Abstract
Background
Probiotics may help to prevent symptoms of anxiety and depression through several putative mechanisms.
Objective
The aim of this study was to evaluate the effect of Lactobacillus rhamnosus HN001 (HN001) given in pregnancy and postpartum on symptoms of maternal depression and anxiety in the postpartum period. This was a secondary outcome, the primary outcome being eczema in the offspring at 12 months of age.
Design, Setting, Participants
A randomised, double-blind, placebo-controlled trial of the effect of HN001 on postnatal mood was conducted in 423 women in Auckland and Wellington, New Zealand. Women were recruited at 14–16 weeks gestation.
Intervention
Women were randomised to receive either placebo or HN001 daily from enrolment until 6 months postpartum if breastfeeding.
Outcome Measures
Modified versions of the Edinburgh Postnatal Depression Scale and State Trait Anxiety Inventory were used to assess symptoms of depression and anxiety postpartum.
Trial Registration
Australia NZ Clinical Trials Registry: ACTRN12612000196842.
Findings
423 women were recruited between December 2012 and November 2014. 212 women were randomised to HN001 and 211 to placebo. 380 women (89.8%) completed the questionnaire on psychological outcomes, 193 (91.0%) in the treatment group and 187 (88.6%) in the placebo group. Mothers in the probiotic treatment group reported significantly lower depression scores (HN001 mean = 7·7 (SD = 5·4), placebo 9·0 (6·0); effect size -1·2, (95% CI -2·3, -0·1), p = 0·037) and anxiety scores (HN001 12·0 (4·0), placebo 13·0 (4·0); effect size -1·0 (-1·9, -0·2), p = 0·014) than those in the placebo group. Rates of clinically relevant anxiety on screening (score > 15) were significantly lower in the HN001 treated mothers (OR = 0·44 (0·26, 0·73), p = 0·002).
Interpretation
Women who received HN001 had significantly lower depression and anxiety scores in the postpartum period. This probiotic may be useful for the prevention or treatment of symptoms of depression and anxiety postpartum.
Funding Source
Health Research Council of New Zealand (11/318) and Fonterra Co-operative Group Ltd.
Keywords: Probiotic, Depression, Anxiety, Randomised controlled trial, Microbiome-gut-brain axis
Highlights
-
•
The microbiome-gut-brain axis may be important for mental health.
-
•
We conducted a study of probiotic supplementation in pregnancy and 6 months after delivery if breastfeeding.
-
•
The probiotic treatment group reported significantly lower depression and anxiety scores than those in the placebo group.
There is mounting evidence from animal studies that the microbiome-gut-brain axis may be important for mental health. Depression and anxiety in pregnancy and after birth affects 10–15% of women, although many are not recognised or treated. We conducted a double-blind placebo-controlled study of probiotic (Lactobacillus rhamnosus HN001) supplementation (from early pregnancy through to 6 months after delivery if breastfeeding) on postnatal symptoms of depression and anxiety in a group (n = 380) of healthy women. Mothers in the probiotic treatment group reported significantly lower depression and anxiety scores than those in the placebo group.
1. Introduction
Major depression in pregnancy and after birth occurs in 10–15% of women in New Zealand, a rate comparable to other western countries (Abbott and Williams, 2006). Postnatal depression (PND) is associated with persistent depression, and even, in a few cases each year, death from suicide (PMMRC, 2014). This disorder may affect a mother's ability to care for and bond with her new infant, as well as her quality of life and daily functioning (Da Costa et al., 2006). In addition, maternal depression can produce long-lasting effects on children's cognitive, social-emotional and health outcomes (Tronick and Reck, 2009; Grace et al., 2003). In addition to depressed mood, PND is associated with hopelessness, excessive fatigue, psychomotor agitation, appetite and sleep disturbances, guilt, or feelings of inadequacy, particularly regarding one's ability to care for the newborn and co-morbid anxiety. Anxiety often coexists with depression and like PND, the prevalence varies widely depending on the timing and the type of disorder (generalised anxiety disorder (GAD) vs obsessive compulsive disorder (OCD)) and the way it is measured (self-report vs structured interview). Despite this, most women with PND are either not recognised as being depressed, are unable to access psychological therapy or are reluctant to take antidepressant medication in pregnancy or while breastfeeding. Furthermore it takes several weeks for the therapeutic effect of antidepressants to appear and there is a 15–30% discontinuation rate (Gartlehner et al., 2005). Safe and effective therapies to prevent and treat PND are needed (Battle et al., 2003).
A “healthy” diet comprising higher intakes of fruit and vegetables, whole grains and lean meat and fish is associated with a reduced likelihood of depression (Lai et al., 2013). These observational studies are supported by a recent randomised controlled trial (RCT) showing that a dietary improvement intervention was effective as an adjuvant therapy in patients with moderate to severe depression who were being treated with psychotherapy or antidepressants (Jacka et al., 2017). Furthermore, it has been suggested that fermented foods alter dietary items before they are ingested, resulting in phytochemical transformation into bioactive chemicals which reduce oxidative stress and inflammation (Selhub et al., 2014).
There is a growing literature linking the gut microbiota to brain chemistry and behaviour via multiple bi-directional pathways (the microbiome-gut-brain axis), including the immune system, neuroendocrine, hypothalamic pituitary adrenal axis (HPA axis), short chain fatty acids or tryptophan and sympathetic and parasympathetic arms of the autonomic nervous system including the enteric nervous system, the vagus nerve, and the gut microbiota (Wang and Kasper, 2014; Dinan and Cryan, 2015). Microbial dysbiosis is associated with many health problems including neuropsychiatric disorders, such as autism spectrum disorder, depression and anxiety, and are associated with elevated levels of pro-inflammatory cytokines, increased oxidative stress, altered gastrointestinal (GI) function, and lowered micronutrient and omega-3 fatty acid status (Dawson et al., 2016). Intriguingly, alterations in the pattern of gut microbial composition in healthy adults influences mood (Li et al., 2016).
Probiotics are live microorganisms that when consumed in adequate amounts provide health benefits to the host (Hill et al., 2014). In 2005 it was first suggested that probiotics might be an adjuvant therapy for major depression (Logan and Katzman, 2005) and others have also suggested that probiotic enhancement of gut microbiota may improve mood outcomes (Dinan and Cryan, 2016). Pre-clinical studies have demonstrated that the anxiety phenotype of mice can be changed with fecal transplantation and that the changes in microbiota are accompanied by changes in brain chemistry (Collins et al., 2013). Furthermore, probiotic treatment has also been shown to have a positive effect on anxiety-like and depressive-like behaviour in animal studies (Bravo et al., 2011; Desbonnet et al., 2010), with mediating mechanisms including GABA receptor expression in specific locations of the central nervous system (Bravo et al., 2011), the HPA axis (Desbonnet et al., 2010) and the vagus nerve which transmits information from the gut luminal environment to the CNS (Carabotti et al., 2015). Interestingly, probiotic supplementation in adults has not been found to substantially alter their gut microbiota as sampled by fecal samples (Kristensen et al., 2016), although this does not exclude their potential effect higher up in the small intestine or on the adherent mucosal gut microbiome.
Clinical trials of probiotic treatment have yielded mixed results, and systematic reviews of human trials concluded that the evidence for beneficial effects of probiotics on mood may not be as strong as some recent narrative studies purport (Romijn and Rucklidge, 2015). A recent systematic review identified 10 clinical trials of the effect of probiotics on symptoms of depression (Wallace and Milev, 2017). Seven studies were in healthy subjects, 2 in chronic fatigue syndrome and one in depression. Three of 5 studies reported improved mood with probiotics, and 5 of 7 studies reported improvements in stress and anxiety. A recent study that was published after these reviews reported that obese women treated with a weight-reduction programme and probiotic had reduced symptoms of depression compared with the comparison group, but this effect was not seen in men (Sanchez et al., 2017). There was no effect on anxiety. Both reviews suggested further RCTs were needed. To date probiotic effects on postnatal depression have not been studied in a clinical trial.
Our aim was to evaluate whether probiotic supplementation with Lactobacillus rhamnosus HN001 (HN001) had a beneficial effect on postnatal symptoms of depression and anxiety in a group of healthy women. This was a secondary outcome, the primary outcome being eczema in the offspring at 12 months of age.
2. Methods and Materials
2.1. Study Design
The Probiotics in Pregnancy Study (PIP Study) is a two-centre (Wellington and Auckland, New Zealand) randomised double-blind placebo-controlled trial testing the effect of the probiotic HN001 on the development of eczema and atopic sensitization in offspring (the primary outcome) and pregnancy outcomes (secondary outcomes) in women. The full protocol is published (Barthow et al., 2016).
2.2. Study Population
The selection of participants, randomisation process and quality control measures have been described in detail previously (Barthow et al., 2016). In brief, 423 women were recruited at 14–16 weeks gestation between December 2012 and November 2014. 212 women were randomised to HN001 and 211 to placebo. Women were considered eligible if they were English-speaking, planning to breastfeed, and if either they or the unborn child's biological father had a history of asthma, hayfever or eczema requiring medication. Women were excluded from the study if aged < 16 years, planning to move outside the study centres during study duration, planning on taking probiotics, or if they had serious medical or health problems related to the pregnancy.
2.3. The Intervention
Women were randomised to receive either HN001 at a dose of 6 × 109 colony-forming units (cfu) or placebo (corn-derived maltodextrin), to be taken daily from enrolment until birth and, from birth up till six months post-birth whilst breastfeeding. The capsules were indistinguishable. Both researchers and participants were blinded to treatment assignation of participants. To assess adherence, capsule bottles were collected at regular intervals and counts of remaining capsules were completed by an independent person.
2.4. Randomisation
Randomisation was managed by Fonterra Co-operative Group Ltd. and concealed from all study staff and participants. Randomisation was stratified by study centre and performed in blocks of random lengths according to a computer-generated randomisation list. Research staff screened and enrolled eligible participants, and provided enrolled participants with the next available sequentially-numbered capsule container.
2.5. Data Collection
Mothers were interviewed at baseline (14–16 weeks gestation) to collect information about maternal characteristics and demographics. When children were aged 6 months and approximately 12 months old, mothers visited the research centres or were visited at home and were invited to complete a questionnaire about their psychological wellbeing, thinking back to when their child was 1–2 months of age. If children were older than 12 months when the questionnaire was being used, mothers were posted the questionnaire or invited to complete it online via a secure link. Mothers and researchers remained blind to treatment assignation of participants at all follow-up stages of the study.
2.6. Primary Outcomes
Edinburgh Postnatal Depression Scale (EPDS): The EPDS is a 10 item screening questionnaire widely used to assess maternal mood (Cox et al., 1987). For the purposes of analysis, the standard cut-off of > 12 was used to identify mothers at higher risk of postnatal depression.
State Trait Anxiety Inventory 6 item version (STAI6): The STAI6 is a short 6 item scale validated as an anxiety screening questionnaire based on the longer State Trait Anxiety Inventory (Marteau and Bekker, 1992). A cut-off score > 15 was used as an indicator of clinically significant levels of anxiety.
For both the EPDS and the STAI6 the questions were altered to use the past tense as mothers were asked to remember back to when their child was 1–2 months old and complete the questions based on how they were feeling at that time. It should be noted that the modified questionnaires have not been validated.
2.7. Explanatory Variable
Infant Colic: Infant colic was assessed at the 6 month interview when mothers were asked if they had contacted a health professional because their child had colic at any time between birth and six months of age.
2.8. Sample Size and Statistical Analysis
With a sample size of 200 in each group and 13% drop-out rate the study had a 79% power to detect a 26% reduction in EPDS at the 5% level of significance.
Data were analysed as intent-to-treat. Adherence was calculated as the number of capsules taken as a proportion of the expected number taken. Statistical analysis was conducted in SAS 9.4 using a generalised linear model for the continuous outcomes and logistic regression for categorical outcomes. Multivariable analysis of the relationship between probiotic supplementation and postnatal depression and anxiety scores, adjusted for the time since birth at which the questionnaires were completed and infant colic.
The trial was registered at the Australia New Zealand Clinical Trials Registry: ACTRN12612000196842.
2.9. Ethics
The study received ethical approval from the New Zealand Multiregional Ethics Committee (MEC/11/09/77). Participants gave written informed consent. The study conforms to the standards indicated by the Declaration of Helsinki.
2.10. Role of Funding Sources
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit.
3. Results
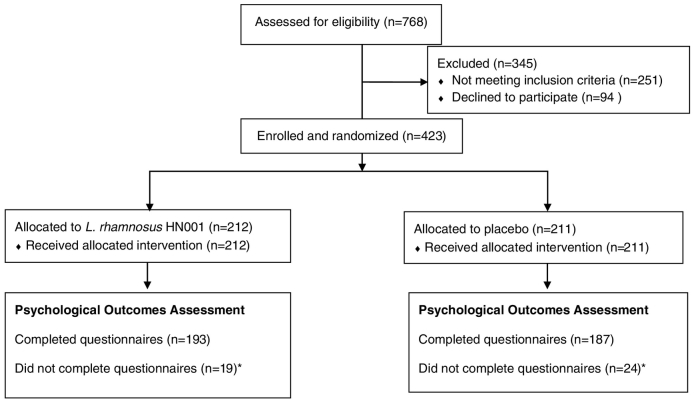
Fig. 1 shows the numbers assessed for eligibility, the number excluded, and the number eligible, recruited and allocated in the PIP Study. Of the 423 randomised women, 380 (89.8%) completed the psychological outcome measures, 193 (91.0%) were in the treatment group and 187 (88.6%) in the placebo group. Of the 380 participants in this study, 11 completed the questionnaires at the 6 month infant visit, 112 completed them at the 12 month infant visit and the remaining 257 completed the measures online at a median child age of 2·1 years (IQR 1·8–2·4). One participant did not complete all questions in the anxiety questionnaire, and the STAI6 score was recorded as missing.
Fig. 1.
Participant flow showing the numbers of participants who were randomly assigned, received intended treatment and were analysed for the psychological outcomes.
Table 1 shows the maternal characteristics of the study participants. Of particular note there was little difference in the use of medications for psychological problems prior to the index pregnancy.
Table 1.
Characteristics of study population at enrolment.
| HN001 (N = 193) | Placebo (N = 187) | |
|---|---|---|
| Previous pregnancy | ||
| 0 | 66 (34.2%) | 52 (27.8%) |
| 1 | 46 (23.8%) | 68 (36.4%) |
| 2 | 43 (22.3%) | 40 (21.4%) |
| 3 | 24 (12.4%) | 11 (5.9%) |
| > 3 | 14 (7.3%) | 16 (8.6%) |
| Age (years), mean (SD) | 33.5 (4.24) | 33.7 (4.44) |
| Weight (kg), median (interquartile range) | 69.2 (63.1–79.1) | 71 (63.5–81.7) |
| BMI, median (interquartile range) | 25.1 (23.0–28.6) | 25.9 (23.0–29.5) |
| Parental allergies | ||
| Mother only | 70 (36.3%) | 64 (34.2%) |
| Father only | 26 (13.5%) | 28 (15.0%) |
| Both | 97 (50.3%) | 95 (50.8%) |
| Ethnicity | ||
| Maori | 20 (10.4%) | 29 (15.5%) |
| Pacific | 5 (2.6%) | 3 (1.6%) |
| Asian | 13 (6.7%) | 14 (7.5%) |
| European | 155 (80.3%) | 140 (74.9%) |
| Other | 0 (0.0%) | 1 (0.5%) |
| Household income ($NZ) | ||
| 0–49k | 15 (7.8%) | 10 (5.4%) |
| 50–99k | 59 (30.6%) | 63 (33.7%) |
| 100–149k | 69 (35.8%) | 66 (35.3%) |
| 150 + k | 50 (25.9%) | 48 (25.7%) |
| Maternal smoking | 42 (21.8%) | 36 (19.3%) |
| Maternal education | ||
| School education | 23 (11.9%) | 25 (13.4%) |
| Post school education | 26 (13.5%) | 17 (9.1%) |
| University education | 144 (74.6%) | 145 (77.5%) |
| Medication for psychological problem ever taken prior to index pregnancy | ||
| Yes | 41 (21.2%) | 36 (19.3%) |
| No | 152 (78.8%) | 151 (80.7%) |
| Maternal antibiotics from enrolment (14–16 weeks gestation) to birth | ||
| Yes | 57 (30.3%) | 58 (31.2%) |
| No | 131 (69.7%) | 128 (68.8%) |
| Maternal antibiotics from birth to 3 months postpartum | ||
| Yes | 64 (33.2%) | 72 (38.5%) |
| No | 129 (66.8%) | 115 (61.5%) |
The median adherence to allocated treatment did not differ (probiotic 92·3% (IQR = 83·9–97·2%), placebo 91·1% (IQR = 81·8–96·9%; p = 0·30)).
Depression and anxiety scores tended to increase with increasing interval between delivery and when the questionnaire was completed (depression score increased by 0·85 per year, p = 0·065; anxiety score increased by 0·66 per year, p = 0·060). Infant colic was associated with higher depression (multivariable p < 0·0001) and anxiety (multivariable p < 0·0001) scores, but was not significantly associated with probiotic supplementation group (p = 0·456).
Table 2 shows the mean and standard deviation of depression and anxiety scores in the probiotic treatment and placebo groups. Mothers in the probiotic treatment group reported significantly lower depression scores (HN001 mean = 7·7 (SD = 5·4), placebo 9·0 (6·0), effect size -1·2, (95% CI -2·4, -0·1), p = 0·035) and anxiety scores (HN001 12·0 (4·0) placebo 13·0 (4·3), effect size -1·1 (-1·9, -0·2), p = 0·014) than those in the placebo group. After controlling for infant colic and time since birth that questionnaires were completed, probiotic supplementation remained significantly associated with reduced depression (p = 0·037) and anxiety (p = 0·014) scores.
Table 2.
Depression and anxiety scores in the probiotic treatment (HN001) and placebo groups.
| Mean | Standard deviation | Univariable effect size (95%CI), p-value | Multivariable† effect size (95%CI), p-value | ||
|---|---|---|---|---|---|
| Depression scoresa | |||||
| HN001 | N = 194 | 7.7 | 5.4 | -1.2 (-2.4, -0.1) | -1.2 (-2.3, -0.1) |
| Placebo | N = 187 | 9.0 | 6.0 | p = 0.035 | p = 0.037 |
| Anxiety Scoresa | |||||
| HN001 | N = 192 | 12.0 | 4.0 | -1.1 (-1.9, -0.2) | -1.0 (-1.9, -0.2) |
| Placebo | N = 187 | 13.0 | 4.3 | p = 0.014 | p = 0.014 |
Three participants had incomplete anxiety data on the STAI6 and one had incomplete depression data on the EPDS, therefore scores could not be calculated.
Adjusted for infant colic and time since birth that questionnaires were completed.
Table 3 shows the number of women in the probiotic treatment and placebo groups who reported clinically significant depression or anxiety scores (that is above the cut-off points). The number of women reporting depression scores above the cut-off point did not differ significantly between the probiotic treatment and placebo groups (OR = 0.64 (0.38, 1.07), p = 0·086). However, women in the probiotic treatment group were significantly less likely to have anxiety scores above the cut-off point than the placebo group (OR = 0.44 (0.27, 0.73) p = 0·001), this association remained statistically significant after controlling for infant colic and time since birth at questionnaire completion (p = 0·002).
Table 3.
Number and percentage of participants scoring at or above the cut-off point for depression and anxiety in the treatment (HN001) and placebo groups.
| Univariable odds ratio (95% CI), p-value | Multivariable† odds ratio (95% CI), p-value | |||
|---|---|---|---|---|
| Depression scorea | Depressed N (%) |
Not depressed N (%) |
||
| HN001 | 32 (16.5) | 162 (83.5) | 0.64 (0.39, 1.07) p = 0.086 |
0.65 (0.38, 1.10) p = 0.11 |
| Placebo | 44 (23.5) | 143 (76.5) | Reference | Reference |
| Anxiety scorea | Anxious N (%) |
Not anxious N (%)s |
||
| HN001 | 30 (15.6) | 162 (84.4) | 0.44 (0.27, 0.73) p = 0.001 |
0.44 (0.26, 0.73) p = 0.002 |
| Placebo | 55 (29.4) | 132 (70.6) | Reference | Reference |
Three participants had incomplete anxiety data on the STAI6 and one had incomplete depression data on the EPDS, therefore scores could not be calculated.
Adjusted for infant colic and time since birth that questionnaires were completed.
Use of medications for psychological problems during the index pregnancy was low and did not differ between treatment groups (HN001 3.6% vs. placebo 3.2%).
4. Discussion
This study demonstrated a significantly lower prevalence of symptoms of depression and anxiety postpartum in women supplemented with the probiotic HN001 during and after pregnancy than in those given a placebo. Furthermore, the number of women reporting clinically significant levels of anxiety on screening was significantly lower in the probiotic group. To our knowledge this is the first double-blind RCT of probiotics that has evaluated symptoms of depression and anxiety in the postpartum period. In addition, our sample size was substantially larger than many previously reported RCTs of probiotics on mood and behaviour.
The finding that women supplemented with probiotics had fewer symptoms of postnatal anxiety and depression is consistent with two previous clinical studies of the effect of probiotics on mood in different populations. A RCT of 40 people with major depressive disorder treated with a combination of three probiotics (Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum) or placebo also found a significant reduction in symptoms of depression on the Beck Depression Inventory (BDI) in the treatment group (Akkasheh et al., 2016). A reduction in anxiety symptoms in a sample of 39 chronic fatigue patients randomised to receive Lactobacillus casei or placebo has also been reported, but the same study did not find a reduction in symptoms of depression on the BDI in the treatment group (Rao et al., 2009). However, not all studies have demonstrated a significant positive effect of probiotic treatment on mood outcomes (Romijn and Rucklidge, 2015; Romijn et al., 2017). A recent study involving 69 subjects who successfully completed the study found no evidence that a probiotic preparation (containing Lactobacillus helveticus and Bifidobacterium longum) for 8 weeks was effective in treating adults with moderate scores on self-reported symptoms of depression, stress and anxiety (Rao et al., 2009). The diversity of study populations, including those with schizophrenia (Dickerson et al., 2014) smokers(Reale et al., 2012), and irritable bowel syndrome patients (Dapoigny et al., 2012), the range of probiotic strains used, varying length of treatment, small sample sizes and varying measures of mood make it difficult, if not impossible, to undertake any meta-analysis of these studies. It is clear that more large studies of probiotic treatment are needed including those that measure psychological outcomes.
In our study infant colic was associated with higher depression and anxiety scores. There has been a suggestion in the literature that probiotic supplementation may benefit maternal mood by reducing infant colic. One study reported that direct probiotic supplementation of infants reduced infant colic and this in turn was associated with lower rates of maternal depression (Mi et al., 2015). While infants in our study are likely to have been exposed to a small amount of probiotic indirectly, either in utero or via breastmilk, they were not administered the probiotic directly; furthermore, we found that prevalence of infant colic did not differ between the probiotic and placebo groups and hence there was little difference in the effect size when adjusted for infant colic. Multivariable analysis showed that probiotic supplementation and absence of infant colic were independently associated with lower postnatal depression and anxiety scores.
The prevalence of scores for depression and anxiety above the cut-off at 1–2 months post-partum in this study was higher than the 10% to 15% usually reported. In part, this may be due to the mothers and or fathers having a history of asthma, hayfever or eczema requiring medication, as it is known that those with allergies are at higher risk of mental health problems (Scott et al., 2007). It may also be due to mothers in our study completing the questionnaire retrospectively. Possibly when mothers reflect back to how they felt 1 to 2 months after delivery, they realise how tiring caring for a newborn infant can be. In studies that survey prevalence of PND at an early time point, women may be less likely to rate themselves as depressed or anxious because they are expecting to feel exhausted.
Despite the prevalence of high symptom scores, the number of women who had medication for psychological problems in pregnancy was low (3.4% of total study population). Our study did not explore the reasons for this; however, it does support the contention that depression and anxiety during pregnancy is unrecognised and not treated.
Mechanisms by which probiotic supplementation influence the physiology of the brain, and thereby anxiety and depression, have been proposed using animal models. Changes in the expression of the neurotransmitter GABA receptors in regions of the mouse brain have been demonstrated along with changes in anxiety-related behaviour with L. rhamnosus treatment (Bravo et al., 2011). However, this relationship was not evident in mice that had had their vagus nerve removed, indicating that the vagus nerve might be the link between events in the gut and altered GABA receptor expression. However, in another study L. rhamnosus was not superior to placebo in modifying stress-related measures in healthy adult human males highlighting the challenge of extrapolating from animal to human studies (Kelly et al., 2017). Biochemical changes have been demonstrated in the HPA axis in rats when exposed to maternal separation, which in turn were associated with anxiety-related behaviours in the Forced Swim Test; these behaviours were reversed when the animals were treated with Bifidobacterium infantis (Collins et al., 2013).
Postnatal depression occurs more frequently in socio-economically disadvantaged populations. In a previous study we found that PND was more likely to occur in women who were single, < 20 years of age, Maori, were unhappy with their relationship with their partner, or had a history of previous psychiatric hospitalisation (Webster et al., 1994). National Health and Nutrition Examination Survey was an observational study and found that probiotic foods or supplements were associated with a reduced risk of depression, but this was attenuated and non-significant when adjusted for factors that were associated with depression and probiotic exposure (Cepeda et al., 2017). This may be a consequence of misclassification, as probiotic food and supplement use over the 24 h period preceding data collection was used to define exposure and may not reflect usual intake. Our study was a RCT and therefore the socioeconomic and other factors are randomly distributed between the intervention and placebo group.
There are many different species and strains of probiotics. L. rhamnosus HN001 was chosen for the beneficial effect on the primary outcome, namely eczema (Wickens et al., 2008; Wickens et al., 2012; Wickens et al., 2013). Many Lactobacillus and Bifidobacterium strains have been studied with respect to mental health and these genera seem to show the most beneficial effects (Mayer et al., 2014).
Limitations of the study need to be considered. Firstly, the EPDS and STAI6 are screening tools for PND and anxiety, but are not diagnostic. In this report we have studied symptoms of PND and anxiety, as clinical assessment and diagnosis of depression and anxiety were not undertaken. However, when the scores were categorised into higher risk of PND and indicators of clinically significant levels of anxiety, similar findings were seen. Secondly, the information regarding symptoms of PND and anxiety was collected retrospectively, and neither the EPDS nor the STAI6 has been validated using the questions phrased in the past tense. While this may have resulted in an increase in measurement error, it would not be expected to have introduced a differential bias in responses. Furthermore, measurement error, if introduced, would be more likely to move the results towards the null hypothesis of there being no effect of probiotic on mood outcomes. The strength of the study is the design (double-blind placebo-controlled study) with substantial group size. It is a simple, cost effective and easily implemented treatment. Furthermore, we have shown that this probiotic is safe and well tolerated in pregnancy and infants (Dekker et al., 2009).
This study provides evidence that probiotic supplementation with L. rhamnosus HN001 in pregnancy and postpartum reduces the prevalence of symptoms of PND and anxiety postpartum. Not all probiotic strains have the same effect on health and it is possible that the results found using HN001 are not generalisable to other probiotic strains, or at lower doses than those used in this study. Furthermore, in a recent study higher levels of anxiety-like behaviour and stress, as measured by plasma corticosterone levels, were seen in young rats treated with Lactobacillus casei or inulin (a prebiotic) compared with controls (Barrera-Bugueño et al., 2017). Those treated with a L. casei and inulin combination (synbiotic) had no anxiety-like behaviours. There are many unanswered questions, including the choice of probiotic, the dose and the duration of treatment. Can probiotics prevent the onset of symptoms? Could probiotics be used as the primary treatment for maternal mental health problems or should it be used as an adjuvant treatment to standard therapy? Such studies might incorporate inflammatory markers, cortisol, or other objective markers,
If replicated by other studies, this probiotic may be useful for the prevention or treatment of symptoms of depression and anxiety postpartum.
Funding
This study was funded by grants and support from the: Health Research Council of New Zealand (HRC 11/318); Fonterra Co-operative Group Ltd., New Zealand; University of Otago, Wellington, New Zealand. Fonterra contributed funds, provided and maintained quality control of the study capsules and performed the participant randomization for the study. Fonterra had no role in the design, analysis or writing of this article. RFS, JMDT and EAM were supported by Cure Kids.
Conflict of Interests
Dr. Wickens, Ms. Hood, Ms. Barthow, Ms. Kang, Professor Crane, Dr. Stanley, Dr. Abels, Mr. Purdie and Dr. Maude report grants from Health Research Council of New Zealand (HRC 11/318), grants and non-financial support from Fonterra Co-operative Group Ltd. and grants from University of Otago, Wellington. Dr. Slykerman, Associate Professor Thompson and Professor Mitchell report grants from Health Research Council of New Zealand (HRC 11/318), grants and non-financial support from Fonterra Co-operative Group Ltd., and financial support from Cure Kids (3627252). Associate Professor Murphy, Ms. Rowden and Professor Stone report grants from Health Research Council of New Zealand (HRC 11/318) and grants and non-financial support from Fonterra Co-operative Group Ltd.
Authors' Contributions
TS first suggested examining the psychological effects of probiotics.
RFS independently suggested probiotics might influence mood, designed the psychological outcome measures, advised on data analysis and wrote the first draft of the manuscript.
JMDT analysed the data.
FH designed the data collection tool and was responsible for collecting the data in Wellington.
RM critically reviewed the manuscript.
CB contributed to the development of the main study and data collection in Wellington.
JK assisted with data collection in Wellington.
JR was project manager of the study in Auckland and collected the data.
KW conceived the main study, raised the funds for both the main study and this component and managed the main project.
JC had overall responsibility for the main study.
EAM conceived, designed and led this component of the study, led the Auckland arm of the main study, advised on data analysis and drafted the manuscript. EAM takes responsibility for the content of the manuscript.
In addition PS, RM, RMM and PA contributed to the design of the main study.
All authors reviewed and approved the submitted manuscript.
Acknowledgments
Acknowledgements
We sincerely thank the women who participated in the study. Dr. Penny Fitzharris is a member of the Probiotics in Pregnancy Study Group and contributed to the design of the main study.
References
- Abbott M.W., Williams M.M. Postnatal depressive symptoms among Pacific mothers in Auckland: prevalence and risk factors. Aust. N. Z. J. Psychiatry. 2006;40:230–238. doi: 10.1080/j.1440-1614.2006.01779.x. [DOI] [PubMed] [Google Scholar]
- Akkasheh G., Kashani-Poor Z., Tajabadi-Ebrahimi M. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Barrera-Bugueño C., Realini O., Escobar-Luna J., Sotomayor-Zarate R., Gotteland M., Julio-Pieper M., Bravo J.A. Anxiogenic effects of a lactobacillus, inulin and the synbiotic on healthy juvenile rats. Neuroscience. 2017;359:18–29. doi: 10.1016/j.neuroscience.2017.06.064. [DOI] [PubMed] [Google Scholar]
- Barthow C., Wickens K., Stanley T. The Probiotics in Pregnancy Study (PIP Study): rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth. 2016;16:133. doi: 10.1186/s12884-016-0923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle C.L., Salisbury A.L., Schofield C.A., Ortiz-Hernandez S. Perinatal antidepressant use: understanding women's preferences and concerns. J. Psychiatr. Pract. 2003;19:443–453. doi: 10.1097/01.pra.0000438183.74359.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J.A., Forsythe P., Chew M.V. Ingestion of Lactobacillus strain regulates emotional behaviour and central GABA receptor expression in a mouse via the vagus nerve. PNAS. 2011;108:16050–16059. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- Cepeda M.S., Katz E.G., Blacketer C. Microbiome-gut-brain axis: probiotics and their association with depression. J. Neuropsychiatr. Clin. Neurosci. 2017 Winter;29(1):39–44. doi: 10.1176/appi.neuropsych.15120410. [DOI] [PubMed] [Google Scholar]
- Collins S.M., Kassam Z., Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr. Opin. Microbiol. 2013;16:240–245. doi: 10.1016/j.mib.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry. 1987;150(6):782. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Da Costa D., Dritsa M., Rippen N., Lowensteyn Khalife S. Health-related quality of life in postpartum depressed women. Arch. Women's Ment. Health. 2006;9:95–102. doi: 10.1007/s00737-005-0108-6. [DOI] [PubMed] [Google Scholar]
- Dapoigny M., Piche T., Ducrote P., Lundard B., Cardot J., Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized double-blind study. World J. Gastroenterol. 2012;18:2067–2075. doi: 10.3748/wjg.v18.i17.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S.L., Dash S.R., Jacka F.N. The importance of diet and gut health to the treatment and prevention of mental disorders. Int. Rev. Neurobiol. 2016;131:325–346. doi: 10.1016/bs.irn.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Dekker J.W., Wickens K., Black P.N. Safety aspects of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium animalis subps. lactis HN019 in human infants aged 0 to 2 years. Int. Dairy J. 2009;19:149–154. [Google Scholar]
- Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effect of the probiotic Bifidobacterium Infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Dickerson F.B., Stallings C., Origoni A. Effects of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomised, placebo-controlled trial. Prim. Care Companion CNS Disord. 2014;16:1. doi: 10.4088/PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. The impact of gut microbiota on brain and behavior: implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:552–558. doi: 10.1097/MCO.0000000000000221. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Mood by microbes: towards clinical translation. Genome Med. 2016;6:36–38. doi: 10.1186/s13073-016-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartlehner G., Hansen R.A., Carey T.S., Lohr K.N., Gaynes B.N., Randolph L.C. Discontinuation rates for selective serotonin reuptake inhibitors and other second-generation antidepressants in outpatients with major depressive disorder: a systematic review and meta-analysis. Int. Clin. Psychopharmacol. 2005;20(2):59–69. doi: 10.1097/00004850-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Grace S.L., Evindar A., Stewart D.E. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch. Women's Ment. Health. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H., Salminen S., Calder P.C., Sanders M.E. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Jacka F.N., O'Neil A., Opie R., Itsiopoulos C., Cotton S., Mohebbi M., Castle D., Dash S., Mihalopoulos C., Chatterton M.L., Brazionis L., Dean O.M., Hodge A.M., Berk M. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial) BMC Med. 2017;15(1):23. doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J.R., Allen A.P., Temko A., Hutch W., Kennedy P.J., Farid N., Murphy E., Boylan G., Bienenstock J., Cryan J.F., Clarke G., Dinan T.G. Lost in translation? The potential psychobiotic lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017;61:50–59. doi: 10.1016/j.bbi.2016.11.018. Mar. [DOI] [PubMed] [Google Scholar]
- Kristensen N.B., Bryrup T., Allin K.H., Nielsen T., Hansen T.H., Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.S., Hiles S., Bisquera A., Hure A.J., McEvoy M., Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2013;99(1):181–197. doi: 10.3945/ajcn.113.069880. [DOI] [PubMed] [Google Scholar]
- Li L., Su Q., Xie B., Duan L., Zhao W., Hu D., Wu R., Liu H. Gut microbes in correlation with mood: case study in a closed experimental human life support system. Neurogastroenterol. Motil. 2016 Aug;28(8):1233–1240. doi: 10.1111/nmo.12822. [DOI] [PubMed] [Google Scholar]
- Logan A.C., Katzman M. Major depressive disorder: probiotics may be an adjuvant therapy. Med. Hypotheses. 2005;64(3):533–538. doi: 10.1016/j.mehy.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Marteau T.M., Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br. J. Clin. Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 2014;34(46):15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi G., Zhao L., Qiao D., Kang W., Tang M., Xu J. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: a prospective single blind randomized trial. Antonie Van Leeuwenhoek. 2015;107:154–155. doi: 10.1007/s10482-015-0448-9. [DOI] [PubMed] [Google Scholar]
- PMMRC . Health Quality & Safety Commission; Wellington: 2014. Eighth Annual Report of the Perinatal and Maternal Mortality Review Committee: Reporting Mortality 2012. [Google Scholar]
- Rao A.V., Bested A.C., Beaulne T.M. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1(1):6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M., Boscolo P., Bellante V. Daily intake of Lactobacillus casei Shirota increases natural killer cell activity in smokers. Br. J. Nutr. 2012;108:308–314. doi: 10.1017/S0007114511005630. [DOI] [PubMed] [Google Scholar]
- Romijn A.R., Rucklidge J.J. Systematic review of evidence to support the theory of psychobiotics. Nutr. Rev. 2015;73:675–693. doi: 10.1093/nutrit/nuv025. [DOI] [PubMed] [Google Scholar]
- Romijn A.R., Rucklidge J.J., Kuijer R.G., Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry. 2017 doi: 10.1177/0004867416686694. https://doi.org/10.1177/0004867416686694 published online January 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M., Darimont C., Panahi S., Drapeau V., Marette A., Taylor V.H., Doré J., Tremblay A. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients. 2017;9(3):284. doi: 10.3390/nu9030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K.M., Von Korff M., Ormel J., Zhang M.Y., Bruffaerts R., Alonso J., Kessler R.C., Tachimori H., Karam E., Levinson D., Bromet E.J., Posada-Villa J., Gasquet I., Angermeyer M.C., Borges G., de Girolamo G., Herman A., Haro J.M. Mental disorders among adults with asthma: results from the World Mental Health Survey. Gen. Hosp. Psychiatry. 2007;29:123–133. doi: 10.1016/j.genhosppsych.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub E.M., Logan A.C., ACl Bested. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J. Physiol. Anthropol. 2014;33:2. doi: 10.1186/1880-6805-33-2. Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E., Reck C. Infants of depressed mothers. Harvard Rev. Psychiatry. 2009;17:147–156. doi: 10.1080/10673220902899714. [DOI] [PubMed] [Google Scholar]
- Wallace C.J.K., Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann. General Psychiatry. 2017;16:14. doi: 10.1186/s12991-017-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Kasper L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M.L., Thompson J.M., Mitchell E.A., Werry J.S. Postnatal depression in a community cohort. Aust. N. Z. J. Psychiatry. 1994;28(1):42–49. doi: 10.3109/00048679409075844. [DOI] [PubMed] [Google Scholar]
- Wickens K., Black P.N., Stanley T.V. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Imunol. 2008;122:788–794. doi: 10.1016/j.jaci.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Wickens K., Black P., Stanley T.V. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy. 2012;42:1071–1079. doi: 10.1111/j.1365-2222.2012.03975.x. [DOI] [PubMed] [Google Scholar]
- Wickens K., Stanley T.V., Mitchell E.A. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization? Clin. Exp. Allergy. 2013;43:1048–1057. doi: 10.1111/cea.12154. [DOI] [PubMed] [Google Scholar]