density of the spine synapses via mTOR cascade activation (Fig. 5). It is noteworthy that an mGlu2/3 receptor antagonist exhibited antidepressant efficacy in an animal model of depression that was refractory to current medications [81, 82]. Moreover, it has been reported that LY341495 enhanced antidepressant effects of ketamine [83], raising possibility that mGlu2/3 receptor antagonists may mitigate side effects of ketamine by allowing its doses to be reduced. Recently, a 6-week randomized, double-blind, placebo-controlled study was conducted to examine antidepressant potency of RG1578, a negative allosteric modulator (NAM) of the mGlu2/3 receptor as an adjunctive treatment for MDD patients showing inadequate response to SSRIs or SNRIs; however, no significant antidepressant efficacy of RG1578 as adjunctive treatment was obtained in this study [84]. It should be noted that the antidepressant potential of orthosteric mGlu2/3 receptor antagonists may differ from that of mGlu2/3 receptor NAMs [85], which needs to be examined in future clinical studies.
Fig. (5).
Shared neural mechanisms of mGlu2/3 receptor antagonists and ketamine. The mGlu2/3 receptor antagonists increase glutamate release through blockade of the autoreceptor expressed at the glutamatergic terminals, while ketamine increases glutamate release, presumably by causing disinhibition of the pyramidal neurons via blockade of the NMDA receptor on the GABA interneurons. Increased glutamate release then stimulates the postsynaptic AMPA receptor, leading to BDNF/TrkB and mTOR signaling in the mPFC, eventually resulting in increased synaptogenesis.
Other mGlu receptor ligands, such as mGlu5 receptor antagonists and an mGlu7 receptor positive allosteric potentiator (PAM), have been reported to exhibit antidepressant effects in several animal models [86], although it would appear that the neural mechanisms underlying the antidepressant potential differ between these agents and ketamine. MTEP, an mGlu5 receptor antagonist, failed to show sustained antidepressant effects in the forced swimming test (at 23 h after administration) [87], while MPEP, another mGlu5 receptor antagonist, showed sustained antidepressant effects in a novelty-suppressed feeding test [88]. The antidepressant effects (either rapid or sustained) of the mGlu5 receptor antagonists were not attenuated by an AMPA receptor antagonist, TrkB inhibitor or mTOR inhibitor in either study. Moreover, the mGlu5 receptor antagonist did not increase synaptic protein synthesis or phosphorylation of mTOR signaling molecules in the PFC [87]. Therefore, unlike ketamine and mGlu2/3 receptor antagonists, the antidepressant effects of mGlu5 receptor antagonists may be short-lasting and not dependent on increased synaptogenesis. In one double-blind, randomized controlled trial in which basimglurant, an mGlu5 receptor antagonist, was administered as an adjunctive treat- ment to ongoing SSRI or SNRI treatment, an antidepressant effect was observed in patients with MDD, as evaluated by secondary and exploratory endpoints (patient-rated Montgomery-Asberg Depression Scale (MADRS), Quick Inventory of Depressive Symptomatology-Self-Report), although the effect did not reach statistical significance when clinician-rated MADRS was used to assess the primary endpoint [89]. Therefore, it is still questionable as to whether mGlu5 receptor antagonists exert as potent antidepressant effects as ketamine. Moreover, like mGlu5 receptor antagonists, AMN082, an mGlu7 receptor agoPAM, was reported to show rapid, but not sustained antidepressant effects [87]. However, in contrast to the mechanisms involved in the actions of the mGlu5 receptor antagonists, the rapid antidepressant effects of AMN082 were mediated through mTOR signaling, because the effect of AMN082 was abolished by rapamycin, and AMN082 increased phosphorylation of mTOR signaling molecules in the PFC [87]. Thus, the neural mechanisms underlying the rapid antidepressant effects of mGlu7 receptor agonists may resemble those of ketamine. However, it should be noted that while in the aforementioned study, the antidepressant effects of AMN082 were not abrogated by an AMPA receptor antagonist, another study indicated AMPA receptor dependence of the antidepressant effects of AMN082 [90]. In any case, mGlu7 receptor agoPAMs do not have sustained antidepressant effects, indicating that the neural mechanisms underlying the antidepressant effects of the mGlu7 receptor agoPAM may differ from those of ketamine. The antidepressant actions and their underlying mechanisms of other mGlu7 receptor agonists or PAMs should be examined further in future studies, considering possible non-specific actions of AMN082 in vivo [91].
7. Conclusions and future directions
Discovery of the antidepressant effects of ketamine has triggered numerous research efforts to understand the mechanisms underlying these effects of ketamine, aimed especially at finding equally potent yet safer antidepressants than ketamine. These research activities have led to the identification of several promising drug candidates that exert rapid and sustained antidepressant effects similar to those of ketamine, but are devoid of the latter’s undesirable. In contrast, these research activities have also raised important, yet unanswered questions in relation to the development of better treatments for depression. These include identification of active substance(s) responsible for the antidepressant effects of ketamine after ketamine administration. Moreover, the synaptic and neural mechanisms underlying antidepressant effects of ketamine still remain to be elucidated. The following issues that have emerged from recent progress in research on ketamine still need to be clarified.
7.1. Active Substance(s) Detected in the Blood after Ketamine Administration
Although (S)-ketamine has long been considered to be the active stereoisomer based on its affinity for the NMDA receptor and anesthetic actions, and indeed its rapid anti- depressant effects demonstrated in patients with TRD [31], recent studies have suggested that (R)-ketamine is also an active stereoisomer, at least in rodents [14, 33]. Interestingly and unexpectedly, (R)-ketamine exerted longer-lasting and more potent antidepressant effects than (S)-ketamine. Very recently, we also confirmed that (R)-ketamine exerted longer-lasting antidepressant effects in rodents than (S)-ketamine [92]. Although we have to wait to draw any conclusions until the antidepressant effects of (R)-ketamine are confirmed in patients with depression, it is possible that both (S)-ketamine and (R)-ketamine exhibit antidepressant effects, and (R)-ketamine may have a more important role in the sustained antidepressant effects of ketamine. Moreover, given that (S)-ketamine and (R)-ketamine show different affinities for the NMDA receptor, the neural mechanisms underlying the antidepressant effects of the two compounds may differ. Yet, on the basis of the findings obtained to date, increase in synaptogenesis triggered by AMPA receptor stimulation appears to be shared by both compounds in that (R)-ketamine has more potent actions than (S)-ketamine [14], consistent with the duration of the effects in behavioral tests.
Another important question is the roles of metabolites in the antidepressant effects of ketamine. Because ketamine is rapidly and stereoselectively metabolized after administration, involvement of metabolites in the long-lasting antidepressant effects of ketamine has been speculated. Indeed, anti- depressant effects of some of the metabolites, which can be detected in reasonable amounts in the human plasma after administration of ketamine, have been reported in animal models [15, 43]. In particular, (2R,6R)-HNK has recently been identified as one of the active substances after ketamine administration, because the antidepressant effects of ketamine were no longer observed following inhibition of degradation of ketamine to (2S,6S; 2R,6R)-HNK, and the antidepressant effects of (2R,6R)-HNK are much more potent than those of (2S,6S)-HNK [15]. However, it is hardly conceivable that (2R,6R)-HNK is solely responsible for the actions of ketamine, because (2R,6R)-HNK is transformed from only (R)-ketamine, and (S)-ketamine also did show antidepressant effects in patients with TRD. To date, reports on the antidepressant activities of (R)-ketamine and its metabolites are limited, and these results have been reported from restricted laboratories. Therefore, more extensive research across laboratories is needed to identify the active components/metabolites of ketamine. Moreover, the anti- depressant potencies of racemic ketamine, (S)-ketamine, (R)-ketamine and their metabolites should be compared in the same animal models under the same conditions, with a thorough understanding of the pharmacokinetic profiles of each metabolite, including its brain exposure. It should be noted that very recently, we demonstrated that antidepressant effects of (2R,6R)-HNK is much weaker than those of (R)-ketamine in rodent models [93]. Therefore, roles of (2R,6R)-HNK in the antidepressant effects of ketamine would be questionable.
7.2. Role of NMDA Receptor Blockade in the Antidepressant Effects of Ketamine
The above studies have also raised the possibility that NMDA receptor blockade may not be the sole mechanism involved in the antidepressant actions of ketamine, because (R)-ketamine has a lower affinity for the NMDA receptor (Ki value for inhibition of [3H]MK-801 binding= 1.4 μM) than (S)-ketamine (Ki value for inhibition of [3H]MK-801 binding= 0.3 μM) [42], and (2R,6R)-HNK show very weak activity [15]. These findings suggest that neural mechanisms other than NMDA receptor blockade may be involved. As described in the text above, Dr. Hashimoto’s group recently conducted direct comparison studies between (R)-ketamine and agents acting on the NMDA receptors, such as MK-801 and GLYX-13, and clearly showed that (R)-ketamine had more sustained antidepressant effects than these agents in the chronic social defeat stress model [34, 35]. In contrast, agents acting on NMDA receptor, including GLYX-13 and GluN2B antagonists, exerted ketamine-like rapid antidepressant effects in patients with TRD [20], indicating that NMDA receptor blockade did have an important role in eliciting the antidepressant effects of these compounds. It is necessary to examine whether sustained antidepressant effects of ketamine can be preserved in a genetically manipulated model with deletion of the NMDA receptor subunit gene in certain cell populations. In addition, it is necessary to examine whether agents having low or no activity on the NMDA receptor, including (R)-ketamine and (2R,6R)-HNK, may also have as potent and long-lasting effects as ketamine in patients with MDD.
Another question to be solved would be contradictory results of agents acting on the glycine modulatory sites at the NMDA receptor. Indeed, both stimulation and inhibition of the glycine modulatory sites have been reported to lead antidepressant effects. In any case, AMPA receptor stimulation has been reported to be involved in the antidepressant effects of agents acting on the glycine modulatory sites. Therefore, they may activate the AMPA receptor in different manners, yet, eventually lead to antidepressant effects, as depicted in Fig. (4), although studies on how these agents stimulate AMPA receptor need to be investigated in further detail.
Nonetheless, several reports have suggested the roles of mechanisms other than NMDA receptor blockade, as pointed out in a review article [94]. Because NMDA receptor blockade may also be involved in the undesirable side effects of ketamine, in particular, transient psychotomimetic/ dissociative symptoms, it would be of interest to search for NMDA receptor blockade-independent mechanisms during the search for safer antidepressants. Recent studies using several ketamine-like agents with sustained antidepressant actions have pointed to a shared mechanism, namely, increased synaptogenesis in discrete brain regions such as the mPFC or hippocampus triggered by AMPA receptor stimulation. In this regard, mGlu2/3 receptor antagonists are of interest, because they showed similar efficacy, with involvement of the same neural mechanism, in rodent models, even though they do not act on the NMDA receptor. Moreover, negative allosteric modulators of GABA GABAA receptors containing α5 subunits are of interest, because they have been reported to exert similar rapid and sustained antidepressant effects in rodents via increased excitatory synaptic strength [95].
Elucidation of above mentioned issues may provide sufficient insight to identify safer means to stimulate the pathway that is responsible for the rapid and sustained antidepressant effects of ketamine, and to ultimately develop safer antidepressants with the same antidepressant potency as ketamine, but without the undesirable effects.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENT
Declared none.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [http://dx.doi.org/ 10.1001/archpsyc.62.6.593]. [PMID: 15939837]. [DOI] [PubMed] [Google Scholar]
- 2.Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., McGrath P.J., Rosenbaum J.F., Sackeim H.A., Kupfer D.J., Luther J., Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [http:// dx.doi.org/10.1176/ajp.2006.163.11.1905]. [PMID: 17074942]. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J., Shores-Wilson K., Biggs M.M., Balasubramani G.K., Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [http://dx.doi.org/10.1176/appi.ajp.163.1.28]. [PMID: 16390886]. [DOI] [PubMed] [Google Scholar]
- 4.Sanacora G., Zarate C.A., Krystal J.H., Manji H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [http://dx.doi.org/10.1038/nrd2462]. [PMID: 18425072]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolnick P., Popik P., Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009;30(11):563–569. doi: 10.1016/j.tips.2009.09.002. [http://dx.doi.org/10.1016/j.tips.2009.09.002]. [PMID: 19837463]. [DOI] [PubMed] [Google Scholar]
- 6.Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [http:// dx.doi.org/10.1016/S0006-3223(99)00230-9]. [PMID: 10686270]. [DOI] [PubMed] [Google Scholar]
- 7.Krystal J.H., Sanacora G., Duman R.S. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol. Psychiatry. 2013;73(12):1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [http://dx.doi.org/10. 1016/j.biopsych.2013.03.026]. [PMID: 23726151]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarate C.A., Jr, Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [http://dx.doi.org/10.1001/archpsyc.63.8.856]. [PMID: 16894061]. [DOI] [PubMed] [Google Scholar]
- 9.Adams J.D., Jr, Baillie T.A., Trevor A.J., Castagnoli N., Jr Studies on the biotransformation of ketamine. 1-Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed. Mass Spectrom. 1981;8(11):527–538. doi: 10.1002/bms.1200081103. [http://dx.doi.org/ 10.1002/bms.1200081103]. [PMID: 7317567]. [DOI] [PubMed] [Google Scholar]
- 10.Cohen M.L., Chan S.L., Way W.L., Trevor A.J. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;39(4):370–376. doi: 10.1097/00000542-197310000-00003. [http://dx. doi.org/10.1097/00000542-197310000-00003]. [PMID: 4758343]. [DOI] [PubMed] [Google Scholar]
- 11.Woolf T.F., Adams J.D. Biotransformation of ketamine, (Z)-6-hydroxyketamine, and (E)-6-hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica. 1987;17(7):839–847. doi: 10.3109/00498258709043993. [http://dx.doi.org/10.3109/00498258709043993]. [PMID: 3660854]. [DOI] [PubMed] [Google Scholar]
- 12.Domino E.F. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113(3):678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [PMID: 20693870]. [DOI] [PubMed] [Google Scholar]
- 13.Kohrs R., Durieux M.E. Ketamine: teaching an old drug new tricks. Anesth. Analg. 1998;87(5):1186–1193. doi: 10.1097/00000539-199811000-00039. [PMID: 9806706]. [DOI] [PubMed] [Google Scholar]
- 14.Yang C., Shirayama Y., Zhang J.C., Ren Q., Yao W., Ma M., Dong C., Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [http://dx.doi.org/10.1038/tp.2015.136]. [PMID: 26327690]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S., Fang Y., Huang X.P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Jr, Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [http://dx.doi.org/10.1038/nature17998]. [PMID: 27144355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhard D.M., Wohleb E.S., Duman R.S. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov. Today. 2016;21(3):454–464. doi: 10.1016/j.drudis.2016.01.016. [http://dx. doi.org/10.1016/j.drudis.2016.01.016]. [PMID: 26854424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgdorf J., Zhang X.L., Nicholson K.L., Balster R.L., Leander J.D., Stanton P.K., Gross A.L., Kroes R.A., Moskal J.R. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38(5):729–742. doi: 10.1038/npp.2012.246. [http:// dx.doi.org/10.1038/npp.2012.246]. [PMID: 23303054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgdorf J., Zhang X.L., Weiss C., Gross A., Boikess S.R., Kroes R.A., Khan M.A., Burch R.M., Rex C.S., Disterhoft J.F., Stanton P.K., Moskal J.R. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience. 2015;308:202–211. doi: 10.1016/j.neuroscience.2015.09.004. [http://dx.doi.org/10.1016/ j.neuroscience.2015.09.004]. [PMID: 26343295]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preskorn S.H., Baker B., Kolluri S., Menniti F.S., Krams M., Landen J.W. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [http://dx.doi.org/10.1097/JCP.0b013 e31818a6cea]. [PMID: 19011431]. [DOI] [PubMed] [Google Scholar]
- 20.Preskorn S., Macaluso M., Mehra D.O., Zammit G., Moskal J.R., Burch R.M. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 2015;21(2):140–149. doi: 10.1097/01.pra.0000462606.17725.93. [http://dx.doi.org/10.1097/01.pra.0000462606.17725.93]. [PMID: 25782764]. [DOI] [PubMed] [Google Scholar]
- 21.Spielmans G.I., Berman M.I., Linardatos E., Rosenlicht N.Z., Perry A., Tsai A.C. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. doi: 10.1371/journal.pmed.1001403. [http:// dx.doi.org/10.1371/journal.pmed.1001403]. [PMID: 23554581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.aan het Rot, M.; Collins, K.A.; Murrough, J.W.; Perez, A.M.; Reich, D.L.; Charney, D.S.; Mathew, S.J. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [http://dx.doi. org/10.1016/j.biopsych.2009.08.038]. [PMID: 19897179]. [DOI] [PubMed] [Google Scholar]
- 23.Murrough J.W., Perez A.M., Pillemer S., Stern J., Parides M.K. aan het Rot, M.; Collins, K.A.; Mathew, S.J.; Charney, D.S.; Iosifescu, D.V. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry. 2013;74(4):250–256. doi: 10.1016/j.biopsych.2012.06.022. [http://dx.doi.org/10.1016/j. biopsych.2012.06.022]. [PMID: 22840761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen K.G., Lineberry T.W., Galardy C.W., Kung S., Lapid M.I., Palmer B.A., Ritter M.J., Schak K.M., Sola C.L., Hanson A.J., Frye M.A. Serial infusions of low-dose ketamine for major depression. J. Psychopharmacol. (Oxford) 2013;27(5):444–450. doi: 10.1177/0269881113478283. [http://dx.doi.org/10.1177/0269881113478283]. [PMID: 23428794]. [DOI] [PubMed] [Google Scholar]
- 25.Ionescu D.F., Swee M.B., Pavone K.J., Taylor N., Akeju O., Baer L., Nyer M., Cassano P., Mischoulon D., Alpert J.E., Brown E.N., Nock M.K., Fava M., Cusin C. Rapid and sustained reductions in current suicidal ideation following repeated doses of intravenous ketamine: secondary analysis of an open-label study. J. Clin. Psychiatry. 2016;77(6):e719–e725. doi: 10.4088/JCP.15m10056. [http://dx.doi.org/10. 4088/JCP.15m10056]. [PMID: 27232360]. [DOI] [PubMed] [Google Scholar]
- 26.Price R.B., Mathew S.J. Does ketamine have anti-suicidal properties? Current status and future directions. CNS Drugs. 2015;29(3):181–188. doi: 10.1007/s40263-015-0232-4. [http://dx.doi.org/10.1007/s40263-015-0232-4]. [PMID: 25715884]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.du Jardin K.G., Liebenberg N., Müller H.K., Elfving B., Sanchez C., Wegener G. Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology (Berl.) 2016;233(14):2813–2825. doi: 10.1007/s00213-016-4327-5. [http://dx.doi.org/10.1007/s00213-016-4327-5]. [PMID: 27236785]. [DOI] [PubMed] [Google Scholar]
- 28.Refsgaard L.K., Pickering D.S., Andreasen J.T. Investigation of antidepressant-like and anxiolytic-like actions and cognitive and motor side effects of four N-methyl-D-aspartate receptor antagonists in mice. Behav. Pharmacol. 2017;28(1):37–47. doi: 10.1097/FBP.0000000000000266. [http:// dx.doi.org/10.1097/FBP.0000000000000266]. [PMID: 27740963]. [DOI] [PubMed] [Google Scholar]
- 29.Paul R., Schaaff N., Padberg F., Möller H.J., Frodl T. Comparison of racemic ketamine and S-ketamine in treatment-resistant major depression: report of two cases. World J. Biol. Psychiatry. 2009;10(3):241–244. doi: 10.1080/15622970701714370. [http://dx.doi.org/10.1080/ 15622970701714370]. [PMID: 19224412]. [DOI] [PubMed] [Google Scholar]
- 30.Segmiller F., Rüther T., Linhardt A., Padberg F., Berger M., Pogarell O., Möller H.J., Kohler C., Schüle C. Repeated S-ketamine infusions in therapy resistant depression: a case series. J. Clin. Pharmacol. 2013;53(9):996–998. doi: 10.1002/jcph.122. [http://dx.doi.org/10.1002/ jcph.122]. [PMID: 23893490]. [DOI] [PubMed] [Google Scholar]
- 31.Singh J.B., Fedgchin M., Daly E., Xi L., Melman C., De Bruecker G., Tadic A., Sienaert P., Wiegand F., Manji H., Drevets W.C., Van Nueten L. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol. Psychiatry. 2016;80(6):424–431. doi: 10.1016/j.biopsych.2015.10.018. [http://dx.doi.org/10.1016/j.biopsych.2015.10.018]. [PMID: 26707087]. [DOI] [PubMed] [Google Scholar]
- 32.Monteggia L.M., Gideons E., Kavalali E.T. The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol. Psychiatry. 2013;73(12):1199–1203. doi: 10.1016/j.biopsych.2012.09.006. [http://dx. doi.org/10.1016/j.biopsych.2012.09.006]. [PMID: 23062356]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J.C., Li S.X., Hashimoto K.R. (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol. Biochem. Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [http://dx.doi.org/10.1016/j.pbb.2013.11.033]. [PMID: 24316345]. [DOI] [PubMed] [Google Scholar]
- 34.Yang B., Ren Q., Ma M., Chen Q.X., Hashimoto K. Antidepressant effects of (+)-MK-801 and (-)-MK-801 in the social defeat stress model. Int. J. Neuropsychopharmacol. 2016;19(12):pyw080. doi: 10.1093/ijnp/pyw080. [http://dx.doi.org/10.1093/ijnp/pyw080]. [PMID: 27608811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang B., Zhang J.C., Han M., Yao W., Yang C., Ren Q., Ma M., Chen Q.X., Hashimoto K. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl.) 2016;233(19-20):3647–3657. doi: 10.1007/s00213-016-4399-2. [http://dx.doi.org/10.1007/s00213-016-4399-2]. [PMID: 27488193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirayama Y., Hashimoto K. Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267(2):177–182. doi: 10.1007/s00406-016-0718-1. [http://dx.doi.org/10.1007/s00406-016-0718-1]. [PMID: 27480092]. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto K., Kakiuchi T., Ohba H., Nishiyama S., Tsukada H. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267(2):173–176. doi: 10.1007/s00406-016-0692-7. [http://dx.doi.org/10.1007/s00406-016-0692-7]. [PMID: 27091456]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C., Han M., Zhang J.C., Ren Q., Hashimoto K. Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res. 2016;239:281–283. doi: 10.1016/j.psychres.2016.03.034. [http://dx.doi. org/10.1016/j.psychres.2016.03.034]. [PMID: 27043274]. [DOI] [PubMed] [Google Scholar]
- 39.Mion G., Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther. 2013;19(6):370–380. doi: 10.1111/cns.12099. [http://dx.doi.org/10.1111/ cns.12099]. [PMID: 23575437]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desta Z., Moaddel R., Ogburn E.T., Xu C., Ramamoorthy A., Venkata S.L., Sanghvi M., Goldberg M.E., Torjman M.C., Wainer I.W. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42(11):1076–1087. doi: 10.3109/00498254.2012.685777. [http://dx.doi.org/10.3109/00498254.2012.685777]. [PMID: 22612619]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarate C.A., Jr, Brutsche N., Laje G., Luckenbaugh D.A., Venkata S.L., Ramamoorthy A., Moaddel R., Wainer I.W. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry. 2012;72(4):331–338. doi: 10.1016/j.biopsych.2012.03.004. [http://dx.doi.org/10.1016/j.biopsych.2012. 03.004]. [PMID: 22516044]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebert B., Mikkelsen S., Thorkildsen C., Borgbjerg F.M. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur. J. Pharmacol. 1997;333(1):99–104. doi: 10.1016/s0014-2999(97)01116-3. [http://dx.doi.org/ 10.1016/S0014-2999(97)01116-3]. [PMID: 9311667]. [DOI] [PubMed] [Google Scholar]
- 43.Sałat K., Siwek A., Starowicz G., Librowski T., Nowak G., Drabik U., Gajdosz R., Popik P. Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor. Neuropharmacology. 2015;99:301–307. doi: 10.1016/j.neuropharm.2015.07.037. [http://dx.doi.org/10.1016/j.neuropharm.2015. 07.037]. [PMID: 26240948]. [DOI] [PubMed] [Google Scholar]
- 44.Leung L.Y., Baillie T.A. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J. Med. Chem. 1986;29(11):2396–2399. doi: 10.1021/jm00161a043. [http://dx.doi.org/10.1021/jm00161a043]. [PMID: 3783598]. [DOI] [PubMed] [Google Scholar]
- 45.Moaddel R., Abdrakhmanova G., Kozak J., Jozwiak K., Toll L., Jimenez L., Rosenberg A., Tran T., Xiao Y., Zarate C.A., Wainer I.W. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2013;698(1-3):228–234. doi: 10.1016/j.ejphar.2012.11.023. [http://dx.doi.org/10.1016/j.ejphar.2012.11.023]. [PMID: 23183107]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh N.S., Zarate C.A., Jr, Moaddel R., Bernier M., Wainer I.W. What is hydroxynorketamine and what can it bring to neurotherapeutics? Expert Rev. Neurother. 2014;14(11):1239–1242. doi: 10.1586/14737175.2014.971760. [http://dx.doi.org/10.1586/14737175.2014.971760]. [PMID: 25331415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul R.K., Singh N.S., Khadeer M., Moaddel R., Sanghvi M., Green C.E., O’Loughlin K., Torjman M.C., Bernier M., Wainer I.W. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology. 2014;121(1):149–159. doi: 10.1097/ALN.0000000000000285. [http://dx.doi.org/ 10.1097/ALN.0000000000000285]. [PMID: 24936922]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258(5082):597–603. doi: 10.1126/science.1329206. [http://dx.doi.org/10.1126/science.1329206]. [PMID: 1329206]. [DOI] [PubMed] [Google Scholar]
- 49.Hardingham G.E., Fukunaga Y., Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [PMID: 11953750]. [DOI] [PubMed] [Google Scholar]
- 50.Vanhoutte P., Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr. Opin. Neurobiol. 2003;13(3):366–371. doi: 10.1016/s0959-4388(03)00073-4. [http://dx.doi.org/10.1016/S0959-4388(03)00073-4]. [PMID: 12850222]. [DOI] [PubMed] [Google Scholar]
- 51.Li N., Lee B., Liu R.J., Banasr M., Dwyer J.M., Iwata M., Li X.Y., Aghajanian G., Duman R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [http://dx.doi.org/ 10.1126/science.1190287]. [PMID: 20724638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibrahim L., Diaz Granados N., Jolkovsky L., Brutsche N., Luckenbaugh D.A., Herring W.J., Potter W.Z., Zarate C.A., Jr A. Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J. Clin. Psychopharmacol. 2012;32(4):551–557. doi: 10.1097/JCP.0b013e31825d70d6. [http://dx.doi.org/10.1097/JCP.0b013e31825d70d6]. [PMID: 22722512]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garner R., Gopalakrishnan S., McCauley J.A., Bednar R.A., Gaul S.L., Mosser S.D., Kiss L., Lynch J.J., Patel S., Fandozzi C., Lagrutta A., Briscoe R., Liverton N.J., Paterson B.M., Vornov J.J., Mazhari R. Preclinical pharmacology and pharmacokinetics of CERC-301, a GluN2B-selective N-methyl-D-aspartate receptor antagonist. Pharmacol. Res. Perspect. 2015;3:e00198. doi: 10.1002/prp2.198. [http://dx.doi.org/10.1002/prp2.198]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mealing G.A., Lanthorn T.H., Murray C.L., Small D.L., Morley P. Differences in degree of trapping of low-affinity uncompetitive N-methyl-D-aspartic acid receptor antagonists with similar kinetics of block. J. Pharmacol. Exp. Ther. 1999;288(1):204–210. [PMID: 9862772]. [PubMed] [Google Scholar]
- 55.Sanacora G., Smith M.A., Pathak S., Su H.L., Boeijinga P.H., McCarthy D.J., Quirk M.C. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol. Psychiatry. 2014;19(9):978–985. doi: 10.1038/mp.2013.130. [http://dx.doi.org/10.1038/mp.2013.130]. [PMID: 24126931]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zarate C.A., Jr, Mathews D., Ibrahim L., Chaves J.F., Marquardt C., Ukoh I., Jolkovsky L., Brutsche N.E., Smith M.A., Luckenbaugh D.A. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol. Psychiatry. 2013;74(4):257–264. doi: 10.1016/j.biopsych.2012.10.019. [http://dx.doi. org/10.1016/j.biopsych.2012.10.019]. [PMID: 23206319]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanacora G., Johnson M., Khan A., Atkinson S., Riesenberg R., Schronen J., Burke M.A., Zajecka J., Barra L., Su H.L., Posener J.A., Bui K., Quirk M., Piser T., Mathew S.J., Pathak S. Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology. 2017;42(4):844–853. doi: 10.1038/npp.2016.224. [http://dx.doi. org/10.1038/npp.2016.224]. [PMID: 27681442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moskal J.R., Kuo A.G., Weiss C., Wood P.L., O’Connor Hanson A., Kelso S., Harris R.B., Disterhoft J.F. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49(7):1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [http://dx.doi.org/10.1016/j.neuropharm.2005.06.006]. [PMID: 16051282]. [DOI] [PubMed] [Google Scholar]
- 59.Liu R.J., Duman C., Kato T., Hare B., Lopresto D., Bang E., Burgdorf J., Moskal J., Taylor J., Aghajanian G., Duman R.S. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42(6):1231–1242. doi: 10.1038/npp.2016.202. [http://dx.doi.org/ 10.1038/npp.2016.202]. [PMID: 27634355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Y., Wang C., Xue Z., Li C., Zhang J., Zhao X., Liu A., Wang Q., Zhou W. PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int. J. Neuropsychopharmacol. 2014;18(5):pyu110. doi: 10.1093/ijnp/pyu110. [http://dx.doi.org/ 10.1093/ijnp/pyu110]. [PMID: 25542689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donello J., Li Y.X., Guo Y.X., Vu C., Banerjee P., Kroes R., Gross A., Moskal J. Abstracts of the 55th Annual meeting of American College of Neuropsychopharmacology; Hollywood, USA. 2016. [Google Scholar]
- 62.Kemp J.A., Foster A.C., Leeson P.D., Priestley T., Tridgett R., Iversen L.L., Woodruff G.N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc. Natl. Acad. Sci. USA. 1988;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [http://dx.doi.org/10.1073/pnas.85.17.6547]. [PMID: 2842779]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu W.L., Wang S.J., Liu M.M., Shi H.S., Zhang R.X., Liu J.F., Ding Z.B., Lu L. Glycine site N-methyl-D-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats. J. Psychiatry Neurosci. 2013;38(5):306–316. doi: 10.1503/jpn.120228. [http://dx.doi.org/10.1503/jpn.120228]. [PMID: 23611177]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu B.B., Luo L., Liu X.L., Geng D., Liu Q., Yi L.T. 7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress. Psychopharmacology (Berl.) 2015;232(3):541–550. doi: 10.1007/s00213-014-3690-3. [http://dx.doi.org/10.1007/s00213-014-3690-3]. [PMID: 25034119]. [DOI] [PubMed] [Google Scholar]
- 65.Li C.F., Chen X.M., Chen S.M., Mu R.H., Liu B.B., Luo L., Liu X.L., Geng D., Liu Q., Yi L.T. Activation of hippocampal BDNF signaling is involved in the antidepressant-like effect of the NMDA receptor antagonist 7-chlorokynurenic acid. Brain Res. 2016;1630:73–82. doi: 10.1016/j.brainres.2015.11.005. [http://dx.doi.org/10.1016/j.brainres.2015.11. 005]. [PMID: 26562663]. [DOI] [PubMed] [Google Scholar]
- 66.Wu H.Q., Salituro F.G., Schwarcz R. Enzyme-catalyzed production of the neuroprotective NMDA receptor antagonist 7-chlorokynurenic acid in the rat brain in vivo. Eur. J. Pharmacol. 1997;319(1):13–20. doi: 10.1016/s0014-2999(96)00829-1. [http://dx.doi.org/10.1016/S0014-2999(96) 00829-1]. [PMID: 9030892]. [DOI] [PubMed] [Google Scholar]
- 67.Zanos P., Piantadosi S.C., Wu H.Q., Pribut H.J., Dell M.J., Can A., Snodgrass H.R., Zarate C.A., Jr, Schwarcz R., Gould T.D., Jr, Schwarcz R., Gould T.D. The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineB-site inhibition. J. Pharmacol. Exp. Ther. 2015;355(1):76–85. doi: 10.1124/jpet.115.225664. [http://dx.doi.org/10.1124/jpet.115.225664]. [PMID: 26265321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C.C., Wei I.H., Huang C.L., Chen K.T., Tsai M.H. ; Tsai P., Tun R., Huang K.H., Chang Y.C., Lane H.Y., Tsai G.E. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol. Psychiatry. 2013;74(10):734–741. doi: 10.1016/j.biopsych.2013.02.020. [http://dx.doi.org/10.1016/j.biopsych.2013.02.020]. [PMID: 23562005]. [DOI] [PubMed] [Google Scholar]
- 69.Chen K.T., Tsai M.H., Wu C.H., Jou M.J., Wei I.H., Huang C.C. AMPA receptor-mTOR activation is required for the antidepressant-like effects of sarcosine during the forced swim test in rats: insertion of AMPA receptor may play a role. Front. Behav. Neurosci. 2015;9:162. doi: 10.3389/fnbeh.2015.00162. [http://dx.doi.org/10.3389/fnbeh.2015. 00162]. [PMID: 26150775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen K.T., Wu C.H., Tsai M.H., Wu Y.C., Jou M.J., Huang C.C., Wei I.H. Antidepressant-like effects of long-term sarcosine treatment in rats with or without chronic unpredictable stress. Behav. Brain Res. 2017;316:1–10. doi: 10.1016/j.bbr.2016.06.004. [http://dx.doi.org/10.1016/ j.bbr.2016.06.004]. [PMID: 27555541]. [DOI] [PubMed] [Google Scholar]
- 71.Schoepp D.D., Conn P.J. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol. Sci. 1993;14(1):13–20. doi: 10.1016/0165-6147(93)90107-u. [http://dx.doi.org/10.1016/0165-6147(93)90107-U]. [PMID: 7680175]. [DOI] [PubMed] [Google Scholar]
- 72.Chaki S., Yoshikawa R., Hirota S., Shimazaki T., Maeda M., Kawashima N., Yoshimizu T., Yasuhara A., Sakagami K., Okuyama S., Nakanishi S., Nakazato A. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46(4):457–467. doi: 10.1016/j.neuropharm.2003.10.009. [http://dx.doi.org/10.1016/j.neuropharm.2003.10.009]. [PMID: 14975669]. [DOI] [PubMed] [Google Scholar]
- 73.Chaki S., Ago Y., Palucha-Paniewiera A., Matrisciano F., Pilc A. mGlu2/3 and mGlu5 receptors: potential targets for novel antidepressants. Neuropharmacology. 2013;66:40–52. doi: 10.1016/j.neuropharm.2012.05.022. [http://dx.doi.org/10.1016/j.neuropharm.2012.05.022]. [PMID: 22640631]. [DOI] [PubMed] [Google Scholar]
- 74.Karasawa J., Shimazaki T., Kawashima N., Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042(1):92–98. doi: 10.1016/j.brainres.2005.02.032. [http://dx.doi.org/10.1016/j.brainres.2005. 02.032]. [PMID: 15823257]. [DOI] [PubMed] [Google Scholar]
- 75.Koike H., Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav. Brain Res. 2014;271:111–115. doi: 10.1016/j.bbr.2014.05.065. [http://dx.doi.org/10.1016/j.bbr.2014.05.065]. [PMID: 24909673]. [DOI] [PubMed] [Google Scholar]
- 76.Koike H., Fukumoto K., Iijima M., Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav. Brain Res. 2013;238:48–52. doi: 10.1016/j.bbr.2012.10.023. [http://dx.doi. org/10.1016/j.bbr.2012.10.023]. [PMID: 23098797]. [DOI] [PubMed] [Google Scholar]
- 77.Koike H., Iijima M., Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011;61(8):1419–1423. doi: 10.1016/j.neuropharm.2011.08.034. [http://dx.doi.org/10. 1016/j.neuropharm.2011.08.034]. [PMID: 21903115]. [DOI] [PubMed] [Google Scholar]
- 78.Dong C., Zhang J.C., Yao W., Ren Q., Ma M., Yang C., Chaki S., Hashimoto K. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int. J. Neuropsychopharmacol. 2016;•••:pyw089. doi: 10.1093/ijnp/pyw089. [http://dx.doi.org/10.1093/ijnp/pyw089]. [PMID: 27765808]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dwyer J.M., Lepack A.E., Duman R.S. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J. Mol. Psychiatry. 2013;1(1):15. doi: 10.1186/2049-9256-1-15. [http://dx.doi.org/10.1186/2049-9256-1-15]. [PMID: 25408908]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dwyer J.M., Lepack A.E., Duman R.S. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int. J. Neuropsychopharmacol. 2012;15(4):429–434. doi: 10.1017/S1461145711001702. [http://dx.doi.org/ 10.1017/S1461145711001702]. [PMID: 22114864]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ago Y., Yano K., Araki R., Hiramatsu N., Kita Y., Kawasaki T., Onoe H., Chaki S., Nakazato A., Hashimoto H., Baba A., Takuma K., Matsuda T. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology. 2013;65:29–38. doi: 10.1016/j.neuropharm.2012.09.008. [http://dx.doi. org/10.1016/j.neuropharm.2012.09.008]. [PMID: 23022081]. [DOI] [PubMed] [Google Scholar]
- 82.Koike H., Iijima M., Chaki S. Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol. Biochem. Behav. 2013;107:20–23. doi: 10.1016/j.pbb.2013.03.017. [http://dx.doi. org/10.1016/j.pbb.2013.03.017]. [PMID: 23578563]. [DOI] [PubMed] [Google Scholar]
- 83.Podkowa K., Pochwat B., Brański P., Pilc A., Pałucha-Poniewiera A. Group II mGlu receptor antagonist LY341495 enhances the antidepressant-like effects of ketamine in the forced swim test in rats. Psychopharmacology (Berl.) 2016;233(15-16):2901–2914. doi: 10.1007/s00213-016-4325-7. [http://dx.doi.org/10.1007/s00213-016-4325-7]. [PMID: 27286960]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Umbricht D., Niggli M., Sanwald-Ducray P., Deptula D., Moore R., Gru¨nbauer W., Boak L., Gatti S., Fontoura P., Fava M. Abstracts of the 54th Annual meeting of American College of Neuropsychopharmacology; Hollywood, USA. 2015. [Google Scholar]
- 85.Campo B., Kalinichev M., Lambeng N., El Yacoubi M., Royer-Urios I., Schneider M., Legrand C., Parron D., Girard F., Bessif A., Poli S., Vaugeois J.M., Le Poul E., Celanire S. Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. J. Neurogenet. 2011;25(4):152–166. doi: 10.3109/01677063.2011.627485. [http://dx.doi.org/10.3109/01677063.2011.627485]. [PMID: 22091727]. [DOI] [PubMed] [Google Scholar]
- 86.Pilc A., Chaki S., Nowak G., Witkin J.M. Mood disorders: regulation by metabotropic glutamate receptors. Biochem. Pharmacol. 2008;75(5):997–1006. doi: 10.1016/j.bcp.2007.09.021. [http://dx.doi.org/10.1016/ j.bcp.2007.09.021]. [PMID: 18164691]. [DOI] [PubMed] [Google Scholar]
- 87.Pałucha-Poniewiera A., Szewczyk B., Pilc A. Activation of the mTOR signaling pathway in the antidepressant-like activity of the mGlu5 antagonist MTEP and the mGlu7 agonist AMN082 in the FST in rats. Neuropharmacology. 2014;82:59–68. doi: 10.1016/j.neuropharm.2014.03.001. [http://dx. doi.org/10.1016/j.neuropharm.2014.03.001]. [PMID: 24631968]. [DOI] [PubMed] [Google Scholar]
- 88.Iijima M., Fukumoto K., Chaki S. Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav. Brain Res. 2012;235(2):287–292. doi: 10.1016/j.bbr.2012.08.016. [http://dx.doi.org/10.1016/j.bbr.2012.08.016]. [PMID: 22921929]. [DOI] [PubMed] [Google Scholar]
- 89.Quiroz J.A., Tamburri P., Deptula D., Banken L., Beyer U., Rabbia M., Parkar N., Fontoura P., Santarelli L. Efficacy and safety of basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(7):675–684. doi: 10.1001/jamapsychiatry.2016.0838. [http://dx.doi.org/10.1001/jamapsychiatry.2016.0838]. [PMID: 27304433]. [DOI] [PubMed] [Google Scholar]
- 90.Bradley S.R., Uslaner J.M., Flick R.B., Lee A., Groover K.M., Hutson P.H. The mGluR7 allosteric agonist AMN082 produces antidepressant-like effects by modulating glutamatergic signaling. Pharmacol. Biochem. Behav. 2012;101(1):35–40. doi: 10.1016/j.pbb.2011.11.006. [http://dx. doi.org/10.1016/j.pbb.2011.11.006]. [PMID: 22138407]. [DOI] [PubMed] [Google Scholar]
- 91.Sukoff Rizzo S.J., Leonard S.K., Gilbert A., Dollings P., Smith D.L., Zhang M.Y., Di L., Platt B.J., Neal S., Dwyer J.M., Bender C.N., Zhang J., Lock T., Kowal D., Kramer A., Randall A., Huselton C., Vishwanathan K., Tse S.Y., Butera J., Ring R.H., Rosenzweig-Lipson S., Hughes Z.A., Dunlop J. The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise? J. Pharmacol. Exp. Ther. 2011;338(1):345–352. doi: 10.1124/jpet.110.177378. [http://dx.doi.org/10.1124/jpet.110.177378]. [PMID: 21508084]. [DOI] [PubMed] [Google Scholar]
- 92.Fukumoto K., Toki H., Iijima M., Hashihayata T., Yamaguchi J.I., Hashimoto K., Chaki S. Antidepressant potential of (R)-ketamine in rodent models: Comparison with (S)-ketamine. J. Pharmacol. Exp. Ther. 2017;361(1):9–16. doi: 10.1124/jpet.116.239228. [http://dx.doi.org/ 10.1124/jpet.116.239228]. [PMID: 28115553]. [DOI] [PubMed] [Google Scholar]
- 93.Yang C., Qu Y., Abe M., Nozawa D., Chaki S., Hashimoto K. (R)-Ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol. Psychiatry. 33157;S0006-3223(16):9–33154. doi: 10.1016/j.biopsych.2016.12.020. [http://dx.doi.org/10.1016/j.biopsych.2016.12.020] [PMID: 28104224] [DOI] [PubMed] [Google Scholar]
- 94.Chaki S., Fukumoto K. Potential of glutamate-based drug discovery for next generation antidepressants. Pharmaceuticals (Basel) 2015;8(3):590–606. doi: 10.3390/ph8030590. [http://dx.doi.org/10.3390/ph8030590]. [PMID: 26393618]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fischell J., Van Dyke A.M., Kvarta M.D., LeGates T.A., Thompson S.M. Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharmacology. 2015;40(11):2499–2509. doi: 10.1038/npp.2015.112. [http://dx.doi. org/10.1038/npp.2015.112]. [PMID: 25900119]. [DOI] [PMC free article] [PubMed] [Google Scholar]