Abstract
Schistosoma japonicum is stubbornly persistent in China and the Philippines. Fast and accurate diagnostic tools are required to monitor effective control measures against schistosomiasis japonica. Promising antigen candidates for the serological diagnosis of schistosomiasis japonica have generally been identified from the Chinese strain of S. japonicum. However, the Chinese (SjC) and Philippine (SjP) strains of S. japonicum express a number of clear phenotypic differences, including aspects of host immune responses. This feature thereby emphasized the requirement to determine whether antigens identified as having diagnostic value for SjC infection are also suitable for the diagnosis of SjP infection. In the current study, 10 antigens were selected for comparison of diagnostic performance of the SjP infection using ELISA. On testing of sera from 180 subjects in the Philippines, SjSAP4 exhibited the best diagnostic performance with 94.03% sensitivity and 98.33% specificity using an optimized serum dilution. In another large scale testing with 412 serum samples, a combination (SjSAP4 + Sj23-LHD (large hydrophilic domain)) provided the best diagnostic outcome with 87.04% sensitivity and 96.67% specificity. This combination could be used in future for serological diagnosis of schistosomiasis in the Philippines, thereby representing an important component for monitoring integrated control measures.
Keywords: Schistosomiasis, Schistosoma japonicum, Philippine strain, Serological diagnosis, SjSAP4, Sj23-LHD
Highlights
-
•
Sj23-LHD was the most promising antigen candidate for early diagnosis of schistosomiasis japonica in a murine model.
-
•
SjSAP4 + Sj23-LHD had the highest diagnostic value when probed with sera from a human cohort with low infection intensity.
-
•
We have developed a novel diagnostic tool that can aid in the integrated control of schistosomiasis in the Philippines.
Schistosomiasis japonica remains a major public health concern in China and the Philippines. Development of accurate and affordable diagnostic tools is a necessity for the control and elimination of schistosomiasis. The differences in the mammalian host immunological responses to Chinese (SjC) and Philippine (SjP) strains of S. japonicum necessitated validation of proven SjC serological markers for application in the diagnosis of SjP infections. Ten antigens were selected for comparison, in ELISA, for their potential of the diagnosis of SjP infection. The results provide the basis for developing an affordable and easy-to-operate tool for the diagnosis of schistosomiasis in the Philippines.
1. Introduction
Schistosomiasis japonica is a disease of poverty and remains as a public health issue in China, the Philippines and Indonesia. In China, the epidemiology of schistosomiasis is changing due to extensive integrated control efforts (Collins et al., 2012; Li et al., 2014b). The estimated number of infected people dropped from ~ 840,000 in 2004 to 185,000 in 2013 (Xu et al., 2016). A number of endemic areas are nearing schistosomiasis transmission interruption (Li et al., 2014b; Xu et al., 2016). In contrast, as of 2010, an estimated 580,000 individuals were reported infected in the Philippines (Rollinson et al., 2013), with very high prevalences recently reported in a number of endemic provinces (Olveda et al., 2016). For China, the need for improved diagnostic tools is urgently required for effective surveillance and determination of elimination; for the Philippines, it is imperative to develop affordable and accurate field diagnostic tools for schistosomiasis control.
There are four major types of methods available for the diagnosis of schistosomiasis: parasitological detection (e.g. the Kato-Katz (KK) method), antibody-detection (AbD), antigen-detection (AgD) and circulating nucleic acids (DNA and RNA) detection (CNAD) (Cai et al., 2016a; Weerakoon et al., 2015). The KK method shows low sensitivity, while AbD based on crude extracted antigens, such as soluble egg antigen (SEA), exhibits cross-reactivity with other helminth infections, which is particularly relevant in many schistosomiasis-endemic areas. There has been considerable focus on the detection of the presence of schistosome ova, or SEA-specific antibodies but these approaches are limited for early diagnosis. More sensitive methods based on PCR technology, including qPCR (Gordon et al., 2015), droplet digital PCR (Weerakoon et al., 2016), and LAMP (Wang et al., 2011a; Xu et al., 2015) have been developed as diagnostic procedures for schistosomiasis. However, the cost of these CNAD tools may limit their practical application for large-scale surveillance of schistosomiasis. Of the available methods, ELISA-based AgD detection has several advantages, such as being affordable, easy-to-operate, there are no requirements for advanced equipment, there is less chance for cross-contamination as reported with PCR methods, such as nested-PCR (Li et al., 2014a) and LAMP (Karthik et al., 2014), and provides a balance of sensitivity and specificity once an appropriate antigen is identified and employed.
In this post-genomics era, an increasing number of high-throughput immunological studies have been carried out on schistosomes. These reports have identified a panel of tegumental and excretory-secretory antigens as potential diagnostic targets with high levels of sensitivity and specificity (Chen et al., 2014; Lu et al., 2012; McWilliam et al., 2014; Sangfuang et al., 2016; Xu et al., 2014). For example, Xu et al. identified a saposin-like protein, SjSP-13, as a potential diagnostic candidate based on a genome-wide screening of secretory proteins (Xu et al., 2014), whereas Liu et al. identified other saposin members showing better diagnostic performance than SjSP-13 (Liu et al., 2016). Since different groups employ different human cohorts with variable infection intensity and use different experimental assay conditions, a comparative study was required to compare the clinical diagnostic performance of different candidate S. japonicum antigens.
The majority of studies undertaken on the serological diagnosis of schistosomiasis japonica have been carried out using the Chinese strain of S. japonicum. However, the Chinese (SjC) and Philippine (SjP) strains of S. japonicum show clearly different phenotypes in terms of virulence, fecundity, pathology, drug sensitivity and immunology (Hope et al., 1996; Moertel et al., 2006; Weerakoon et al., 2017). Here, we carried out a comparative study with ten antigens to validate the most promising candidate for diagnosis of schistosomiasis in the Philippines. An optimized formula for serological diagnosis of SjP infection was further suggested based on this parallel comparative study.
2. Materials and Methods
2.1. Ethical Statement
All animal work was conducted according to the Australian Code for the Care and Use of Animals for Scientific Purposes (8th edition) and with the approval of the QIMR Berghofer Medical Research Institute Animal Ethics Committee (Ethics Approval: Project P288). Serum samples from the study participants in the Philippines were collected with informed written consent, and ethical approval was provided by the Institutional Review Board of the Research Institute for Tropical Medicine, Department of Health, Manila, the Philippines (Institutional Review Board Numbers 2012-13-0 and 2015-12) and the Human Research Ethics Committee, QIMR Berghofer Medical Research Institute, Brisbane, Australia (Ethics Approval: Project P524). All serum samples from healthy humans were obtained with informed written consent, and the protocol was approved by the ethics committee of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (Beijing, China).
2.2. Mice and Parasites
Eight-week-old female BALB/c mice were percutaneously infected with 14 S. japonicum cercariae (Chinese mainland strain, Anhui population) or 25 S. japonicum cercariae (Philippine strain, Sorsogon population). Blood samples were taken from animals at 4, 6, 7, 9 and 11 weeks post infection (p.i.). Blood samples from five naive mice were used as controls. The liver tissues were collected at 11 weeks post infection. Eggs per gram of liver were calculated as a measure of hepatic egg burden and general infection level, as described (Cai et al., 2015). Six Swiss mice were infected with approximately 25 S. japonicum cercariae (Philippine strain). After 6 weeks, these mice were orally administered 150, 200, 250, 300 and 350 mg/kg praziquantel prepared in 2.5% (v/v) Cremophor EL (Sigma, USA) for 5 consecutive days (Chuah et al., 2016). Serum samples were collected before infection and at 2, 4 and 6 weeks post-infection as well as at 1, 2 3, 4, 5, 6 and 7 months after chemotherapy.
2.3. Gene Expression Analysis
A next-generation oligonucleotide microarray was used to determine the expression patterns of the obtained selected target proteins in four developmental stages of S. japonicum (eggs, cercariae, hepatic schistosomula and adult worms). The design and construction of the microarray, as well as the hybridization procedures and feature extraction, have been reported previously (Cai et al., 2016b; Cai et al., 2017). Raw data and normalized gene level data from the array have been deposited in the public database Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers for the platform GPL18617, and series GSE57143. The expression pattern of the genes encoding target antigen candidates in the four developmental stages was extracted from the above dataset. A heatmap was generated based on the relative signal intensities of forward probes against the egg stage using HemI 1.0 software.
2.4. Cloning, Expression and Purification of Recombinant Proteins
Primers were designed to amplify a specific region of the target proteins (Supplementary Table 1). The DNA fragments were amplified by PCR from cDNA isolated from adult worms of S. japonicum (Philippine strain). After digestion with restriction enzymes, DNA fragments were cloned into the pET-28a vector. Recombinant plasmids were confirmed by sequencing and transformed into E. coli BL21 (DE3). Expression of the recombinant proteins was induced by 0.5 mM IPTG. Recombinant proteins were purified under native or denaturing conditions using Ni-NTA agarose (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Purified recombinant proteins were analyzed by 12% (w/v) SDS-PAGE.
2.5. Western Blotting
Purified recombinant protein samples were loaded onto 12% (w/v) SDS-PAGE and transferred to 0.2-μm PVDF membranes at 100 V for 10 min and then at 100 mA for 1 h using a wet western blotting system. After blocking with 5% (v/v) non-fat milk in PBST for 90 min, the membrane was incubated with a mouse anti-His monoclonal antibody (Sigma-Aldrich Co, MO, USA) at 4 °C overnight. After washing, the membrane was incubated with an HRP-conjugated goat anti-mouse IgG (H + L) antibody (ThermoFisher Scientific, MA, USA) for 1 h at room temperature. SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher Scientific, MA, USA) was used to detect signals.
2.6. Human Serum Samples
Serum samples from schistosomiasis patients were obtained from Northern Samar, the Philippines. For the first serum cohort (n = 180), 67 and 113 patients were confirmed as positively and negatively infected, respectively, in 2012 by examining eggs in stool samples using the Kato-Katz method. The second serum batch (n = 412) were obtained from the same endemic area in 2015, two years after a program of mass drug (i.e., 40 mg/kg praziquantel) administration. Of these, 108 and 304 patients were confirmed as positive and negative infection, respectively, using the Kato-Katz method (Olveda et al., 2017). Serum samples of healthy individuals were obtained from Heilongjiang Province, a non-endemic area for schistosomiasis in China.
2.7. Evaluation of Diagnostic Candidates for Schistosomiasis Japonica by ELISA
Recombinant proteins were quantified by the bicinchoninic acid assay (BCA assay) (ThermoFisher Scientific, MA, USA). For determining IgG levels induced in SjC or SjP-infected BALB/c mice, all recombinant proteins were diluted to a final concentration of 1 μg/mL with coating buffer overnight at 4 °C with 100 μL added per well. All wells were blocked by blocking buffer (1% BAS in PBST) at 37 °C for 1 h. All tested serum samples were diluted at 1:102 with blocking buffer (100 μL/well) and incubated at 37 °C for 1 h. A biotin-SP-conjugated goat anti-mouse IgG (Fc specific)-biotin antibody (Jackson ImmunoResearch, PA, USA) was used as secondary antibody (1:10,000, 100 μL/well) and samples were incubated for 1 h at 37 °C. Streptavidin-HRP (BD Pharmingen, CA, USA) (1:10,000) was then applied to each well (100 μL/well). PBST washes were applied 5 times after each step, 2 min between each step. Reactions were developed using TMB substrate (100 μL/well) for 5 min and stopped using 2 M sodium hydroxide (50 μL/well). Optical density (OD) values were read at 450 nm using a microplate reader, and all tests were run in duplicate on each test plate. A positive antibody response was defined as an OD value higher than 2.1 times the mean of OD values of the serum samples from control mice. To determine IgG titers against the ten antigens in SjP-infected BALB/c mice at 7 weeks p.i., the same procedure was applied except that a series of serum dilutions (1:102, 1:103, 1:104, 1:105, and 1:106) were used.
For small scale assays, determining the level of human IgG generated against these antigens, all procedures were similar to those described above except that all recombinant proteins were diluted to a final concentration of 1 μg/mL, serum samples were used at two dilutions (1:102 and 1:103) and a mouse monoclonal anti-human IgG (Fc specific)-biotin antibody (Sigma-Aldrich Co, MO, USA) was employed as secondary antibody (1:20,000, 100 μL/well). In large scale assays with the first human cohort, four dilutions of serum samples (1:100, 1:250, 1:500 and 1:1000) were tested for the detection of IgG antibodies against Sj23-LHD, SjSAP4, SjSAP5, SjSAP4 plus Sj23-LHD, and SjSAP5 plus Sj23-LHD. For the latter two combinations, 50 ng of each antigen were mixed per well. With the second human cohort, only one optimized serum dilution (1:250) was used to detect IgG antibodies against these antigens or antigen combinations. A positive antibody response was defined as an OD value higher than 2.1 times the mean of OD values of the control serum samples from healthy individuals.
2.8. Statistical Analysis
All results are reported as means ± SEM (standard error of the mean). For analysis of the IgG levels generated against the ten antigens during the SjC- or SjP-infection course, one-way ANOVA followed by Holm-Sidak multiple comparison was used. For analysis of IgG levels at each time point between the SjC- and SjP-infection in the BALB/c mice, two-way ANOVA followed by Holm-Sidak multiple comparisons were used to compare statistical differences. For analysis of the worm burden between the SjC and SjP-infections in the BALB/c mice, the Man-Whitney test was used. P-values of < 0.05 were considered statistically significant. We defined sensitivity and specificity according to the following formulae: sensitivity = number of true positives / (number of true positive + number of false negatives) and specificity = number of true negatives / (number of false positives + number of true negatives).
3. Results
3.1. General Information for the 10 Selected Proteins
Five secretory (SjSP-13, SjSAP4, SjSAP5, SjSP-163 and SjSP-189) and five tegumental-associated (Sj23-LHD, SjSTIP1 (stress-induced phosphoprotein 1), SjPPase (pyrophosphatase), SjPGM (phosphoglycerate mutase) and SjRAD23 (radiation sensitive 23)) proteins were selected for comparison as antigen candidates based on previous studies of SjC (Table 1). The DNA sequences encoding these antigens were cloned from a cDNA library of SjP and protein sequences deduced. Comparative sequence analysis indicated SjSP-189 shared 90% with the SjC homologue, whereas the other nine antigens shared 98–100% identity between the two strains (Table 1).
Table 1.
Ten S. japonicum antigen candidates selected for comparison.
| Antigen | Accession No. | Sequence identitya | References |
|---|---|---|---|
| SjSP-13 | AY222880 | 98%b | (Liu et al., 2016; Xu et al., 2014) |
| SjSAP4 | FN315320 | 99%b | (Liu et al., 2016) |
| SjSAP5 | AY222887 | 99%b | (Liu et al., 2016) |
| SjSP-163 | AY814943 | 98%b | (Xu et al., 2014) |
| SjSP-189 | AY815838 | 90%b | (Xu et al., 2014) |
| Sj23-LHD | M63706 | 100% | (Jiang et al., 2010; Jin et al., 2010; Wang et al., 2011b) |
| SjSTIP1 | AY816102 | 99% | (Chen et al., 2014) |
| SjPPase | AY814211 | 99% | (Chen et al., 2014) |
| SjPGM | FN315287 | 100% | (Zhang et al., 2015) |
| SjRAD23 | FN314619 | 99% | (Zhang et al., 2015) |
Between the SjP and SjC orthologs.
Signal peptide sequence excluded.
3.2. Transcriptional Profiling of the ten Antigens in four Developmental Stages
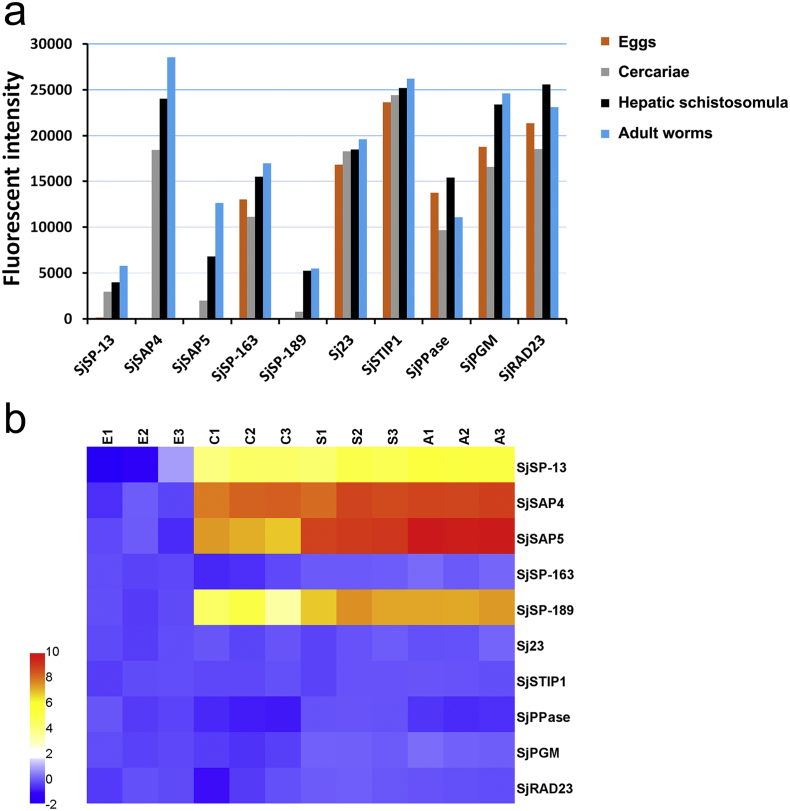
The expression levels of the 10 S. japonicum antigen candidates in eggs, cercariae, hepatic schistosomula and adult worms were further analyzed based on data extracted from a next-generation schistosome DNA microarray dataset. SjSP-13 and SjSAP4 were mainly expressed in cercariae, hepatic schistosomula and adults; SjSAP5 and SjSP-189 were most highly expressed in hepatic schistosomula and adults; while one secretory protein, SjSP-163, and five tegumental-associated antigens were consistently expressed across all four stages (Fig. 1a,b).
Fig. 1.
Expression profiles of the 10 S. japonicum antigens in the four developmental stages. (a) The transcriptional profiles of the antigen candidates. The data were extracted from an available next-generation schistosome microarray dataset (Cai et al., 2017). The column represents the mean of normalized fluorescent intensity value. (b) Heatmap showing the relative transcriptional levels of the 10 S. japonicum antigen candidates in: E, eggs; C, cercariae; S, hepatic schistosomula; A, adult worms.
3.3. Production of Recombinant Proteins
The gene sequences of the ten proteins were confirmed as correct by DNA sequencing. The ten recombinant proteins were successfully expressed in E. coli. Four and six recombinant proteins were purified under native and denaturing conditions, respectively (Supplementary Fig. 1). The ten purified antigens were further probed by Western blotting with an anti-his-tag antibody.
3.4. Kinetics of Specific IgG Antibodies in the Sera of S. japonicum-infected Mice
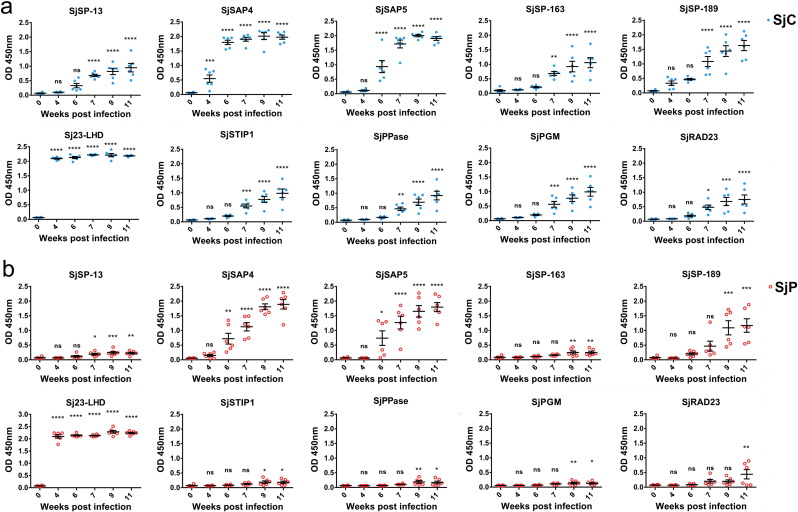
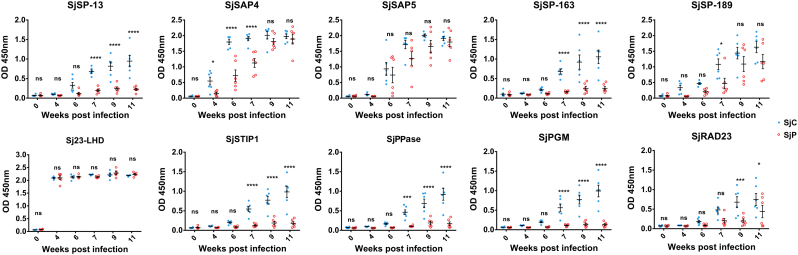
Serum samples from six infected mice were collected prior to infection (0 week) and at 4, 6, 7, 9 and 11 weeks p.i. with SjC or SjP cercariae. The time point when specific IgG became elevated during the course of infection against the ten antigens varied among the different candidates. In SjC-infected mice, significant increases in IgG antibody levels against Sj23-LHD and SjSAP4 were detected from 4 weeks p.i. (1-Way ANOVA, P < 0.0001 and P < 0.001, respectively) and subsequently, and a significant rise in IgG antibody level against SjSAP5 occurred from 6 weeks p.i. onwards (1-Way ANOVA, P < 0.0001); with the remaining seven antigens, a significant increase in specific IgG was observed from 7 weeks p.i. (Fig. 2a). During the course of SjP infection, the IgG antibody level against Sj23-LHD rose significantly from 4 weeks p.i. onwards (1-Way ANOVA, P < 0.0001). A significant increase in SjSAP4- and SjSAP5-specific IgG antibody levels was observed at 6 weeks p.i. (1-Way ANOVA P < 0.01, and P < 0.05, respectively) (Fig. 2b). The IgG levels against SjSP-13 and SjRAD23 increased significantly from 7 weeks p.i. (1-Way ANOVA P < 0.05) and 11 weeks p.i. (1-Way ANOVA P < 0.01), respectively. With the remaining antigens, a significant increase in specific IgG levels was found at 9 weeks p.i. onwards (Fig. 2b). We carried out comparative analysis of IgG responses during the course of the SjC and SjP infections. These were no differences detected in IgG responses against either Sj23-LHD or SjSAP5 in mice infected with SjC or SjP for any of the time points selected. SjSAP4 elicited a significantly higher level of IgG in the SjC-infected mice from 4 to 7 weeks p.i. Significantly higher IgG levels were induced against SjSP-189 and SjRAD23 in SjC-infected mice at 7 and 9 weeks p.i., while with the other antigens, significantly higher IgG levels were induced in these mice from 7 weeks p.i. onwards (Fig. 3). There was no difference in the worm burden between the SjC- and SjP-infections (Supplementary Fig. 2).
Fig. 2.
IgG responses against the 10 antigens during the course of S. japonicum infection in BALB/c mice. (a) SjC and (b) SjP infection. Statistical significance between infected and naive mice was determined using 1-Way ANOVA. (* = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001, ns = no significant difference). Serum dilution: 1:100.
Fig. 3.
Comparison of IgG responses against the 10 antigens in BALB/c mice infected with SjC or SjP. Statistical significance was determined using 2-Way ANOVA. (* = P < 0.05, *** = P < 0.001, **** = P < 0.0001, ns = no significant difference).
3.5. Determination of IgG Titers against the 10 Antigens in the Sera of SjP-infected BALB/c Mice
We then determined the IgG titer against the 10 antigens in the sera of SjP-infected BALB/c mice at 7 weeks p.i. We found that the highest IgG titer (between 1:104 and 1:106) was raised against Sj23-LHD. High IgG titers (between 1:103 and 1:104) were also induced by SjSAP4 and SjSAP5. With SjSP-189, the IgG titer was between 1:102 and 1:103. Consistent with the results obtained for the IgG kinetics during SjP infection of BALB/c mice, the remaining antigens elicited low IgG titers. Accordingly, Sj23-LHD appeared to be the most sensitive antigen candidate for the diagnosis of schistosomiasis japonica (Supplementary Table 2).
3.6. Initial Evaluation of the Sensitivity of the 10 Antigens for the Diagnosis of Human SjP Infection
For initial screening of the 10 antigens, we used nine serum samples from SjP-infected patients and three healthy individuals as controls. Six antigens exhibited seropositivity being detected at a serum dilution of 1:100, among which SjSAP4 and SjSAP5 exhibited the highest sensitivity, followed by SjSP-13. Unexpectedly, Sj23-LHD-ELISA showed a moderate sensitivity of 55.5%, and four other tegumental proteins, SjSTIP1, SjPPase, SjPGM and SjRAD23, were seronegative with these serum samples in ELISA (Table 2).
Table 2.
Reactivity of the six selected proteins with 9 serum samples from SjP infected patients with varying infection intensities.
| No. | EPG | Antigens (R value) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SjSP-13 | SjSAP4 | SjSAP5 | SjSP-163 | SjSP-189 | Sj23-LHD | ||||||||
| 1 | 43.3 | 3.92 | 2.75 | 20.79 | 3.36 | 11.67 | 5.71 | 1.66 | 0.99 | 1.62 | 1.03 | 1.51 | 0.91 |
| 2 | 10.0 | 4.73 | 2.33 | 31.81 | 16.47 | 9.62 | 5.38 | 2.09 | 1.10 | 7.82 | 1.65 | 5.82 | 1.23 |
| 3 | 6.7 | 1.72 | 1.06 | 31.51 | 11.01 | 4.93 | 3.70 | 1.27 | 1.01 | 15.65 | 3.68 | 9.70 | 1.47 |
| 4 | 2750.0 | 2.75 | 1.39 | 31.51 | 19.14 | 9.96 | 5.42 | 1.12 | 1.07 | 9.31 | 1.79 | 20.20 | 2.64 |
| 5 | 323.3 | 1.69 | 1.07 | 27.32 | 6.00 | 11.27 | 3.97 | 1.72 | 1.21 | 1.85 | 1.06 | 1.31 | 0.92 |
| 6 | 23.3 | 2.09 | 1.23 | 27.28 | 6.55 | 5.00 | 1.98 | 0.81 | 0.93 | 0.93 | 1.01 | 0.94 | 0.97 |
| 7 | 3.3 | 4.63 | 1.81 | 27.49 | 6.62 | 12.46 | 5.52 | 2.49 | 1.02 | 2.24 | 1.05 | 1.43 | 0.93 |
| 8 | 186.7 | 5.47 | 3.37 | 31.98 | 24.67 | 13.39 | 9.48 | 3.84 | 1.12 | 3.33 | 1.15 | 4.86 | 1.04 |
| 9 | 650.0 | 5.20 | 3.40 | 28.13 | 14.70 | 12.41 | 5.34 | 3.44 | 1.05 | 3.54 | 1.33 | 11.58 | 1.78 |
| Sensitivity | 66.7% | 44.4% | 100% | 100% | 100% | 88.9% | 33.3% | 0% | 66.7% | 11.1% | 55.5% | 11.1% | |
| Serum dilution | 1:102 | 1:103 | 1:102 | 1:103 | 1:102 | 1:103 | 1:102 | 1:103 | 1:102 | 1:103 | 1:102 | 1:103 | |
R value = OD450patient/Mean of OD450healthy.
Serum samples with R value ≥ 2.1 were considered as seropositive.
EPG: eggs per gram of faeces.
3.7. Comparison of Sj23-LHD-, SjSAP4-, SjSAP5-, SjSAP4 + Sj23-LHD- and SjSAP5 + Sj23-LHD-ELISA for Diagnosis of SjP Infection in the First Patient Cohort
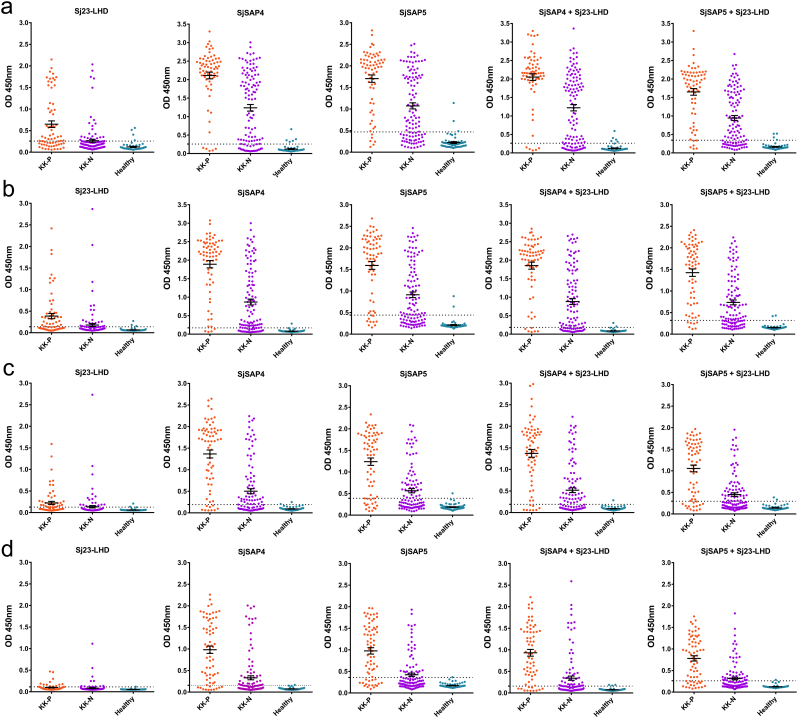
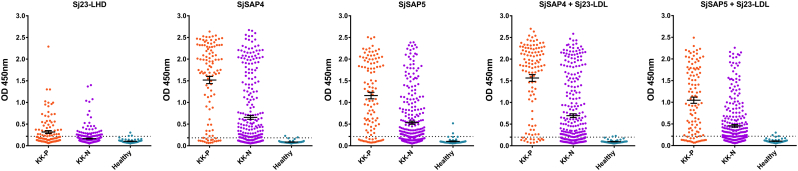
Three antigens (Sj23-LHD, SjSAP4 and SjSAP5) were thus selected for diagnosing clinical serum samples on a larger scale. Sj23-LHD was included due to its earlier diagnostic performance, whereas SjSAP4 and SjSAP5 were selected as a result of their high sensitivity in the diagnosis of SjP infection. Also, SjSAP4 + Sj23-LHD and SjSAP5 + Sj23-LHD, were employed to determine whether these two combinations could achieve a better diagnostics performance than the single antigens employed alone. To obtain optimized assay conditions, a series of serum dilutions (1:100, 1:250; 1:500 and 1:1000) was used, since a highly sensitive biotin-streptavidin system was employed in the ELISA (Fig. 4a,b,c,d). After testing the serum samples from the first cohort, we found that 1:250 was the best dilution to achieve a balance of sensitivity and specificity of the assays (Table 3). With this dilution, the SjSAP4-ELISA showed the best performance with a sensitivity of 94.03% for KK-positives and of 72.57% for KK-negatives, and a specificity of 98.33%. This was followed by the SjSAP4 + Sj23-LHD-ELISA, exhibiting 91.04% sensitivity for KK-positives and 67.26% sensitivity for KK-negatives, and a specificity of 96.67%. The Sj23-LHD-ELISA showed quite moderate sensitivity (53.73% for KK-positives and 21.24% for KK-negatives), yet a high specificity (95.00%) (Fig. 4b, Table 3).
Fig. 4.
Scatter plots showing the responses of Sj23-LHD-, SjSAP4-, SjSAP5-, SjSAP4 + Sj23-LHD- and SjSAP5 + Sj23-LHD-ELISA for the diagnosis of schistosomiasis japonica. Different serum dilution (a) 1:100, (b) 1:250; (c) 1:500; (d) 1:1000 were used for optimization. ‘KK-P’: Kato-Katz positive; ‘KK-N’: Kato-Katz negative.
Table 3.
Sensitivity and specificity of Sj23-LHD-, SjSAP4-, SjSAP5-, SjSAP4 + Sj23-LHD- and SjSAP5 + Sj23-LHD-ELISA tested against 180 serum samples from the first SjP patient cohort.
| Serum dilution | Antigens | Sensitivity (KK-positive n = 67) | Sensitivity (KK-negative n = 113) | Specificity |
|---|---|---|---|---|
| 1:100 | Sj23-LHD | 61.19% | 22.12% | 93.33% |
| SjSAP4 | 92.54% | 75.22% | 91.67% | |
| SjSAP5 | 91.04% | 66.37% | 95.00% | |
| SjSAP4 + Sj23-LHD | 92.54% | 76.99% | 91.67% | |
| SjSAP5 + Sj23-LHD | 91.04% | 68.14% | 93.33% | |
| 1:250 | Sj23-LHD | 53.73% | 21.24% | 95.00% |
| SjSAP4 | 94.03% | 72.57% | 98.33% | |
| SjSAP5 | 85.07% | 61.95% | 96.67% | |
| SjSAP4 + Sj23-LHD | 91.04% | 67.26% | 96.67% | |
| SjSAP5 + Sj23-LHD | 89.55% | 65.49% | 96.67% | |
| 1:500 | Sj23-LHD | 46.27% | 16.81% | 96.67% |
| SjSAP4 | 86.57% | 50.44% | 96.67% | |
| SjSAP5 | 76.12% | 45.13% | 98.33% | |
| SjSAP4 + Sj23-LHD | 88.06% | 53.10% | 98.33% | |
| SjSAP5 + Sj23-LHD | 79.10% | 46.90% | 96.67% | |
| 1:1000 | Sj23-LHD | 19.40% | 10.62% | 98.33% |
| SjSAP4 | 83.58% | 38.05% | 98.33% | |
| SjSAP5 | 74.63% | 33.63% | 98.33% | |
| SjSAP4 + Sj23-LHD | 82.09% | 39.82% | 95.00% | |
| SjSAP5 + Sj23-LHD | 73.13% | 37.17% | 98.33% |
3.8. Comparison of Sj23-LHD-, SjSAP4-, SjSAP5-, SjSAP4 + Sj23-LHD- and SjSAP5 + Sj23-LHD-ELISA for Diagnosis of SjP Infection in the Second Patient Cohort
To validate the results, we analyzed serum samples from a larger size cohort with lower intensity S. japonicum infections. A comparison of the infection intensities based on EPG between the first and the second cohort is presented in Table 4. With the second cohort, we observed reduced sensitivity, regardless of which antigen or antigen combination was used. In contrast to the results obtained with sera from the first cohort, the SjSAP4 + Sj23-LHD-ELISA exhibited the best diagnostic performance with a sensitivity of 87.04% for KK-positives and 58.55% for KK-negatives, and a specificity of 96.67%, followed by the SjSAP4-ELISA, which showed a sensitivity of 84.26% for KK-positives and of 56.25% for NN-negatives, and a specificity of 96.67% (Fig. 5, Table 5). For KK-negatives, a relatively high positivity rate was observed with the SjSAP4-ELISA (56.25%), the SjSAP5-ELISA (51.64%), the SjSAP4 + Sj23-LHD-ELISA (58.55%) and the SjSAP5 + Sj23-LHD-ELISA (49.01%) (Fig. 5, Table 5). Stool and serum samples, collected from the same cohort, were subjected to a sensitive droplet digital PCR (ddPCR) assay, which detects Sjnad1, a cell-free DNA marker for diagnosis of SjP infection (Weerakoon et al., 2017). A positive ddPCR rate of 66.12% and 57.57% were obtained for KK-negatives when testing the stool and serum samples, respectively (Supplementary Table 3).
Table 4.
Comparison of S. japonicum infection intensity in the two patient cohorts.
| EPG | 1st cohort (KK positive n = 67) n (percentage) |
2nd cohort (KK positive n = 108) n (percentage) |
|---|---|---|
| > 400 | 5 (7.46%) | 0 (0.00%) |
| 100–400 | 13 (19.40%) | 4 (3.70%) |
| 40–99 | 5 (7.46%) | 8 (7.41%) |
| 10–39 | 21 (31.34%) | 18 (16.67%) |
| < 10 | 23 (34.33%) | 78 (72.22%) |
EPG: eggs per gram of faeces.
Fig. 5.
Scatter plots showing the responses of the Sj23-LHD-, SjSAP4-, SjSAP5-, Sj23-LHD + SjSAP4- and Sj23-LHD + SjSAP5-ELISAs for the diagnosis of schistosomiasis japonica with serum samples from the second patient cohort. A serum dilution of 1:250 was used in the assays. ‘KK-P’: Kato-Katz positive; ‘KK-N’: Kato-Katz negative.
Table 5.
Sensitivity and specificity of the Sj23-LHD-, SjSAP4-, SjSAP5-, Sj23-LHD + SjSAP4- and Sj23-LHD + SjSAP5-ELISA for diagnosis of 412 serum samples from the second patient cohort.
| Serum dilution | Antigens | Sensitivity (KK-positive n = 108) |
Sensitivity (KK-negative n = 304) |
Specificity |
|---|---|---|---|---|
| 1:250 | Sj23-LHD | 42.59% | 18.09% | 95.00% |
| SjSAP4 | 84.26% | 56.25% | 96.67% | |
| SjSAP5 | 80.56% | 51.64% | 96.67% | |
| SjSAP4 + Sj23-LHD | 87.04% | 58.55% | 96.67% | |
| SjSAP5 + Sj23-LHD | 80.56% | 49.01% | 95.00% |
3.9. Antibody Responses against Sj23-LHD, SjSAP4 and SjSAP5 before and after Drug Treatment in a Mouse Model
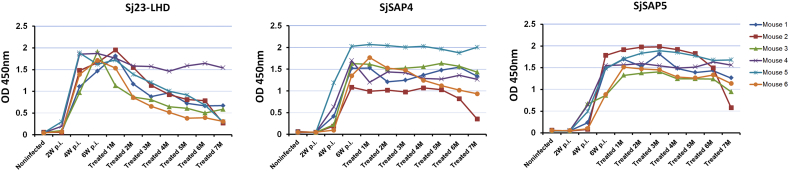
To compare the duration of Sj23-LHD-, SjSAP4- and SjSAP5-specific IgG antibodies post chemotherapy, six Swiss mice were infected with cercariae of the SjP strain, and after 6 weeks, all mice were treated with praziquantel for 5 consecutive days. The levels of IgG antibodies to the three antigens were determined at 2, 4, and 6 weeks p.i. and 1–7 months after completion of the drug treatment in the mice. In five of the six mice, the Sj23-LHD-specific IgG antibody started to drop at 2 months post drug treatment and decreased to relatively low levels at 7 months post-chemotherapy. In only one out of six mice, did the levels of SjSAP4- and SjSAP5-specific IgG antibodies decrease at 7 months post-chemotherapy (Fig. 6).
Fig. 6.
ELISA detection of Sj23-LHD, SjSAP4- and SjSAP5-specific IgG antibodies in the sera of Swiss mice prior to and post drug treatment (Serum dilution for Sj23-LHD-, SjSAP4- and SjSAP5-ELISA: 1:10,000).
4. Discussion
While remarkable success has been achieved in combating schistosomiasis japonica in China, it is still a devastating disease in the Philippines with high prevalences and morbidity reported in several provinces (Olveda et al., 2016; Rollinson et al., 2013; Xu et al., 2016). A major obstacle preventing its effective management in the Philippines may, in part, be due to the scarcity of affordable and accurate diagnostic tools and ELISA-based serological diagnosis remains an attractive option to achieve this goal. Currently, antigens for diagnosis of S. japonicum infection predominantly use antigens identified in the SjC strain. A validation was thus required before applying SjC antigen candidates for the diagnosis of SjP infections which we report here.
In this study, we used serum samples from mice and patients to compare the diagnostic performance of 10 antigen candidates selected based on studies undertaken on SjC. However, murine models of schistosomiasis cannot completely reflect authentic immunoreactivities in patients. Firstly, the levels of IgG antibodies induced by the 10 antigens were only observed for 11 weeks p.i. since the BALB/c mouse infections were terminated for ethical reasons. Secondly, in mice, the presence of only one worm pair represents a high infection dose if body weight is taken into consideration. In murine models of schistosomiasis, the dynamic IgG levels against different antigens vary during the course of infection. Sj23, being a tegumental antigen, is exposed to the host directly after invasion and induces a rapid immune response as was observed here and in previous investigations (Jiang et al., 2010; Krautz-Peterson et al., 2017), and has been suggested as a potential diagnostic target for early detection (Wang et al., 2011b). We further showed that, following drug treatment, that the level of Sj23-LHD-specific IgG antibodies dropped more rapidly than those generated against SjSAP4 and SjSAP5. Theoretically, the diagnostic performance of Sj23-LHD should have been superior to SjSAP4 and SjSAP5 based on the results obtained in mice. However, this was not the case when clinical serum samples were tested. The low sensitivity of the Sj23-LHD-ELISA, using serum samples from KK-positive subjects, indicates that Sj23-LHD-specific IgG levels may decline in patients during the late course of an infection, since the infection intensity is lower than in mice. In contrast, the two saposin family members, SjSAP4 and SjSAP5, being intestinal tract secretory proteins (Liu et al., 2016), may be released slowly into the host circulation thereby inducing longer lasting antibody responses.
Differing results were obtained when testing serum samples from the two human Philippines cohorts. Firstly, diminished sensitivity was observed when the second cohort was tested, regardless of which antigen or antigen combination was used. This is likely due to the comparatively lower S. japonicum infection intensity levels of the second cohort compared with the first cohort based on the egg burden of those individuals positive by the Kato-Katz procedure. In contrast to the first cohort, where about 30% KK-positive individuals had an EPG < 10, this number was > 70% in the second cohort. Secondly, with the first cohort, the SjSAP4-ELISA exhibited the best diagnostic performance, followed by the SjSAP4 + Sj23-LHD-ELISA, whereas this diagnostic outcome was reversed with the second cohort. The reason for including Sj23-LHD as a diagnostic marker was to contribute to the detection of individuals with early infections of S. japonicum. We did find individuals in the second cohort showing positive serological reactivity against Sj23-LHD, but negative against SjSAP4 and SjSAP5. Mass praziquantel drug administration had been applied to the second cohort 2 years prior to blood collection, but re-infection may have occurred subsequently in these individuals. This reflects the current epidemiological picture in S. japonicum-endemic areas of the Philippines (Inobaya et al., 2015), which strengthens the case for including Sj23-LHD to increase the sensitivity of available diagnostic assays and the earlier detection of schistosomiasis which no doubt will contribute to its monitoring and control.
For KK-negative individuals, relatively high positivity rates were observed in the ELISAs, which necessitated using an alternative diagnostic tool to validate these serological results. Detection of cell-free DNA (cfDNA) has proven a reliable and sensitive diagnostic tool for human parasitic infections (Weerakoon and McManus, 2016). Accordingly, we thus employed a novel ddPCR developed by our group (Weerakoon et al., 2017) to probe serum samples from the second cohort, and found similar positivity rates when the serum samples were tested by this method (Supplementary Table 3), which substantiates the serology data we report. It is noteworthy that conflicting results regarding the duration of detectable schistosome cfDNA in mammalian hosts after drug treatment have been reported. It has been shown by one group that DNA (a 230-bp fragment of the highly repetitive retrotransposon SjR2 of S. japonicum) was undetectable by conventional PCR in the serum of SjC-infected rabbits 10 weeks after a single PZQ treatment (Xia et al., 2009). In contrast, another group reported that the time to total elimination of Schistosoma-derived cell-free DNA fragments from plasma following treatment was projected to exceed one year (Wichmann et al., 2009). These conflicting studies are cautionary, suggesting that the time point when the detection of parasite-derived cfDNA becomes negative after chemotherapy depends on multiple factors, such as infection intensity, the concentration of the target cfDNA, the sample volume for DNA extraction, and the sensitivity of the PCR method used. We propose that the KK-negatives may have harboured a low intensity infection (likely due to unsatisfactory treatment/drug compliance issues (Olveda et al., 2016)), in the early stage of a re-infection or antibodies against the target antigens remained from a previous infection.
For several of the selected antigens we found inconsistent diagnostic performance for detecting SjC or SjP infection both with mouse and human serum samples. We found that in mice there was no significant difference in worm burden between the SjC and SjP infections, but the IgG responses induced by SjSP-13, SjSP-163, SjSTIP1, SjPPase and SjPGM were significantly different from 7 weeks p.i. onwards in the two types of infection (Fig. 3). Given these antigens are expressed in the gastrodermis or tegument, the process of vesicle-mediated transport may play an important role in the localization of these proteins. Previously, it has been shown that some of the components associated with vesicle-mediated transport have lower copy number in SjP compared with SjC worms (Gobert et al., 2013), which may impact on the exposure of these antigens to the host. Further, the rSjSTIP1- and rSjPPase-ELISAs showed a sensitivity of 80% and 90%, respectively, on testing a small number of patient sera with SjC-infection (Chen et al., 2014). rSjPGM- and rSjRAD23-ELISA achieved a sensitivity of 91.35% and 88.46%, respectively, when sera from SjC-infected buffaloes were tested (Zhang et al., 2015). However, these antigens had no diagnostic value for detecting SjP infection. These differing results may have been due to the variable S. japonicum infection intensities among the different samples. But it is more likely to reflect differences in the host response against the two S. japonicum strains. In addition, the rSj23-LHD-ELISA exhibited 89.9% sensitivity when probing sera of SjC-infected bovines (Jin et al., 2010), but here, a sensitivity of only 42.59–53.73% was achieved when sera from SjP patients were tested. The reactivity of SjSP-13 with sera from humans infected with SjC parasites previously showed circa 90% sensitivity (Liu et al., 2016; Xu et al., 2014), whereas a sensitivity of only 66.7% was achieved when tested with 9 sera from SjP-infected individuals. These findings emphasize the importance of validating SjC antigen homologues for the diagnosis of SjP infections.
Further, the poor diagnostic performance of SjSP-163, SjSP-189, SjSTIP1, SjPPase, SjPGM and SjRAD23 triggered us to re-consider the criteria for candidate antigen selection based on their subcellular localization, relative expression levels and biological functions. SjSP-163, being a putative orthologue of the endoplasmic reticulum-residing protein, nucleotide exchange factor SIL1, acting as a molecular chaperone facilitating protein folding, translocation and degradation (Yan et al., 2011), may have less chance to be exposed to the host immune system. The transcript level of SjSP-189 in the intravascular stage was similar to that of SjSP-13, but lower than those of other antigens (Fig. 1). STIP1 acts as a co-chaperone of Hsp70 and Hsp90 (Cross et al., 2016), and the subcellular localization of schistosome TIP1 was suggested by the results of a previous proteomic study showing that Hsp70 and Hsp90 are cytosolic proteins in Schistosoma bovis (Perez-Sanchez et al., 2008). Inorganic PPases are highly conserved enzymes responsible for catalyzing the hydrolysis of inorganic pyrophosphate (PPi) into two molecules of orthophosphate (Pi), providing a thermodynamic driving force for a variety of biosynthetic reactions, such as DNA, RNA, protein, polysaccharide, and lipid synthesis (Guimier et al., 2016). There are two groups of PPases: soluble PPases and membrane-bound PPases, but the latter only occurs in algae, plants, and selected species of protozoan parasites, bacteria and archaea (Nita et al., 2016). PGM is involved in the energy metabolism required for parasite activity and survival, and it has been found that the PGM ortholog in S. bovis is localized to the cytosol (Perez-Sanchez et al., 2008). RAD23 is known to be associated with DNA-damage repair (Ng et al., 2003), so it seems most likely that SjRAD23 may be located in the nuclei of the subtegumental cell bodies of S. japonicum. Although these latter four antigens (SjSTIP1, SjPPase, SjPGM and SjRAD23) have been suggested to be tegument-associated (Chen et al., 2014; Zhang et al., 2015), they may not be directly exposed to the host vascular system. Tegumental debris shed from the blood flukes may be the source of these antigens resulting in the induction of low levels of antibodies. Consequently, the most important selection criteria for diagnosis are that a candidate antigen should be continuously released into or exposed to the bloodstream of the host by an intact viable parasite and that it has a relatively high level of expression and antigenicity.
An inevitable dilemma in using serology for schistosomiasis diagnosis is its limited ability to distinguish between ongoing and previous schistosome infections. Here we investigated IgG titers generated against Sj23-LHD, SjSAP4 and SjSAP5 over several months in PZQ-cured mice previously infected with S. japonicum. While IgG responses to Sj23-LHD declined with time after treatment, those to SjSAP4 and SjSAP5 in the main did not (Fig. 6). As mentioned earlier, clinical infections in the Philippine are now mostly low intensity, so IgG titers against SjSAP4 and SjSAP5 in cured patients may not last as long as those observed in mice, but this still needs to be evaluated. In this context, further steps (such as adjusting the ratio of antigens in the combination ELISA) to optimize the assay to improve the ability to distinguish active from past infections are warranted.
In summary, our findings highlight the fact that during the course of a SjC and SjP infection, different IgG levels may be induced by antigen orthologs. Three antigens, Sj23-LHD, SjSAP4 and SjSAP5, elicited high IgG titers in both SjC- and SjP-infected BALB/c mice. SjSAP4 exhibited the best diagnostic performance when probing sera from the first SjP patient cohort with relatively high infection intensity. With the second cohort, where the majority of infections were of low intensity and early stage infection may have occurred, the combination of SjSAP4 + Sj23-LHD yielded the best diagnostic performance. This combination could be used in future for serological diagnosis of schistosomiasis japonica in the Philippines, thereby representing an important component for monitoring and sustaining integrated control measures.
Financial Support
This research was funded by the National Health and Medical Research Council (NHMRC) of Australia (ID APP1102926, APP1037304 and APP1098244). DPM is a NHMRC Senior Principal Research Fellow and Senior Scientist at QIMRB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
All authors: No reported conflicts.
Author Contribution
P.C. and D.P.M. conceptualized the study design and directed the project; P.C. and Y.M. performed all the experiments; P.C., S.L., X.P. and Q.C. contributed to the DNA chip data; K.G.W., D.U.O., X.P., R.M.O., Q.C. and A.G.R. contributed to the collection of clinical samples; P.C., K.G.W., Y.M. and D.P.M. analyzed, reviewed and interpreted the data; P.C. drafted and D.P.M. revised the manuscript. All authors approve of the final version of the manuscript.
Acknowledgments
Acknowledgements
We thank Mary Duke for maintenance of the S. japonicum lifecycle at QIMR Berghofer Medical Research Institute (QIMRB). We also thank the local field and clinical staff in Palapag, Northern Samar for their kind assistance in the collection of the clinical samples.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.09.011.
Contributor Information
Pengfei Cai, Email: Pengfei.Cai@qimrberghofer.edu.au.
Donald P. McManus, Email: Don.McManus@qimrberghofer.edu.au.
Appendix A. Supplementary data
Supplementary material
References
- Cai P., Gobert G.N., You H., Duke M., McManus D.P. Circulating miRNAs: potential novel biomarkers for hepatopathology progression and diagnosis of schistosomiasis japonica in two murine models. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai P., Gobert G.N., McManus D.P. MicroRNAs in parasitic helminthiases: current status and future perspectives. Trends Parasitol. 2016;32:71–86. doi: 10.1016/j.pt.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Cai P., Liu S., Piao X., Hou N., Gobert G.N., McManus D.P., Chen Q. Comprehensive transcriptome analysis of sex-biased expressed genes reveals discrete biological and physiological features of male and female Schistosoma japonicum. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai P., Liu S., Piao X., Hou N., You H., McManus D.P., Chen Q. A next-generation microarray further reveals stage-enriched gene expression pattern in the blood fluke Schistosoma japonicum. Parasit. Vectors. 2017;10:19. doi: 10.1186/s13071-016-1947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.H., Zhang T., Ju C., Xu B., Lu Y., Mo X.J., Chen S.B., Fan Y.T., Hu W., Zhou X.N. An integrated immunoproteomics and bioinformatics approach for the analysis of Schistosoma japonicum tegument proteins. J. Proteome. 2014;98:289–299. doi: 10.1016/j.jprot.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Chuah C., Jones M.K., McManus D.P., Nawaratna S.K., Burke M.L., Owen H.C., Ramm G.A., Gobert G.N. Characterising granuloma regression and liver recovery in a murine model of schistosomiasis japonica. Int. J. Parasitol. 2016;46:239–252. doi: 10.1016/j.ijpara.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Collins C., Xu J., Tang S. Schistosomiasis control and the health system in P.R. China. Infect. Dis. Poverty. 2012;1:8. doi: 10.1186/2049-9957-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Klepzig E., Dallaston M., Young N.D., Bailey U.M., Mason L., Jones M.K., Gasser R.B., Hofmann A. Exploring the molecular mechanisms of parasite-host interactions with a view towards new therapeutics and vaccines. Postepy Biochem. 2016;62:370–376. [PubMed] [Google Scholar]
- Gobert G.N., You H., Jones M.K., McInnes R., McManus D.P. Differences in genomic architecture between two distinct geographical strains of the blood fluke Schistosoma japonicum reveal potential phenotype basis. Mol. Cell. Probes. 2013;27:19–27. doi: 10.1016/j.mcp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Gordon C.A., Acosta L.P., Gobert G.N., Olveda R.M., Ross A.G., Williams G.M., Gray D.J., Harn D., Li Y., McManus D.P. Real-time PCR demonstrates high prevalence of Schistosoma japonicum in the Philippines: implications for surveillance and control. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimier A., Gordon C.T., Godard F., Ravenscroft G., Oufadem M., Vasnier C., Rambaud C., Nitschke P., Bole-Feysot C., Masson C., Dauger S., Longman C., Laing N.G., Kugener B., Bonnet D., Bouvagnet P., Di Filippo S., Probst V., Redon R., Charron P., Rotig A., Lyonnet S., Dautant A., de Pontual L., di Rago J.P., Delahodde A., Amiel J. Biallelic PPA2 mutations cause sudden unexpected cardiac arrest in infancy. Am. J. Hum. Genet. 2016;99:666–673. doi: 10.1016/j.ajhg.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope M., Duke M., McManus D.P. A biological and immunological comparison of Chinese and Philippine Schistosoma japonicum. Int. J. Parasitol. 1996;26:325–332. doi: 10.1016/0020-7519(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Inobaya M.T., Olveda R.M., Tallo V., McManus D.P., Williams G.M., Harn D.A., Li Y., Chau T.N., Olveda D.U., Ross A.G. Schistosomiasis mass drug administration in the Philippines: lessons learnt and the global implications. Microbes Infect. 2015;17:6–15. doi: 10.1016/j.micinf.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Jiang N., Cai P., Yin J., Hao L., Lu H., Wang X., Wang H., Chen Q. Characterization of antibody responses to the Sj23 antigen of Schistosoma japonicum after infection and immunization. Acta Trop. 2010;116:9–14. doi: 10.1016/j.actatropica.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Jin Y.M., Lu K., Zhou W.F., Fu Z.Q., Liu J.M., Shi Y.J., Li H., Lin J.J. Comparison of recombinant proteins from Schistosoma japonicum for schistosomiasis diagnosis. Clin. Vaccine Immunol. 2010;17:476–480. doi: 10.1128/CVI.00418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthik K., Rathore R., Thomas P., Arun T.R., Viswas K.N., Dhama K., Agarwal R.K. New closed tube loop mediated isothermal amplification assay for prevention of product cross-contamination. MethodsX. 2014;1:137–143. doi: 10.1016/j.mex.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautz-Peterson G., Debatis M., Tremblay J.M., Oliveira S.C., Da'dara A.A., Skelly P.J., Shoemaker C.B. Schistosoma mansoni infection of mice, rats and humans elicits a strong antibody response to a limited number of reduction-sensitive epitopes on five major tegumental membrane proteins. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Zhao Z., Wang Y., Xing H., Parker D.M., Yang Z., Baum E., Li W., Sattabongkot J., Sirichaisinthop J., Li S., Yan G., Cui L., Fan Q. Nested PCR detection of malaria directly using blood filter paper samples from epidemiological surveys. Malar. J. 2014;13:175. doi: 10.1186/1475-2875-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.Z., Zheng H., Abe E.M., Yang K., Bergquist R., Qian Y.J., Zhang L.J., Xu Z.M., Xu J., Guo J.G., Xiao N., Zhou X.N. Reduction patterns of acute schistosomiasis in the People's Republic of China. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhou X., Piao X., Hou N., Shen Y., Zou Y., Li S., Cao J., Chen Q. Saposin-like proteins, a multigene family of Schistosoma species, are biomarkers for the immunodiagnosis of schistosomiasis japonica. J. Infect. Dis. 2016;214:1225–1234. doi: 10.1093/infdis/jiw188. [DOI] [PubMed] [Google Scholar]
- Lu Y., Xu B., Ju C., Mo X., Chen S., Feng Z., Wang X., Hu W. Identification and profiling of circulating antigens by screening with the sera from schistosomiasis japonica patients. Parasit. Vectors. 2012;5:115. doi: 10.1186/1756-3305-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam H.E., Driguez P., Piedrafita D., McManus D.P., Meeusen E.N. Discovery of novel Schistosoma japonicum antigens using a targeted protein microarray approach. Parasit. Vectors. 2014;7:290. doi: 10.1186/1756-3305-7-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moertel L., McManus D.P., Piva T.J., Young L., McInnes R.L., Gobert G.N. Oligonucleotide microarray analysis of strain- and gender-associated gene expression in the human blood fluke, Schistosoma japonicum. Mol. Cell. Probes. 2006;20:280–289. doi: 10.1016/j.mcp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Ng J.M., Vermeulen W., van der Horst G.T., Bergink S., Sugasawa K., Vrieling H., Hoeijmakers J.H. A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev. 2003;17:1630–1645. doi: 10.1101/gad.260003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita R.S., Vidilaseris K., Henri X., Adrian C. Integral membrane pyrophosphatases: a novel drug target for human pathogens? AIMS Biophys. 2016;3:171–194. [Google Scholar]
- Olveda R.M., Tallo V., Olveda D.U., Inobaya M.T., Chau T.N., Ross A.G. National survey data for zoonotic schistosomiasis in the Philippines grossly underestimates the true burden of disease within endemic zones: implications for future control. Int. J. Infect. Dis. 2016;45:13–17. doi: 10.1016/j.ijid.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Olveda D.U., Inobaya M., Olveda R.M., Vinluan M.L., Ng S.K., Weerakoon K., McManus D.P., Ramm G.A., Harn D.A., Li Y., Lam A.K., Guevarra J.R., Ross A.G. Diagnosing schistosomiasis-induced liver morbidity: implications for global control. Int. J. Infect. Dis. 2017;54:138–144. doi: 10.1016/j.ijid.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Perez-Sanchez R., Valero M.L., Ramajo-Hernandez A., Siles-Lucas M., Ramajo-Martin V., Oleaga A. A proteomic approach to the identification of tegumental proteins of male and female Schistosoma bovis worms. Mol. Biochem. Parasitol. 2008;161:112–123. doi: 10.1016/j.molbiopara.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Rollinson D., Knopp S., Levitz S., Stothard J.R., Tchuem Tchuente L.A., Garba A., Mohammed K.A., Schur N., Person B., Colley D.G., Utzinger J. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Sangfuang M., Chusongsang Y., Limpanont Y., Vanichviriyakit R., Chotwiwatthanakun C., Sobhon P., Preyavichyapugdee N. Schistosoma mekongi cathepsin B and its use in the development of an immunodiagnosis. Acta Trop. 2016;155:11–19. doi: 10.1016/j.actatropica.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Wang C., Chen L., Yin X., Hua W., Hou M., Ji M., Yu C., Wu G. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasit. Vectors. 2011;4:164. doi: 10.1186/1756-3305-4-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yu C.X., Yin X.R., Zhang W., Qian C.Y., Song L.J., Ke X.D., Xu Y.L., He W., Cao G.Q. Monitoring specific antibody responses against the hydrophilic domain of the 23 kDa membrane protein of Schistosoma japonicum for early detection of infection in sentinel mice. Parasit. Vectors. 2011;4:172. doi: 10.1186/1756-3305-4-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakoon K.G., McManus D.P. Cell-free DNA as a diagnostic tool for human parasitic infections. Trends Parasitol. 2016;32:378–391. doi: 10.1016/j.pt.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Weerakoon K.G., Gobert G.N., Cai P., McManus D.P. Advances in the diagnosis of human schistosomiasis. Clin. Microbiol. Rev. 2015;28:939–967. doi: 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakoon K.G., Gordon C.A., Gobert G.N., Cai P., McManus D.P. Optimisation of a droplet digital PCR assay for the diagnosis of Schistosoma japonicum infection: a duplex approach with DNA binding dye chemistry. J. Microbiol. Methods. 2016;125:19–27. doi: 10.1016/j.mimet.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Weerakoon K.G., Gordon C.A., Cai P., Gobert G.N., Duke M., Williams G.M., McManus D.P. A novel duplex ddPCR assay for the diagnosis of schistosomiasis japonica: proof of concept in an experimental mouse model. Parasitology. 2017:1–11. doi: 10.1017/S003118201700021X. [DOI] [PubMed] [Google Scholar]
- Wichmann D., Panning M., Quack T., Kramme S., Burchard G.D., Grevelding C., Drosten C. Diagnosing schistosomiasis by detection of cell-free parasite DNA in human plasma. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.M., Rong R., Lu Z.X., Shi C.J., Xu J., Zhang H.Q., Gong W., Luo W. Schistosoma japonicum: a PCR assay for the early detection and evaluation of treatment in a rabbit model. Exp. Parasitol. 2009;121:175–179. doi: 10.1016/j.exppara.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Xu X., Zhang Y., Lin D., Zhang J., Xu J., Liu Y.M., Hu F., Qing X., Xia C., Pan W. Serodiagnosis of Schistosoma japonicum infection: genome-wide identification of a protein marker, and assessment of its diagnostic validity in a field study in China. Lancet Infect. Dis. 2014;14:489–497. doi: 10.1016/S1473-3099(14)70067-2. [DOI] [PubMed] [Google Scholar]
- Xu J., Guan Z.X., Zhao B., Wang Y.Y., Cao Y., Zhang H.Q., Zhu X.Q., He Y.K., Xia C.M. DNA detection of Schistosoma japonicum: diagnostic validity of a LAMP assay for low-intensity infection and effects of chemotherapy in humans. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Steinman P., Maybe D., Zhou X.N., Lv S., Li S.Z., Peeling R. Evolution of the national schistosomiasis control programmes in the People's Republic of China. Adv. Parasitol. 2016;92:1–38. doi: 10.1016/bs.apar.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Yan M., Li J., Sha B. Structural analysis of the Sil1-Bip complex reveals the mechanism for Sil1 to function as a nucleotide-exchange factor. Biochem. J. 2011;438:447–455. doi: 10.1042/BJ20110500. [DOI] [PubMed] [Google Scholar]
- Zhang M., Fu Z., Li C., Han Y., Cao X., Han H., Liu Y., Lu K., Hong Y., Lin J. Screening diagnostic candidates for schistosomiasis from tegument proteins of adult Schistosoma japonicum using an immunoproteomic approach. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material