Abstract
Background:
Understanding the complexities associated with the ischemic condition and identifying therapeutic targets in ischemia is a continued challenge in stroke biology. Emerging evidence reveals the potential involvement of epigenetic mechanisms in the incident and outcome of stroke, suggesting novel therapeutic options of targeting different molecules related to epigenetic regulation.
Objective:
This review summarizes our current understanding of ischemic pathophysiology, describes various in vivo and in vitro models of ischemia, and examines epigenetic modifications associated with the ischemic condition.
Method:
We focus on microRNAs, DNA methylation, and histone modifying enzymes, and present how epigenetic studies are revealing novel drug target candidates in stroke.
Conclusion:
Finally, we discuss emerging approaches for the prevention and treatment of stroke and post-stroke effects using pharmacological interventions with a wide therapeutic window.
Keywords: HDAC inhibitors, JMJD2 inhibitors, micro RNA, stroke, neurogenesis, angiogenesis, REST complex
1. Introduction: Stroke and its patho- physiology
Stroke stands as one of the leading causes of death and long-term disability (http://www.who.int/mediacentre/factsheets/fs310/en/index.html). In view of present day lifestyle and socioeconomic burden, stroke continues to be one of the most challenging diseases. Stroke is considered a multifactorial and polygenomic condition [1-3]. It is affected by both modifiable, viz., diabetes, hypercholesterolemia, smoking, hypertension, atrial fibrillation, valvular heart disease, ischemic cardiomyopathy, and carotid stenosis, and non-modifiable factors, viz., age, gender, ethnic background and family history of cerebrovascular diseases [4]. Stroke is a pathophysiological condition that arises due to the blood flow interruption (ischemia) either by a thrombus or embolism or from a ruptured blood vessel (hemorrhage), resulting in depletion of oxygen and other essential nutrients that are vital for neural function and survival. This energy imbalance eventually induces dysfunction or cell death of brain cells that often results in neurological impairments depending on the size and location of the ischemic brain area.
The pathophysiology of cerebral ischemia is very complex and involves multiple interlinked processes like excitotoxicity associated ionic imbalance, oxidative stress, inflammation, apoptosis and mitochondrial dysfunction which leads to neural cell death. During cerebral ischemia, cell death occurs in the core region within minutes to hours via a necrotic pathway and in the penumbra region within days to weeks via an apoptotic pathway [5].
1.1. Ionic Imbalance and Glutamate-mediated Excitotoxicity
The brain is ceaselessly dependent upon the steady flow of glucose and oxygen for energy production. During the onset of ischemia, reduced blood flow depletes glucose and oxygen levels, leading to decreased ATP production and functional disruption of Na+/K+ and Ca2+ ATPase ionic pumps present on the plasma membrane of neurons, which in turn results in membrane depolarization via the release of sodium and entry of calcium ions into the cell. Consequently, excessive calcium triggers activation of proteases, lipases, and DNAses that eventually leads to cell death in ischemic core. Moreover, an excitatory neurotransmitter glutamate plays a vital role in the membrane depolarization and pathophysiology of cerebral ischemia. Excessive synaptic glutamate, as a result of reduced uptake in ischemic insult, causes prolonged activation of NMDA and AMPA receptors, that in turn results in excessive calcium influx and membrane depolarization, leading to excitotoxicity [6].
1.2. Oxidative Stress
Oxidative stress is considered as an imbalance between the formation of reactive oxygen species (ROS), reactive nitrogen species (RNS) and antioxidant enzymes. This imbalance leads to the accumulation of oxidative damage via DNA, protein, and lipid oxidative modifications and impaired redox signaling, thus contributing to ischemic brain injury [7-12]. During cerebral ischemia, an increase in the influx of calcium and sodium ions and adenosine diphosphate (ADP) induces mitochondrial activity that can increase the production of ROS, further causing a destruction of cellular macromolecules and activation of apoptotic pathways. Various studies report that in mitochondria a DNA repair enzyme, apurinic/apyrimidinic endonuclease/redox factor-1 (APE/Ref-1), is a major target for redox signaling, leading to cerebral ischemic cell death [13, 14]. Nuclear factor erythroid 2-related factor 2 (NRF2), a key regulator of antioxidant-responsive genes, is altered in ischemia [15], and p21, a stabilizer of NRF2, is known to be associated with the apoptotic pathways induced post-ischemia [16]. Also, redox state of the cell regulates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) transcription factor activation by pro-inflammatory cytokines, and the antioxidants can block this NF-kB activation by inhibiting the phosphorylation of nuclear factor of kappa light poly-peptide gene enhancer in B-cells inhibitor (IkB) in serine residue [17, 18].
1.3. Inflammation
Ischemic injury triggers a rapid activation of the inflammatory cascade immediately after the onset of cerebral ischemia. Within first few hours of the stroke, an array of upregulated pro-inflammatory mediators like chemokines and cytokines are released from the damaged tissue, with increased expression of cell adhesion molecule (CAM) and subsequent transendothelial migration of inflammatory cells. Essentially interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α) and Toll-like receptors (TLRs) perform an important role in stroke-induced neuro-inflammation [19, 20]. However, microglia and astrocytes play an essential role in stroke-induced neuro-inflammation, especially within the penumbra region. Microglial activation produces pro-inflammatory cytokines, toxic metabolites, and enzymes in the penumbra region. The overall role of glia including microglial and astrocytic products may vary at different time periods following cerebral ischemia with either protective or harmful factors occurring within hours to days to even weeks following the onset of cerebral ischemia [21].
1.4. Apoptosis
Cerebral ischemia-induced cell death is a complex interplay of necrosis and apoptosis [22]. Within the ischemic core, due to ATP depletion, necrosis is the predominant form of cell death and in the penumbra region since it can sustain a mild injury and can preserve ATP, apoptosis predominates [23]. Ischemia induces apoptosis through alteration of various gene expression patterns of apoptotic mediators like Bcl-2-associated X protein (BAX), B-cell lymphoma 2 (BCL2), Bcl-2-associated death promoter (BAD), BH3 interacting domain death agonist (BID), etc. [24]. Cytochrome c (cyt c) released from the outer mitochondrial membrane, ionic imbalance, inflammation, mitochondrial and DNA damage can also initiate apoptosis [25].
1.5. Mitochondrial Dysfunction
The Mitochondrion is an organelle for energy production and calcium storage. There is a massive entry of Ca2+ ions into the cytoplasm as a result of a series of events and also from the endoplasmic reticulum (ER) via both inositol triphosphate receptors (IP3R) and ryanodine receptors. Thus, an increased inflow of Ca2+ ions into the mitochondrial matrix alters its permeability [26]. The Mitochondria Permeability Transition (MPT) pore opening [27] and the cyt c and pro-apoptotic factors’ release into the cytoplasm result in the activation of caspase pathway causing neuronal cell death [28]. Heat shock proteins 70 (HSP70) family members have been shown to modulate the conductance of IP3R and ryanodine receptor Ca2+ channels [29].
Regulation of this entire ischemic cascade principally occurs at the transcriptional, post-transcriptional and post-translational level, involving numerous epigenetic factors.
2. Experimental models of cerebral ischemia
2.1. In Vivo Models of Ischemia
Till date, many experimental models have been developed to mimic human stroke conditions. Rodent models are used to study the possible mechanisms of brain injury following several hours to days after ischemic events, and for in-depth studies of potential neuroprotection. Moreover, with rodents, there is a possibility of observing/measuring sensory and motor behavioral outcomes post-cerebral ischemia. There are two main categories of cerebral ischemic models reported, global ischemia and focal ischemia. Global cerebral ischemia occurs due to reduced blood flow throughout or most of the brain, while in focal cerebral ischemia, a minute blood flow is allowed to the central core of the ischemic region, with some blood reaching the brain via vertebral arteries [30]. The principal theme of these models is to reduce glucose and oxygen supply to brain tissue and study the possible mechanisms in the pathophysiologic condition.
Focal cerebral ischemia condition simulates human cerebral stroke condition whereas global ischemia is clinically more relevant to cardiac arrest and asphyxia. Focal cerebral ischemia model typically involves middle cerebral artery occlusion (MCAO), a major cerebral blood vessel, in rodents [31, 32] and large mammals [33]. The following are a few of the models that are extensively used to produce focal cerebral ischemia in rodents, dissect out mechanisms underlying the pathophysiology, and discover novel therapeutic targets and test the efficacy of novel compounds.
2.1.1. Transient Middle Cerebral Artery Occlusion
Among all the available ischemic stroke models, the transient MCAO rodent model is the most frequently used due to lesser invasiveness, ease of performance, and reproducible territory infarct volume. In this model, MCA is occluded by the insertion of monofilament of varying length into the internal carotid artery up to the branch of MCA to block the blood flow as much as possible for 30-120 minutes followed by reperfusion by retracting the suture [34, 35]. This occlusion results in quite extensive damage to specific brain areas like striatum, hippocampus, and cortex, which subsequently leads to neuronal cell death by exacerbating the inflammatory response during reperfusion. This model has an advantage over permanent MCA occlusion model as lesion induced by more than 3-4 hours of ischemia cannot be reversed [36].
2.1.2. Photothrombosis Model
This model represents an alternative approach to producing a blood vessel occlusion. To produce cortical infarct, thrombosis within a specific blood vessel is induced by systemic injection of photoactive dye especially Rose Bengal or erythrosin B in combination with irradiating of beam light at a particular wavelength [37]. The reaction between the light of the correct wavelength and the photoactive dye generates oxygen radicals, which cause peroxidation of endothelial lipids and blood elements that will lead to platelets activation and thrombosis within the irradiated area [38]. This model has an advantage as it can regulate the degree of damage and eventually ischemic lesion size, through the manipulation of both intensity of beam light and the concentration of photoactive dye. A major disadvantage of this model is that it lacks penumbra area due to vasogenic edema and the blood brain barrier (BBB) breakdown in the ischemic lesion area occurs within minutes [39]. However, photothrombotic ring model with varied beam light intensity and duration seems to induce cortical lesion along with penumbra [40].
2.1.3. Endothelin-1 Stroke Model
Endothelin-1 produced by vascular endothelial cells is a potent vasoconstrictor [41]. The vasoconstrictor is injected stereotaxically and intracerebrally at the MCA site or adjacent to it in a dose-dependent manner [42, 43]. Reversibility of ischemia and less invasiveness are the advantages of this model. Moreover, it can induce ischemia comparable to MCAO model by controlling the extent and duration of ischemia.
2.1.4. Thromboembolic Model
This model has higher relevance to human stroke conditions, as thromboembolism is the principal cause of stroke in humans [44]. Here, an autologous blood clot is injected in the extracranial artery to induce thromboembolic ischemia. A human blood clot is introduced into the common carotid artery or internal carotid artery to produce embolism [45, 46]. In most of the studies, multiple fibrin-rich autologous clots are injected simultaneously into the external carotid artery to avoid the problem of spontaneous recanalization after 3hr of embolism and to reduce cerebral blood flow which leads to histological damage in MCA territory [47].
2.2. In Vitro Models of Ischemia
Even with the limitations like absence of blood vessels, blood flow and the differences in the composition and responsiveness of glial cells [48], isolated cells in vitro behave under substrate stress conditions strikingly similar to cells in vivo during stroke condition. In vitro condition provides a highly controlled experimental system and helps in generating detail information on how cells respond to oxygen and glucose deprivation.
2.2.1. Glucose Deprivation (GD) and Combined Oxygen-glucose Deprivation (OGD)
GD and OGD are general in vitro models of brain ischemia. In GD model, primary brain cell cultures and cultured slices are subjected to media lacking glucose. In OGD model, along with the media lacking glucose, a very low level of oxygen is supplied to the chamber for a fixed period followed by their restoration to imitate reperfusion [49-51]. Further, individual cell types and intact circuitry in the slices can be studied in vitro.
3. Epigenetic Machinery
Chromatin biology is one of the most exciting fields of biomedical science and translational research at present. Chromatin mostly comprises DNA, histone proteins and associated factors that together form a highly complex, compact and coiled superstructure that is dynamic in nature [52]. The basic unit of chromatin is the nucleosome, composed of DNA wrapped around an octamer of histone proteins H2A, H2B, H3, and H4, each in dimer form. The dynamic nature of chromatin structure involves local and global level modifications. Replacement of histone proteins in the octamer with its variants (e.g., H2A.Z) can alter the biophysical and biochemical properties of a nucleosome, influencing various processes like gene activation, chromosome segregation, and cell cycle progression [53]. All these modifications which are beyond genomics are called epigenetic and can modulate the gene expression by altering the chromatin structure in and around the genes, unfolding the information or repressing it.
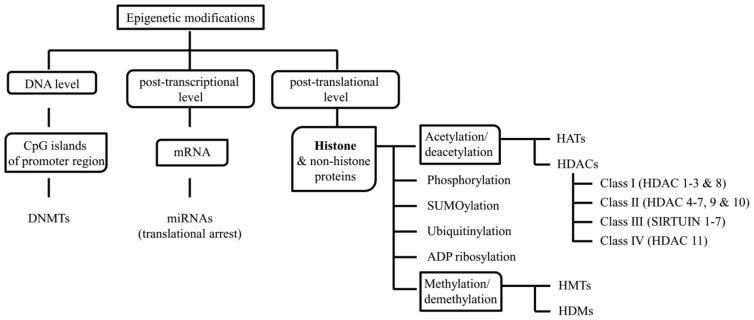
Epigenetic changes can occur at various levels including DNA, post-transcriptional and post-translational levels via DNA methylation, microRNAs (miRNAs), and histone acetylation, methylation, phosphorylation, sumoylation, ubiquitination or ADP-ribosylation respectively (Fig. 1). In the promoter region, DNA methyltransferases (DNMTs) methylate DNA at CpG islands and confers localized gene repression by recruiting chromatin modifiers [54]. At post-transcriptional level a class of short (≈ 20-25 nucleotides) RNA molecules, miRNAs, can alter the translation of the mRNA by binding to it using the seed sequence (≈ 5-7 nucleotide long), which confers specificity to miRNA for the mRNA, and either impede or hasten its degradation. Mostly, miRNA binding sites are present at 3’ untranslated region (UTR) of the mRNA but for a few in other regions of the mRNA [55, 56]. At post-translational level, at which mostly histones and a few non-histone proteins have been studied, acetylation and methylation are the most commonly reported modifications. Histone acetyltransferases (HATs) and histone deacetylases (HDACs) maintain the acetylated and deacetylated state of histone proteins respectively. HATs and HDACs add and remove an acetyl group respectively from the lysine group of a lysine-rich amino-terminal tail of histones. Acetylation of histone proteins results in charge neutralization and reduction in association with the negatively charged DNA, resulting in a more relax and open form of chromatin that can allow free binding of transcription factors and co-activators to facilitate active transcription. Based on the structure, sequence homology and domain organization, HDACs are categorized into four classes. Class I (HDAC 1-3 and 8), class II (HDAC 4-7, 9, and 10), and class IV (HDAC 11) HDACs are zinc-dependent while class III (Sirtuin 1-7) HDACs are nicotinamide adenine dinucleotide (NAD) dependent. Similar to acetylation-deacetylation, methylation and demethylation are also maintained by histone methyltransferases (HMTs) and histone demethylases (HDMs) respectively. However, contrary to acetylation, methylation either induces or represses gene expression based on the type of methylated residue (lysine or arginine) in histones and the number and the position of methyl groups added to the residue [57].
Fig. (1).
Epigenetic modifications at the DNA level, post-transcriptional level and post-translational level and the molecules and enzymes involved at each level.
Similar to the “one to many and many to one” relationship, a single modification can recruit many effector molecules and can engage in multifaceted response, while a crosstalk between the modifications mentioned above can form a hierarchy “code” and determine the functional genomics [58]. Many effector molecules identify the specific histone modifications through their protein domains (e.g., Tudor, PHD fingers, chromodomains, and bromodomains), and can alter the interaction between the chromatin and the multimeric complexes which promote nucleosome repositioning [e.g., SWItch/Sucrose NonFermentable (SWI/SNF)] and higher order chromatin remodeling (e.g., PcG) [59]. The histone-modifying enzymes, transcription factors, and effectors can act alone or together as part of the macro- molecular epigenetic regulatory complexes, such as the Repressor Element-1 Silencing Transcription factor (REST)/ Neuron-Restrictive Silencer Factor (NRSF) complex [60].
4. Epigenetics and cerebral ischemia
Accumulating evidence suggests that epigenetic modifications play a role in the etiology and progression of neuropsychiatric diseases [61, 62], neurodegenerative disorders [63, 64], and neurodevelopmental processes [65, 66]. However, for cell survival and homeostasis, a regulated and coordinated expression of genes is imperative. Pathophysiological condition (e.g., ischemia) may induce and/or repress genes and thereby lead to activation of protective and/or harmful pathways in the cell [67]. Recent investigations have implicated the role of epigenetic mechanisms in ischemia-induced brain damage. Molecular studies using various models of stroke in rodents have shown that the global amount of DNA methylation increases after an ischemic insult, and this increase correlates with the augmented brain injury [68, 69]. At the post-transcriptional level, it has been estimated that miRNAs regulate at least 30% of protein-coding genes, and several recent studies have demonstrated the alterations in the cerebral “miRNA-ome” following ischemia/reperfusion [70-72]. At post-translational level, acetylation of histone proteins has been extensively studied, and to an extent their methylation in ischemic condition. A general decrease in H3 and H4 acetylation has been observed in and around the ischemic core region. Histone 2AX is phosphorylated upon oxidative stress and accumulates with the progressing injury [73]. The macromolecular complex RESTis a master transcription and epigenetic regulator that has been shown to be deregulated following ischemia and reperfusion, resulting in altered expression of an extensive repertoire of protein-coding [60, 74] and non-coding genes [75, 76]. Studies of epigenetic mechanisms in stroke are in their infancy but offer a great promise for a better unders- tanding of stroke pathology and the potential viability of new strategies for its treatment. The potential involvements of epigenetic mechanisms at the onset and during the stroke recovery at different levels of epigenetic machinery are described below.
4.1. DNA Methylation
Cerebral ischemia leads to a massive alteration in gene expression [67, 77] to restore the cellular damage in the ischemic core region. The DNA methylation based epigenetic mechanism responsible for an increase in CpG island methylation regulates specific and global gene expression by transcriptional inhibition. There are reports of an increase in DNA methylation after an ischemic insult causing transcriptional repression of a large number of genes leading to an augmented brain injury [68, 69]. Mice expressing reduced levels of DNA methyltransferase 1 (DNMT1) in postmitotic neurons were protected from the ischemic brain injury while a complete absence failed to lead a functional recovery [68]. Reduced DNMT1 levels influence the chromatin structure and allow transcription factors (e.g. HIF-1) to bind and up-regulate expression of genes involved in neuroprotection. DNA methylation changes can occur at gene specific, promoter specific, or global level [78].
Recent studies also show that ischemia-induced production of ROS/RNS appears to modify CpG island methylation (which correlates with transcriptional inhibition) by converting 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC), and alternatively regulates the methylation pattern by inhibiting the binding of Dnmt1 and MBP to DNA [79-81]. According to a recent clinical study, 5hmC was found to be increased in blood cell DNA from stroke patients [82]. Besides, peroxides also modify nucleobase that mimics 5-mC and induce improper Dnmt1 methylation that leads to gene silencing [83, 84].
4.2. MicroRNA (miRNA)
Few studies have reported the role of miRNA in the modulation of dendritic plasticity and neural growth [85-87] while others have reported altered miRNA expression profile in chronic neurological diseases like Alzheimer’s [88] and schizophrenia [89]. It will be interesting to study the miRNA profile in acute cell stress condition, oxidative stress and stroke condition. Recent efforts in miRNA profiling have revealed the changes in miRNAs in the rat MCAO model [70, 71, 90], forebrain ischemia model [91], neural stem and progenitor cells (NSPCs) associated with stroke-induced neurogenesis [92], and in stroke patients [72]. NSPCs from the subventricular zone (SVZ) of ischemic animals showed a significant change in the top 10 enriched miRNAs compared to non-ischemic animals. A reduced level of the novel miRNA pc-17172 is suggested to be involved in stroke-induced neurogenesis [92]. In the rat MCAO model, there was an increase in miR-146a density in the subventricular zone and corpus callosum of the ischemic hemisphere [93]. In another rat MCAO model, an increase in miR-140, miR-145 and miR-331 was observed at three hours following reperfusion [90]. In an ischemic preconditioning study, about eight miRNAs, namely miR-200a, miR-200b, miR-200c, miR-141, miR-429, miR-182, miR-183 and miR-96, were found upregulated following three hours of reperfusion [94]. In a subsequent in vitro study, miR-200b, miR-200c and miR-429 were identified to be cytoprotective. Neuro-2a cells transfected with miR-200b and miR-200c resulted in increased neural cell survival when subjected to oxygen-glucose deprivation [94]. Levels of miR-15a were inversely related to the oxygen, glucose deprivation induced cerebral vascular endothelial cell death [95]. In vivo repression of miR-497 reduced MCAO model induced infarct volume and improved the neurological deficits [96]. miR-210 was found to be a salient component in the hypoxia response coordinated by HIF. Also, miR-210 is positively correlated with better prognosis in stroke patients [97]. miR-424 has shown neuroprotection against transient cerebral ischemia-reperfusion injury via reducing oxidative stress [98]. Also, vagus nerve stimulation can modulate redox state of the cell and show neuroprotection against cerebral ischemia via activating neuronal and astrocyte α7n acetylcholine receptor and most probably in association with increased miR-210 expression [99]. Further, miRNAs have also been suggested to regulate aquaporin gene expression in ischemic conditions, the molecule implicated in stroke pathophysiology [100].
Further to add complexity, spatial expression of miRNAs was observed, such as a regional expression of miR-121 in the ischemic penumbra [101] and an upregulation of miR-181a in hippocampus following 30 min of reperfusion. However, no change in brain miR-181 level was observed following either permanent focal ischemia [71] or transient focal ischemia [90]. Complementary expression of miR-181a and GRP78 protein, a chaperone that protects primary cultured astrocytes against ischemic injury, was found in both the core and penumbra. In vitro experiments show that miR-181a mimic increases GRP78 protein expression while miR-181a inhibitor decreases the level of the chaperone [102]. miR-15a and miR-497 contribute to the pathogenesis of ischemic injury by inhibiting the antiapoptotic gene BCL2 at translational level [95, 103]. In another study, miR-181a levels were negatively correlated with BCL2 and MCL1 protein levels, the pro-survival proteins [104]. A study based on luciferase reporter assay confirmed that BCL2 and MCL1 are the direct targets of mouse miR-181a [105]. Based on the computational miRNA target prediction algorithms (http://targetscan.org, Release 6.2), four miRNAs were found to target both HSP70 and BCL2 family members. miR-181 can target GRP78, BCL2, BIM, and MCL1, miR-30 can target GRP78, BCL2, and BIM, miR-200 can target GRP75 and BCL2 and miR-17 can target HSP73 and BCLw [104] (Table 1). Further, there is a differential response between sexes in miR-23a levels in rats subjected to MCAO [106]. So, miRNA profiling studies might help in explaining the gender-based differences in ischemic response and outcome. Thus, miRNAs can be considered to serve as both biomarkers and therapeutic targets of ischemia.
Table 1.
miRNAs and their predicted targets [107].
| miRNAs | Targets | ||
|---|---|---|---|
|
Chaperones
(HSP70 Family) |
BCL2 Family | ||
| Pro-survival | Pro-apoptotic | ||
| miRNA-181 | GRP78 (Endoplasmic reticulum form) | BCL2, MCL1 | BIM |
| miRNA-30 | GRP78 (Endoplasmic reticulum form) | BCL2 | BIM |
| miRNA-200 | GRP75 (Mitochondrial form) | BCL2 | |
| miRNA-17 | HSP73 (Constitutive) | BCLw | |
4.3. Histone Acetylation/Deacetylation
Global transcriptional repression, as reflected by a general decrease in H3 as well as H4 acetylation levels, has been reported in ischemic condition [108-117]. Therefore, activating HATs or inhibiting HDACs, the strategy to restore the acetylation of histones and gene transcription, may possibly ameliorate the condition.
A decrease in the substrate acetyl-CoA due to anaerobic metabolism in hypoxic condition might lead to inhibition of HAT activation [118]. However, there is also a report that HAT activity does not appear to be modified by cerebral ischemia [108]. To add to the complexity, HATs, CBP/p300, and SRC-1 are known to be involved in hypoxia-mediated transactivation of hypoxia inducible factor-1 (HIF-1) [118, 119]. On the contrary, genetic inhibition of a HAT, CBP/p300, shows neuroprotective effects after experimental global cerebral ischemia [120].
In MCAO, upregulation of HDAC 3, 6 and 11 in cortex occurs within hours after the stroke that lasts during early phases [121]. After a week, HDAC 1, 2 and 3 are still upregulated in the peri-infarct region, but not in the ischemic core [122]. In in vitro studies, 60 minutes after the OGD there is an increase in HDAC1, 2 and 3 in glial cells [122]. Whereas in both MCAO and OGD models there is an NADPH oxidase-mediated downregulation of other HDACs like HDAC4 and HDAC5 [123]. In the approaches to pin down the role of single HDAC isoforms in rodent models, HDAC 3 and 6 were identified as potential mediators of neurotoxicity [121]. HDACs play different roles in oxidative stress following stroke. Cerebral ischemia/reperfusion injury deteriorated the HDAC2-FOXO3a interaction via reducing phosphorylation at Ser 394 of HDAC2. In addition to this, H2O2 also reduced this interaction in cerebellar granule neurons and resulted in increased H4K16 Ac in the p21 promoter region and upregulated its expression, leading to oxidative stress-induced neuronal cell death [124]. Transient MCAO and prolonged OGD resulted in a reduction in the expression of one of the Class III family of HDACs, i.e. SIRT1 [125, 126], whereas, short OGD and moderate ischemic insults failed to alter SIRT1 expression [127]. On the other hand, there is a protective effect of SIRT1 upregulation in stroke condition [126] which might be through its non-histone targets [128]. In a genome-wide association study in human, a new association was found between HDAC 9 and the large vessel stroke, one of the brain infarct subtypes. Accordingly, carriers of the A-allele (about 9% of the population) as well as homozygous carriers (1% of the population) have an increased chance of stroke [129, 130].
4.4. Histone Methylation/Demethylation
In ischemia, the glutamate receptor 2 (GluR2) gene is repressed and is associated with H3K9 deacetylation and rise in H3K9 dimethylation over its promoter in hippocampal CA1 neurons. Recently it has been reported that inhibition of transcriptional repressor at the level of histone methylation can promote neuronal survival in cerebral ischemia in vitro model. Also, pharmacological inhibition of histone methyl- transferases like SUV39H1 and G9a can significantly improve neuronal survival after OGD in in vitro ischemia model [131, 132].
Our own study using mild ischemia model in CD1 mouse shows dysregulation of many histone lysine methylases (HMTs) and few of histone lysine demethylases (HDMs) at various time intervals post-ICAO (internal carotid artery occlusion) [133]. This causes significant attenuation in the transcriptionally repressive epigenetic modification H3K9me2, in the affected striatal region 1d post-ICAO. Sudden decrease in this transcriptionally repressive H3K9me2 in early hours of acute ischemic insult also appears to be due to an increase in the transcription of one of the JMJD2 or HDM4 family member of HDMs. Administration of few inhibitors of JMJD2/ HDM4 lysine demethylases during the stroke induction in mice significantly improved the stroke-induced neurological deficit and reduced the apoptotic cell death by restoring the perturbed H3K9me2 level in the affected striatum. These findings [133] suggest a critical role of epigenetic regulatory mechanisms involving histone lysine methylation and demethylation in stroke-induced damage and subsequent repair.
Jumonji C (JmjC) domain-containing histone demethylases belong to the Fe(II) and 2-oxoglutarate-dependent oxygenase family [134]. These HDMs catalyze the demethylation of histone lysine residue and require molecular oxygen for their function. However, various reports show that in hypoxic condition, JmjC activity is still maintained under reduced oxygen tension, and even its gene expression is strongly induced by HIF-1α in in vitro hypoxia model [135]. It has been suggested that this maintenance property of JmjC domain containing histone demethylase enzyme is due to the ferrous state of the enzyme, which contributes to its conserved activity in hypoxic condition too. Otherwise, the activation of ROS, superoxide, H2O2, and peroxynitrite can readily oxidize ferrous to the ferric state. Ascorbate, an essential cofactor of this enzyme, also has a crucial role in maintaining it in the ferrous state in a reduced condition, which is required for the enzymatic activity [136]. However, ascorbate levels can change with the cellular redox state and show a redox switch for JmjC histone demethylase enzymatic activity. This JmjC domain-containing histone demethylase is regulated by cellular redox state and directs antioxidant protection under oxidative stress conditions and apoptosis [137].
4.5. REST, PcG & TrxG Proteins
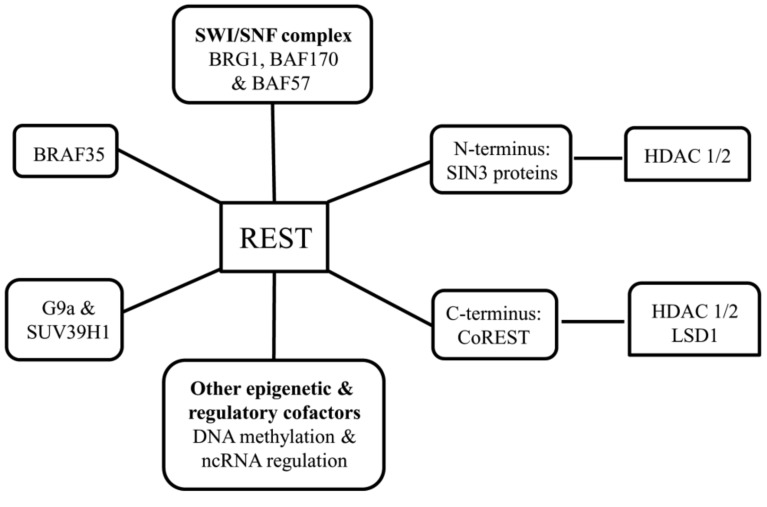
One of the most investigated epigenetic molecule in ischemia, Repressor Element-1 Silencing Transcription factor (REST) protein, also known as NRSF (Neuron-Restrictive Silencer Factor], binds to SIN3 proteins (i.e., SIN3A and SIN3B) at its N-terminus and to the corepressor for element-1 silencing transcription factor (CoREST) protein at its C-terminus. SIN3 proteins are scaffolds and corepressors that, in turn, recruit HDAC1/2. Similarly, CoREST can recruit various factors, including HDAC1/2 and the histone H3K4 lysine demethylase, LSD1. REST also associates with the histone H3K9 methyltransferases, G9a, and SUV39H1; the SWI/SNF chromatin remodeling complex components, BRG1, BAF170, and BAF57 and the high mobility group protein BRAF35. REST can also associate with other epigenetic and regulatory cofactors implicated in DNA methylation and ncRNA regulation [138] (Fig. 2). This large machinery helps in changing histone modifications and higher-order chromatin remodeling. REST complex has been shown to be associated with the pathophysiology of ischemia and other disease conditions. In the neurons destined to die, REST complex was found upregulated, and the knockdown of REST rescued these post-ischemic neurons from cell death [131]. REST represses the expression of AMPA receptor subunit GluR2 and µ opioid receptor 1 (MOR-1) through deacetylation of histone H3 and H4 [131], deacetylation of histone H3 and H4 and also dimethylation of H3K9 [139] at their respective promoter regions. The deacetylation and methylation activity is probably mediated by HDAC1/2 and G9a respectively. SWI/SNF chromatin remodeling complexes serve as regulators of HIF and are considered critical mediators of cellular responses to ischemia [140].
Fig. (2).
REST complex with its associated proteins and enzymes.
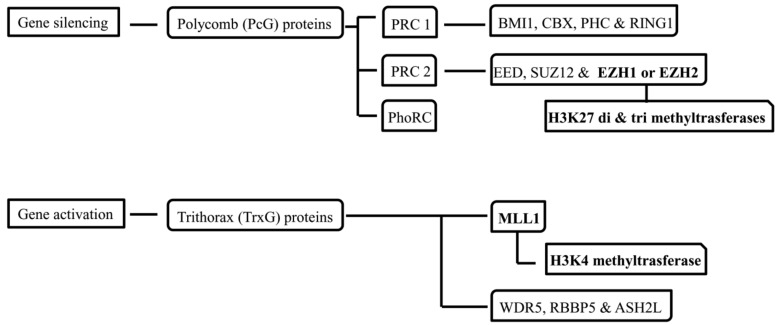
Polycomb (PcG) and Trithorax (TrxG) group proteins form combinatorially assembled multi-subunit complexes with respective inhibitory and activating histone modifications. Polycomb repressor complexes (PRC) formed from PcG proteins is associated with gene silencing through di- and tri- methylation of H3K27 using histone methyltransferase activity of either the Enhancer of Zeste 1 or 2 (EZH1 or EZH2) catalytic subunits [141] (Fig. 3). In a study, proteomic analysis of ischemic preconditioned brain tissue found an increase in the levels of PcG proteins [142]. TrxG proteins assemble into complexes associated with gene activation using the H3K4 methyltransferase activity of MLL1 catalytic subunit [143]. These PRC and TrxG complexes are also functionally interrelated with multiple interactors, comprising those involved in DNA methylation and ncRNA regulation, in a cell type- and gene-specific manner [144].
Fig. (3).
Polycomb (PcG) and Trithorax (TrxG) group proteins with their associated proteins and enzymes.
So, from the recent epigenetic investigations in ischemia models, it can be concluded that targeting the key epigenetic molecules using pharmacological intervention may possibly rescue or mitigate the neural cell damage by activating or repressing the protective or harmful pathways respectively.
5. Endogenous Recovery
In response to ischemic insult, the innate system activates mechanisms which can prevent, reduce or reverse the damage induced by ischemia. Neurogenesis, angiogenesis, and synaptic plasticity are part of the post-ischemic recovery mechanisms activated in response to ischemia.
5.1. Neurogenesis
In adults, new neurons are generated from the endogenous neural stem and progenitor cells (NSPCs) in the subventricular zone (SVZ) lining the lateral ventricles, which migrate to the olfactory bulb to get integrated into the neuronal network. Neurogenesis is not only modified by physiological stimuli, but also by brain insults like stroke, traumatic brain injury, and epilepsy. Stroke not only causes ischemic cell death, but it also triggers regenerative processes in the tissue adjacent to the area of cell death. Stroke induces proliferation of endogenous NSPCs. These newly born cells migrate into the damaged area and differentiate into cells with phenotypic markers of mature neurons [145]. Understanding the cellular and molecular mechanisms that play an important role in this neuronal regeneration can lead to novel therapeutics for brain injury in stroke and other conditions [146].
Most studies have focused on the activation of NSPC self-renewal, proliferation, migration, survival and synaptic plasticity by using growth factor such as BDNF [147]. However, epigenetic mechanisms also play a pivotal role in the neural cell proliferation and differentiation. Both in vitro and in vivo studies have implicated histone methylation and acetylation in neural lineage specification and maturation. Histone demethylase LSD1 has been demonstrated to regulate Neural Stem and Progenitor Cell (NSPCs) proliferation [148]. Of the PcG proteins, BMI1 mediates NSPC self-renewal, and proliferation [149] while EZH2 regulates the differentiation of NSPCs into oligodendrocytes (OLs) [150]. The TrxG protein, MLL1, has been shown to be required for neurogenesis, but not gliogenesis [151].
Several studies have also shown that modulation of histone acetylation by administering HDAC inhibitors (HDACi) such as Sodium Butyrate (SB), Valproate (VPA), and Trichostatin A (TSA) can modulate proliferation, differentiation, and associated processes. SB induces BDNF-receptor tyrosine kinase (TrkB)- dependent cell proliferation, migration, and differentiation [152]. VPA induces various proneuronal genes like NeuroD, Math1, Ngn1 and p15 to promote neural differentiation of hippocampal neural progenitor cells [153, 154] and TSA suppresses oligodendrocyte (OL) specific gene expression programs and prevents the progression of OL progenitor cells to post-mitotic OLs. These observations have led to the speculation that HDACi can enhance NSPC proliferation, migration, and differentiation in the brain after ischemic injury to help recover fast from the ischemic damage [152].
5.2. Angiogenesis
Post-stroke angiogenesis is an endogenous recovery process associated with the increase in vascular endothelial growth factor (VEGF) mediated by transcription factor HIF-1. In this short-lived event, the expression of thrombospondin-1 (TSP1), an endogenous angiostatic factor, is downregulated by DNA methylation at its promoter region [155]. There is also an alteration in the expression of the matrix metalloprotease (MMP) family members MMP 2 and 9, which have pro-angiogenic [114] and blood-brain barrier (BBB) disruption [156] properties, depending on the time point post-stroke. In post-stroke HDACi treatment, enhanced microvessel density and increased relative cerebral blood flow are attributed to HIF-1 activation and its downstream VEGF. Additionally, MMP 2 and 9 protein levels are also found increased [114].
5.3. Synaptic Plasticity
Ischemia can induce plasticity through cortical reorganization at structural and functional levels, leading to functional recovery [157, 158]. The increase in excitability around cortical ischemic lesions possibly results from a decrease in GABAergic inhibitory activity [159]. Results from non-invasive techniques support the hypothesis that an increase in neuronal excitability in non-injured brain areas may contribute to clinical recovery after a stroke [160]. Preconditioning with sub-lethal ischemia induces a neuroprotective form of neuronal plasticity which provides tolerance to subsequent lethal ischemia [161]. In gerbils, preconditioning ischemia a few days before a longer period of forebrain ischemia protects against neuronal death [162]. Ischemia-induced long-term potentiation (LTP) mimics physiological LTP induction by the expression of calcium-permeable AMPARs (CP-AMPARs) at synapses and activation of adenosine A2A receptors (A2ARs) [163]. Epigenetic dysregulation is a common theme in disorders of synaptic plasticity and cognition including neurodegenerative disorders [Parkinson’s disease (PD) and ischemia] and mood disorders (depression and anxiety). Evidence exists to suggest that the epigenetic regulator REST actively represses a large array of neural-specific genes essential for synaptic plasticity including synaptic vesicle proteins, structural proteins, voltage-gated channels, and ligand-gated receptors [164, 165].
6. Pharmacological intervention
Various compounds are currently known to mitigate ischemia-induced damage in experimental animal models. However, clinical trial results have not been reassuring with regard to their potential toxic side-effects [166]. As of now, the only globally approved treatment for stroke is the time-dependent thrombolytic therapy, i.e. intravenous administration of tissue plasminogen activator (tPA) within 4.5hr post-injury. Its beneficial effects warrant recovery bypassing the risk of hemorrhage [167, 168]. Therefore, there is a requisite for intense research to find the potential targets and drugs towards surpassing the limitations posed by the complexity of the condition and the limited therapeutic window, and towards negligible side-effects. Here, we review the molecules that have shown potential to treat ischemic conditions through targeting different components of epigenetic machinery.
6.1. DNA Methylation
There are reports which suggest that pharmacological inhibition of DNA methyltransferase by 5-aza-2′-deoxycytidine alters the transcriptional processes at the epigenetic level and can reduce neurobehavioral deficits in models of ischemia [69].
6.2. Micro RNA
In ischemic condition, a few of the numerous altered miRNAs target chaperone and BCL2 families of proteins. The miRNA levels can be altered using mimics and inhibitors or antagomirs which can increase and decrease the effective levels of a miRNA, respectively. An anti-miRNA (miravirsen or SPC3649) targeting a liver miRNA, miR-122, is being developed as a hepatitis C therapy. SPC3649 is in Phase IIa clinical trials (sataris.com). In other conditions like lung cancer and ischemic heart disease, miR-34a [169] and miR-210 [170] are targeted, respectively. Early miR-124 injection has been found to increased the polarization to M2-like microglia/macrophages from M1-like microglia/ macrophages, resulting in a significantly increased neuronal survival with reduced lesion core [171]. Overexpression of miR-146a in NSPCs upon transfection of its mimics is known to significantly increase the percentage of neuronal marker positive neuroblasts [93]. Further studies are warranted on the potential use of miRNAs as therapeutic targets in ischemic condition, towards developing novel treatment strategy with higher specificity.
6.3. Histone Modifications
Application of HDAC inhibitors (HDACi) can modulate the expression and alter the outcome at different levels of the ischemic pathophysiologic cascade, as these inhibitors have also been shown to protect oxidative neuronal cell death induced by peroxide addition/glutathione depletion [110] and ameliorate post-ischemic condition [109, 110, 172]. Other HDACi like hydroxamate-based HDACi act via HDAC-independent mechanism and reduce neuronal cell death induced by oxidative stress. This mechanism involves in situ formation of hydroxamate-iron complexes that catalyze the decomposition of H2O2 [173]. Pre-treatment with the HDACi VPA has been shown to prevent glutamate-induced excitotoxicity in cortical neurons [174] and cerebellar granular neurons in an in vitro model [175, 176]. HDACi administration has also been found to preserve the white matter functioning and to stimulate astrocytic glutamate uptake in an OGD model [177]. Treatment of astrocytes with the HDACi TSA is known to result in an increased glutamate uptake, with an increase in the GluR1 and GluR2 expression [178]. Pretreatment with various HDACi increases the expression of NRF2 and the associated antioxidant-responsive genes in an in vitro model of stroke by repressing the NRF2 suppressor Keap1 [15]. It also enhances p21 promoter acetylation and its expression [110]. HDAC4 activity appears to be required for the suppression of p21 activity, suggesting that it acts as a potential and a selective target of HDACi [179]. Resveratrol, a modulator of sirtuin activity, acetylates NRF2 protein, a critical transcription factor targeting antioxidant genes and responses, and thereby provides cell protection [180].
Administration of the HDACi VPA or sodium 4-phenylbutyrate (SB) suppresses microglial activation and other inflammatory markers like monocytes and macro- phages in stroke [109, 115]. VPA treatment induces HSP70 transcription, as reflected by an increase in H3K4 methylation at its promoter region [181]. HSP70 participates in protein folding and is involved in both pro and anti- inflammatory processes depending on the cell type, context, and location [182]. HDACi treatment upregulates the pro-survival proteins like BCL-2 and BCL-xL [108, 109, 111] and downregulates the pro-apoptotic players like BAX [183] and Caspase 3 (CASP3) [112]. Further, administration of SB has been shown to decrease the phosphorylation of eIF2α and endoplasmic reticulum stress-induced cell death, like C/EBP homology protein (CHOP) [172].
Suberoylanilide hydroxamic acid (SAHA) and TSA treatments have shown neuroprotection in ischemic models, as reflected by a decrease in infarct volume and an increase in histone acetylation levels [108, 116]. Moreover, SAHA and TSA cause a dose-dependent effect, with upregulation of the neuroprotective genes BCL-2 and HSP70 [112] and protein gelsolin [116]. Besides increasing histone acetylation levels, HDACis also affect histone methylation levels and miRNA pathways [184]. Our own recent study also suggests that SAHA plays a neuroprotective role in ICAO, a mild to moderate ischemia model in mouse. In this model, SAHA attenuates the increased expression of IL-1α and CASP3, with a concomitant increase in H3K9K14ac level in the affected striatum region, 1d post-ICAO [117].
Pan-HDACi and Class I HDACi increase histone 3 lysine 4 (H3K4) di- and trimethylation (me2/me3) and decrease the repressive H3K9me2 marks, a typical epigenetic change reflecting transcriptional activation [181, 185]. Notably, HDACis do not always confer protection as HDAC1 inhibition or loss also induces p25/CDK5 activation, DNA damage and neuronal cell death [186]. Specific knockdown of HDAC 3 and 6 in an in vitro model of stroke, the oxygen-glucose deprivation (OGD) model, has been shown to enhance the survival of cortical neurons [121]. In an oxidative stress model, selective inhibition of HDAC 6 alone has been found to result in attenuation of cell damage, comparable to the effect of pan-HDAC inhibition [187]. Therefore, inhibitors which can selectively target individual HDAC need to be developed to reduce the side-effects of pan HDACi. It is notable here that effects of HDACi also depend on dosage, timing, and route of administration.
6.4. REST, PcG & TrxG Proteins
2-aminothiazole derivatives inhibit REST activity and cause upregulation expression of the target genes [188-190]. LSD1, a member of amine oxidase enzyme superfamily, is a histone lysine demethylase, the activity of which is inhibited by monoamine oxidase (MAO) inhibitors [191, 192] and polyamine analogs [193, 194]. The activity of histone methyltransferase G9a and SUV39H1 is inhibited by BIX-01294, UNC0224 and UNC0321 [125, 195, 196], and Chaetocin [197], in that order. The S-adenosylhomocysteine hydrolase inhibitor 3-deazaneplanocin causes a reduction in cellular levels of the PRC2 components, EZH2, SUZ12, and EED, an inhibition of H3K27 methylation, and an upregulation of PRC2 target genes [198]. Similarly, dietary omega-3 polyunsaturated fatty acids (PUFAs) downregulate EZH2, probably through post-translational mechanisms decreasing H3K27me3 and upregulating EZH2 target genes [199, 200]. Levels of MLL1 are increased and decreased by deoxynivalenol [201] and radicicol [202], respectively. Interaction with WDR5 is important for H3K4 methyl- transferase activity of MLL1, and two 3-mer peptides, Ac-ARA-NH2, and Ac-ART-NH2 are known to inhibit the interaction between MLL1 and WDR5 [203] (Table 2).
Table 2.
REST complex, PcG & TrxG proteins, and their modifying agents [138].
| Macromolecular Complex | Protein | Modifying Agent |
|---|---|---|
| REST complex | REST | 2-aminothiazole derivatives |
| LSD1 | Monoamine oxidase (MAO) inhibitors Polyamine analogs |
|
| G9a |
Small molecules: BIX-01294, UNC0224 and, UNC0321 |
|
| SUV39H1 | Chaetocin (Mycotoxin) | |
| Polycomb (PcG) proteins | PRC2 (EZH2, SUZ12 & EED) | 3-deazaneplanocin (DZNep) Omega-3 polyunsaturated fatty acids (PUFAs) |
| Trithorax (TrxG) group proteins | MLL1 and WDR5 | Deoxynivalenol (Mycotoxin) Radicicol 3-mer peptides (Ac-ARA-NH2 & Ac-ART-NH2) |
Conclusion
Targeting epigenetic machinery by repressing or activating a few critical epigenetic regulators using enzyme inhibitors, small molecules, mimics and antagomirs of miRNA, etc. may not only prevent and mitigate the damage caused by the ischemic insult and oxidative stress following the stroke but may also ameliorate the post-stroke conditions by inducing neurogenesis, angiogenesis, and synaptic plasticity. The Neuron-damaging effects of oxygen radicals strengthen the evidence supporting a role of redox signaling, and its targets like mitochondrial cytochrome c release, DNA repair enzymes, and transcription factors, in neuronal apoptotic cell death. Several HDACis have shown promising results in medical conditions like cancer, and are at various phases of clinical trials, with a few making it to the clinics (just one, Vorinostat). HDACis have also shown beneficial effects in ischemic condition, in in vitro and in vivo models. Validation of these results in clinical trials would be crucial towards the potential therapeutic use of HDACi in human neurological condition. As of now, clinical trials with HDACi have not been conducted in stroke (sources: www.clinicaltrials.gov, www.ncbi.nlm.nih.gov/pubmed/). Using computational biology tools, small molecules can be designed against specific targets to reduce the off-target effects and associated side-effects. Cumulatively, epigenetic mechanism offers a promising new therapeutic target in ischemia. Future studies are warranted to advance our understanding of epigenetic regulation in ischemic condition and in redox state following acute stroke.
ACKNOWLEDGEMENT
This work was initiated under a Neuro grant of the Department of Biotechnology [BT/PR14338/MED/30/495/ 2010 to SC] and supported by the Council of Scientific and Industrial Research (CSIR) network project (BSC0103-UNDO to SC and AK). KBC wish to thank CSIR, India for the award of research fellowship. We also would like to thank Dr. Abhay Sharma, Scientist CSIR-IGIB and Mr. Dwaipayan Bhattacharya for their invaluable contributions in critical reading and comments on the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Lanktree M.B., Dichgans M., Hegele R.A. Advances in genomic analysis of stroke what have we learned and where are we headed? Stroke. 2010;41(4):825–832. doi: 10.1161/STROKEAHA.109.570523. [http://dx.doi.org/10.1161/ STROKEAHA.109.570523]. [DOI] [PubMed] [Google Scholar]
- 2.Marnellos G. High-throughput SNP analysis for genetic association studies. Curr. Opin. Drug Discov. Devel. 2003;6(3):317–321. [PubMed] [Google Scholar]
- 3.Meschia J.F., Worrall B.B., Rich S.S. Genetic susceptibility to ischemic stroke. Nat. Rev. Neurol. 2011;7(7):369–378. doi: 10.1038/nrneurol.2011.80. [http://dx.doi.org/10.1038/nrneurol.2011.80]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moskowitz M.A., Lo E.H., Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [http://dx.doi.org/10.1016/j.neuron.2010.07.002]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith W.S. Pathophysiology of focal cerebral ischemia: a therapeutic perspective. J. Vasc. Interv. Radiol. 2004;15(1):S3–S12. doi: 10.1097/01.rvi.0000108687.75691.0c. [http://dx.doi.org/10.1097/01.RVI.0000108687.75691.0C]. [DOI] [PubMed] [Google Scholar]
- 6.Ankarcrona M., Dypbukt J.M., Bonfoco E., Zhivotovsky B., Orrenius S., Lipton S.A., Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–973. doi: 10.1016/0896-6273(95)90186-8. [http://dx. doi.org/10.1016/0896-6273(95)90186-8]. [DOI] [PubMed] [Google Scholar]
- 7.Hall E., Braughler J. Free radicals in CNS injury. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1992;71:81–105. [PubMed] [Google Scholar]
- 8.Keyer K., Gort A.S., Imlay J.A. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 1995;177(23):6782–6790. doi: 10.1128/jb.177.23.6782-6790.1995. [http://dx.doi.org/10.1128/jb.177.23.6782-6790.1995]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambeth J.D., Neish A.S. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [http://dx.doi.org/10.1146/annurev-pathol-012513-104651]. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa T., Edelstein D., Du X.L., Yamagishi S-i., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H-P. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [http://dx.doi.org/10.1038/35008121]. [DOI] [PubMed] [Google Scholar]
- 11.Thomas S.R., Witting P.K., Drummond G.R. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2008;10(10):1713–1766. doi: 10.1089/ars.2008.2027. [http://dx.doi.org/10.1089/ars.2008.2027]. [DOI] [PubMed] [Google Scholar]
- 12.Zaleska M.M., Floyd R.A. Regional lipid peroxidation in rat brain in vitro: possible role of endogenous iron. Neurochem. Res. 1985;10(3):397–410. doi: 10.1007/BF00964608. [http://dx.doi.org/10.1007/BF00964608]. [DOI] [PubMed] [Google Scholar]
- 13.Fujimura M., Morita-Fujimura Y., Murakami K., Kawase M., Chan P.H. Cytosolic redistribution of cytochrome c after transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1998;18(11):1239–1247. doi: 10.1097/00004647-199811000-00010. [http://dx.doi.org/10.1097/00004647-199811000-00010]. [DOI] [PubMed] [Google Scholar]
- 14.Fujimura M., Morita-Fujimura Y., Narasimhan P., Copin J-C., Kawase M., Chan P.H. Copper-zinc superoxide dismutase prevents the early decrease of apurinic/apyrimidinic endonuclease and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 1999;30(11):2408–2415. doi: 10.1161/01.str.30.11.2408. [http://dx. doi.org/10.1161/01.STR.30.11.2408]. [DOI] [PubMed] [Google Scholar]
- 15.Wang B., Zhu X., Kim Y., Li J., Huang S., Saleem S., Li R-c., Xu Y., Dore S., Cao W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic. Biol. Med. 2012;52(5):928–936. doi: 10.1016/j.freeradbiomed.2011.12.006. [http://dx. doi.org/10.1016/j.freeradbiomed.2011.12.006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W., Sun Z., Wang X-J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct interaction between Nrf2 and p21 Cip1/WAF1 upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34(6):663–673. doi: 10.1016/j.molcel.2009.04.029. [http://dx.doi.org/10.1016/j.molcel.2009.04. 029]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton T.P., Shertzer H.G., Puga A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999;39(1):67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [http://dx.doi.org/10.1146/annurev.pharmtox.39.1.67]. [DOI] [PubMed] [Google Scholar]
- 18.Traenckner E., Pahl H.L., Henkel T., Schmidt K., Wilk S., Baeuerle P. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14(12):2876. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinig T.J., Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr. Opin. Neurol. 2009;22(3):294–301. doi: 10.1097/wco.0b013e32832b4db3. [http://dx.doi.org/10.1097/WCO.0b013e32832b4db3]. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R., Chopp M., Zhang Z., Jiang N., Powers C. The expression of P-and E-selectins in three models of middle cerebral artery occlusion. Brain Res. 1998;785(2):207–214. doi: 10.1016/s0006-8993(97)01343-7. [http://dx. doi.org/10.1016/S0006-8993(97)01343-7]. [DOI] [PubMed] [Google Scholar]
- 21.Schilling M., Besselmann M., Leonhard C., Mueller M., Ringelstein E.B., Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2003;183(1):25–33. doi: 10.1016/s0014-4886(03)00082-7. [http://dx.doi.org/10.1016/S0014-4886(03)00082-7]. [DOI] [PubMed] [Google Scholar]
- 22.Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14(4):469–477. doi: 10.1007/s10495-008-0304-8. [http://dx. doi.org/10.1007/s10495-008-0304-8]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonfoco E., Krainc D., Ankarcrona M., Nicotera P., Lipton S.A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl. Acad. Sci. USA. 1995;92(16):7162–7166. doi: 10.1073/pnas.92.16.7162. [http://dx.doi.org/10.1073/pnas.92. 16.7162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams J.M., Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr. Opin. Immunol. 2007;19(5):488–496. doi: 10.1016/j.coi.2007.05.004. [http://dx.doi.org/10.1016/j.coi.2007.05.004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H., Rafiuddin-Shah M., Tu H-C., Jeffers J.R., Zambetti G.P., Hsieh J.J., Cheng E. H Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006;8(12):1348–1358. doi: 10.1038/ncb1499. [http://dx.doi.org/10.1038/ncb1499]. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz A., Matute C., Alberdi E. Endoplasmic reticulum Ca 2+ release through ryanodine and IP 3 receptors contributes to neuronal excitotoxicity. Cell Calcium. 2009;46(4):273–281. doi: 10.1016/j.ceca.2009.08.005. [http://dx.doi.org/10.1016/j.ceca.2009.08.005]. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang Y.B., Kuroda S., Kristián T., Siesjö B.K. Release of mitochondrial aspartate aminotransferase (mAST) following transient focal cerebral ischemia suggests the opening of a mitochondrial permeability transition pore. Neurosci. Res. Commun. 1997;20(3):167–173. [http://dx.doi.org/10.1002/(SICI) 1520-6769(199705)20:3<167:AID-NRC198>3.0.CO;2-3]. [Google Scholar]
- 28.Ouyang Y-B., Tan Y., Comb M., Liu C-L., Martone M., Siesjö B.K., Hu B-R. Survival-and death-promoting events after transient cerebral ischemia & colon; phosphorylation of Akt, release of cytochrome C, and activation of caspase-like proteases. J. Cereb. Blood Flow Metab. 1999;19(10):1126–1135. doi: 10.1097/00004647-199910000-00009. [http://dx.doi.org/ 10.1097/00004647-199910000-00009]. [DOI] [PubMed] [Google Scholar]
- 29.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175(6):901–911. doi: 10.1083/jcb.200608073. [http://dx.doi.org/10.1083/jcb.200608073]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traystman R.J. Animal models of focal and global cerebral ischemia. ILAR J. 2003;44(2):85–95. doi: 10.1093/ilar.44.2.85. [http://dx.doi.org/10.1093/ ilar.44.2.85]. [DOI] [PubMed] [Google Scholar]
- 31.J.H. Experimental ischemie stroke: A review. Stroke. 1984;15(1):5–14. doi: 10.1161/01.str.15.1.5. [http://dx.doi.org/10.1161/01.STR.15.1.5]. [DOI] [PubMed] [Google Scholar]
- 32.Takizawa S., Hakim A. Animal models of cerebral ischemia. 2. Rat models. Cerebrovasc. Dis. 1991;1(Suppl. 1):16–21. [http://dx. doi.org/10.1159/000108876]. [Google Scholar]
- 33.Bose B., Osterholm J.L., Berry R. A reproducible experimental model of focal cerebral ischemia in the cat. Brain Res. 1984;311(2):385–391. doi: 10.1016/0006-8993(84)90106-9. [http://dx.doi.org/10.1016/0006-8993(84)90106-9]. [DOI] [PubMed] [Google Scholar]
- 34.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [http://dx.doi.org/10.1161/01.STR.20.1.84]. [DOI] [PubMed] [Google Scholar]
- 35.Belayev L., Busto R., Zhao W., Fernandez G., Ginsberg M.D. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833(2):181–190. doi: 10.1016/s0006-8993(99)01528-0. [http://dx.doi.org/10. 1016/S0006-8993(99)01528-0]. [DOI] [PubMed] [Google Scholar]
- 36.Memezawa H., Minamisawa H., Smith M-L., Siesjö B. Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp. Brain Res. 1992;89(1):67–78. doi: 10.1007/BF00229002. [http://dx.doi.org/ 10.1007/BF00229002]. [DOI] [PubMed] [Google Scholar]
- 37.Watson B.D., Dietrich W.D., Busto R., Wachtel M.S., Ginsberg M.D. Induction of reproducible brain infarction by photo- chemically initiated thrombosis. Ann. Neurol. 1985;17(5):497–504. doi: 10.1002/ana.410170513. [http://dx.doi.org/10.1002/ana.410170513]. [DOI] [PubMed] [Google Scholar]
- 38.Ginsberg M., Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20(12):1627–1642. doi: 10.1161/01.str.20.12.1627. [http://dx.doi.org/10.1161/01.STR. 20.12.1627]. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich W., Busto R., Watson B., Scheinberg P., Ginsberg M. Photochemically induced cerebral infarction. Acta Neuropathol. 1987;72(4):326–334. doi: 10.1007/BF00687263. [http://dx.doi.org/10.1007/BF00687263]. [DOI] [PubMed] [Google Scholar]
- 40.Hilger T., Blunk J.A., Hoehn M., Mies G., Wester P. Characterization of a novel chronic photothrombotic ring stroke model in rats by magnetic resonance imaging, biochemical imaging, and histology. J. Cereb. Blood Flow Metab. 2004;24(7):789–797. doi: 10.1097/01.WCB.0000123905.17746.DB. [http://dx.doi.org/10.1097/01.WCB.0000123905.17746.DB]. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimoto S., Ishizaki Y., Kurihara H., Sasaki T., Yoshizumi M., Yanagisawa M., Yazaki Y., Masaki T., Takakura K., Murota S-i. Cerebral microvessel endothelium is producing endothelin. Brain Res. 1990;508(2):283–285. doi: 10.1016/0006-8993(90)90407-3. [http://dx.doi.org/10.1016/ 0006-8993(90)90407-3]. [DOI] [PubMed] [Google Scholar]
- 42.Robinson M., Macrae I., Todd M., Reid J., McCulloch J. Reduction of local cerebral blood flow to pathological levels by endothelin-1 applied to the middle cerebral artery in the rat. Neurosci. Lett. 1990;118(2):269–272. doi: 10.1016/0304-3940(90)90644-o. [http://dx.doi.org/10.1016/ 0304-3940(90)90644-O]. [DOI] [PubMed] [Google Scholar]
- 43.Sharkey J. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J. Cereb. Blood Flow Metab. 1993;13(5):865–871. doi: 10.1038/jcbfm.1993.108. [http://dx.doi.org/10.1038/jcbfm. 1993.108]. [DOI] [PubMed] [Google Scholar]
- 44.Overgaard K. Thrombolytic therapy in experimental embolic stroke. Cerebrovasc. Brain Metab. Rev. 1993;6(3):257–286. [PubMed] [Google Scholar]
- 45.Papadopoulos S.M., Chandler W.F., Salamat M.S., Topol E.J., Sackellares J.C. Recombinant human tissue-type plasminogen activator therapy in acute thromboembolic stroke. J. Neurosurg. 1987;67(3):394–398. doi: 10.3171/jns.1987.67.3.0394. [http://dx.doi.org/10.3171/jns.1987.67.3.0394]. [DOI] [PubMed] [Google Scholar]
- 46.Kaneko D., Nakamura N., Ogawa T. Cerebral infarction in rats using homologous blood emboli: development of a new experimental model. Stroke. 1985;16(1):76–84. doi: 10.1161/01.str.16.1.76. [http://dx.doi.org/10.1161/01. STR.16.1.76]. [DOI] [PubMed] [Google Scholar]
- 47.Busch E., Krüger K., Hossmann K-A. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res. 1997;778(1):16–24. doi: 10.1016/s0006-8993(97)01008-1. [http://dx.doi.org/10.1016/S0006-8993(97)01008-1]. [DOI] [PubMed] [Google Scholar]
- 48.del Zoppo G.J., Milner R., Mabuchi T., Hung S., Wang X., Berg G.I., Koziol J.A. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38(2):646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [http://dx.doi.org/10.1161/01.STR.0000254477.34231.cb]. [DOI] [PubMed] [Google Scholar]
- 49.Ouyang Y-B., Giffard R.G. Bcl-XL maintains mitochondrial function in murine astrocytes deprived of glucose. J. Cereb. Blood Flow Metab. 2003;23(3):275–279. doi: 10.1097/01.WCB.0000055774.06337.F6. [http://dx.doi.org/10.1097/01. WCB.0000055774.06337.F6]. [DOI] [PubMed] [Google Scholar]
- 50.Ouyang Y-B., Xu L., Giffard R.G. Geldanamycin treatment reduces delayed CA1 damage in mouse hippocampal organotypic cultures subjected to oxygen glucose deprivation. Neurosci. Lett. 2005;380(3):229–233. doi: 10.1016/j.neulet.2005.01.055. [http://dx.doi.org/10.1016/j.neulet.2005.01. 055]. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang Y-B., Xu L-J., Sun Y-J., Giffard R.G. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11(2):180. doi: 10.1379/CSC-182R.1. [http://dx.doi.org/10.1379/CSC-182R.1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [http://dx.doi.org/10.1016/j.cell.2007.02.005]. [DOI] [PubMed] [Google Scholar]
- 53.Zlatanova J., Thakar A. H2A. Z: view from the top. Structure. 2008;16(2):166–179. doi: 10.1016/j.str.2007.12.008. [http://dx.doi.org/10.1016/j.str.2007.12.008]. [DOI] [PubMed] [Google Scholar]
- 54.Jurkowska R.Z., Jurkowski T.P., Jeltsch A. Structure and function of mammalian DNA methyltransferases. ChemBioChem. 2011;12(2):206–222. doi: 10.1002/cbic.201000195. [http://dx.doi.org/10.1002/cbic.201000195]. [DOI] [PubMed] [Google Scholar]
- 55.Ørom U.A., Nielsen F.C., Lund A.H. MicroRNA-10a binds the 5′ UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell. 2008;30(4):460–471. doi: 10.1016/j.molcel.2008.05.001. [http://dx.doi.org/10.1016/j.molcel. 2008.05.001]. [DOI] [PubMed] [Google Scholar]
- 56.Trujillo R.D., Yue S.B., Tang Y., O’Gorman W.E., Chen C.Z. The potential functions of primary microRNAs in target recognition and repression. EMBO J. 2010;29(19):3272–3285. doi: 10.1038/emboj.2010.208. [http://dx.doi.org/10.1038/emboj.2010.208]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chakravarty S., Pathak S.S., Maitra S., Khandelwal N., Chandra K.B., Kumar A. Epigenetic regulatory mechanisms in stress-induced behavior. Int. Rev. Neurobiol. 2014;115:117–154. doi: 10.1016/B978-0-12-801311-3.00004-4. [http:// dx.doi.org/10.1016/B978-0-12-801311-3.00004-4]. [DOI] [PubMed] [Google Scholar]
- 58.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [http://dx.doi.org/10.1126/science. 1063127]. [DOI] [PubMed] [Google Scholar]
- 59.Ruthenburg A.J., Li H., Patel D.J., Allis C.D. Multivalent engagement of chromatin modifications by linked binding modules. Nat. Rev. Mol. Cell Biol. 2007;8(12):983–994. doi: 10.1038/nrm2298. [http://dx.doi. org/10.1038/nrm2298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otto S.J., McCorkle S.R., Hover J., Conaco C., Han J-J., Impey S., Yochum G.S., Dunn J.J., Goodman R.H., Mandel G. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J. Neurosci. 2007;27(25):6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [http://dx.doi.org/10.1523/JNEUROSCI.0091-07.2007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar A., Choi K-H., Renthal W., Tsankova N.M., Theobald D.E., Truong H-T., Russo S.J., LaPlant Q., Sasaki T.S., Whistler K.N. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [http://dx.doi.org/10.1016/j.neuron.2005.09.023]. [DOI] [PubMed] [Google Scholar]
- 62.Tsankova N.M., Berton O., Renthal W., Kumar A., Neve R.L., Nestler E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9(4):519–525. doi: 10.1038/nn1659. [http://dx.doi.org/10.1038/nn1659]. [DOI] [PubMed] [Google Scholar]
- 63.Ferrante R.J., Ryu H., Kubilus J.K., D’Mello S., Sugars K.L., Lee J., Lu P., Smith K., Browne S., Beal M.F. Chemotherapy for the brain: the antitumor antibiotic mithramycin prolongs survival in a mouse model of Huntington’s disease. J. Neurosci. 2004;24(46):10335–10342. doi: 10.1523/JNEUROSCI.2599-04.2004. [http://dx.doi.org/10.1523/JNEUROSCI. 2599-04.2004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marambaud P., Wen P.H., Dutt A., Shioi J., Takashima A., Siman R., Robakis N.K. A CBP binding transcriptional repressor produced by the PS1/ϵ-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114(5):635–645. doi: 10.1016/j.cell.2003.08.008. [http://dx.doi.org/ 10.1016/j.cell.2003.08.008]. [DOI] [PubMed] [Google Scholar]
- 65.Alarcón J.M., Malleret G., Touzani K., Vronskaya S., Ishii S., Kandel E.R., Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42(6):947–959. doi: 10.1016/j.neuron.2004.05.021. [http://dx.doi.org/10.1016/j.neuron.2004.05.021]. [DOI] [PubMed] [Google Scholar]
- 66.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23(2):185–188. doi: 10.1038/13810. [http://dx.doi.org/10.1038/13810]. [DOI] [PubMed] [Google Scholar]
- 67.Papadopoulos M.C., Giffard R.G., Bell B.A. An introduction to the changes in gene expression that occur after cerebral ischaemia. Br. J. Neurosurg. 2000;14(4):305–312. doi: 10.1080/026886900417261. [http://dx.doi.org/10.1080/ 026886900417261]. [DOI] [PubMed] [Google Scholar]
- 68.Endres M., Fan G., Meisel A., Dirnagl U., Jaenisch R. Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport. 2001;12(17):3763–3766. doi: 10.1097/00001756-200112040-00032. [http://dx.doi.org/10.1097/00001756-200112040-00032]. [DOI] [PubMed] [Google Scholar]
- 69.Endres M., Meisel A., Biniszkiewicz D., Namura S., Prass K., Ruscher K., Lipski A., Jaenisch R., Moskowitz M.A., Dirnagl U. DNA methyltransferase contributes to delayed ischemic brain injury. J. Neurosci. 2000;20(9):3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeyaseelan K., Lim K.Y., Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008 doi: 10.1161/STROKEAHA.107.500736. [http://dx.doi.org/ 10.1161/STROKEAHA.107.500736]. [DOI] [PubMed] [Google Scholar]
- 71.Liu D-Z., Tian Y., Ander B.P., Xu H., Stamova B.S., Zhan X., Turner R.J., Jickling G., Sharp F.R. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J. Cereb. Blood Flow Metab. 2010;30(1):92–101. doi: 10.1038/jcbfm.2009.186. [http://dx.doi.org/10.1038/jcbfm.2009.186]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan K.S., Armugam A., Sepramaniam S., Lim K.Y., Setyowati K.D., Wang C.W., Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4(11):e7689. doi: 10.1371/journal.pone.0007689. [http:// dx.doi.org/10.1371/journal.pone.0007689]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crowe S.L., Movsesyan V.A., Jorgensen T.J., Kondratyev A. Rapid phosphorylation of histone H2A. X following ionotropic glutamate receptor activation. Eur. J. Neurosci. 2006;23(9):2351–2361. doi: 10.1111/j.1460-9568.2006.04768.x. [http://dx.doi.org/10.1111/j.1460-9568.2006.04768.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mortazavi A., Thompson E.C., Garcia S.T., Myers R.M., Wold B. Comparative genomics modeling of the NRSF/REST repressor network: from single conserved sites to genome-wide repertoire. Genome Res. 2006;16(10):1208–1221. doi: 10.1101/gr.4997306. [http://dx.doi.org/10.1101/ gr.4997306]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson R., Teh C.H., Jia H., Vanisri R.R., Pandey T., Lu Z-H., Buckley N.J., Stanton L.W., Lipovich L. Regulation of neural macroRNAs by the transcriptional repressor REST. RNA. 2009;15(1):85–96. doi: 10.1261/rna.1127009. [http://dx.doi.org/10.1261/rna.1127009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu J., Xie X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 2006;7(9):R85. doi: 10.1186/gb-2006-7-9-r85. [http://dx.doi.org/ 10.1186/gb-2006-7-9-r85]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kogure K., Kato H. Altered gene expression in cerebral ischemia. Stroke. 1993;24(12):2121–2127. doi: 10.1161/01.str.24.12.2121. [http://dx.doi.org/10.1161/01. STR.24.12.2121]. [DOI] [PubMed] [Google Scholar]
- 78.Huang Y., Myers S.J., Dingledine R. Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat. Neurosci. 1999;2(10):867–872. doi: 10.1038/13165. [http://dx.doi.org/10. 1038/13165]. [DOI] [PubMed] [Google Scholar]
- 79.Chia N., Wang L., Lu X., Senut M-C., Brenner C.A., Ruden D.M. Hypothesis: environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics. 2011;6(7):853–856. doi: 10.4161/epi.6.7.16461. [http://dx. doi.org/10.4161/epi.6.7.16461]. [DOI] [PubMed] [Google Scholar]
- 80.Valinluck V., Sowers L.C. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltrans-ferase DNMT1. Cancer Res. 2007;67(3):946–950. doi: 10.1158/0008-5472.CAN-06-3123. [http://dx.doi. org/10.1158/0008-5472.CAN-06-3123]. [DOI] [PubMed] [Google Scholar]
- 81.Valinluck V., Tsai H-H., Rogstad D.K., Burdzy A., Bird A., Sowers L.C. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2). Nucleic Acids Res. 2004;32(14):4100–4108. doi: 10.1093/nar/gkh739. [http://dx.doi.org/10.1093/nar/gkh739]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miao Z., He Y., Xin N., Sun M., Chen L., Lin L., Li J., Kong J., Jin P., Xu X. Altering 5-hydroxymethylcytosine modification impacts ischemic brain injury. Hum. Mol. Genet. 2015;24(20):5855–5866. doi: 10.1093/hmg/ddv307. [http://dx.doi.org/10.1093/hmg/ddv307]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lao V.V., Herring J.L., Kim C.H., Darwanto A., Soto U., Sowers L.C. Incorporation of 5-chlorocytosine into mammalian DNA results in heritable gene silencing and altered cytosine methylation patterns. Carcinogenesis. 2009;30(5):886–893. doi: 10.1093/carcin/bgp060. [http://dx.doi.org/10.1093/carcin/bgp060]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valinluck V., Sowers L.C. Inflammation-mediated cytosine damage: a mechanistic link between inflammation and the epigenetic alterations in human cancers. Cancer Res. 2007;67(12):5583–5586. doi: 10.1158/0008-5472.CAN-07-0846. [http://dx.doi.org/10.1158/0008-5472.CAN-07-0846]. [DOI] [PubMed] [Google Scholar]
- 85.Lugli G., Torvik V.I., Larson J., Smalheiser N.R. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J. Neurochem. 2008;106(2):650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [http:// dx.doi.org/10.1111/j.1471-4159.2008.05413.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siegel G., Obernosterer G., Fiore R., Oehmen M., Bicker S., Christensen M., Khudayberdiev S., Leuschner P.F., Busch C.J., Kane C. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat. Cell Biol. 2009;11(6):705–716. doi: 10.1038/ncb1876. [http://dx. doi.org/10.1038/ncb1876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White R.E., Giffard R.G. MicroRNA-320 induces neurite outgrowth by targeting ARPP-1. Neuroreport. 2012;23(10):590. doi: 10.1097/WNR.0b013e3283540394. [http://dx.doi.org/10.1097/WNR.0b013e3283540394]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hébert S.S., Horré K., Nicolaï L., Papadopoulou A.S., Mandemakers W., Silahtaroglu A.N., Kauppinen S., Delacourte A., De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc. Natl. Acad. Sci. USA. 2008;105(17):6415–6420. doi: 10.1073/pnas.0710263105. [http://dx.doi.org/10.1073/pnas.0710263105]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beveridge N.J., Tooney P.A., Carroll A.P., Gardiner E., Bowden N., Scott R.J., Tran N., Dedova I., Cairns M.J. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum. Mol. Genet. 2008;17(8):1156–1168. doi: 10.1093/hmg/ddn005. [http://dx.doi.org/10.1093/hmg/ ddn005]. [DOI] [PubMed] [Google Scholar]
- 90.Dharap A., Bowen K., Place R., Li L-C., Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J. Cereb. Blood Flow Metab. 2009;29(4):675–687. doi: 10.1038/jcbfm.2008.157. [http://dx.doi.org/10.1038/jcbfm.2008.157]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yuan Y., Wang J.Y., Xu L.Y., Cai R., Chen Z., Luo B.Y. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J. Clin. Neurosci. 2010;17(6):774–778. doi: 10.1016/j.jocn.2009.10.009. [http://dx.doi.org/10.1016/j.jocn.2009.10.009]. [DOI] [PubMed] [Google Scholar]
- 92.Liu X., Chopp M., Pan W., Fan B., Wang X., Li C., Levin A.M., Zhang R., Zhang Z. Abstract TMP60: RNA sequencing reveals novel MicroRNA profiles in neural progenitor cells after stroke. Stroke. 2016;47(Suppl 1):ATMP60–ATMP60. [Google Scholar]
- 93.Liu X., Michael C., Wang X., Zhang L., Cui Y., Zhang Y., Zhang R., Zhang Z. Abstract W MP86: Microrna-146a modulates neurogenesis and oligodendrogenesis after stroke. Stroke. 2015;46(Suppl 1):AWMP86–AWMP86. [Google Scholar]
- 94.Lee S-T., Chu K., Jung K-H., Yoon H-J., Jeon D., Kang K-M., Park K-H., Bae E-K., Kim M., Lee S.K. MicroRNAs induced during ischemic preconditioning. Stroke. 2010;41(8):1646–1651. doi: 10.1161/STROKEAHA.110.579649. [http://dx.doi.org/10.1161/STROKEAHA.110.579649]. [DOI] [PubMed] [Google Scholar]
- 95.Yin K-J., Deng Z., Hamblin M., Xiang Y., Huang H., Zhang J., Jiang X., Wang Y., Chen Y.E. Peroxisome proliferator-activated receptor δ regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J. Neurosci. 2010;30(18):6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.0780-10.2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin K-J., Deng Z., Huang H., Hamblin M., Xie C., Zhang J., Chen Y.E. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol. Dis. 2010;38(1):17–26. doi: 10.1016/j.nbd.2009.12.021. [http://dx.doi.org/10.1016/j.nbd.2009.12.021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeng L., Liu J., Wang Y., Wang L., Weng S., Tang Y., Zheng C., Cheng Q., Chen S., Yang G-Y. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front. Biosci. (Elite Ed.) 2011;3:1265–1272. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- 98.Liu P., Zhao H., Wang R., Wang P., Tao Z., Gao L., Yan F., Liu X., Yu S., Ji X. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46(2):513–519. doi: 10.1161/STROKEAHA.114.007482. [http://dx.doi.org/ 10.1161/STROKEAHA.114.007482]. [DOI] [PubMed] [Google Scholar]
- 99.Jiang Y., Li L., Tan X., Liu B., Zhang Y., Li C. miR‐210 mediates vagus nerve stimulation‐induced antioxidant stress and anti‐apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J. Neurochem. 2015;134(1):173–181. doi: 10.1111/jnc.13097. [http://dx.doi. org/10.1111/jnc.13097]. [DOI] [PubMed] [Google Scholar]
- 100.Sepramaniam S., Armugam A., Lim K.Y., Karolina D.S., Swaminathan P., Tan J.R., Jeyaseelan K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J. Biol. Chem. 2010;285(38):29223–29230. doi: 10.1074/jbc.M110.144576. [http://dx.doi.org/ 10.1074/jbc.M110.144576]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buller B., Liu X., Wang X., Zhang R.L., Zhang L., Hozeska‐Solgot A., Chopp M., Zhang Z.G. MicroRNA‐21 protects neurons from ischemic death. FEBS J. 2010;277(20):4299–4307. doi: 10.1111/j.1742-4658.2010.07818.x. [http://dx.doi.org/10.1111/j.1742-4658.2010.07818.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ouyang Y-B., Lu Y., Yue S., Xu L-J., Xiong X-X., White R.E., Sun X., Giffard R.G. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol. Dis. 2012;45(1):555–563. doi: 10.1016/j.nbd.2011.09.012. [http://dx.doi.org/10.1016/j.nbd.2011. 09.012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yadav S., Pandey A., Shukla A., Talwelkar S.S., Kumar A., Pant A.B., Parmar D. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J. Biol. Chem. 2011;286(43):37347–37357. doi: 10.1074/jbc.M111.235531. [http://dx.doi.org/10. 1074/jbc.M111.235531]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ouyang Y-B., Lu Y., Yue S., Giffard R.G. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12(2):213–219. doi: 10.1016/j.mito.2011.09.001. [http://dx.doi.org/10.1016/j.mito.2011.09.001]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen G., Zhu W., Shi D., Lv L., Zhang C., Liu P., Hu W. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol. Rep. 2010;23(4):997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- 106.Siegel C., Li J., Liu F., Benashski S.E., McCullough L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. USA. 2011;108(28):11662–11667. doi: 10.1073/pnas.1102635108. [http://dx.doi.org/10.1073/pnas.1102635108]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ouyang Y-B., Stary M.C., Yang G-Y., Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr. Drug Targets. 2013;14(1):90–101. doi: 10.2174/138945013804806424. [http://dx.doi.org/10.2174/ 138945013804806424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Faraco G., Pancani T., Formentini L., Mascagni P., Fossati G., Leoni F., Moroni F., Chiarugi A. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol. Pharmacol. 2006;70(6):1876–1884. doi: 10.1124/mol.106.027912. [http://dx.doi.org/10.1124/mol.106.027912]. [DOI] [PubMed] [Google Scholar]
- 109.Kim H.J., Rowe M., Ren M., Hong J-S., Chen P-S., Chuang D-M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007;321(3):892–901. doi: 10.1124/jpet.107.120188. [http://dx.doi.org/10.1124/jpet.107.120188]. [DOI] [PubMed] [Google Scholar]
- 110.Langley B., D’Annibale M.A., Suh K., Ayoub I., Tolhurst A., Bastan B., Yang L., Ko B., Fisher M., Cho S. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21waf1/cip1 in cell cycle-independent neuro-protection. J. Neurosci. 2008;28(1):163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [http://dx.doi.org/ 10.1523/JNEUROSCI.3200-07.2008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lanzillotta A., Pignataro G., Branca C., Cuomo O., Sarnico I., Benarese M., Annunziato L., Spano P., Pizzi M. Targeted acetylation of NF-kappaB/RelA and histones by epigenetic drugs reduces post-ischemic brain injury in mice with an extended therapeutic window. Neurobiol. Dis. 2013;49:177–189. doi: 10.1016/j.nbd.2012.08.018. [http://dx. doi.org/10.1016/j.nbd.2012.08.018]. [DOI] [PubMed] [Google Scholar]
- 112.Ren M., Leng Y., Jeong M., Leeds P.R., Chuang D.M. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J. Neurochem. 2004;89(6):1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02406.x]. [DOI] [PubMed] [Google Scholar]
- 113.Wang Z., Leng Y., Tsai L-K., Leeds P., Chuang D-M. Valproic acid attenuates blood–brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J. Cereb. Blood Flow Metab. 2011;31(1):52–57. doi: 10.1038/jcbfm.2010.195. [http://dx.doi.org/10.1038/jcbfm.2010.195]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z., Tsai L-K., Munasinghe J., Leng Y., Fessler E.B., Chibane F., Leeds P., Chuang D-M. Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke. 2012;43(9):2430–2436. doi: 10.1161/STROKEAHA.112.652545. [http://dx.doi.org/10.1161/STROKEAHA.112.652545]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xuan A., Long D., Li J., Ji W., Hong L., Zhang M., Zhang W. Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sci. 2012;90(11):463–468. doi: 10.1016/j.lfs.2012.01.001. [http://dx.doi.org/ 10.1016/j.lfs.2012.01.001]. [DOI] [PubMed] [Google Scholar]
- 116.Yildirim F., Gertz K., Kronenberg G., Harms C., Fink K.B., Meisel A., Endres M. Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp. Neurol. 2008;210(2):531–542. doi: 10.1016/j.expneurol.2007.11.031. [http://dx.doi.org/10. 1016/j.expneurol.2007.11.031]. [DOI] [PubMed] [Google Scholar]
- 117.Jhelum P., Maitra S., Shailaja K., Butola T., Kumar A., Chakravarty S. Role of histone based epigenetic modifications in cerebral ischemia induced striatal damage in internal carotid artery occlusion (ICAO) rodent models.; Proceedings of the International Conference on New Dimensions in Chemistry & Chemical Technologies- Applications in Pharma Industry (NDCT-2014); 2014. pp. 375–381. [Google Scholar]
- 118.Johnson A.B., Barton M.C. Hypoxia-induced and stress-specific changes in chromatin structure and function. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007;618(1):149–162. doi: 10.1016/j.mrfmmm.2006.10.007. [http://dx.doi.org/10. 1016/j.mrfmmm.2006.10.007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Melvin A., Rocha S. Chromatin as an oxygen sensor and active player in the hypoxia response. Cell. Signal. 2012;24(1):35–43. doi: 10.1016/j.cellsig.2011.08.019. [http://dx.doi.org/10.1016/j.cellsig.2011.08.019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raz L., Zhang Q-g., Han D., Dong Y., De Sevilla L., Brann D.W. Acetylation of the pro-apoptotic factor, p53 in the hippocampus following cerebral ischemia and modulation by estrogen. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0027039. [http://dx.doi.org/10.1371/journal. pone.0027039]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Y.T., Zang X.F., Pan J., Zhu X.L., Chen F., Chen Z.B., Xu Y. Expression patterns of histone deacetylases in experimental stroke and potential targets for neuroprotection. Clin. Exp. Pharmacol. Physiol. 2012;39(9):751–758. doi: 10.1111/j.1440-1681.2012.05729.x. [http://dx.doi.org/10.1111/j.1440-1681.2012.05729.x]. [DOI] [PubMed] [Google Scholar]
- 122.Baltan S., Bachleda A., Morrison R.S., Murphy S.P. Expression of histone deacetylases in cellular compartments of the mouse brain and the effects of ischemia. Transl. Stroke Res. 2011;2(3):411–423. doi: 10.1007/s12975-011-0087-z. [http://dx.doi.org/10.1007/s12975-011-0087-z]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He M., Zhang B., Wei X., Wang Z., Fan B., Du P., Zhang Y., Jian W., Chen L., Wang L. HDAC4/5‐HMGB1 signalling mediated by NADPH oxidase activity contributes to cerebral ischaemia/reperfusion injury. J. Cell. Mol. Med. 2013;17(4):531–542. doi: 10.1111/jcmm.12040. [http://dx.doi.org/10.1111/jcmm.12040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng S., Zhao S., Yan F., Cheng J., Huang L., Chen H., Liu Q., Ji X., Yuan Z. HDAC2 selectively regulates FOXO3a-mediated gene transcription during oxidative stress-induced neuronal cell death. J. Neurosci. 2015;35(3):1250–1259. doi: 10.1523/JNEUROSCI.2444-14.2015. [http://dx.doi.org/10.1523/JNEUROSCI.2444-14.2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu F., Chen X., Allali-Hassani A., Quinn A.M., Wasney G.A., Dong A., Barsyte D., Kozieradzki I., Senisterra G., Chau I. Discovery of a 2, 4-diamino-7-aminoalkoxyquinazoline as a potent and selective inhibitor of histone lysine methyltransferase G9a. J. Med. Chem. 2009;52(24):7950–7953. doi: 10.1021/jm901543m. [http://dx.doi.org/10.1021/ jm901543m]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yan W., Fang Z., Yang Q., Dong H., Lu Y., Lei C., Xiong L. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. J. Cereb. Blood Flow Metab. 2013;33(3):396–406. doi: 10.1038/jcbfm.2012.179. [http://dx.doi.org/10.1038/jcbfm.2012.179]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang L.M., Wang Y.J., Cui M., Luo W.J., Wang X.J., Barber P.A., Chen Z.Y. A dietary polyphenol resveratrol acts to provide neuroprotection in recurrent stroke models by regulating AMPK and SIRT1 signaling, thereby reducing energy requirements during ischemia. Eur. J. Neurosci. 2013;37(10):1669–1681. doi: 10.1111/ejn.12162. [http://dx. doi.org/10.1111/ejn.12162]. [DOI] [PubMed] [Google Scholar]
- 128.Haigis M.C., Sinclair D.A. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 2010;5:253. doi: 10.1146/annurev.pathol.4.110807.092250. [http://dx.doi.org/10.1146/annurev.pathol.4.110807.092250]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bellenguez C., Bevan S., Gschwendtner A., Spencer C.C., Burgess A.I., Pirinen M., Jackson C.A., Traylor M., Strange A., Su Z. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat. Genet. 2012;44(3):328–333. doi: 10.1038/ng.1081. [http://dx.doi.org/10.1038/ng.1081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hacke W., Grond-Ginsbach C. Commentary on a GWAS: HDAC9 and the risk for ischaemic stroke. BMC Med. 2012;10(1):70. doi: 10.1186/1741-7015-10-70. [http://dx.doi.org/10.1186/1741-7015-10-70]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Calderone A., Jover T., Noh K-m., Tanaka H., Yokota H., Lin Y., Grooms S.Y., Regis R., Bennett M.V., Zukin R.S. Ischemic insults derepress the gene silencer REST in neurons destined to die. J. Neurosci. 2003;23(6):2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noh K-M., Hwang J-Y., Follenzi A., Athanasiadou R., Miyawaki T., Greally J.M., Bennett M.V., Zukin R.S. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc. Natl. Acad. Sci. USA. 2012;109(16):E962–E971. doi: 10.1073/pnas.1121568109. [http://dx.doi.org/10.1073/pnas.1121568109]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chakravarty S., Jhelum P., Bhat U.A., Rajan W.D., Maitra S., Pathak S.S., Patel A.B., Kumar A. Insights into the epigenetic mechanisms involving histone lysine methylation and demethylation in ischemia induced damage and repair has therapeutic implication. Biochimica et Biophysica Acta (BBA). Molecular Basis of Disease; 2016. [DOI] [PubMed] [Google Scholar]
- 134.Monfort A., Wutz A. Breathing‐in epigenetic change with vitamin C. EMBO Rep. 2013;14(4):337–346. doi: 10.1038/embor.2013.29. [http://dx.doi.org/10.1038/ embor.2013.29]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chervona Y., Costa M. The control of histone methylation and gene expression by oxidative stress, hypoxia, and metals. Free Radic. Biol. Med. 2012;53(5):1041–1047. doi: 10.1016/j.freeradbiomed.2012.07.020. [http://dx.doi.org/10. 1016/j.freeradbiomed.2012.07.020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang T., Chen K., Zeng X., Yang J., Wu Y., Shi X., Qin B., Zeng L., Esteban M.A., Pan G. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. 2011;9(6):575–587. doi: 10.1016/j.stem.2011.10.005. [http://dx.doi.org/10.1016/j.stem.2011.10.005]. [DOI] [PubMed] [Google Scholar]
- 137.Polytarchou C., Pfau R., Hatziapostolou M., Tsichlis P.N. The JmjC domain histone demethylase Ndy1 regulates redox homeostasis and protects cells from oxidative stress. Mol. Cell. Biol. 2008;28(24):7451–7464. doi: 10.1128/MCB.00688-08. [http://dx.doi.org/10.1128/MCB. 00688-08]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qureshi I.A., Mehler M.F. Chromatin-modifying agents for epigenetic reprogramming and endogenous neural stem cell-mediated repair in stroke. Transl. Stroke Res. 2011;2(1):7–16. doi: 10.1007/s12975-010-0051-3. [http://dx.doi.org/10.1007/s12975-010-0051-3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Formisano L., Noh K-M., Miyawaki T., Mashiko T., Bennett M.V., Zukin R.S. Ischemic insults promote epigenetic reprogramming of μ opioid receptor expression in hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2007;104(10):4170–4175. doi: 10.1073/pnas.0611704104. [http://dx.doi.org/10. 1073/pnas.0611704104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dekanty A., Romero N.M., Bertolin A.P., Thomas M.G., Leishman C.C., Perez-Perri J.I., Boccaccio G.L., Wappner P. Drosophila genome-wide RNAi screen identifies multiple regulators of HIF-dependent transcription in hypoxia. PLoS Genet. 2010;6(6):e1000994. doi: 10.1371/journal.pgen.1000994. [http://dx.doi.org/10.1371/journal.pgen. 1000994]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Herz H-M., Shilatifard A. The JARID2–PRC2 duality. Genes Dev. 2010;24(9):857–861. doi: 10.1101/gad.1921610. [http://dx.doi.org/10.1101/gad.1921610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stapels M., Piper C., Yang T., Li M., Stowell C., Xiong Z-g., Saugstad J., Simon R.P., Geromanos S., Langridge J. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Sci. Signal. 2010;3(111) doi: 10.1126/scisignal.2000502. [http://dx.doi.org/ 10.1126/scisignal.2000502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dou Y., Milne T.A., Ruthenburg A.J., Lee S., Lee J.W., Verdine G.L., Allis C.D., Roeder R.G. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat. Struct. Mol. Biol. 2006;13(8):713–719. doi: 10.1038/nsmb1128. [http://dx.doi.org/10.1038/ nsmb1128]. [DOI] [PubMed] [Google Scholar]
- 144.Tsai M-C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–693. doi: 10.1126/science.1192002. [http://dx.doi.org/10.1126/science.1192002]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lichtenwalner R.J., Parent J.M. Adult neurogenesis and the ischemic forebrain. J. Cereb. Blood Flow Metab. 2006;26(1):1–20. doi: 10.1038/sj.jcbfm.9600170. [http://dx.doi.org/10.1038/sj.jcbfm.9600170]. [DOI] [PubMed] [Google Scholar]
- 146.Jhelum P., Reddy R.G., Kumar A., Chakravarty S. Natural product based novel small molecules with promising neurotrophic, neurogenic and anti-neuroinflammatory actions can be developed as stroke therapeutics. Neural Regen. Res. 2016;11(6):916. doi: 10.4103/1673-5374.184486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferrer I., Krupinski J., Goutan E., Marti E., Ambrosio S., Arenas E. Brain-derived neurotrophic factor reduces cortical cell death by ischemia after middle cerebral artery occlusion in the rat. Acta Neuropathol. 2001;101(3):229–238. doi: 10.1007/s004010000268. [DOI] [PubMed] [Google Scholar]
- 148.Sun G., Alzayady K., Stewart R., Ye P., Yang S., Li W., Shi Y. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol. Cell. Biol. 2010;30(8):1997–2005. doi: 10.1128/MCB.01116-09. [http://dx.doi.org/10. 1128/MCB.01116-09]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y., Guan Y., Wang F., Huang A., Wang S., Zhang Y.A. Bmi-1 regulates self-renewal, proliferation and senescence of human fetal neural stem cells in vitro. Neurosci. Lett. 2010;476(2):74–78. doi: 10.1016/j.neulet.2010.04.006. [http://dx.doi.org/10.1016/j.neulet.2010.04.006]. [DOI] [PubMed] [Google Scholar]
- 150.Sher F., Rößler R., Brouwer N., Balasubramaniyan V., Boddeke E., Copray S. Differentiation of neural stem cells into oligodendrocytes: involvement of the polycomb group protein Ezh2. Stem Cells. 2008;26(11):2875–2883. doi: 10.1634/stemcells.2008-0121. [http://dx.doi.org/10. 1634/stemcells.2008-0121]. [DOI] [PubMed] [Google Scholar]
- 151.Lim D.A., Huang Y-C., Swigut T., Mirick A.L., Garcia-Verdugo J.M., Wysocka J., Ernst P., Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458(7237):529–533. doi: 10.1038/nature07726. [http://dx.doi.org/10.1038/nature07726]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim H.J., Leeds P., Chuang D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009;110(4):1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [http://dx.doi.org/10.1111/j. 1471-4159.2009.06212.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hsieh J., Nakashima K., Kuwabara T., Mejia E., Gage F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA. 2004;101(47):16659–16664. doi: 10.1073/pnas.0407643101. [http://dx.doi.org/10.1073/pnas. 0407643101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu I.T., Park J-Y., Kim S.H., Lee J-s., Kim Y-S., Son H. Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology. 2009;56(2):473–480. doi: 10.1016/j.neuropharm.2008.09.019. [http://dx.doi.org/10.1016/ j.neuropharm.2008.09.019]. [DOI] [PubMed] [Google Scholar]
- 155.Hu C-J., Chen S-D., Yang D-I., Lin T-N., Chen C-M., Huang T.H., Hsu C.Y. Promoter region methylation and reduced expression of thrombospondin-1 after oxygen–glucose deprivation in murine cerebral endothelial cells. J. Cereb. Blood Flow Metab. 2006;26(12):1519–1526. doi: 10.1038/sj.jcbfm.9600304. [http://dx.doi.org/10.1038/sj.jcbfm. 9600304]. [DOI] [PubMed] [Google Scholar]
- 156.Yang Y., Estrada E.Y., Thompson J.F., Liu W., Rosenberg G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [http://dx.doi.org/10.1038/ sj.jcbfm.9600375]. [DOI] [PubMed] [Google Scholar]
- 157.Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res. Brain Res. Rev. 2001;36(2):169–174. doi: 10.1016/s0165-0173(01)00092-3. [http://dx. doi.org/10.1016/S0165-0173(01)00092-3]. [DOI] [PubMed] [Google Scholar]
- 158.Rijntjes M., Weiller C. Recovery of motor and language abilities after stroke: the contribution of functional imaging. Prog. Neurobiol. 2002;66(2):109–122. doi: 10.1016/s0301-0082(01)00027-2. [http://dx.doi.org/10.1016/S0301-0082(01)00027-2]. [DOI] [PubMed] [Google Scholar]
- 159.Hagemann G., Redecker C., Neumann‐Haefelin T., Freund H.J., Witte O.W. Increased long‐term potentiation in the surround of experimentally induced focal cortical infarction. Ann. Neurol. 1998;44(2):255–258. doi: 10.1002/ana.410440217. [http://dx.doi.org/10.1002/ana.410440217]. [DOI] [PubMed] [Google Scholar]
- 160.Rossini P.M., Calautti C., Pauri F., Baron J-C. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2(8):493–502. doi: 10.1016/s1474-4422(03)00485-x. [http://dx.doi.org/10.1016/S1474-4422(03)00485-X]. [DOI] [PubMed] [Google Scholar]
- 161.Kirino T. Ischemic tolerance. J. Cereb. Blood Flow Metab. 2002;22(11):1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [http://dx.doi.org/10.1097/01.WCB.0000040942. 89393.88]. [DOI] [PubMed] [Google Scholar]
- 162.Kawai K., Nakagomi T., Kirino T., Tamura A., Kawai N. Preconditioning in vivo ischemia inhibits anoxic long-term potentiation and functionally protects CA1 neurons in the gerbil. J. Cereb. Blood Flow Metab. 1998;18(3):288–296. doi: 10.1097/00004647-199803000-00007. [http://dx. doi.org/10.1097/00004647-199803000-00007]. [DOI] [PubMed] [Google Scholar]
- 163.Dias R.B., Rombo D.M., Ribeiro J.A., Sebastião A.M. Ischemia-induced synaptic plasticity drives sustained expression of calcium-permeable AMPA receptors in the hippocampus. Neuropharmacology. 2013;65:114–122. doi: 10.1016/j.neuropharm.2012.09.016. [http://dx.doi.org/10.1016/ j.neuropharm.2012.09.016]. [DOI] [PubMed] [Google Scholar]
- 164.Ballas N., Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 2005;15(5):500–506. doi: 10.1016/j.conb.2005.08.015. [http://dx.doi.org/10.1016/j.conb.2005.08.015]. [DOI] [PubMed] [Google Scholar]
- 165.Roopra A., Huang Y., Dingledine R. Neurological disease: listening to gene silencers. Mol. Interv. 2001;1(4):219. [PubMed] [Google Scholar]
- 166.Ginsberg M.D. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55(3):363–389. doi: 10.1016/j.neuropharm.2007.12.007. [http://dx. doi.org/10.1016/j.neuropharm.2007.12.007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.del Zoppo G.J., Saver J.L., Jauch E.C., Adams H.P. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–2948. doi: 10.1161/STROKEAHA.109.192535. [http://dx.doi.org/10.1161/ STROKEAHA.109.192535]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hacke W., Kaste M., Bluhmki E., Brozman M., Dávalos A., Guidetti D., Larrue V., Lees K.R., Medeghri Z., Machnig T. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [http://dx. doi.org/10.1056/NEJMoa0804656]. [DOI] [PubMed] [Google Scholar]
- 169.Wiggins J.F., Ruffino L., Kelnar K., Omotola M., Patrawala L., Brown D., Bader A.G. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70(14):5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [http://dx.doi.org/10.1158/0008-5472.CAN-10-0655]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu S., Huang M., Li Z., Jia F., Ghosh Z., Lijkwan M.A., Fasanaro P., Sun N., Wang X., Martelli F. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122(11) Suppl. 1:S124–S131. doi: 10.1161/CIRCULATIONAHA.109.928424. [http://dx.doi.org/10.1161/ CIRCULATIONAHA.109.928424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Taj S.H., Kho W., Riou A., Wiedermann D., Hoehn M. MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials. 2016;91:151–165. doi: 10.1016/j.biomaterials.2016.03.025. [http://dx.doi.org/10.1016/j.biomaterials.2016.03.025]. [DOI] [PubMed] [Google Scholar]
- 172.Qi X., Hosoi T., Okuma Y., Kaneko M., Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol. Pharmacol. 2004;66(4):899–908. doi: 10.1124/mol.104.001339. [http://dx.doi.org/10.1124/mol. 104.001339]. [DOI] [PubMed] [Google Scholar]
- 173.Olson D.E., Sleiman S.F., Bourassa M.W., Wagner F.F., Gale J.P., Zhang Y-L., Ratan R.R., Holson E.B. Hydroxamate-based histone deacetylase inhibitors can protect neurons from oxidative stress via a histone deacetylase-independent catalase-like mechanism. Chem. Biol. 2015;22(4):439–445. doi: 10.1016/j.chembiol.2015.03.014. [http://dx.doi.org/10.1016/j. chembiol.2015.03.014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hashimoto R., Hough C., Nakazawa T., Yamamoto T., Chuang D.M. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J. Neurochem. 2002;80(4):589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [http://dx.doi.org/10.1046/ j.0022-3042.2001.00728.x]. [DOI] [PubMed] [Google Scholar]
- 175.Kanai H., Sawa A., Chen R., Leeds P., Chuang D. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. 2004;4(5):336–344. doi: 10.1038/sj.tpj.6500269. [http://dx. doi.org/10.1038/sj.tpj.6500269]. [DOI] [PubMed] [Google Scholar]
- 176.Leng Y., Chuang D-M. Endogenous α-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J. Neurosci. 2006;26(28):7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [http://dx.doi.org/10.1523/ JNEUROSCI.0096-06.2006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Baltan S., Murphy S.P., Danilov C.A., Bachleda A., Morrison R.S. Histone deacetylase inhibitors preserve white matter structure and function during ischemia by conserving ATP and reducing excitotoxicity. J. Neurosci. 2011;31(11):3990–3999. doi: 10.1523/JNEUROSCI.5379-10.2011. [http://dx. doi.org/10.1523/JNEUROSCI.5379-10.2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu J-Y., Niu F-n., Huang R., Xu Y. Enhancement of glutamate uptake in 1-methyl-4-phenylpyridinium-treated astrocytes by trichostatin A. Neuroreport. 2008;19(12):1209–1212. doi: 10.1097/WNR.0b013e328308b355. [http://dx. doi.org/10.1097/WNR.0b013e328308b355]. [DOI] [PubMed] [Google Scholar]
- 179.Mottet D., Pirotte S., Lamour V., Hagedorn M., Javerzat S., Bikfalvi A., Bellahcene A., Verdin E., Castronovo V. HDAC4 represses p21WAF1/Cip1 expression in human cancer cells through a Sp1-dependent, p53-independent mechanism. Oncogene. 2009;28(2):243–256. doi: 10.1038/onc.2008.371. [http://dx.doi.org/10.1038/onc.2008.371]. [DOI] [PubMed] [Google Scholar]
- 180.Raval A.P., Dave K.R., Pérez-Pinzón M.A. Resveratrol mimics ischemic preconditioning in the brain. J. Cereb. Blood Flow Metab. 2006;26(9):1141–1147. doi: 10.1038/sj.jcbfm.9600262. [http://dx.doi.org/10.1038/sj. jcbfm.9600262]. [DOI] [PubMed] [Google Scholar]
- 181.Marinova Z., Leng Y., Leeds P., Chuang D-M. Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology. 2011;60(7):1109–1115. doi: 10.1016/j.neuropharm.2010.09.022. [http://dx. doi.org/10.1016/j.neuropharm.2010.09.022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Giffard R.G., Han R-Q., Emery J.F., Duan M., Pittet J.F. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia-the complex roles of heat shock protein 70. Anesthesiology. 2008;109(2):339. doi: 10.1097/ALN.0b013e31817f4ce0. [http://dx.doi.org/10.1097/ ALN.0b013e31817f4ce0]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Uo T., Veenstra T.D., Morrison R.S. Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms. J. Neurosci. 2009;29(9):2824–2832. doi: 10.1523/JNEUROSCI.6186-08.2009. [http://dx.doi.org/10.1523/ JNEUROSCI.6186-08.2009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hunsberger J.G., Fessler E.B., Wang Z., Elkahloun A.G., Chuang D-M. Post-insult valproic acid-regulated microRNAs: potential targets for cerebral ischemia. Am. J. Transl. Res. 2012;4(3):316–332. [PMC free article] [PubMed] [Google Scholar]
- 185.Marinova Z., Ren M., Wendland J.R., Leng Y., Liang M.H., Yasuda S., Leeds P., Chuang D.M. Valproic acid induces functional heat‐shock protein 70 via class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J. Neurochem. 2009;111(4):976–987. doi: 10.1111/j.1471-4159.2009.06385.x. [http://dx.doi.org/10.1111/ j.1471-4159.2009.06385.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim D., Frank C.L., Dobbin M.M., Tsunemoto R.K., Tu W., Peng P.L., Guan J-S., Lee B-H., Moy L.Y., Giusti P. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60(5):803–817. doi: 10.1016/j.neuron.2008.10.015. [http://dx.doi.org/10.1016/j.neuron.2008.10. 015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rivieccio M.A., Brochier C., Willis D.E., Walker B.A., D’Annibale M.A., McLaughlin K., Siddiq A., Kozikowski A.P., Jaffrey S.R., Twiss J.L. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc. Natl. Acad. Sci. USA. 2009;106(46):19599–19604. doi: 10.1073/pnas.0907935106. [http://dx.doi.org/10. 1073/pnas.0907935106]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leone S., Mutti C., Kazantsev A., Sturlese M., Moro S., Cattaneo E., Rigamonti D., Contini A. SAR and QSAR study on 2-aminothiazole derivatives, modulators of transcriptional repression in Huntington’s disease. Bioorg. Med. Chem. 2008;16(10):5695–5703. doi: 10.1016/j.bmc.2008.03.067. [http://dx.doi.org/10.1016/j.bmc.2008.03.067]. [DOI] [PubMed] [Google Scholar]
- 189.Rigamonti D., Bolognini D., Mutti C., Zuccato C., Tartari M., Sola F., Valenza M., Kazantsev A.G., Cattaneo E. Loss of huntingtin function complemented by small molecules acting as repressor element 1/neuron restrictive silencer element silencer modulators. J. Biol. Chem. 2007;282(34):24554–24562. doi: 10.1074/jbc.M609885200. [http://dx. doi.org/10.1074/jbc.M609885200]. [DOI] [PubMed] [Google Scholar]
- 190.Rigamonti D., Mutti C., Zuccato C., Cattaneo E., Contini A. Turning REST/NRSF dysfunction in Huntington’s disease into a pharmaceutical target. Curr. Pharm. Des. 2009;15(34):3958–3967. doi: 10.2174/138161209789649303. [http://dx.doi.org/10.2174/138161209789649303]. [DOI] [PubMed] [Google Scholar]
- 191.Culhane J.C., Wang D., Yen P.M., Cole P.A. Comparative analysis of small molecules and histone substrate analogues as LSD1 lysine demethylase inhibitors. J. Am. Chem. Soc. 2010;132(9):3164–3176. doi: 10.1021/ja909996p. [http://dx.doi.org/10.1021/ja909996p]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schmidt D.M., McCafferty D.G. -2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46(14):4408–4416. doi: 10.1021/bi0618621. [http://dx.doi.org/10.1021/ bi0618621]. [DOI] [PubMed] [Google Scholar]
- 193.Huang Y., Marton L.J., Woster P.M., Casero R.A. Polyamine analogues targeting epigenetic gene regulation. Essays Biochem. 2009;46:95–110. doi: 10.1042/bse0460007. [http://dx.doi.org/10.1042/bse0460007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Huang Y., Stewart T.M., Wu Y., Baylin S.B., Marton L.J., Perkins B., Jones R.J., Woster P.M., Casero R.A. Novel oligoamine analogues inhibit lysine-specific demethylase 1 and induce reexpression of epigenetically silenced genes. Clin. Cancer Res. 2009;15(23):7217–7228. doi: 10.1158/1078-0432.CCR-09-1293. [http://dx.doi.org/10.1158/1078-0432.CCR-09-1293]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kubicek S., O’Sullivan R.J., August E.M., Hickey E.R., Zhang Q., Teodoro M.L., Rea S., Mechtler K., Kowalski J.A., Homon C.A. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Mol. Cell. 2007;25(3):473–481. doi: 10.1016/j.molcel.2007.01.017. [http://dx.doi.org/10.1016/j.molcel.2007.01.017]. [DOI] [PubMed] [Google Scholar]
- 196.Liu F., Chen X., Allali-Hassani A., Quinn A.M., Wigle T.J., Wasney G.A., Dong A., Senisterra G., Chau I., Siarheyeva A. Protein lysine methyltransferase G9a inhibitors: design, synthesis, and structure activity relationships of 2, 4-diamino-7-aminoalkoxy-quinazolines. J. Med. Chem. 2010;53(15):5844–5857. doi: 10.1021/jm100478y. [http://dx. doi.org/10.1021/jm100478y]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Greiner D., Bonaldi T., Eskeland R., Roemer E., Imhof A. Identification of a specific inhibitor of the histone methyltrans-ferase SU (VAR) 3-9. Nat. Chem. Biol. 2005;1(3):143–145. doi: 10.1038/nchembio721. [http://dx.doi.org/10.1038/nchembio721]. [DOI] [PubMed] [Google Scholar]
- 198.Tan J., Yang X., Zhuang L., Jiang X., Chen W., Lee P.L., Karuturi R.M., Tan P.B., Liu E.T., Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21(9):1050–1063. doi: 10.1101/gad.1524107. [http://dx.doi.org/10.1101/gad.1524107]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dimri M., Bommi P.V., Sahasrabuddhe A.A., Khandekar J.D., Dimri G.P. Dietary omega-3 polyunsaturated fatty acids suppress expression of EZH2 in breast cancer cells. Carcinogenesis. 2010;31(3):489–495. doi: 10.1093/carcin/bgp305. [http://dx.doi.org/10.1093/carcin/bgp305]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Niemoller T.D., Bazan N.G. Docosahexaenoic acid neuro- lipidomics. Prostaglandins Other Lipid Mediat. 2010;91(3):85–89. doi: 10.1016/j.prostaglandins.2009.09.005. [http://dx.doi.org/10.1016/j.prostaglandins.2009.09.005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ansari K.I., Hussain I., Das H.K., Mandal S.S. Overexpression of human histone methylase MLL1 upon exposure to a food contaminant mycotoxin, deoxynivalenol. FEBS J. 2009;276(12):3299–3307. doi: 10.1111/j.1742-4658.2009.07055.x. [http://dx.doi.org/10.1111/j.1742-4658.2009.07055.x]. [DOI] [PubMed] [Google Scholar]
- 202.Tariq M., Nussbaumer U., Chen Y., Beisel C., Paro R. Trithorax requires Hsp90 for maintenance of active chromatin at sites of gene expression. Proc. Natl. Acad. Sci. USA. 2009;106(4):1157–1162. doi: 10.1073/pnas.0809669106. [http://dx.doi.org/10.1073/pnas.0809669106]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Karatas H., Townsend E.C., Bernard D., Dou Y., Wang S. Analysis of the binding of mixed lineage leukemia 1 (MLL1) and histone 3 peptides to WD repeat domain 5 (WDR5) for the design of inhibitors of the MLL1− WDR5 interaction. J. Med. Chem. 2010;53(14):5179–5185. doi: 10.1021/jm100139b. [http://dx.doi.org/10.1021/j m100139b]. [DOI] [PMC free article] [PubMed] [Google Scholar]