Abstract
Background:
Posttraumatic stress disorder (PTSD) is a severe problem among soldiers with combating experience difficult to treat. The pathogenesis is still not fully understood at the psychological level. Therefore, genetic research became a focus of interest. The identification of single nucleotide polymorphisms (SNPs) may help to predict, which persons are at high risk to develop PTSD as a starting point to develop novel targeted drugs for treatment.
Methods:
We conducted a systematic review on SNPs in genes related to PTSD pathology and development of targeted pharmacological treatment options based on PubMed database searches. We focused on clinical trials with military personnel.
Results:
SNPs in 22 human genes have been linked to PTSD. These genes encode proteins acting as neurotransmitters and receptors, downstream signal transducers and metabolizing enzymes. Pharmacological inhibitors may serve as drug candidates for PTSD treatment, e.g. β2 adrenoreceptor antagonists, dopamine antagonists, partial dopamine D2 receptor agonists, dopamine β hydroxylase inhibitors, fatty acid amid hydrolase antagonists, glucocorticoid receptor agonists, tropomyosin receptor kinase B agonists, selective serotonin reuptake inhibitors, catechol-O-methyltransferase inhibitors, gamma-amino butyric acid receptor agonists, glutamate receptor inhibitors, monoaminoxidase B inhibitors, N-methyl-d-aspartate receptor antagonists.
Conclusion:
The combination of genetic and pharmacological research may lead to novel target-based drug developments with improved specificity and efficacy to treat PTSD. Specific SNPs may be identified as reliable biomarkers to assess individual disease risk. Focusing on soldiers suffering from PTSD will not only help to improve treatment options for this specific group, but for all PTSD patients and the general population.
Keywords: DNA, genetics, mental diseases, pharmacology, single nucleotide polymorphisms, gene-environment interactions
1. INTRODUCTION
Posttraumatic stress disorder (PTSD) may develop after a person has been exposed to one or more traumatic events, such as sexual assault, warfare, serious injury, or threats of imminent death. The diagnosis may be given, if symptoms such as disturbing recurring flashbacks, avoidance or numbing of memories of the event, and hyperarousal continue for more than a month after the occurrence of a traumatic event. Symptoms may include disturbing thoughts, feelings, or dreams related to the events, mental or physical distress to trauma-related cues, attempts to avoid trauma-related cues, alterations in how a person thinks and feels, and increased arousal [1].
PTSD diagnosis is closely associated to people with war experiences. Indeed, this disease became first aware to a wider public during the Vietnam War and the anti-war movement. According to the psychiatrist Jonathan Shay, PTSD was already mentioned in the literature of the 16th century. Lady Percy's monologue in Henry IV, written around 1597, represents one of the first unusually accurate descriptions of PTSD symptoms [2]. In 1952, the diagnosis appeared as “gross stress reaction” in Diagnostic and Statistical Manual of Mental Disorders-I (DSM-I), a system of psychiatric classification of the American Psychiatric Association. In the mid-1970s, the term “posttraumatic stress disorder” was created, in part through the efforts of anti-Vietnam War activists. Chaim F. Shatan, who worked with Vietnam Veterans, coined the term “post-Vietnam Syndrome”. It was added to the DSM-III, under the name posttraumatic stress disorder in 1980 [3].
Finding its way into DSM-III and understanding PTSD as a severe disease was a result of trial and tribulation. In World War I, combat-related traumata were described as “shell shock”, “garnet fever” or a dither disease (= war shakers). At this time, researchers questioned, whether these symptoms were caused by a physical injury or solely result from traumatic experiences [4]. Although, it was recognized by physicians as a distinct disease, the population often greeted psychologically traumatized war-returnees with profound contempt. Affected soldiers were declared cowards and sometimes even killed by the army leadership for cowardice. Many people supposed organic factors as underlying causes, such as shrapnel penetrated into the brain or simple simulation. Only as very similar symptoms appeared again during World War II, first systematic examinations were initiated [5].
Independent of the nature of traumatic experience, the risk for women is two to three times higher than for men, indicating that traumatic events alone are not sufficient, whether or not a person will develop PTSD [6]. The U.S. National Comorbidity Survey (NCS) estimated 7.8% (5% for males and 10.4% for females) lifetime prevalence for the U.S. population aged between 15 and 55 years. In Germany, the PTSD prevalence rate among the entire population is lower than in the U.S. (between 1% and 3%) [7]. Less economically developed states show higher PTSD prevalence rates. Many of these estimates have been derived following wars and political riots. For example, PTSD rates are about 15.8% and 17.8% in Ethiopia and Gaza [8]. In a Palestine study, PTSD rates of 21.5% in women and 13.2% in men have been reported [8].
In general, the chance is lower for victims of accidental events such as natural or anthropogenic catastrophes [9]. By contrast, the situation is different for military people. They are usually under special occupational risk. Recent studies suggest that an increasing number of soldiers develop PTSD, depending on the intensity of their mission. PTSD figures increased to more than 20% among U.S. soldiers in Afghanistan and Iraq [10]. The number of treated U.S. veterans rose during 13 years (1997-2010) by approximately 9% per year and the newly diagnosed cases rose by 19.2% annually. By contrast, other mental diseases showed an incidence of only 5.4% [11]. These numbers indicate both a growing burden of war operations and an increased awareness of the disease.
The posttraumatic stress disorder is a specific form of trauma disorders. Since the release of DSM-5 in May 2013, PTSD moved from the class of “anxiety disorders” into a new class of “trauma and stressor-related disorders.” All conditions included in this classification require exposure to a traumatic or stressful event as diagnostic criterion. The rationale for the creation of this new class is based upon clinical recognition of variable expressions of distress as result of traumatic experience. DSM-5 includes the addition of two subtypes: PTSD in children younger than 6 years and PTSD with prominent dissociative symptoms [12]. Depending on the time of onset and the duration of stress symptoms, PTSD can be divided in the acute form, where the duration of the symptoms is between 1 to 3 months. In the chronic form, symptoms last more than 3 months. With delayed onset, symptoms develop more than 6 months after the traumatic event. A trauma-associated stress activation, caused by chronic PTSD, may influence the course of physical disorders [13].
Various scales to measure the severity and frequency of PTSD symptoms exist. Standardized screening tools such as Trauma Screening Questionnaire and PTSD Symptom Scale can be used to detect possible symptoms of posttraumatic stress disorder. The main treatments for people with PTSD are counselling and medication. Cognitive behavioral therapy (CBT) is one type of counseling [14], which seems to be the most effective type of counseling for PTSD. Other types are exposure therapy and eye movement desensitization and reprocessing (EMDR). Antidepressants of the selective serotonin reuptake inhibitor type (e.g. fluoxetine) are first-line medications for PTSD. The use of benzodiazepines, however, is controversial [15]. A combination of different psychotherapies and medication seem to be most useful [16]. Nevertheless, existing treatment options are often not sufficiant for many people. This is the reason why new therapies are urgently needed.
Many factors contribute to PTSD development, e.g. disposing factors and characteristics of traumatic experiences and protective factors. Protective and disposing factors always interact with the social environment persons are embedded in, its self-conception as well as neurobiological factors.
Anatomical features (e.g. lower hippocampus volumes) as well as genetic factors (e.g. polymorphisms in neuro- receptors or neurotransmitter transporter genes) influence the vulnerability towards traumatic situations [17, 18]. Cross-talk between different brain areas represents an indicator for emotional dysregulation. Hypoactivity in the prefrontal cortex and corresponding hyperactivity in the amygdala are important markers for functional imbalance [19]. Neuro- imaging studies of PTSD can therefore be used to focus on elucidating the brain circuits that mediate this disorder. They reported significant findings in the amygdala, medial prefrontal cortex, hippocampus and insula. These findings complement our understanding of the wide-ranging neurobiological changes in trauma survivors who develop post-traumatic stress disorder [20, 21].
Traumatic events also cause morphological changes in brain areas. Epigenetic modifications such as histone modification [22] occur as result of trauma even affecting subsequent generations [23].
Twin and heritability studies performed among military personnel implied that 30%70% variation in PTSD risk may be determined by genetic factors [24, 25]. The sequencing of the human genome enabled new approaches to analyze the disease. In recent years, genetic research has made considerable progress and important functions of mutations for PTSD here unraveled. The relations between genetic differences and treatment outcomes may lead to the identification of new targets for treatments. Soldiers and military personnel are interesting research subjects for different reasons. On the one hand, they generally exert higher risks of developing PTSD, because they are more frequently exposed to hazardous situations. On the other hand, sufficiently large numbers of
PTSD patients are available for research purposes among soldiers and veterans. Another aim of this kind of studies is to identify, which persons are at high risk to develop PTSD in combat situations. In this overview, we focus on genetic investigations among military personnel and veterans and refer to scientific and clinical problems specifically related to soldiers. The impact of genetic research for drug development represents a central aspect of this review.
1.1. Genes and Society
In public media and the general public, questions are coming up at times such as: How much are we determined by our genes and are genes really predictive for our future? Is there a danger to categorize people according to their genomic status - especially military personnel? Could genetic information on predicting PTSD risk be misused by employers, health insurance companies and other institutions? Do soldiers returning from war with PTSD symptoms deserve the same treatment independent of their genetic status? What are the implications of false negative or false positive genetic test results? An open discourse between the public and scientists is necessary to discuss the various aspects in an unbiased and facts-based manner to foster the development of novel treatment options for affected patients. Progress in genetic research has much impact in military and civil health care. Efforts may lead to improved clinically pragmatic test systems for risk assessment, diagnosis and prognosis as well as treatment planning. Such findings may ultimately result in better prevention and outcome of PTSD - not only in military but also in civil life.
2. STUDIES ON GENETIC POLYMORPHISMS AMONG MILITARY PERSONNEL
2.1. Methods
2.1.1. Search Strategy
The electronic database Pubmed was searched on March 15th 2015 for the search terms: (1) single nucleotide poly- morphisms/SNP, (2) posttraumatic stress disorder/PTSD and (3) veterans/soldiers/combat-related. Only English articles were taken into account. The following inclusion and exclusion criteria were applied. Inclusion criteria: 1. Studies on SNPs or gene variants in relation to PTSD. 2. Studies performed among military personnel (active duty soldiers or veterans). Exclusion criteria: 1. Studies not conducted among military personnel. 2. Studies examining only comorbidities in PTSD, but not PTSD itself. 3. Meta-analyses or reviews.
2.2. Search Results
The search resulted in 32 articles. Twenty-eight met inclusion criteria. Four additional references were included by screening reference lists of the initially found 28 studies. These were not found by entering the search terms in the databases, probably because the title and abstract included several of the search terms, but not all.
Table 1 summarizes the studies among military personnel on single nucleotide polymorphisms published to date. Twenty-two candidate genes and their PTSD-associated SNPs have been investigated. If not declared otherwise,
Table 1.
Genetic loci and SNPs examined in military veterans.
| Gene | Protein | Function | Polymorphism Name or dbSNP |
Description of
Collective |
Sex
Female/ Male |
Size | Country | Refs. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNPs with strong evidence | ||||||||||||||||||
| BDNF | Brain derived neurotrophic factor | Survival of existing neurons; encourage growth and differentiation of new neurons and synapses. | rs6265 | US. Army soldiers from Iraq and Afghanistan wars | - | 461 | U.S. | [27] | ||||||||||
| rs6265 | Caucasian | male | 576 | Croatia | [28] | |||||||||||||
| COMT | Catechyl-O- methyl- transferase |
Degrades catecholamines such as dopamine, epinephrine, and norepinephrine. | rs4680 | Caucasian Iraq War veterans | male | 236 | U.S. | [29] | ||||||||||
| rs4680 | Caucasian and non- Caucasian Vietnam and Persian Gulf War veterans | 7/92 | 99 | U.S. | [30] | |||||||||||||
| D2DR | Dopamine receptor D2 | Dopamine receptor, which directly inhibits the formation of cAMP by inhibiting the enzyme adenylyl cyclase. | rs1800497 | Caucasian Vietnam combat veterans | male | 57 | Australia | [31] | ||||||||||
| rs1800497 | Caucasian Vietnam combat veterans | male | 63 | Australia | [32] | |||||||||||||
| rs1800497 | Non- hispanic white combat veterans | - | 56 | U.S. | [33] | |||||||||||||
| rs6277, rs1799732, rs1800497 | Caucasian combat veterans | male | 127 | Australia | [34] | |||||||||||||
| DRD3 | Dopamine D3 receptor | Dopamine receptor, which directly inhibits the formation of cAMP by inhibiting the enzyme adenylyl cyclase. | rs2134655, rs201252087, rs4646996, rs9868039 | White, non-Hispanic U.S. veterans | 172/319 | 491 | U.S. | [35] | ||||||||||
| SLC6A4 | Sodium-dependent serotonin transporter | Transportation of serotonin from the synaptic cleft to the presynaptic neuron. | rs4795541 | Veterans from Iraq and Afghanistan war | - | 186 | U.S. | [36] | ||||||||||
| rs16965628, rs25531 | Combat veterans | - | 44 | U.S. | [37] | |||||||||||||
| rs25531 | OEF/OIF/OND Veterans | male | 67 | U.S. | [38] | |||||||||||||
| rs25531 | Combat veterans (70% Caucasian) |
49/339 | 388 | U.S. | [39] | |||||||||||||
| rs25531, rs4795541 | Infantry soldiers (99% Jewish 25% Ashkenazi, 27% Sephardic, 44% mixed, and 3% of African- Ethiopian origin]) | male | 1085 | Israel | [40] | |||||||||||||
| NR3C1 | Glucocorticoid receptor | Nuclear receptor for cortisol and other glucocorticoids. | rs10052957, rs6189, rs6190, rs6195, rs41423247, rs6198 | >95% Caucasian veterans | male | 448 | Netherlands | [41] | ||||||||||
| rs41423247 | 74 Iraq or Afghanistan veterans, 32 Vietnam veterans and 7 Gulf War I veterans or other veterans | 9/104 | 113 | Australia | [42] | |||||||||||||
| rs41423247, rs6195 | Vietnam veterans | male | 160 | Australia | [43] | |||||||||||||
| Gene | Protein | Function | Polymorphism Name or dbSNP |
Description of Collective |
Sex Female/ Male |
Size | Country | Refs. | ||||||||||
| SNPs with strong evidence | ||||||||||||||||||
| CRHR-2 | Corticotropin releasing hormone receptor- 2 | Receptors for corticotropin-releasing hormone (CRH); HPA axis activation. | rs8192496, rs2190242, rs2267715, rs2284218 | Caucasian non-Hispanic veterans | 172 /319 | 491 | U.S. | [44] | ||||||||||
| SNPs with weak evidence | ||||||||||||||||||
| ADCY8 | Adenylate cyclase 8 (brain) | Membrane bound enzyme that catalyzes the formation of cyclic AMP from ATP. | rs263232 | White, non-Hispanic veterans | 170/314 | 484 | U.S. | [45] | ||||||||||
| ADRB2 | β-2-adrenergic receptor | G protein-coupled receptor, mediates the catecholamine-induced activation of adenylate cyclase through the action of G proteins. |
rs2400707 | Active duty Ohio National Guard soldiers | Predominantly male | 810 | U.S. | [46] | ||||||||||
| ANK3 | Ankyrin- 3 | Ankyrin-G is required for the normal clustering of voltage-gated sodium channels. | rs9804190, rs28932171, rs11599164, rs17208576 | White, non-Hispanic combat veterans | - | 554 | U.S. | [47] | ||||||||||
| APOE | Apolipoprotein E | Lipoproteins are responsible for packaging cholesterol and other fats and carrying them through the bloodstream. | Apo-ε2 | Korean veterans of the Vietnam War | male | 256 | Korea | [48] | ||||||||||
| Apo-ε4 | 92.3% White, 4.2% African-American, and 3.5% “other” | male | 1237 | U.S. | [49] | |||||||||||||
| DBH | Dopamine β- hydroxylase | Catalyzes the chemical reaction from dopamine to noradrenaline. | rs1611115 | Croatian- Caucasian | - | 167 | Croatia | [50] | ||||||||||
| DPP6 | Dipeptidyl-peptidase 6 | Binds specific voltage-gated potassium channels and alters their expression and biophysical properties. | rs71534169 | White, non-Hispanic veterans | 170/314 | 484 | U.S. | [45] | ||||||||||
| FAAH | Fatty acid amide hydrolase | Principal catabolic enzyme for a class of bioactive lipids called the fatty acid amides. | rs2295633 | Caucasian Vietnam war veterans | male | 115 | U.S. | [51] | ||||||||||
| FΚBP5 | FΚ506 binding protein 5 | Co-chaperone of the glucocorticoid receptor. | rs3800373, rs1360780 | >95% Caucasian veterans | male | 448 | Netherlands | [41] | ||||||||||
| GABRB3 | GABAA receptor β3 subunit | GABAA is an ionotropic receptor and ligand-gated ion channel. | dinucleotide repeat polymorphisms | Caucasian veterans | male | 86 | Australia | [52] | ||||||||||
| MAOB | Monoamine oxidase B | Catalyzes the oxidative deamination of biogenic and xenobiotic amines. | rs1799836 | Croatian Caucasian veterans | male | 386 | Croatia | [53] | ||||||||||
| NOS1AP | Nitric oxide synthase 1 adaptor protein | Ligand of neuronal nitric oxide synthase protein. | rs4531275, rs386231 | Caucasian veterans | male | 121 | Australia | [54] | ||||||||||
| PRKCΑ | Protein kinase C α |
PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. | rs4790904 | Caucasian veterans African- American veterans |
15% female 31% female |
428 533 |
U.S. | [55] | ||||||||||
| Gene | Protein | Function |
Polymorphism Name or dbSNP |
Description of Collective |
Sex Female/ Male |
Size | Country | Refs. | ||||||||||
| SNPs with weak evidence | ||||||||||||||||||
| PRTFDC1 | Phosphoribosyl transferase domain containing 1 | Enzyme with transferase acticvity. | GWAS, rs6482463, rs2148269, rs1033962 | Marines and Sailors scheduled for combat deployment to Iraq or Afghanistan, 85.5% white 75.5% non-Hispanic |
male | 3494 | U.S. | [56] | ||||||||||
| RORA | Retinoic acid receptor-related orphan receptor | Nuclear receptor, amongst others development of the cerebellum and lymph nodes, lipid metabolism, immune response, maintenance of bone. | rs8042149 | African- American veterans | - | 84 | U.S. | [57] | ||||||||||
| SLC1A1 | Excitatory amino-acid transporter 3 (EAAT3) or high affinity glutamate transporter | Transportation of glutamate across plasma membranes. | rs10814987, rs10739062, rs10758629, rs2228622, rs301435, rs12682807, rs3780412, rs2072657, rs301430, rs301979, rs301434, rs3087879, rs301443 | Caucasian (69.9%) | 80.9% male | 418 | U.S. | [58] | ||||||||||
5HT= serotonin, 5-HT2= serotonin receptor 2, Ad= adrenaline, AC8= adenylyle cyclase 8, APOE 4= apolipoprotein E 4, ANK3= ankyrin3, BDNF= brain derived neurotrophic factor, Ca2+= calcium ion, Cl-= chloride ion, COMT= catechol-=-methyltransferase, CRCHR2= corticotropin releasing hormone receptor, DA= dopamine, DAT= dopamine transporter, DBH= dopamine beta hydroxylase, D2= dopamine receptor D2, D3= dopamine receptor D3, EAAT= excitatory amino acid transporter, FAAH= fatty acid amide hydroxylase, DPP6= dipeptidyle peptidase 6 FKBP5= FK560 binding protein 5, Gi= G protein i, Gq= G protein q, GS= G protein S, GABA= gamma-aminobutyric acid, GABAA= gamma-aminobutyric acid receptor type A, GR= glucocorticoid receptor, HSP90= heat shock protein 90, iNOS= inducible NO synthase, MAO
B= monoamine oxidase B, NA= noradrenaline, NMDA-R= N-methyl-D-aspartate receptor, nNOS= neuronal NO synthase, NO= nitric oxide, PKC= protein kinase C pathway, RORA= RAR-related orphan receptor A, SERT= serotonin transporter, Trk-R= tyrosine kinase receptor.
Glucocorticoids (e.g. cortisol) from the adrenal cortex bind to receptors in the amygdala, hippocampus and the cortex. Here, differential gene transcription and repression result in higher levels of dopamine, glutamate and serotonin. Dopamine receptor 2 and 3 are inhibitory receptors acting via inhibition of adenylyl cyclase. These receptors have been associated with startle reactivity, sensorimotor gating, stress-related behaviors, memory, social recognition and responding, and cognitive impairment. In the HPA axis, FKBP5 plays a role as a glucocorticoid receptor (GR)-regulating co-chaperone molecule of heat shock protein 90 by binding to GRs in the cytosol and decreasing GR nuclear translocation. FKBP5 thereby inhibits the function of GRs which regulate adrenocortical secretion of glucocorticoids during stress-induced HPA axis activity [59]. The NMDA receptor is involved in normal memory encoding processes, while overstimulation of the NMDA receptor leads to strongly ingrained emotional memories via excessive mobilization of free cytosolic Ca2+. Glutamatergic stimulation of NMDA receptors activates various enzymes including NOS. The activity of constitutive NOS depends on Ca2+ and calmodulin, whereas inducible NOS is independent of Ca2+. Neuronal nNOS is located in neuronal cells, while inducible iNOS is located in macrophages and glial cells. Excessive NO release inhibits GABA release and therefore disrupting glutamate GABA balance. NO promotes cellular processes of plasticity and memory either by itself, or by the synthesis of cGMP as second messenger. 5HT released as a consequence of stress acts on 5-HT2 receptors activating constitutive nNOS by the protein kinase C (PKC) pathway [60]. APOE supports injury repair in the brain by transporting cholesterol and other lipids to neurons [49]. Growth factors like the brain-derived neurotrophic factor (BDNF) regulate cell birth and foster the cell maturation process and survival, wherefore they are crucial as regulating factors in the neoplastic process. Certain pathways can be strengthened through the development of new dendrites or additional synapses [61].
structures of the gene have been drawn with Variation Viewer from NCBI [26].
Fig. 1 shows an overview of the signaling cascades, which are controlled by these genes. In addition, the drugs are shown with their targets. Various pre- and postsynaptic receptors as well as metabolic enzymes have been shown to be important targets.
Fig. (1).
(a) Localization of hippocampus in brain, (b) two hippocampal synapses, c) pharmacological targets and signaling cascades contributing to PTSD vulnerability.
In the following chapters, we describe the 22 genes and their SNPs associated with PTSD among military personnel. Depending on the number of studies performed and the number of participants, six genes emphasize a high correlation with PTSD (BDNF, COMT, DRD2, DRD3, SLC6A4 and NR3C1). We splitted the following part into two groups emphasizing those first, where the genetic link is the most robust.
2.3. Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor (BDNF) encoded by the BDNF gene belongs to the group of neurotrophin family growth factors [62]. These factors are localized in the central peripheral nervous systems. The receptor of BDNF is the tropomyosin-related kinase B (TrkB) receptor. BDNF maintains neuronal survival and fosters outgrowth and differentiation of new neurons [63]. Brain areas with high BDNF activity are hippocampus, cortex, and basal forebrain. These areas are essential for learning, memory, and complex thinking. BDNF is also important for the shaping of long-term memory [64].
2.3.1. SNPs
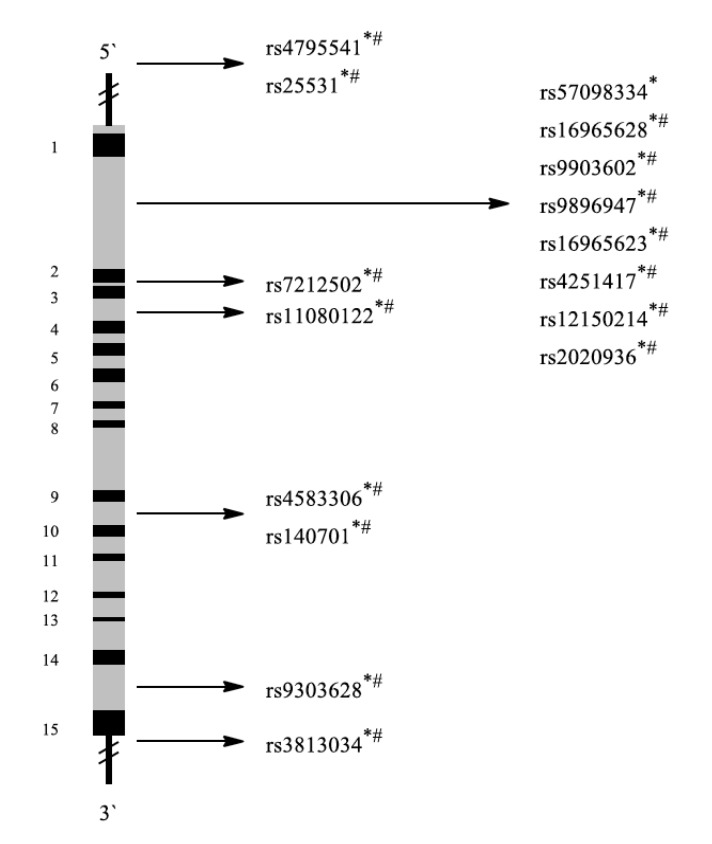
BDNF regulates stress responses. A common SNP in the BDNF gene is rs6265 (amino acid valine in position 66 replaced by methionine, (Fig. 2)). This SNP causes inefficient BDNF trafficking and reduced BDNF secretion. It also influences the hippocampal volume and memory and is related to a variety of neuropsychiatric disorders, including PTSD. Moreover, PTSD patients with rs6265 showed reduced responsivity to exposure-based therapies [65].
Fig. (2).
Schematic representation of the BDNF gene with exons (black 1-2) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases.
Zhang et al. analyzed genetic variations in 461 and BDNF plasma levels in 68 U.S. Army Special Operations soldiers deployed during Iraq and Afghanistan wars. PTSD was diagnosed using DSM-IV criteria. In the PTSD group, the frequency of the Met/Met genotype was nearly threefold higher than in control subjects. BDNF plasma levels of PTSD patients were significantly higher than those in healthy control persons. These results support a role of BDNF in PTSD. People carrying the Met allele had smaller hippocampus volumes and revealed poorer memory tasks performances than control persons with the wild-type Val/Val allele. Additionally, veterans with psychotic PTSD carried more frequently Met alleles than non-psychotic veterans with or without PTSD [27].
Another study assessed, whether the Met allele was overrepresented in unrelated Caucasian male veterans with psychotic PTSD compared to veteran controls. The rs6265 variants were genotyped in 576 veterans: 206 veterans without PTSD and 370 veterans with PTSD subdivided into groups with or without psychotic features. Veterans with psychotic PTSD were more frequently carriers of one or two Met alleles of this polymorphism than veterans with PTSD without psychotic features and veterans without PTSD [28].
2.3.2. Drugs
These findings suggest that BDNF signaling may be an important common signaling pathway in fear-related disorders and worthy to target for therapy. The tropomyosin receptor kinase B (TrkB) agonist 7, 8-dihydroxyflavone (DHF) can augment fear extinction, which underscores the potent of the BDNF-TrkB signaling pathway as target for cognitive enhancing therapy [66]. BDNF injected into rat brains induced extinction of 14 days old as well as recent fear memories [67]. Although poor blood-brain-barrier permeability [68] limits the therapeutic use of BDNF TrkB receptor agonists such as DHF may be promising candidates for therapy in anxiety disorders and PTSD.
2.4. Catechol-O-Methyltransferase
Catechol-O-methyltransferase (COMT) methylates various catecholamines, including natural neurotransmitters and neuroactive drugs and thereby inactivates them. This enzyme is part of the xenobiotic metabolism. COMT inactivates neuro-transmitters such as noradrenaline, adrenaline and dopamine in sympathetic nerve endings. The inactivation of these neurotransmitters is mediated by COMT-mediated methyl group transfer from S-adenosylmethionine (SAM) to a phenolic hydroxyl group, followed by oxidative deamination by monoamine oxidase (MAO). Dopamine is one of the main neurotransmitters inactivated by COMT. It plays a key role in prefrontal cortical function, including working memory [69]. Dopamine is also involved in the hippocampal consolidation of long-term memories [70].
2.4.1. SNPs
The rs4680 or Val158Met SNP in the COMT gene is well examined. The replacement of adenine by guanine in the DNA leads to an amino acid exchange at position 158 in the protein, resulting in substitution of valine with methionine. Methionine influences thermostability of the protein at physiological temperatures, which leads to reduced COMT activity [71]. Met158 carriers showed approximate one-third to one-fourth of the activity compared to Val158 carries. High COMT activity leads to low dopamine levels and vice versa. The Val158 and Met158 alleles are co-dominant. Hence, heterozygosity leads to intermediate COMT activity [72]. Dopamine affects cognitive functions of prefrontal inverted-U-shaped rather than linear kinetics, illustrating that the cognitive functions operate best at moderate levels of dopamine and are impaired at both high and low levels (Fig. 3) [73].
Fig. (3).
Inverted U-shaped kinetics of dopamine function. Relation between Val158Met genotype, dopamine level and function of the prefrontal cortex [73].
People with the homozygous Met-allele had levels of dopamine near the top of the U-shaped curve and perform better on many cognitive tasks. This seemed to be the optimal dopamine level for effective cognitive functioning. However, subjects with the Val/Val genotype showing lowest dopamine levels appeared to perform better on affective tasks and emotional processing [74]. The differing risks associated with both alleles suggest that people carrying the heterozygous allele may have a differential risk profile for PTSD compared to the homozygous group.
A prospective study on Iraq War veterans (n = 236) examined the interaction between COMT genotype and traumatic experiences to predict the subsequent development of PTSD symptoms. The assessments were done before and during deployment. Interestingly, the interaction between trauma load and COMT genotype was a significant predictor of PTSD symptoms. Those patients with heterozygous genotype (Val/Met) showed fewer symptoms associated with trauma exposure compared to those with homozygous (Met/Met and Val/Val) genotypes. This interaction remained significant even after adjusting for other PTSD risk factors. The COMT genotype Val/Met clearly increased the risk for PTSD after trauma exposure [29].
Schulz-Heik et al. conducted a clinical trial among 99 veterans of the U.S. military, who served in the Vietnam War or the first Persian Gulf War and who experienced substantial military operational stress. The Val158Met polymorphism in the COMT gene, which substantially influences dopamine inactivation in the frontal lobe in general and in the anterior cingulate cortex (ACC) in particular, may modulate integrity in PTSD. This study suggested that Val158Met moderates the relationship between PTSD and ACC volume. Homozygous Val genotype and presumably the associated decrease in intrasynaptic dopamine may increase the vulnerability to dystrophic effects of trauma [30].
Survivors of the Rwandan genocide with homozygous Met allele were at much higher risk to develop PTSD independently of the level of trauma exposure compared to those with Val/Val or Val/Met genotypes [75]. Two other studies showed similar results, although the stressors were quite different [76, 77]. These findings are in contrast to the above mentioned study among Iraq war veterans [29]. The question arises, how environmental factors such as the type of trauma interact with SNPs. COMT variations influence the capability of coping with traumatic memories and fear, but which of these alleles are risk factors seems to be dependent on the type of trauma. This aggravated the comparison of studies with different sample cohorts, e.g. results from a veteran’s cohort are hardly matchable with results from cohorts with childhood abuse.
Fig. (4) illustrates the location of the SNP described in the studies above. It is placed in exon 6. SNP rs6267 is connected to the pathogenesis of schizophrenia [78]. Rs174677 is an example for a non-pathogenic SNP located in an intron region. Association of the COMT synonymous polymorphism Leu136Leu and missense variant Val158Met with mood disorders [79].
Fig. (4).
Schematic representation of the COMT gene [80] with exons (black 1-6) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases.
2.4.2. Drugs
COMT is also relevant for other diseases. COMT inhibitors are used to treat Parkinson’s disease. This disease is characterized by a loss of dopaminergic neurons in the brain. COMT inhibitors (entacapone and tolcapone) can counteract the progression of dopamine deficiency. Therefore, the question rises whether COMT may also serve as target for PTSD therapy. High levels of dopamine and serotonin upon treatment with selective serotonin reuptake inhibitors (SSRIs) reduce PTSD symptoms. Therefore, it is reasonable to address the question whether other classes of drugs may also inhibit the degradation of catecholamines. In a rat model of attentional set shifting, tolcapone significantly improved extradimensional set shifting and potentiated the increase of extracellular dopamine in the medial prefrontal cortex. These data suggested a link between COMT activity and prefrontal cortex function in rats. Tolcapone seems to ameliorate the availability of dopamine in the prefrontal cortex [81]. Animal models of depression [82] also suggested a therapeutic value of tolcapone. A clinical trial with patients suffering from major depression confirmed these in vivo results and showed significant reductions of depression after tolcapone treatment [83]. In addition to their potential therapeutic value for Parkinson's disease, COMT inhibitors might also be an attractive option for PTSD treatment.
2.5. Dopamine Receptor D2
The etiology of PTSD is closely connected to the dopaminergic system. Dopamine plays an important role as neurotransmitter in the central nervous system. It binds to dopamine receptors, which can be divided into five subgroups. All of them are G protein-coupled receptors exerting their effects by a complex second messenger systems [84].
2.5.1. SNPs
To date, more than 1500 SNPs in the dopamine D2 receptor (DRD2) gene are known [86]. DRD2 polymorphisms affect DRD2 protein expression at the surface of neuronal cells, reduce D2 receptor binding and dopamine synthesis [87]. Hence, DRD2 polymorphisms may be determinants for PTSD. Fig. (5) illustrates the structure of DRD2 including PTSD-associated SNPs. DRD2 mutations are generally associated with various behavioral, psychiatric or neurological disease. For example, the polymorphism rs1079597 is associated with alcohol dependency and PTSD in military personnel [88].
Fig. (5).
Schematic representation of the DRD2 gene [85] with exons (black 1-7) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases
Polymorphisms such as 957C>T (rs6277) and the deletion polymorphism, 141delC (rs1799732) in the DRD2 gene are associated with schizophrenia, which reveals some symptoms comparable to PTSD. Specifically, rs1799732 affects mRNA stability and protein translation of the DRD2 receptor and determines striatal dopamine D2 binding in healthy subjects. The `T` allele in rs6277 was associated with decreased DRD2 mRNA stability, decreased DRD2 translation and diminished dopamine binding [89]. One hundred twenty-seven war veterans with PTSD and 228 control individuals without PTSD were investigated for their mutational DRD2 status. In addition to the two poly- morphisms mentioned above, a third one has been found, the Taq1A polymorphism (adenine replaced by thymine; rs1800497). It is located more than 10,000 bp downstream of the gene). The authors did not find significant associations of PTSD to the Taq1A or 141delC polymorphisms, but to the 957C>T polymorphism [34].
Lawford et al. examined the Taq1A polymorphism in 63 unrelated male Caucasian patients with PTSD. All subjects were Vietnam combat veterans, who had served in the Australian armed forces. The authors compared veterans with the A1+ (adenine positive) allele to veterans with the A1- (adenine negative) allele. The psychopathology score was greater in DRD2 A1+ than in A1− allelic patients. In particular A1+ compared to A1− allelic individuals showed significantly higher levels of social dysfunction, anxiety and depression [32].
2.5.2. Drugs
DRD2 represents an interesting target for PTSD therapy. In schizophrenia, DRD2 antagonists and partial agonists are used to reduce disease symptoms. Therefore, these drugs might also be used for PTSD treatment, because schizophrenia and PTSD share approximately 60% symptoms. The above mentioned paper of Lawford et al. also reports on the use of paroxetine for PTSD treatment. After paroxetine treatment for 8 weeks, three of the four GHQ-28 subscales showed significant reductions. GHQ-28 is the General Health-Questionnaire with subscales measuring somatic symptoms, anxiety/insomnia, social dysfunction and depression [90]. Furthermore, the social dysfunction subscale showed a significant allele by time interaction. Social dysfunction was significantly reduced in A1+ allelic patients compared to those with A1– allelic status. At the end of treatment, there were, however, no significant differences between these two allelic groups in any of the GHQ scores measured [32].
Drugs such as aripiprazole act as partial agonists and are known as second generation antipsychotics used against schizophrenia. This class of drugs exerts lower side effects compared to older antagonists. Aripiprazole was tested among military personnel as adjunctive therapy, if anti- depressants were ineffective [91, 92]. The outcomes of these studies have been positive regarding reduction of overall symptoms and safety, but have to be supported by further studies with larger numbers of participants and placebo controls.
The natural product yohimbine represents another drug, which could be an option for the treatment of PTSD. Yohimbine occurs mainly in the leaves and bark of the yohimbe tree (Pausinystalia yohimbe) and belongs to the group of indole alkaloids. It was tested in an animal model to investigate its function on dopaminergic neurotransmission. Yohimbine had a significant affinity towards DRD2 and increased dopamine levels, implying that it may be a possible candidate for pharmacotherapy of depression [93]. Further investigations should address the translation of these results to PTSD.
2.6. Dopamine D3 Receptor
Dopamine receptor D3 (DRD3) belongs to the same subtype of receptors DRD2. Activation of D2-like family receptors is coupled to the G protein Gαi, which prevents the formation of cyclic adenosine monophosphate (CAMP) by inhibiting the enzyme adenylyl cyclase [94].
2.6.1. SNPs
The distribution of SNPs in the DRD3 gene has been investigated in 852 subjects (590 white, non-Hispanic U.S. veterans and 262 intimate partners). Four SNPs (Fig. 6) were significantly associated with lifetime PTSD: rs2134655 (adenine replaced by guanine), rs201252087 (adenine replaced by guanine), rs4646996 (adenine replaced by guanine), and rs9868039 (adenine replaced by guanine). For each of these SNPs, the minor allele was less common among individuals with PTSD, suggesting a protective effect against risk for PTSD. The prevalence of lifetime PTSD among participants with one copy of the protective allele on rs2134655 (the most significant SNP) was 33.3%. It was 62.0% for those with no copies of the minor allele (there were no participants without copies of the minor allele) [35].
Fig. (6).
Schematic representation of the DRD3 gene with exons (black 1-8) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.6.2. Drugs
Abnormal dopamine receptor signaling and dopaminergic nerve function is implicated in several neuropsychiatric disorders. Thus, dopamine receptors are important neurologic drug targets. Antipsychotics are often dopamine receptor antagonists, while psychostimulants are typically indirect agonists of dopamine receptors [95]. The prefrontal cortex and DRD3 also play a role in working memory and executive functions [96]. Moreover, the Ser9Gly DRD3 polymorphism was associated with perseverative errors [97] and other indices of executive functioning [98]. PTSD also is associated with executive function deficits, decreased activation of prefrontal brain regions, and impaired concentration [99], this raises the possibility that DRD3 dysfunction at least partially accounts for cognitive deficits in PTSD. More research is needed to prove this hypothesis.
Aripiprazole, which possesses a high affinity for DRD3 is used to treat schizophrenia and bipolar disorder. It is also the most effective antipsychotic drug increasing the response to second-generation antidepressant drugs in patients with treatment-resistant depression [95]. Aripiprazole might also be used to treat PTSD. Polymorphisms in DRD3 are associated with Tourette (rs6280) [100], alcoholism [101] and schizophrenia [102].
2.7. Serotonin Transporters
Serotonin transporters (5-HTTLPR) are sodium- and chloride-dependent members of the solute carrier family 6 (SLC6), which are widely distributed throughout the brain. They are responsible for re-uptake of serotonin (5-HT) from the synaptic cleft back into the pre-synaptic terminal for further reutilization. Serotonin plays an important role in the regulation of cognitive functions, including memory, learning and sleep.
2.7.1. SNPs
The genetic structure of SLC6A4 (serotonin transporter gene) and PTSD-associated SNPs in military personnel are shown in Fig. (7).
Fig. (7).
Schematic representation of the SLC6A4 gene [37] with exons (black 1-15) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel.
The association of SNPs in the serotonin transporter gene with PTSD has been controversially discussed in the literature. Animal models and studies among people with anxiety disorders (comparable symptoms to PTSD) indicated some SNPs as risk factors for the disease, but rather seemed to be protective factors for soldiers in combat situations. A functional 44-base pair insertion/deletion polymorphism (rs4795541) of the SLC6A4 gene, located in the promoter region results in two main alleles, the short ´S´ and the long ‘L’ allele. Furthermore, an additional SNP can occur in the 44-base pair region of the ´L´ allele (adenine replaced by guanine, rs25531). As a consequence, the ´L´ allele can be further divided into ´LA´ and ´LG´ depending on the base. The ´LA´ allele is associated with higher transcriptional rates of the serotonin transporter, while the ´LG´ and S alleles reveal equivalent expression levels [103]. The ‘S’ allele impairs transcriptional activity of SLC6A4 and reduces the transporter activity [104], thereby increasing the risk for depression. Increased serotonergic neurotransmission contributed to the generation of anxiety as a consequence of reduced 5-HT uptake capacity found in individuals with the short allele of the 5-HTT polymorphism. Reduced 5-HT uptake was associated with depression and several anxiety disorders. These findings seem to be controversial with the fact that the only approved pharmacotherapy against PTSD are SSRIs such as sertraline, which selectively inhibit serotonin reuptake [105]. In a longitudinal prospective study with 1085 male Israeli defense infantry soldiers, Wald et al. examined how threat-related attention and serotonin-related gene polymorphisms rs4795541 and rs25531 interacted with combat exposure and predicted PTSD symptoms. The primary outcome parameters were post-combat symptoms measured in the combat theater (area for simulation of military actions) after six months of deployment. Baseline and pre-deployment symptoms were also measured. The authors defined three functionally relevant genetic categories according to the presumed efficacy of serotonin neuro- transmission: low efficacy of serotonin neurotransmission (´SS´, ´SLG´ and ´LGLG´), intermediate efficacy (´SLA´ and ´LALG´) and high efficacy (´LALA´). These findings suggest that military deployment induced shifts in threat-related attention related to PTSD risk. Low transcription rates of SLC6A4 may have protected from extreme stress of military deployment, where vigilance towards minor danger was crucial for survival. On the other hand, low SLC6A4 expression can cause enhanced maladaptive emotional response and elevated anxiety in a safe environment [40]. A study on the associations between the ´SS´ variant of SLC6A4 and PTSD among adults with histories of childhood emotional abuse confirmed this conclusion. The ´SS´ variant of SLC6A4 protected against re-experiencing and arousal symptoms of PTSD [106].
Kimbrel et al. genotyped 186 returning Iraq and Afghanistan veterans for the rs4795541 polymorphism. Typical PTSD symptoms, such as depression, general stress, and anxiety were assessed along with quality of life. After controlling for combat exposure, age, sex of the participant, and race, rs4795541 had a significant effect on post-deployment adjustment. S' carriers reported more post-deployment adjustment problems and worse quality of life than veterans with homozygous L' allele. This effect was even larger, if the analysis was restricted to veterans of European ancestry [36].
Wang et al. examined the relationship between the rs4795541 polymorphism and PTSD diagnostic status and severity, among a combat exposed sample of veterans. The hypothesis was that the low transcriptionally efficient variant (S′) of the rs4795541 would be associated with PTSD. Indeed, each copy of the S′ allele was associated with a 1.77-fold increased risk of being in the PTSD group. Additionally, the S′S′ genotype was associated with greater PTSD severity compared to the L′L′ genotype [39].
SLC6A4 promoter polymorphisms (rs4795541, rs25531) and several downstream single nucleotide polymorphisms modulated the activity of brain regions involved in the cognitive control of emotion in post-9/11 veterans with PTSD. In patients with PTSD, rs16965628 (associated with serotonin transporter gene expression) modulated task-related ventrolateral prefrontal cortex activation, if compared to trauma-exposed controls. Furthermore, rs4795541 tended to modulate left amygdala activation [37]. Taken together, the level of stress in a particular environmental condition may determine, whether a specific SNP protects from anxiety disorders or rather represents a risk factor.
SNP rs57098334 (variable number tandem repeat of several bases) has only been connected to PTSD in the general population as of yet, but not to PTSD among military personnel. The study was conducted at an emergency department in Turkey and the experimental subjects had experienced mild physical injury [107]. The presence of different SNPs in diverse cohorts could eventually be explained by the observations already mentioned above, that for some SNPs the type of trauma may predict, whether or not a point mutation is a risk factor for PTSD.
2.7.2. Drugs
Selective serotonin reuptake inhibitors are frequently prescribed for anxiety disorders, such as social anxiety disorder, panic disorders, obsessive–compulsive disorder, eating disorders and chronic pain. As described above, SSRIs are the first-choice treatment for PTSD. They increase the level of serotonin in the synaptic cleft by inhibiting its reuptake into the presynaptic cell leading to increased postsynaptic receptor activation [108].
2.8. Glucocorticoid Receptor
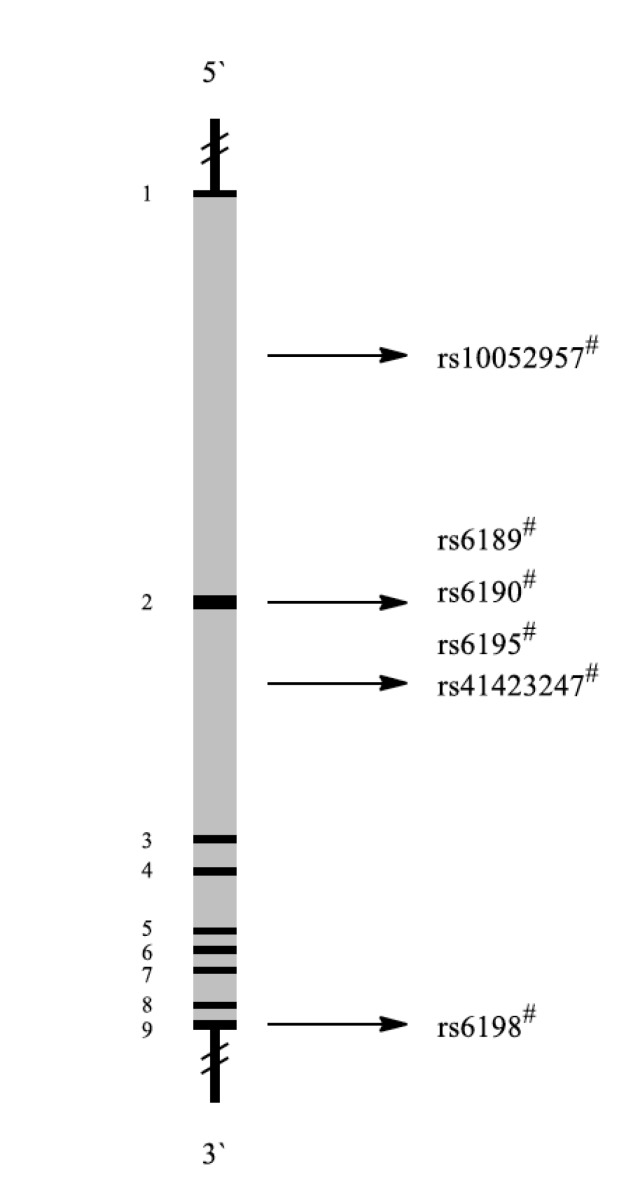
The glucocorticoid receptor (GR) also known as NR3C1 (nuclear receptor subfamily 3, group C, member 1) binds cortisol and other glucocorticoids. Glucocorticoids are key molecules in stress adaption [109]. Glucocorticoid is known to regulate neuronal survival, neuronal excitability, neurogenesis and memory acquisition [110]. Thus, high glucocorticoid levels may contribute to depressive symptoms by impairing these brain functions. Glucocorticoids regulate the HPA axis through negative feedback inhibition thereby reducing the production of glucocorticoids [111]. During depression, impaired GR function has been suggested to lead to HPA axis hyperactivity. The structure of the NR3C1 gene is illustrated in Fig. 8.
Fig. (8).
Schematic representation of the NR3C1 gene with exons (black 1-9) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.8.1. SNPs
Yehuda et al. examined BCLI, a single nucleotide polymorphism (rs41423247, C>G polymorphism) located in intron 2 of the NR3C1 gene in response to psychotherapy. Fifty-two male and female veterans with PTSD were randomized 2:1 to receive either prolonged exposure therapy or weekly minimal attention interventions for 12 consecutive weeks. Psychological and biological assessments were obtained prior to and following treatment and after 12-week follow-up. The genotypes were divided into two groups to designate ‘carriers’ of the G-allele (both homozygous GG and heterozygous CG) and ‘non-carriers’ (homozygous wild-type). Responders were more likely to carry the GG or GC genotype than the CC genotype [42].
Five common polymorphisms of the GR gene (rs10052957, rs6189/90, rs6195, rs41423247, and rs6198) have been investigated by van Zuiden et al. Predeployment GR pathway components were vulnerability factors for the subsequent development of PTSD symptoms [41].
Another study reported on the N363S and BClI poly- morphisms in 118 combat-exposed Vietnam veterans from a nationally accredited inpatient and outpatient PTSD treatment program in Brisbane, Australia. PTSD patients with the BclI GG genotype tended to have higher Clinician Administered PTSD Scale scores that were significantly negatively correlated with basal plasma cortisol levels. However, the number of subjects with the BclI GG genotype was small and statistical significance was not reached [43].
2.8.2. Drugs
Antipsychotics, such as clozapine may suppress HPA activity by reducing cortisol levels [112]. This activity may be exploited for PTSD treatment.
A novel antidepressant triple reuptake inhibitor (TRI) named RO-05 (4-[1-[1-(benzoyloxy)cyclohexyl]-2-(dimethylamino) ethyl]-phenyl benzoate) has been investigated in a mouse tail suspension, forced swimming and chronic mild stress tests. The antidepressant effects of RO-05 may be explained by the modulation of FKBP5 expression, GR activation, inhibition of HPA axis hyperactivity, and increase of BDNF expression [113]. RO-05 is a promising candidate for PTSD treatment, because it combines different targets involved in the development of posttraumatic stress.
2.9. Corticotropin-Releasing Hormone Receptor-2
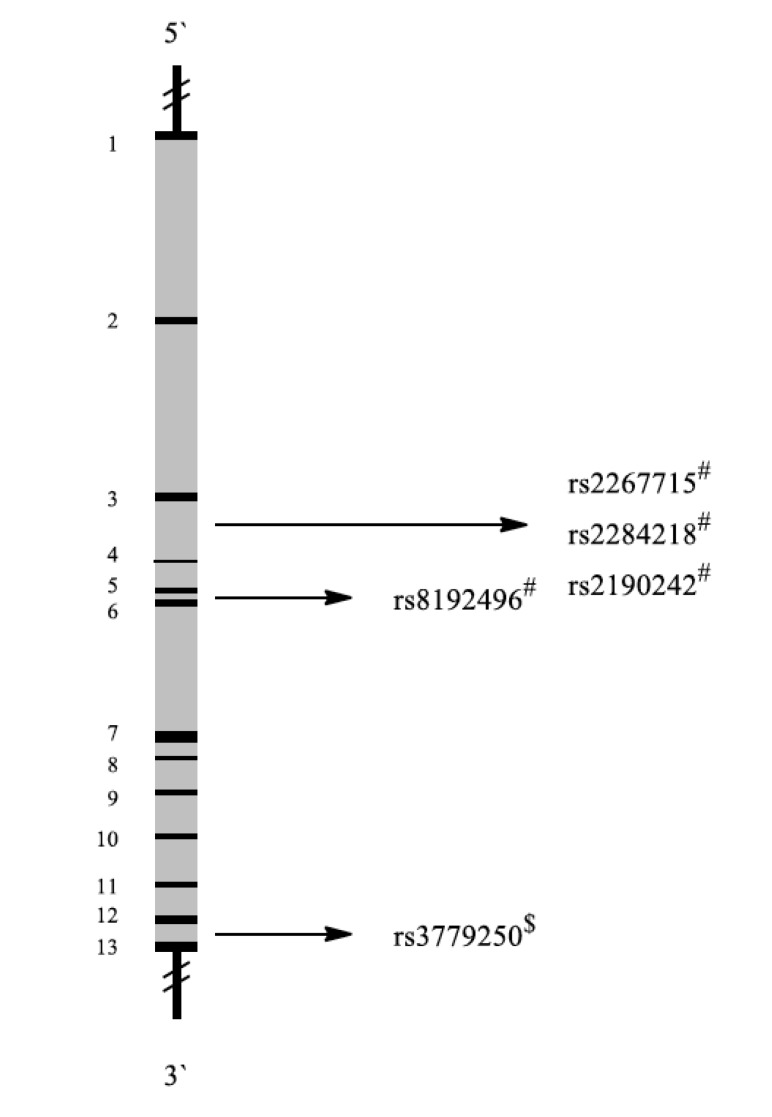
Corticotropin-releasing hormone receptors (CRHR) encoded by the CRHR1 and CRHR2 genes (Fig. 9) are type-2 G protein-coupled receptors for corticotropin-releasing hormone (CRH) that are resident in the plasma membranes of hormone-sensitive cells. CRH is synthesized in the hypothalamus and released following exposure to a stressor. It is the principal neuroregulator of the HPA axis, which modulates the dopamine, serotonin, glutamate, and norepinephrine systems [114]. Furthermore, CRH exerts effects on immune and autonomic processes and plays an important role in coordinating the physiological and behavioral response to stressors [115].
Fig. (9).
Schematic representation of the CRHR2 gene with exons (black 1-13) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.9.1. SNPs
One study examined 491 trauma-exposed white non-Hispanic veterans (n=364) and their cohabitating intimate partners (n=127). Interaction analyses revealed that effects were specific to women and that rs2267715 (adenine replaced by guanine) and rs2284218 (cytosine replaced by thymine) were also significantly associated to PTSD in women only. The minor allele of these SNPs was associated with reduced risk and severity of PTSD symptoms. These findings indicated that the relationship of the CRHR2 genotype to PTSD may be specific to women. This is consistent with prior observations that women are more likely to exhibit anxiety, unipolar depressive disorders and PTSD [44].
Major depressive disorder (MDD) and panic disorder (PD) are common, disabling disorders with both, stress and genetic components. Dysregulated stress response by the HPA axis, including CRHR1- and CRHR2-mediated signaling is considered to play a major role for onset and recurrence in MDD and PD (Fig. 9) [116].
2.9.2. Drugs
Neurotransmission of corticotropin-releasing hormone (CRH) may also be altered in PTSD. CRH levels in cerebrospinal fluid were higher in PTSD patients compared to control subjects [117]. Identifying the underlying mechanisms may therefore be necessary to identify suitable targets for developing novel compounds to treat anxiety disorders such as PTSD.
Clinical trials focused on CRHR1. A phase II trial evaluated the efficacy of a CRHR1 antagonist (GSK561679) in the treatment of PTSD. Untreated women with PTSD of at least three months’ duration, are being enrolled in a parallel-group, double-blind, placebo-controlled, randomized clinical trial evaluating the efficacy and safety of GSK561679 [118]. This is an ongoing study and treatment effects remain to be evaluated.
2.10. Adenylate Cyclase 8
Adenylate cyclase is a membrane-bound enzyme that catalyzes the formation of cyclic adenosine monophosphate (AMP) from adenosine triphosphate (ATP). ADCY8 is presynaptically expressed in many areas of the brain, including cortex, hippocampus, amygdala, thalamus, hypo- thalamus, and cerebellum. Cyclic AMP is integral to long-term potentiation, synaptic plasticity, and hence learning and memory. In the hippocampus, cyclic AMP determines the basal balance between silenced and active synapses [119].
2.10.1. SNPs
Examining the genome-wide distribution of SNPs, a sample of 484 white, non-Hispanic, trauma-exposed veterans and their partners was assessed for lifetime PTSD and dissociation using a structured clinical interview. The SNP with the strongest association to PTSD was rs263232 in the ADCY8 gene (G>T), on chromosome 8 [45]. Failure to encode memories associated with intense emotion or stress is clearly related to PTSD because of psychogenic amnesia, which has been uniquely associated with PTSD. One hypothesis is that dissociation occurs as a neurobiological consequence of hyperarousal in the context of ADCY8 deficiency that prevents encoding and consolidation of contextual information [45].
2.10.2. Drugs
ADCY8 has also been associated with bipolar disorder (SNP rs37500889, Fig. 10) [120], and depression in connection with alcohol dependency in women [121]. Although not available yet, specific ADCY8 antagonists are therefore an interesting target to treat depression and PTSD. Such an inhibitory drugs might help to contextualize information and memory in psychological disorders.
Fig. (10).
Schematic representation of the ADCY8 gene with exons (black 1-17) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases.
2.11. β2-adrenergic Receptor
The β2 adrenergic receptor (β2 adrenoreceptor), also known as ADRB2, is an adrenergic receptor in cell membranes encoded by the ADRB2 gene. The neurotransmitter adrenaline binds to its extracellular domain which causes multiple effects in muscles or organs. For example, it increases mass and contraction speed for fight-or-flight reaction in striated muscles. Adrenergic and noradrenergic abnormalities have long been believed to play a key role in PTSD development contributing to exaggerated physiological reactivity and hyperarousal symptoms [119].
2.11.1. SNPs
Polymorphisms in ADRB2 have been observed in a study among soldiers from the Ohio National Guard Study of Risk and Resilience. The study design was a prospective longitudinal study of 2616 post deployment psychological healthy persons recruited from 6514 randomly selected Ohio National Guard members during predeployment training and assessed over three annual follow-ups. In total, 72.1% of soldiers had been deployed to combat zones, including Iraq, Afghanistan, and other combat zones, such as Bosnia and Somalia, and 42% of soldiers had been exposed to military combat [46]. The genetic associations were demonstrated using a G × E model, which means observation of interaction of environmental as well as genetic factors. The rs2400707 polymorphism was significantly associated with PTSD (adenine replaced by guanine, Fig. 11).
Fig. (11).
Schematic representation of the ADRB2 gene with exon (black) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases, UTR Untranslated region.
The AA (homozygous), AG (heterozygous), or GG genotypes showed different degrees of PTSD, which correlated with adverse childhood experiences. Similar findings have been found in the replication cohort. These results suggest that the ADRB2 gene interacts with childhood adversity, constituting a vulnerability and resilience factor for the development of PTSD symptoms following adult trauma. The rs2400707 homozygotes represented the most resilient group without increase in PTSD symptoms despite the exposure to several types of childhood adversity, while the GG homozygotes showed the greatest vulnerability. The heterozygotes revealed intermediate vulnerability [46].
2.11.2. Drugs
Since the adrenergic system is involved in the etiology of PTSD, it represents an interesting target for pharmacotherapy. Animal experiments showed that the β2 adrenergic receptor blocker propranolol reduced the strength of conditional fear memories through blockade of reconsolidation, if administered shortly after reactivation of such memories [122].
Salbutamol is a β2-adrenergic receptor agonist often used to treat acute asthma attacks and respiratory failure [123]. Interestingly, two SNPs in this gene (rs1800888, rs1042713) are associated with a higher risk for asthma [124].
Salbutamol improved subsequent posttraumatic stress symptoms (PTSS) within a few hours after a motor vehicle accident [125]. Participants receiving salbutamol had less severe overall PTSS and hyper-arousal symptoms at 6 weeks and less severe re-experiencing symptoms at 1 year post-motor vehicle accident than those without salbutamol.
2.12. Ankyrin-3
The ANK3 gene encodes the ankyrin G protein (Fig. 12). It is found at the axonal initial segment and Ranvier nodes of neurons in the central and peripheral nervous systems. It plays an essential role in regulating neuronal activity [126].
Fig. (12).
Schematic representation of the ANK3 gene with exons (black 1-44) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.12.1. SNPs
An association between ANK3 SNPs and PTSD was observed in a cohort of white non-Hispanic combat veterans and their intimate partners (n = 554). Rs9804190 (cytosine replaced by thymine) was associated with PTSD [47]. The minor T allele, which was related with higher ankyrin G expression and lower risk of bipolar disorder and schizophrenia, was significantly correlated with reduced frequencies of PTSD and externalizing psychopathology. Conversely, the rs9804190 C allele was linked to lower ankyrin G expression, higher frequency of PTSD and a greater degree of externalizing behaviors. This corresponded to increased reactivity to stressors and increased behavioral disinhibition as observed in mice with reduced ankyrin G expression [127]. The association was expected based on prior results showing an association between rs9804190 and bipolar disorder and schizophrenia and the behavioral changes in Ank3 knock-out mice [47].
2.12.2. Drugs
SNPs in the ANK3 gene were, however, also associated with schizophrenia (rs10761482 and rs10994336) [128]. It is reasonable to speculate that these findings from schizophrenia might be transferable to PTSD treatment.
2.13. Apolipoprotein E
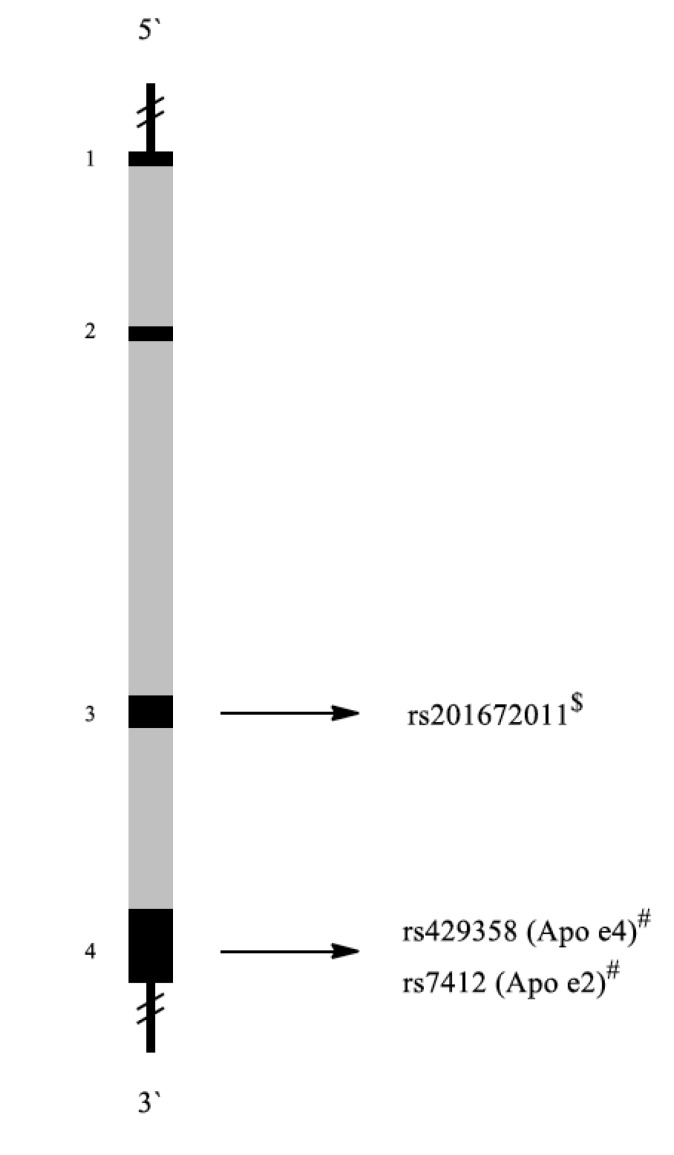
The APOE gene encodes apolipoprotein E. The structure of APOE gene is shown in Fig. 13. This protein associates with lipids to form lipoproteins, which are responsible for packaging cholesterol and other fats and carrying them through the bloodstream. ApoE is important for neuronal repair. It affects neuronal plasticity through the transport of cholesterol and other lipids to neuronal sites undergoing remodeling [129].
Fig. (13).
Schematic representation of the APOE gene with exons (black 1-4) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.13.1. SNPs
APOE is polymorphic, with three major alleles: APOE2 (cys112, cys158), APOE3 (cys112, arg158), and APOE4 (arg112, arg158) [130]. Although these allelic forms differ from each other by only one or two amino acids at positions 112 and 158, these differences alter ApoE structure and function. The rs201672011 polymorphism is associated with Alzheimer`s disease [131] or hyperlipoproteinemia [132].
Kim et al. studied the connection between APOE polymorphisms and PTSD and drinking behavior. All subjects were male veterans serving in the Korean Armed Forces during the Vietnam War. Compared to the controls, PTSD patients consumed greater amounts of alcohol. In addition, there were more ε2-allele carriers in the PTSD group than in the control group. However, no significant differences were observed between groups with respect to the numbers of ε3- and ε4-allele carriers [48].
2.13.2. Drugs
Drug candidates targeting ApoE have been investigated for the treatment of Alzheimer`s disease [133]. However, data for PTSD are missing as of yet.
2.14. Dopamine β-Hydroxylase
Dopamine β-hydroxylase is encoded by the DBH gene (Fig. 14). It catalyzes the reaction from dopamine to norepinephrine. Norepinephrine is an important neuro- transmitter in normal stress reactions as well as in PTSD [134]. DBH is expressed in noradrenergic nerve terminals of the central and peripheral nervous systems [135].
Fig. (14).
Schematic representation of the DBH gene with exons (black 1-12) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. *Association with PTSD in general, #Association with PTSD among military personnel, $Association with other diseases.
2.14.1. SNPs
A study examining DBH polymorphisms included 167 unrelated Croatian Caucasian medication-free subjects from the same military unit. The study population was divided into two groups. The first group consisted of 133 war veterans chronic PTSD, whereas the second group contained 34 combat exposed war veterans with similar traumatic experiences, but who did not develop PTSD. War veterans with PTSD revealed lower DBH activity compared to war veterans without PTSD, which suggests that combat traumatized soldiers can be divided into subjects adaptable to trauma (with higher plasma DBH activity after trauma) and those less resilient and more vulnerable to develop PTSD (with lower plasma DBH activity after a trauma). Another finding was that SNP rs1611115 cytosine replaced by thymine at position 1021 was strongly associated with plasma DBH activity. In this case the genotype CC was associated with higher plasma DBH activity, the CT genotype with intermediate activity and soldiers with TT genotype with low DBH activity, suggesting a co-dominant inheritance. Plasma DBH activity was lower in war veterans with PTSD carrying the CC genotype compared to non-PTSD veterans of the same genotype [50].
SNP rs1611115 has also been investigated in African-American civilians with and without PTSD. The genetic status correlated with the serum activity of DBH and was a significantly associated with the status of depressions, but not with PTSD diagnosis itself [136].
2.14.2. Drugs
The DBH inhibitor nepicastat has been assessed as treatment for PTSD and cocaine dependency [137]. Nepicastat, a potent, competitive, and selective DBH inhibitor, was selected for this study because it decreases norepinephrine. This was a 6-week randomized, double-blind, multisite, placebo-controlled study involving 22 veterans with PTSD. The sample consisted of 14 CC and eight T carriers with 45.5% Caucasian and 54.5% African-American participants. The purpose of this study was to compare the utility of using genetics versus functional norepinephrine physiology to predict, who may respond to nepicastat. The authors found a significantly probability in carriers of the CC genotype in PTSD symptomatology than the carriers of the T genotypes. There was no genotype- related treatment [138].
2.15. Dipeptidyl-Peptidase 6
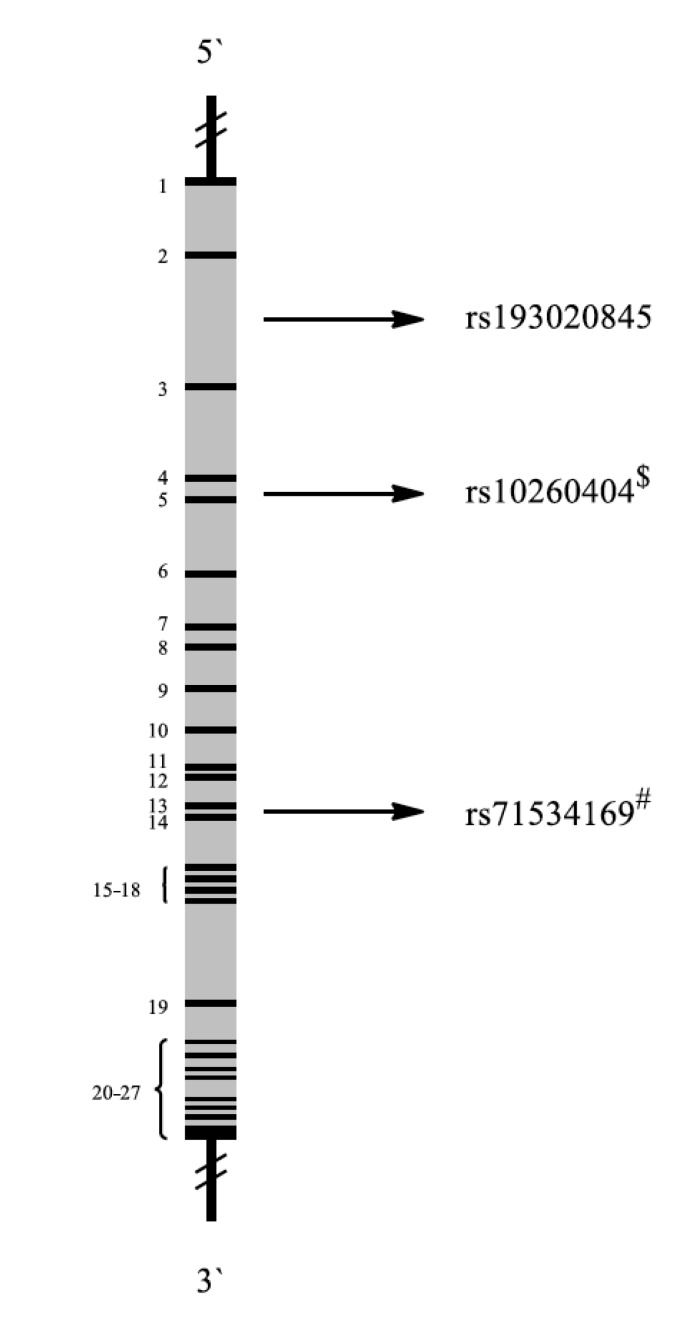
Dipeptidyl-peptidase 6 is encoded by the DPP6 gene (Fig. 15). DPP6 is critical for synaptic integration and excitation. It binds specific voltage-gated potassium channels and alters their expression and biophysical properties [139].
Fig. (15).
Schematic representation of the DPP6 gene with exons (black 1-27) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.15.1. SNPs
A total number of 23240 SNPs have been described for DPP6 [140]. SNP rs71534169 is the only one, which has been correlated with combat-related PTSD until to date.
SNP rs71534169 was stronger associated to PTSD in women compared to men, suggesting gender-specific effects in the development and manifestation of dissociative symptoms [45].
In contrast to PTSD, DPP6 has been most frequently studied in multiple sclerosis [141] and association has also been reported to autism [142] and amyotrophic lateral sclerosis (rs10260404) [143].
2.15.2. Drugs
There are no compounds targeting DPP6 as of yet.
2.16. Excitatory Amino Acid Carrier
SLC1A1 (solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter), member 1, (Fig. 16)) is a gene coding for the excitatory amino acid carrier, a glutamate transporter [144]. Functions of glutamate transporters include regulation of excitatory neurotransmission, maintenance of low ambient extracellular glutamate concentrations (as protection against neurotoxicity) and provision of glutamate by the glutamate-glutamine cycle [145]. Glutamate is abundant in the human body, but particularly in the nervous system and brain's main excitatory neurotransmitter. Glutamate also serves as precursor for GABA, the brain's main inhibitory neurotransmitter [146].
Fig. (16).
Schematic representation of the SLC1A1 gene with exons (black 1-12) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.16.1. SNPs
Zhang et al. performed a study with 418 combat veterans and 63.2% (n = 264) of them met criteria for PTSD. The majority of the participants were males (80.9%) and Caucasians (69.9%). Male gender and the major allele of rs10739062 significantly increased the likelihood of PTSD. Within the sample, the only SNP associated with a PTSD diagnosis was rs10739062, whereas no single SNPs were associated with symptom severity. However, when considering variants within the SLC1A1 gene in an additive manner, they were associated both with the presence of a PTSD diagnosis as well as the symptom severity. Even the severity of combat exposure correlated the sum of SNPs. This indicates the importance of considering multiple SLC1A1 polymorphisms in an additive manner for PTSD [58].
Additionally, SNP rs301430 [147, 148] was associated with obsessive-compulsive disorder.
2.16.2. Drugs
Clinical investigations among combat veterans demonstrated that the non-competitive NMDA glutamate receptor antagonist memantine reduced PTSD symptom severity [149]. Similarly, ketamine, a non-competitive NMDA glutamate receptor antagonist, reduced soldiers’ likelihood of developing PTSD, if provided prior to operations for burns [150]. This drug reduced the severity of PTSD symptoms in a randomized, double-blind, placebo-controlled crossover trial [151]. While recent clinical research mainly focused on the targeting of NMDA receptors, there remains a dearth of genetic data may further assist the development of targeted treatment approaches.
Structure-activity-relationship analyses led to the synthesis of two potent inhibitors, UCPH-101 and UCPH-102 [152]. Animal experiments have to gain information on their activity on the excitatory amino acid transporter (EAAT).
2.17. Fatty Acid Amide Hydrolase
Fatty acid amide hydrolase (FAAH) is an integral membrane hydrolase and is the principal catabolic enzyme for a class of bioactive lipids called fatty acid amides (FAAs) and is responsible for the degradation of endogenous cannabinoids (eCBs) [153]. It is encoded by the FAAH gene [154].
2.17.1. SNPs
Pardini et al. assessed PTSD frequency male Vietnam War veterans, who suffered combat-related penetrating traumatic brain injury (PTBI) and PTSD. Rs2295633 (cytosine replaced by thymine, (Fig. 17)) was significantly associated with PTSD diagnosis in subjects without lesions in the ventromedial prefrontal cortex. Moreover, the presence of the C allele was associated with more severe re-experiencing of trauma and more negative reported childhood experiences. FAAH as a contributor to PTSD after PTBI, possibly through the modulation of aversive memories by extinction processes. These data suggest a role for endocannabinoid signaling in the development and maintenance of PTSD and hint at the therapeutic potential of endocannabinoid systems-modulating drugs for PTSD patients [51]. Another SNP in FAAH is associated with alcoholism (rs324420) [155].
Fig. (17).
Schematic representation of the FAAH gene with exons (black 1-15) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.17.2. Drugs
Due to the ability of FAAH to regulate nociception, it is currently viewed as an attractive drug target for the treatment of pain. FAAH has emerged as promising target for anxiety-related disorders, since FAAH inhibitors are able to increase the levels of anandamide and thereby induce anxiolytic-like effects in rodents [153, 156].
2.18. FΚ506 Binding Protein 5
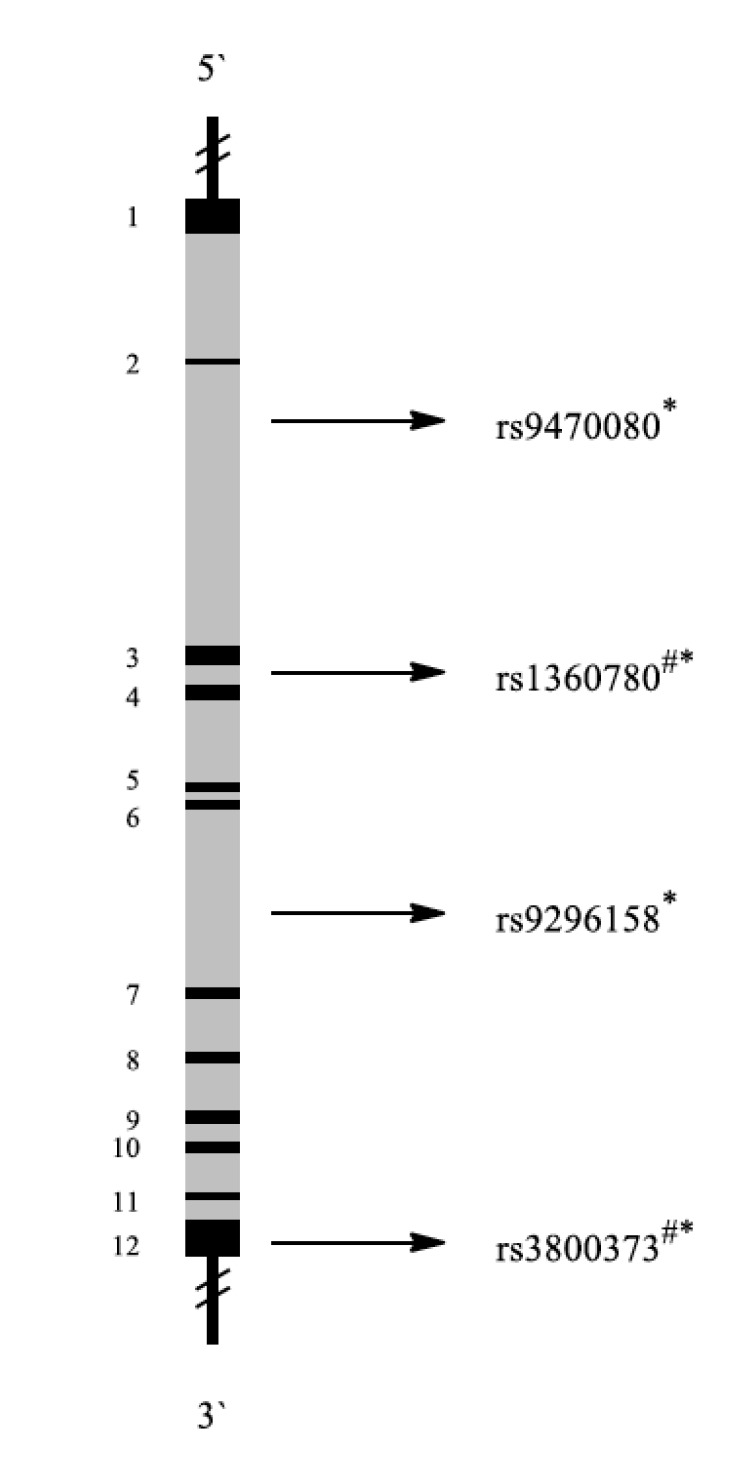
FK506 binding protein 5, also known as FKBP5, is a protein which is encoded by the FKBP5 gene (Fig. 18) [157]. The protein encoded by this gene is a member of the immunophilin protein family, which plays a role in immunoregulation and basic cellular processes involving protein folding and trafficking. It is a co-chaperone of the glucocorticoid receptor (GR). When bound to the complex, FKBP5 functions as inhibitor of glucocorticoid binding to GR. If released, the ligand-bound receptor then translocates to the nucleus and acts as a transcription factor [158].
Fig. (18).
Schematic representation of the FKBP5 gene with exons (black 1-12) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases.
2.18.1. SNPs
Van Zuiden et al. investigated whether predeployment GR and FKBP5 mRNA expression were associated with SNPs in the GR and FKBP5 genes, either alone or in interaction with childhood trauma. Four hundred forty-eight male participants completed the assessments before and 6 months after deployment. The sample had a predominantly Caucasian background (>95%). Two FKBP5 polymorphisms (rs3800373 (guanine replaced by thymine), rs1360780 (cytosine replaced by thymine)) were investigated in 448 male soldiers, of whom 35 reported a high level of PTSD symptoms after return from deployment. Participants in the PTSD group reported more PTSD symptoms than the comparison group before and after deployment. More importantly, the PTSD group revealed a strong increase in PTSD symptoms in response to deployment, while PTSD symptoms did not increase in the comparison group. Childhood trauma was independently associated with increased risk for high levels of PTSD symptoms. This study revealed that multiple GR pathway components measured before deployment are vulnerability factors for development of high levels of PTSD symptoms in response to military deployment [41].
FKBP5 polymorphisms have also been investigated among civilians. Both genetic and environmental factors are contributory, with child abuse providing significant risk liability. Four FKBP5 SNPs (rs9296158, rs3800373, rs1360780, and rs9470080) interacted with severity of child abuse as a predictor of adult PTSD symptoms. There were no main effects of these SNPs on PTSD symptoms and no significant genetic interactions with severity of non-child abuse trauma as predictor of adult PTSD symptoms. This suggests a potential gene-childhood environment interaction for adult PTSD [159, 160].
2.18.2. Drugs
The FK506 binding protein 5 (FKBP5) is of special interest, since it modulates HPA axis signaling reactivity through glucocorticoid receptor [nuclear receptor subfamily 3, group c, member 1 (NR3C1)] [161].
SNPs in FKBP5 influence the response to antidepressant drug. Carriers of rs3800373 and rs1360780 responded better to treatment. The effect was mainly seen in patients treated with different drug combinations or with venlafaxine [156]. These findings could be interesting for PTSD treatment too. SSRIs like venlafaxine and other antidepressants are also used in the treatment of PTSD. The detection of SNPs could help to ameliorate treatment efficacy.
2.19. GABAA Receptor β3 Subunit
The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neuro- transmitter in the central nervous system. Upon activation, the GABAA receptor selectively conducts Cl− through its pore, resulting in neuronal hyperpolarization. This inhibits neurotransmission by diminishing the number of action potentials [162]. There are numerous isoforms of the GABAA receptor and three β-subunits (GABRB1, GABRB2, GABRB3). These are characterized by different agonist binding affinities, their opening and conductance characteristics and other properties [163].
2.19.1. SNPs
Feusner et al. examined a polymorphism in the GABAA receptor beta 3 subunit gene (GABRB3) (Fig. 19). In Caucasian male PTSD patients, dinucleotide repeat polymorphisms were compared to GHQ-28 scores. As the major allele at GABRB3 was G1 (guanine), the alleles were divided into G1 and non-G1 groups. The GHQ-28 score considers somatic symptoms, anxiety/insomnia, social dysfunction and depression subscales. Patients with the G1 non-G1 genotype had a significantly higher score, if compared to both, the G1G1 genotype and non-G1 non-G1 genotypes. No significant differences were found between G1G1 and non-G1 non-G1 genotypes. If G1 non-G1 heterozygotes were compared to combined G1G1 and non-G1 non-G1 homozygotes, significantly higher total GHQ score was found in the heterozygotes. These observations suggest a heterosis effect i.e. improved or increased function in heterozygous individuals. In conclusion, this study indicates that heterozygosity of the GABRB3 major (G1) allele conferred more somatic symptoms, anxiety/insomnia, social dysfunction and depression than found in homozygosity [52]. SNPs in GABRB3 also increase the risk for other mental diseases such as the Asperger syndrome [164].
Fig. (19).
Schematic representation of the GABRB3 gene with exons (black 1-9) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases.
2.19.2. Drugs
Anxiety and fear responses are partly modulated by GABAA receptor-mediated synaptic inhibition. Benzodiazepines potentiate GABAergic inhibition and are effective anxiolytics. An animal model with genetically modified mouse lines tested benzodiazepine activity. Administration of the benzo- diazepines alprazolam, chlordiazepoxide, and diazepam significantly reduced fear-potentiated startle (FPS) in mouse model [165]. FPS represents a conditioned fear test and a useful preclinical tool to study PTSD-like responses. These findings are a first valuable hint for further testing of benzo- diazepines in PTSD patients.
2.20. Monoamine Oxidase B
Monoaminoxidase B encoded by the MAOB gene (Fig. 20) belongs to the flavin monoamine oxidase family. It is an enzyme located in the outer mitochondrial membrane. It catalyzes the oxidative deamination of biogenic and xenobiotic amines and plays an important role in the metabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissue. MAOB mainly metabolizes dopamine (DA).
Fig. (20).
Schematic representation of the MAOB gene with exons (black 1-15) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.20.1. SNPs
Platelet MAOB activity and a MAOB intron 13 polymorphism (G/A substitution) were determined in a study among male war veterans. The MAOB intron 13 polymorphism was not functional, and did not affect platelet MAOB activity. The allele frequencies of the MAOB genotype were similarly distributed among healthy controls and veterans with or without PTSD and/or psychotic symptoms. The results suggest that platelet MAOB activity might be used as marker for psychotic symptoms in PTSD [53]. Polymorphisms in MAOB were also associated with Parkinson’s disease [166]. Other polymorphisms of the MAOB gene have been linked to negative emotionality, and suspected as an underlying factor in depression [167].
2.20.2. Drugs
Alzheimer's disease and Parkinson's disease are both associated with elevated MAOB levels in the brain. The normal activity of MAOB leads to the generation of reactive oxygen species, which damage neuronal cells. Selective MAOB inhibitors such as selegiline and rasagiline are therefore used for the treatment of early-stage Parkinson's disease, depression and dementia [168].
2.21. Nitric Oxidase Synthase 1 Adaptor Protein
The NOS1AP gene encodes the nitric oxide synthase 1 adaptor protein that binds to its signaling molecule, neuronal nitric oxide synthase (nNOS). NOS1AP is involved in pathways promoting PTSD development. Nitric oxide (NO) is the product of NO synthase (NOS). This enzyme has three isoforms, i.e. neuronal (nNOS), endothelial (eNOS), and inducible NOS (iNOS). NO release activates glutamate N-methyl-D-aspartate (NMDA) receptors. NOS1AP competes with postsynaptic density protein-95 (PSD-95) for nNOS binding. NMDA receptor signaling is reduced via PSD-95 and nNOS. Overexpression of NOS1AP leads to a loss of PSD-95/nNOS complexes in transfected cells [169] and results in higher glutamate levels. This neurotransmitter increases the risk of hippocampal atrophy and is released during stress by increased sensitivity of hippocampal glucocorticoid receptors [170]. In animal studies, stress-mediated glucocorticoid release activated NOS leading to down-regulation of hippocampal NMDA receptors and total GABA levels [171]. Furthermore, the NO cascade is involved in PTSD-related hippocampal volume loss and cognitive deficits [172].
2.21.1. SNPs
SNPs in the NOS1AP gene have been studied in Vietnam veterans regarding modulation of stress-evoked NMDA activity [54]. A correlation between an intronic SNP (rs386231, replacement of one or both adenosines by guanine) and manifested PTSD has been observed in these combat veterans. Individuals carrying the GG genotype were more than three times likely to have PTSD compared to those with the AA genotype. The GG genotype is associated with more PTSD symptoms and severe depression [54].
Fig. (21) illustrates the different SNPs supposed to contribute to PTSD vulnerability. All these polymorphisms have only been detected in military personnel as of yet. Investigations among other groups affected by PTSD are necessary to gain more information. Additionally, it would be interesting to investigate if there are hierarchies of polymorphisms according to the strength of their effect on PTSD.
Fig. (21).
Schematic representation of the NOS1AP gene with exons (black 1-10) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. # Association with PTSD among military personnel, $ Association with other diseases.
2.21.2. Drugs
NO is involved in modulating depression [173]. NOS inhibitors such as NG-nitro-l-arginine and its derivatives showed antidepressant effects in animal trials [174]. These findings might be transferable to PTSD. However, the development of pharmacotherapies targeting NOS1AP is a difficult task, because of its role in diverse molecular pathways. Many of the first developed arginine-based NOS inhibitors exhibited potent inhibition of NOS, low toxicity, and reasonable pharmacokinetics, but a major problem is the low selectivity between different NOS isoforms. In fact, this might result in pronounced side effects in vivo such as hypertension and decreased cardiac output as a result of unselective inhibition [175].
Another possibility to inhibit NOS is to target glutamate release, as tested in trials with D-cycloserine (a partial NMDA receptor agonist) or ADX71743, a selective negative allosteric modulator of glutamate receptor (see “The impact of genetic research on drug development”).
2.22. Protein Kinase C α
Protein kinase C α is encoded by the PRKCA gene and belongs to the protein kinase C (PKC) family, a group of serine- and threonine-specific protein kinases that are activated by calcium and the second messenger diacylglycerol. PKC family members phosphorylate a huge number of proteins and are involved in diverse cellular signaling pathways. Protein kinases play an important role in the formation of emotional memory in animals [176], suggesting a linkage between PRKCA and PTSD.
2.22.1. SNPs
SNP rs4790904 (adenine replaced by guanine, (Fig. 22)) in the PRKCA gene was significantly associated with memory for negative pictures, increased PTSD re-experiencing and avoidance symptoms, as well as increased risk of current PTSD diagnosis in genocide survivors from Rwanda [177].
Fig. (22).
Schematic representation of the PRKCA gene with exons (black 1-9) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases, UTR Untranslated region.
This SNP has also been investigated in U.S. Afghanistan and Iraq veterans. A significant correlation of rs4790904 to all three typical PTSD symptoms, but not to current PTSD diagnosis was found in Caucasian soldiers. This relationships was observed for the G allele rather than for the A allele. A significant association of this variant with current PTSD diagnosis was also detected in African-American veterans [55].
2.22.2. Drugs
Despite these correlations, PKCA is a less suitable target for PTSD treatment, as protein kinases occur ubiquitously in the human body. PKCA inhibition would probably also lead to inhibition of other protein kinases causing many side effects.
2.23. Phosphoribosyl Transferase Domain Containing 1
PRTFDC1 (phosphoribosyl transferase domain containing 1, Fig. 23) codes for the phosphoribosyl transferase domain containing 1 protein, which has its highest expression in the brain. It is a homolog with 65% identity to the human hypoxanthine guanine phosphoribosyltransferase (HPRT), which is an important enzyme in the salvage pathway of purine nucleotides [178]. The function of PRTFDC1 is not fully understood until to date, but it is involved in several diseases. A recent study identified this homologue as a gene potentially involved in the development of cancer [179], but as well in childhood obesity [180].
Fig. (23).
Schematic representation of the PRTFDC1 gene with exons (black 1-9) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases.
2.23.1. SNPs
The Marine Resiliency Study (MRS) was a genome wide association study (GWAS), including 3494 trauma-exposed participants. The MRS is a well-characterized, prospective study of Marines and Sailors scheduled for combat deployment to Iraq or Afghanistan, with longitudinal follow-up to track the effect of combat stress. They performed a GWAS across ancestral groups, including subjects of European, African, Hispanic/Native American, and other ancestries. Gene associations found in MRS were reproducibly also detected in an independent cohort with 491 veterans. The study led to the identification of a SNP in the phosphoribosyl transferase domain containing 1 gene (rs6482463) [56]. Further investiga-tions are needed to clarify the influence of this polymorphism on gene function and its role in PTSD.
2.23.2. Drugs
Since the function of PRTFDC1 is not fully understood, pharmacotherapy has not yet been investigated. Until to date 2366 polymorphisms are described for this gene, but rs6482463 is the only one, which has been linked to PTSD [181].
2.24. Retinoic Acid Receptor-Related Orphan Receptor
Retinoic Acid Receptor-Related Orphan Receptor (RORA) plays a role in neuroprotection. The RORA protein is widely expressed in brain regions affected by PTSD such as cerebral cortex, thalamus and hypothalamus [182]. It protects neurons and glial cells from degenerative oxidative stress.
2.24.1. SNPs
People with RORA mutations have an increased risk for developing PTSD due to deficits in initiating neuroprotective processes after trauma [183]. Trauma-exposed white non-Hispanic veterans and their intimate partners (295 cases and 196 controls) were examined for several SNPs in the RORA gene. One SNP (rs8042149, replacement of guanine by thymine) reached genome-wide significance. A high incidence of the disorder (more than 60%) was observed among those with limited histories of trauma exposure, who carried the high-risk allele. PTSD prevalence was elevated in individuals with the high-risk genotype across all levels of exposure. Unfortunately, only trauma-exposed individuals were included into the study and a control group was missing. Several associations between other RORA SNPs and PTSD were found in a subset of African-American veterans in the same study as well as in a second much larger independent African-American cohort [57], although only SNP rs17303244 (either cytosine or thymine) from the African-American cohort was statistically significant after gene-level multiple testing correction.
Fig. (24) illustrates the localization of RORA SNPs. Interestingly, rs8042149 elevated the risk for PTSD across all levels of exposure. This finding is different from SLC6A4 gene, where the impact of polymorphisms on PTSD risk was connected to the level of stress. This could be an explanation, why this SNP is widely distributed among PTSD patients. Rs8042149 and rs17303244 are located in intron regions of the RORA gene. These polymorphisms may affect gene splicing, transcription factor binding, messenger RNA degradation, or sequences of non-coding RNA. Another polymorphism (rs12912233) is associated with depression [184].
Fig. (24).
Schematic representation of the RORA gene with exons (black 1-11) and introns (grey). Localization of representative SNPs in the gene is marked with bolts. * Association with PTSD in general, # Association with PTSD among military personnel, $ Association with other diseases, UTR Untranslated region.
Other SNPs are found in the UTR 3` region. These polymorphisms are not related to pathomechanisms. This downstream region seems to have no impact on the receptor function.
2.24.2. Drugs
It is a matter of discussion, whether or not RORA may be a suitable target for drug development. There is an association of high RORA activity and autoimmune disorders by activation of CD4+ T helper cells [185]. Hence, RORA-targeting therapies might bear the risk of severe side effects.
2.25. The Million Veteran Program
The Million Veteran Program (MVP) is a national, voluntary research program funded by the U.S. Department of Veterans Affairs Office of Research and Development and represents one of the greatest research efforts in American military history. Over a time period of 5-7 years, a genomic database consisting of 1 million veterans is being set up for users of the Department of Veterans Affairs healthcare system. MVP intents to facilitate the development of new diagnostic tests to enable disease prevention and early treatment as well as personalized therapies based on the veteran's individual genetic characteristics and conditions. Furthermore, surveillance is conducted for early detection of trauma exposure and other deployment-related conditions and possibly link them to genetic susceptibilities. Another advantage is that MVP captures information on different populations and genders as well as different types of trauma. By identifying gene-health connections, the program aims to consequent improvement of disease screening, diagnosis, and prognosis, ultimately fostering the development of more effective, personalized therapies [186].
2.26. Difficulties and Chances of Genetic Studies Among Military Personnel
The identification of genetic polymorphisms may facilitate the drug development process and may help to find individualized therapy options for patients. For other complex diseases such as cancer, the identification of genetic risk factors and biomarkers has been successful [187]. Mood disorders are complex in nature and are influenced by multiple diverse factors. Therefore, the determination of parameters for PTSD risk or prediction of response to pharmacotherapy is certainly not a trivial task, but would be of great advantage for PTSD treatment. An advantage of genetic markers is their presence from birth, which allows their detection before a person is exposed to any traumatic event. Genetic testing may help answering the question, why individuals react differently upon trauma exposure.
A possible problem of genetic testing may be the anxiety of affected soldiers to be stigmatized and discriminated by employers and health insurances. This may lead to an ambivalent view in the society towards genetic testing. PTSD patients are less ready to agree with genetic testing of their family members [188]. Furthermore, genetic poly- morphisms are differently distributed among the human populations on earth. Hence, it should be taken into account, whether veterans from different countries might reveal different probabilities to suffer from PTSD. This aspect has not been adequately addressed yet, but warrant thorough attention in the future.
Studies among military personnel yield several methodological advantages. Due to the fact that large numbers of patients can be studied, valid information can be gained. This knowledge may be translated to non-military PTSD patients, where it may be more difficult to set up
clinical trials with sufficiently large numbers of participants for prospective studies. A possible bias is that soldiers may suffer from PTSD due to experiencing a traumatic event before they joined the army. Such patients should be excluded from clinical trials by careful anamnesis [189].
2.27. The Impact of Genetic Research on Drug Development
Without doubt, genetic research will probably lead to innovative pharmacological treatments of PTSD. The potential for future drug development can be illustrated by achievements in the past. Elucidating the role of glutamate-signaling pathways for PTSD led to a novel orally active selective negative allosteric modulator of glutamate receptor, ADX71743. The in vivo efficacy was tested in appropriate rodent animal models. The substance showed an anti-anxiolytic-like profile, but no effect against depression. Further investigations have to clarify the underlying modes of action of this compound [190].
Another example is d-cycloserine, a partial NMDA receptor agonist with approved activity against anxiety symptoms. In combination with exposure therapy, d-cycloserine significantly ameliorated the treatment outcome of PTSD patients [191]. In another randomized, double blind, clinical trial d-cycloserine alleviated numbing and avoidance in combat-related chronic PTSD. The study was an 11-week, double-blind, and crossover trial with randomly selected outpatients with chronic combat-related PTSD. Seventy-six eligible patients were randomly assigned to two groups. One group received either an add-on treatment of d-cycloserine (50 mg daily), or placebo (four weeks). After a washout phase for two weeks, the groups changed the treatment for another four weeks. PTSD scales were performed at baseline and at the end of the 1st, 5th, and 11th week. The overall number of avoidance and numbing symptoms, symptom frequency, and symptom intensity were measured separately. The outcome of the study showed no significant differences between d-cycloserine and placebo regarding overall avoidance and numbing symptom frequency, but significant reductions of overall symptom intensity and impact on function. Another positive aspect is that d-cycloserine did not reveal significant adverse effects and none of the patients dropped out because of side effects [192].
CONCLUSION
During the past two decades, PTSD became more and more recognized as mental disease. From former denunciation of soldiers as dissemblers until the inclusion into the DSM many years have passed and it was only recently that the elucidation of pathomechanisms of PTSD came into the center of interest. Initial psychiatric research has been complemented in recent years by progresses in genetics and molecular biology. This enabled us to develop a more integrative view, how the dysfunction of molecular pathways caused by genetic mutations relate to psychiatric symptoms. Even more, some genetic mutations may serve as predictive factors for the onset of PTSD. Studies with animal models or patients with anxiety disorders indicate that some SNPs may be risk factors for the disease, but seem to be protective factors for soldiers in combat situations. According to these findings, it can be concluded that the level of stress in a particular environmental condition might determine whether certain types of SNPs protect from or predispose for PTSD. Further research is warranted to further specify the predictive character of such polymorphisms.
Genetic mutations associated with higher PTSD risks have been found in several genes encoding as neuro- transmitters and their receptors (ADRB2, BDNF, CRHR-2, DRD2, DRD3, NR3C1 and RORA) or downstream signal transducers and metabolizing enzymes (ADCY8, ANK3, APOE, COMT, DBH, DPP6, FAAH, FKBP5, GABRB3, MAOB, NOS1AP, PRKCA, PRTFDC1, SLC1A1, SLC6A4). Genetic research may identify novel biomarkers to predict new targets, to better predict the patient’s response to existing pharmacotherapy and to novel targets for future drugs. New medications are urgently needed because of the poor response of many PTSD patients. d-cycloserine, a NMDA receptor agonist with anxiety reducing effects and ADX71743, a novel selective negative allosteric modulator of glutamate receptor with anxiolytic activity have shown positive effects. UCPH-101 is an inhibitor of the excitatory amino-acid transporter (EAAT). NG-nitro-l-arginine was developed as an NOS inhibitor and yohimbine inhibited dopamine D2 receptors. Fatty acid amide hydrolase could be targeted by a new inhibitor with the preliminary name OL-135. An agonist of Trk receptor is 7, 8-dihydroxyflavone, which activates gene transcription.
The knowledge from other diseases with symptoms partly comparable to PTSD such as depression or schizophrenia provides the opportunity to test already approved medications in the context of PTSD. Possible candidates are COMT inhibitors (e.g. tolcapone) or partial dopamine agonists (e.g. aripiprazole). Yohimbine may also be an interesting new drug candidate as dopamine antagonist. Other examples are the SSRI sertraline or neuroleptic clozapine, which affects cortisol levels. Salbutamol, a β2 adrenergic receptor agonist normally used to treat asthma has been tested with favorable results in a group of patients surviving a motor cycle accident [125]. Further controlled clinical studies are needed to prove its efficacy and safety for PTSD patients.
One of the biggest research programs among military personnel is MVP. This emphasizes the relevance of military as a valuable resource for basic and clinical studies to gain fundamental knowledge on PTSD.
Despite progress in drug research, challenges remain especially to develop effective, target-oriented drugs. Furthermore, measures for disease prevention should be improved and tests for the assessment of individual PTSD risks have to be implemented in the primary health care of soldiers. Further research on soldiers will improve the health care requirements of traumatized soldiers and other PTSD patients.
ACKNOWLEDGEMENT
This work was funded by the Research Training Program GRK 2015 “Life Science-Life Writing-Liminal Experiences of Human Life” of the Deutsche Forschungsgemeinschaft (DFG).
List of Abbreviations
- 5-HT
5-Hydroxytryptophan = serotonin
- 5-HT2
Serotonin receptor 2
- 5-HTT
5-Hydroxytryptamintransporter = serotonin transporter
- 5-HTTLPR
Serotonin-transporter-linked polymorphic region
- A
Adenine
- Ad
Adrenaline
- ACTH
Adrenocorticotropic hormone
- AMP
Adenosine monophosphate
- ANK3
Ankyrin 3
- APOE
Apolipoprotein E
- ATP
Adenosine triphosphate
- BDNF
Brain-derived neurotrophic factor
- C
Cytosine
- cAMP
Cyclic adenosine monophosphate
- CBT
Cognitive behavioral therapy
- Cl-
Chloride ion
- COMT
Catechol-O-methyltransferase
- CRH
Corticotropin-releasing hormone
- CRHR-2
Corticotropin-releasing hormone receptor 2
- D2
Dopamine receptor 2
- D3
Dopamine receptor 3
- DA
Dopamine
- DAT
Dopamine transporter
- DBH
Dopamine β-hydroxylase
- DHF
7, 8-Dihydroxyflavone
- DNA
Deoxyribonucleic acid
- DRD2
Dopamine receptor D2
- DRD3
Dopamine receptor D3
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- eCBs
Endogenous cannabinoids
- EMDR
Eye movement desensitization and reprocessing
- eNOS
Endothelial nitric oxide synthase
- FAAH
Fatty acid amide hydrolase
- FKBP5
FK506 binding protein 5
- G
Guanine
- GABA
γ-Aminobutyric acid
- GABAA
γ-Aminobutyric acid receptor type A
- GABRB3
γ-Aminobutyric acid receptor subunit β-3
- GHQ
General health questionnaire
- GR
Glucocorticoid receptor
- GWAS
Genome wide association study
- HPA axis
Hypothalamic–pituitary–adrenal axis
- HSP90
Heat shock protein 90
- iNOS
Inducible nitric oxide synthase
- MAO B
Monoaminoxidase B
- Met
Methionine
- MRS
Marine resiliency study
- MVP
Million veteran program
- NA
Noradrenaline
- NCS
United States National comorbidity survey
- NMDA-R
N-methyl-d-aspartic acid receptor
- NO
Nitric oxide = nitrogen monoxide
- NOS1AP
Nitric oxide synthase 1 adaptor protein
- nNOS
Neuronal nitric oxide synthase
- NR3C1
Nuclear receptor subfamily 3, group C, member 1
- PRKCA
Protein kinase C α
- PSD-95
Postsynaptic density protein 95
- PTSD
Posttraumatic stress disorder
- RNA
Ribonucleic acid
- RORA
Related orphan receptor A
- SAM
S-adenosyl methionine
- SERT
Serotonin transporter
- SLC6
Solute carrier 6
- SLC1A1
Solute carrier family 1 (neuronal/ epithelial high affinity glutamate trans- porter, system Xag), member 1
- SLC6A4
Serotonin transporter gene
- SNP
Single nucleotide polymorphism
- SSRI
Selective serotonin reuptake inhibitor
- T
Thymine
- Trk-R
Tyrosine kinase receptor
- VA
Veteran affairs
- Val
Valine
CONFLCIT OS INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Forbes D., Lockwood E., Elhai J.D., Creamer M., Bryant R., McFarlane A., Silove D., Miller M.W., Nickerson A., O’Donnell M. An evaluation of the DSM-5 factor structure for posttraumatic stress disorder in survivors of traumatic injury. J. Anxiety Disord. 2014;29C:43–51. doi: 10.1016/j.janxdis.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Shay J. Achilles in Vietnam: Combat Trauma and the Undoing of Character. Scribner; 1995. p. 272. [Google Scholar]
- 3.Shoshana Ringel J.R. Trauma: Contemporary Directions in Theory, Practice, and Research. SAGE Publications; 2012. p. 272. [Google Scholar]
- 4.Linden S.C., Hess V., Jones E. The neurological manifestations of trauma: lessons from World War I. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262(3):253–264. doi: 10.1007/s00406-011-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreasen N.C. Posttraumatic stress disorder: a history and a critique. Ann. N. Y. Acad. Sci. 2010;1208:67–71. doi: 10.1111/j.1749-6632.2010.05699.x. [DOI] [PubMed] [Google Scholar]
- 6.Perkonigg A., Kessler R.C., Storz S., Wittchen H.U. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr. Scand. 2000;101(1):46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- 7.Maercker A., Forstmeier S., Wagner B., Glaesmer H., Brahler E. Post-traumatic stress disorder in Germany. Results of a nationwide epidemiological study. Nervenarzt. 2008;79(5):577–586. doi: 10.1007/s00115-008-2467-5. [DOI] [PubMed] [Google Scholar]
- 8.Keane T.M., Marshall A.D., Taft C.T. Posttraumatic stress disorder: etiology, epidemiology, and treatment outcome. Annu. Rev. Clin. Psychol. 2006;2:161–197. doi: 10.1146/annurev.clinpsy.2.022305.095305. [DOI] [PubMed] [Google Scholar]
- 9.Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 10.Wittchen H.U., Schonfeld S., Kirschbaum C., Thurau C., Trautmann S., Steudte S., Klotsche J., Hofler M., Hauffa R., Zimmermann P. Traumatic experiences and posttraumatic stress disorder in soldiers following deployment abroad: how big is the hidden problem? Dtsch. Arztebl. Int. 2012;109(35-36):559–568. doi: 10.3238/arztebl.2012.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermes E.D., Rosenheck R.A., Desai R., Fontana A.F. Recent trends in the treatment of posttraumatic stress disorder and other mental disorders in the VHA. Psychiatr. Serv. 2012;63(5):471–476. doi: 10.1176/appi.ps.201100432. [DOI] [PubMed] [Google Scholar]
- 12.Association A.P. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: 2013. [Google Scholar]
- 13.Pollard H.B., Shivakumar C., Starr J., Eidelman O., Jacobowitz D.M., Dalgard C.L., Srivastava M., Wilkerson M.D., Stein M.B., Ursano R.J. “Soldier’s Heart”: A genetic basis for elevated cardiovascular disease risk associated with post-traumatic stress disorder. Front. Mol. Neurosci. 2016;9:87. doi: 10.3389/fnmol.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr. Dis. Treat. 2011;7:167–181. doi: 10.2147/NDT.S10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinician’s Guide to Medications for PTSD 2016 http://www.ptsd.va.gov/professional/treatment/overview/clinicians-guide-to-medicationsfor-ptsd.asp
- 16.Harpaz-Rotem I., Rosenheck R., Mohamed S., Pietrzak R., Hoff R. Initiation of pharmacotherapy for post-traumatic stress disorder among veterans from Iraq and Afghanistan: a dimensional, symptom cluster approach. BJPsych Open. 2016;2(5):286–293. doi: 10.1192/bjpo.bp.115.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherin J.E., Nemeroff C.B. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011;13(3):263–278. doi: 10.31887/DCNS.2011.13.2/jsherin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meaney M.J., Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hull A.M. Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br. J. Psychiatry. 2002;181:102–110. [PubMed] [Google Scholar]
- 21.Hughes K.C., Shin L.M. Functional neuroimaging studies of post-traumatic stress disorder. Expert Rev. Neurother. 2011;11(2):275–285. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stankiewicz A.M., Swiergiel A.H., Lisowski P. Epigenetics of stress adaptations in the brain. Brain Res. Bull. 2013;98:76–92. doi: 10.1016/j.brainresbull.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Klengel T., Mehta D., Anacker C., Rex-Haffner M., Pruessner J.C., Pariante C.M., Pace T.W., Mercer K.B., Mayberg H.S., Bradley B., Nemeroff C.B., Holsboer F., Heim C.M., Ressler K.J., Rein T., Binder E.B. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.True W.R., Rice J., Eisen S.A., Heath A.C., Goldberg J., Lyons M.J., Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch. Gen. Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 25.Stein M.B., Jang K.L., Taylor S., Vernon P.A., Livesley W.J. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am. J. Psychiatry. 2002;159(10):1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Biotechnology Information, U.S. National Library of Medicine. Gene variation viewer. 2015 http://www.ncbi.nlm. nih.gov/variation/view/
- 27.Zhang L., Benedek D.M., Fullerton C.S., Forsten R.D., Naifeh J.A., Li X.X., Hu X.Z., Li H., Jia M., Xing G.Q., Benevides K.N., Ursano R.J. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol. Psychiatry. 2014;19(1):8–10. doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]
- 28.Pivac N., Kozaric-Kovacic D., Grubisic-Ilic M., Nedic G., Rakos I., Nikolac M., Blazev M., Muck-Seler D. The association between brain-derived neurotrophic factor Val66Met variants and psychotic symptoms in posttraumatic stress disorder. World J. Biol. Psychiatry. 2012;13(4):306–311. doi: 10.3109/15622975.2011.582883. [DOI] [PubMed] [Google Scholar]
- 29.Clark R., DeYoung C.G., Sponheim S.R., Bender T.L., Polusny M.A., Erbes C.R., Arbisi P.A. Predicting post-traumatic stress disorder in veterans: interaction of traumatic load with COMT gene variation. J. Psychiatr. Res. 2013;47(12):1849–1856. doi: 10.1016/j.jpsychires.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Schulz-Heik R.J., Schaer M., Eliez S., Hallmayer J.F., Lin X., Kaloupek D.G., Woodward S.H. Catechol-O-methyltransferase Val158Met polymorphism moderates anterior cingulate volume in posttraumatic stress disorder. Biol. Psychiatry. 2011;70(11):1091–1096. doi: 10.1016/j.biopsych.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Lawford B.R., Young R., Noble E.P., Kann B., Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur. Psychiatry. 2006;21(3):180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Lawford B.R., Mc D.Y., Noble E.P., Kann B., Arnold L., Rowell J., Ritchie T.L. D2 dopamine receptor gene polymorphism: paroxetine and social functioning in posttraumatic stress disorder. Eur. Neuropsychopharmacol. 2003;13(5):313–320. doi: 10.1016/s0924-977x(02)00152-9. [DOI] [PubMed] [Google Scholar]
- 33.Comings D.E., Muhleman D., Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol. Psychiatry. 1996;40(5):368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 34.Voisey J., Swagell C.D., Hughes I.P., Morris C.P., van Daal A., Noble E.P., Kann B., Heslop K.A., Young R.M., Lawford B.R. The DRD2 gene 957C>T polymorphism is associated with posttraumatic stress disorder in war veterans. Depress. Anxiety. 2009;26(1):28–33. doi: 10.1002/da.20517. [DOI] [PubMed] [Google Scholar]
- 35.Wolf E.J., Mitchell K.S., Logue M.W., Baldwin C.T., Reardon A.F., Aiello A., Galea S., Koenen K.C., Uddin M., Wildman D., Miller M.W. The dopamine D3 receptor gene and posttraumatic stress disorder. J. Trauma. Stress. 2014;27(4):379–387. doi: 10.1002/jts.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimbrel N.A., Morissette S.B., Meyer E.C., Chrestman R., Jamroz R., Silvia P.J., Beckham J.C., Young K.A. Effect of the 5-HTTLPR polymorphism on posttraumatic stress disorder, depression, anxiety, and quality of life among Iraq and Afghanistan veterans. Anxiety Stress Coping. 2015;28(4):456–466. doi: 10.1080/10615806.2014.973862.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morey R.A., Hariri A.R., Gold A.L., Hauser M.A., Munger H.J., Dolcos F., McCarthy G. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BMC Psychiatry. 2011;11:76. doi: 10.1186/1471-244X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham D.P., Helmer D.A., Harding M.J., Kosten T.R., Petersen N.J., Nielsen D.A. Serotonin transporter genotype and mild traumatic brain injury independently influence resilience and perception of limitations in veterans. J. Psychiatr. Res. 2013;47(6):835–842. doi: 10.1016/j.jpsychires.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z., Baker D.G., Harrer J., Hamner M., Price M., Amstadter A. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depress. Anxiety. 2011;28(12):1067–1073. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wald I., Degnan K.A., Gorodetsky E., Charney D.S., Fox N.A., Fruchter E., Goldman D., Lubin G., Pine D.S., Bar-Haim Y. Attention to threats and combat-related posttraumatic stress symptoms: prospective associations and moderation by the serotonin transporter gene. JAMA Psychiatry. 2013;70(4):401–408. doi: 10.1001/2013.jamapsychiatry.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Zuiden M., Geuze E., Willemen H.L., Vermetten E., Maas M., Amarouchi K., Kavelaars A., Heijnen C.J. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol. Psychiatry. 2012;71(4):309–316. doi: 10.1016/j.biopsych.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Yehuda R., Pratchett L.C., Elmes M.W., Lehrner A., Daskalakis N.P., Koch E., Makotkine I., Flory J.D., Bierer L.M. Glucocorticoid-related predictors and correlates of post-traumatic stress disorder treatment response in combat veterans. Interface Focus. 2014;4(5):20140048. doi: 10.1098/rsfs.2014.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachmann A.W., Sedgley T.L., Jackson R.V., Gibson J.N., Young R.M., Torpy D.J. Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psychoneuroendocrinology. 2005;30(3):297–306. doi: 10.1016/j.psyneuen.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Wolf E.J., Mitchell K.S., Logue M.W., Baldwin C.T., Reardon A.F., Humphries D.E., Miller M.W. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress. Anxiety. 2013;30(12):1161–1169. doi: 10.1002/da.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf E.J., Rasmusson A.M., Mitchell K.S., Logue M.W., Baldwin C.T., Miller M.W. A genome-wide association study of clinical symptoms of dissociation in a trauma-exposed sample. Depress. Anxiety. 2014;31(4):352–360. doi: 10.1002/da.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liberzon I., King A.P., Ressler K.J., Almli L.M., Zhang P., Ma S.T., Cohen G.H., Tamburrino M.B., Calabrese J.R., Galea S. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry. 2014;71(10):1174–1182. doi: 10.1001/jamapsychiatry.2014.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Logue M.W., Solovieff N., Leussis M.P., Wolf E.J., Melista E., Baldwin C., Koenen K.C., Petryshen T.L., Miller M.W. The ankyrin-3 gene is associated with posttraumatic stress disorder and externalizing comorbidity. Psychoneuroendocrinology. 2013;38(10):2249–2257. doi: 10.1016/j.psyneuen.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim T.Y., Chung H.G., Shin H.S., Kim S.J., Choi J.H., Chung M.Y., An S.K., Choi T.K., So H.S., Cho H.S. Apolipoprotein E gene polymorphism, alcohol use, and their interactions in combat-related posttraumatic stress disorder. Depress. Anxiety. 2013;30(12):1194–1201. doi: 10.1002/da.22138. [DOI] [PubMed] [Google Scholar]
- 49.Lyons M.J., Genderson M., Grant M.D., Logue M., Zink T., McKenzie R., Franz C.E., Panizzon M., Lohr J.B., Jerskey B., Kremen W.S. Gene-environment interaction of ApoE genotype and combat exposure on PTSD. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2013;162B(7):762–769. doi: 10.1002/ajmg.b.32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mustapic M., Pivac N., Kozaric-Kovacic D., Dezeljin M., Cubells J.F., Muck-Seler D. Dopamine beta-hydroxylase (DBH) activity and -1021C/T polymorphism of DBH gene in combat-related post-traumatic stress disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B(8):1087–1089. doi: 10.1002/ajmg.b.30526. [DOI] [PubMed] [Google Scholar]
- 51.Pardini M., Krueger F., Koenigs M., Raymont V., Hodgkinson C., Zoubak S., Goldman D., Grafman J. Fatty-acid amide hydrolase polymorphisms and post-traumatic stress disorder after penetrating brain injury. Transl. Psychiatry. 2012;2:e75. doi: 10.1038/tp.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feusner J., Ritchie T., Lawford B., Young R.M., Kann B., Noble E.P. GABA(A) receptor beta 3 subunit gene and psychiatric morbidity in a post-traumatic stress disorder population. Psychiatry Res. 2001;104(2):109–117. doi: 10.1016/s0165-1781(01)00296-7. [DOI] [PubMed] [Google Scholar]
- 53.Pivac N., Knezevic J., Kozaric-Kovacic D., Dezeljin M., Mustapic M., Rak D., Matijevic T., Pavelic J., Muck-Seler D. Monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity in combat-related posttraumatic stress disorder. J. Affect. Disord. 2007;103(1-3):131–138. doi: 10.1016/j.jad.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Lawford B.R., Morris C.P., Swagell C.D., Hughes I.P., Young R.M., Voisey J. NOS1AP is associated with increased severity of PTSD and depression in untreated combat veterans. J. Affect. Disord. 2013;147(1-3):87–93. doi: 10.1016/j.jad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y., Rimmler J., Dennis M.F., Ashley-Koch A.E., Hauser M.A. Mid-Atlantic Mental Illness Research, E.; Clinical Center, W.; Beckham, J. C. Association of variant rs4790904 in protein kinase C alpha with posttraumatic stress disorder in a U.S. caucasian and African-American veteran sample. J. Depress. Anxiety. 2013;2(1):S4001. doi: 10.4172/2167-1044.s4-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nievergelt C.M., Maihofer A.X., Mustapic M., Yurgil K.A., Schork N.J., Miller M.W., Logue M.W., Geyer M.A., Risbrough V.B., O’Connor D.T., Baker D.G. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 57.Logue M.W., Baldwin C., Guffanti G., Melista E., Wolf E.J., Reardon A.F., Uddin M., Wildman D., Galea S., Koenen K.C., Miller M.W. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol. Psychiatry. 2013;18(8):937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J., Sheerin C., Mandel H., Banducci A.N., Myrick H., Acierno R., Amstadter A.B., Wang Z. Variation in SLC1A1 is related to combat-related posttraumatic stress disorder. J. Anxiety Disord. 2014;28(8):902–907. doi: 10.1016/j.janxdis.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Fujii T., Ota M., Hori H., Hattori K., Teraishi T., Matsuo J., Kinoshita Y., Ishida I., Nagashima A., Kunugi H. The common functional FKBP5 variant rs1360780 is associated with altered cognitive function in aged individuals. Sci. Rep. 2014;4:6696. doi: 10.1038/srep06696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oosthuizen F., Wegener G., Harvey B.H. Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): evidence from an animal model. Neuropsychiatr. Dis. Treat. 2005;1(2):109–123. doi: 10.2147/nedt.1.2.109.61049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deppermann S., Storchak H., Fallgatter A.J., Ehlis A.C. Stress-induced neuroplasticity: (mal)adaptation to adverse life events in patients with PTSD--a critical overview. Neuroscience. 2014;283:166–177. doi: 10.1016/j.neuroscience.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 62.Jones K.R., Reichardt L.F. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc. Natl. Acad. Sci. USA. 1990;87(20):8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bekinschtein P., Cammarota M., Katche C., Slipczuk L., Rossato J.I., Goldin A., Izquierdo I., Medina J.H. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. USA. 2008;105(7):2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGuire J.F., Lewin A.B., Storch E.A. Enhancing exposure therapy for anxiety disorders, obsessive-compulsive disorder and post-traumatic stress disorder. Expert Rev. Neurother. 2014;14(8):893–910. doi: 10.1586/14737175.2014.934677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters J., Dieppa-Perea L.M., Melendez L.M., Quirk G.J. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328(5983):1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosas-Vidal L.E., Do-Monte F.H., Sotres-Bayon F., Quirk G.J. Hippocampal--prefrontal BDNF and memory for fear extinction. Neuropsychopharmacology. 2014;39(9):2161–2169. doi: 10.1038/npp.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx. 2005;2(1):120–128. doi: 10.1602/neurorx.2.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aalto S., Bruck A., Laine M., Nagren K., Rinne J.O. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J. Neurosci. 2005;25(10):2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernabeu R., Bevilaqua L., Ardenghi P., Bromberg E., Schmitz P., Bianchin M., Izquierdo I., Medina J.H. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. USA. 1997;94(13):7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutherford K., Le Trong I., Stenkamp R.E., Parson W.W. Crystal structures of human 108V and 108M catechol O-methyltransferase. J. Mol. Biol. 2008;380(1):120–130. doi: 10.1016/j.jmb.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 72.Sheldrick A.J., Krug A., Markov V., Leube D., Michel T.M., Zerres K., Eggermann T., Kircher T. Effect of COMT val158met genotype on cognition and personality. Eur. Psychiatry. 2008;23(6):385–389. doi: 10.1016/j.eurpsy.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Meyer-Lindenberg A., Nichols T., Callicott J.H., Ding J., Kolachana B., Buckholtz J., Mattay V.S., Egan M., Weinberger D.R. Impact of complex genetic variation in COMT on human brain function. Mol. Psychiatry. 2006;11(9):867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- 74.Mier D., Kirsch P., Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol. Psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 75.Kolassa I.T., Kolassa S., Ertl V., Papassotiropoulos A., De Quervain D.J. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol. Psychiatry. 2010;67(4):304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Valente N.L., Vallada H., Cordeiro Q., Bressan R.A., Andreoli S.B., Mari J.J., Mello M.F. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J. Mol. Neurosci. 2011;43(3):516–523. doi: 10.1007/s12031-010-9474-2. [DOI] [PubMed] [Google Scholar]
- 77.Boscarino J.A., Erlich P.M., Hoffman S.N., Rukstalis M., Stewart W.F. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Res. 2011;188(1):173–174. doi: 10.1016/j.psychres.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park B.L., Shin H.D., Cheong H.S., Park C.S., Sohn J.W., Kim B.J., Seo H.K., Kim J.W., Kim K.H., Shin T.M., Choi I.G., Kim S.G., Woo S.I. Association analysis of COMT polymorphisms with schizophrenia and smooth pursuit eye movement abnormality. J. Hum. Genet. 2009;54(12):709–712. doi: 10.1038/jhg.2009.102. [DOI] [PubMed] [Google Scholar]
- 79.Pandolfo G., Gugliandolo A., Gangemi C., Arrigo R., Curro M., La Ciura G., Muscatello M.R., Bruno A., Zoccali R., Caccamo D. Association of the COMT synonymous polymorphism Leu136Leu and missense variant Val158Met with mood disorders. J. Affect. Disord. 2015;177:108–113. doi: 10.1016/j.jad.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 80.2015 http://www.ncbi.nlm.nih.gov/SNP/
- 81.Tunbridge E.M., Bannerman D.M., Sharp T., Harrison P.J. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J. Neurosci. 2004;24(23):5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moreau J.L., Borgulya J., Jenck F., Martin J.R. Tolcapone: a potential new antidepressant detected in a novel animal model of depression. Behav. Pharmacol. 1994;5(3):344–350. [PubMed] [Google Scholar]
- 83.Fava M., Rosenbaum J.F., Kolsky A.R., Alpert J.E., Nierenberg A.A., Spillmann M., Moore C., Renshaw P., Bottiglieri T., Moroz G., Magni G. Open study of the catechol-O-methyltransferase inhibitor tolcapone in major depressive disorder. J. Clin. Psychopharmacol. 1999;19(4):329–335. doi: 10.1097/00004714-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Wen J.L., Xue L., Wang R.H., Chen Z.X., Shi Y.W., Zhao H. Involvement of the dopaminergic system in the consolidation of fear conditioning in hippocampal CA3 subregion. Behav. Brain Res. 2014;278C:527–534. doi: 10.1016/j.bbr.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 85.Grandy D.K., Marchionni M.A., Makam H., Stofko R.E., Alfano M., Frothingham L., Fischer J.B., Burke-Howie K.J., Bunzow J.R., Server A.C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc. Natl. Acad. Sci. USA. 1989;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weizmann Institute of Science. Gene Cards. 2015 http://www.genecards.org/cgi-bin/carddisp. pl?gene=DRD2&search=drd2#snp
- 87.Abi-Dargham A., Rodenhiser J., Printz D., Zea-Ponce Y., Gil R., Kegeles L.S., Weiss R., Cooper T.B., Mann J.J., Van Heertum R.L., Gorman J.M., Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. USA. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doehring A., Kirchhof A., Lotsch J. Genetic diagnostics of functional variants of the human dopamine D2 receptor gene. Psychiatr. Genet. 2009;19(5):259–268. doi: 10.1097/YPG.0b013e32832d0941. [DOI] [PubMed] [Google Scholar]
- 89.Hirvonen M.M., Laakso A., Nagren K., Rinne J.O., Pohjalainen T., Hietala J. C957T polymorphism of dopamine D2 receptor gene affects striatal DRD2 in vivo availability by changing the receptor affinity. Synapse. 2009;63(10):907–912. doi: 10.1002/syn.20672. [DOI] [PubMed] [Google Scholar]
- 90.Goldberg D.P., Hillier V.F. A scaled version of the General Health Questionnaire. Psychol. Med. 1979;9(1):139–145. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 91.Youssef N.A., Marx C.E., Bradford D.W., Zinn S., Hertzberg M.A., Kilts J.D., Naylor J.C., Butterfield M.I., Strauss J.L. An open-label pilot study of aripiprazole for male and female veterans with chronic post-traumatic stress disorder who respond suboptimally to antidepressants. Int. Clin. Psychopharmacol. 2012;27(4):191–196. doi: 10.1097/YIC.0b013e328352ef4e. [DOI] [PubMed] [Google Scholar]
- 92.Naylor J.C., Kilts J.D., Bradford D.W., Strauss J.L., Capehart B.P., Szabo S.T., Smith K.D., Dunn C.E., Conner K.M., Davidson J.R., Wagner H.R., Hamer R.M., Marx C.E. A pilot randomized placebo-controlled trial of adjunctive aripiprazole for chronic PTSD in US military Veterans resistant to antidepressant treatment. Int. Clin. Psychopharmacol. 2015;30(3):167–174. doi: 10.1097/YIC.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 93.Millan M.J., Newman-Tancredi A., Audinot V., Cussac D., Lejeune F., Nicolas J.P., Coge F., Galizzi J.P., Boutin J.A., Rivet J.M., Dekeyne A., Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35(2):79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 94.Neve K.A., Seamans J.K., Trantham-Davidson H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004;24(3):165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 95.Leggio G.M., Salomone S., Bucolo C., Platania C., Micale V., Caraci F., Drago F., Dopamine D. (3) receptor as a new pharmacological target for the treatment of depression. Eur. J. Pharmacol. 2013;719(1-3):25–33. doi: 10.1016/j.ejphar.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 96.Black K.J., Hershey T., Koller J.M., Videen T.O., Mintun M.A., Price J.L., Perlmutter J.S. A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc. Natl. Acad. Sci. USA. 2002;99(26):17113–17118. doi: 10.1073/pnas.012260599. [https://doi.org/10.1073/pnas. 012260599]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lane H.Y., Liu Y.C., Huang C.L., Hsieh C.L., Chang Y.L., Chang L., Chang Y.C., Chang W.H. Prefrontal executive function and D1, D3, 5-HT2A and 5-HT6 receptor gene variations in healthy adults. J. Psychiatry Neurosci. 2008;33(1):47–53. [PMC free article] [PubMed] [Google Scholar]
- 98.Bombin I., Arango C., Mayoral M., Castro-Fornieles J., Gonzalez-Pinto A., Gonzalez-Gomez C., Moreno D., Parellada M., Baeza I., Graell M., Otero S., Saiz P.A., Patino-Garcia A. DRD3, but not COMT or DRD2, genotype affects executive functions in healthy and first-episode psychosis adolescents. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008;147B(6):873–879. doi: 10.1002/ajmg.b.30710. [https://doi.org/10.1002/ajmg.b.30710]. [DOI] [PubMed] [Google Scholar]
- 99.Polak A.R., Witteveen A.B., Reitsma J.B., Olff M. The role of executive function in posttraumatic stress disorder: a systematic review. J. Affect. Disord. 2012;141(1):11–21. doi: 10.1016/j.jad.2012.01.001. [https://doi.org/10. 1016/j.jad.2012.01.001]. [DOI] [PubMed] [Google Scholar]
- 100.He F., Zheng Y., Huang H.H., Cheng Y.H., Wang C.Y. Association between Tourette syndrome and the dopamine D3 receptor gene rs6280. Chin. Med. J. (Engl.) 2015;128(5):654–658. doi: 10.4103/0366-6999.151665. [https://doi.org/10.4103/0366-6999.151665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang S.G., Lee B.H., Lee J.S., Chai Y.G., Ko K.P., Lee H.J., Han D.M., Ji H., Jang G.H., Shin H.E. DRD3 gene rs6280 polymorphism may be associated with alcohol dependence overall and with Lesch type I alcohol dependence in Koreans. Neuropsychobiology. 2014;69(3):140–146. doi: 10.1159/000358062. [https://doi.org/10.1159/ 000358062]. [DOI] [PubMed] [Google Scholar]
- 102.Kukshal P., Kodavali V.C., Srivastava V., Wood J., McClain L., Bhatia T., Bhagwat A.M., Deshpande S.N., Nimgaonkar V.L., Thelma B.K. Dopaminergic gene polymorphisms and cognitive function in a north Indian schizophrenia cohort. J. Psychiatr. Res. 2013;47(11):1615–1622. doi: 10.1016/j.jpsychires.2013.07.007. [https://doi.org/10.1016/j.jpsychires. 2013.07.007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heils A., Mossner R., Lesch K.P. The human serotonin transporter gene polymorphism--basic research and clinical implications. J. Neural Transm. (Vienna) 1997;104(10):1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- 104.Lesch K.P., Bengel D., Heils A., Sabol S.Z., Greenberg B.D., Petri S., Benjamin J., Muller C.R., Hamer D.H., Murphy D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 105.Baldwin D.S., Anderson I.M., Nutt D.J., Allgulander C., Bandelow B., den Boer J.A., Christmas D.M., Davies S., Fineberg N., Lidbetter N., Malizia A., McCrone P., Nabarro D., O’Neill C., Scott J., van der Wee N., Wittchen H.U. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol. 2014;28(5):403–439. doi: 10.1177/0269881114525674. [DOI] [PubMed] [Google Scholar]
- 106.Walsh K., Uddin M., Soliven R., Wildman D.E., Bradley B. Associations between the SS variant of 5-HTTLPR and PTSD among adults with histories of childhood emotional abuse: results from two African American independent samples. J. Affect. Disord. 2014;161:91–96. doi: 10.1016/j.jad.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sayin A., Kucukyildirim S., Akar T., Bakkaloglu Z., Demircan A., Kurtoglu G., Demirel B., Candansayar S., Mergen H. A prospective study of serotonin transporter gene promoter (5-HTT gene linked polymorphic region) and intron 2 (variable number of tandem repeats) polymorphisms as predictors of trauma response to mild physical injury. DNA Cell Biol. 2010;29(2):71–77. doi: 10.1089/dna.2009.0936. [DOI] [PubMed] [Google Scholar]
- 108.Difede J., Olden M., Cukor J. Evidence-based treatment of post-traumatic stress disorder. Annu. Rev. Med. 2014;65:319–332. doi: 10.1146/annurev-med-051812-145438. [DOI] [PubMed] [Google Scholar]
- 109.Aas M., Dazzan P., Mondelli V., Toulopoulou T., Reichenberg A., Di Forti M., Fisher H.L., Handley R., Hepgul N., Marques T., Miorelli A., Taylor H., Russo M., Wiffen B., Papadopoulos A., Aitchison K.J., Morgan C., Murray R.M., Pariante C.M. Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol. Med. 2011;41(3):463–476. doi: 10.1017/S0033291710001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abraham I.M., Meerlo P., Luiten P.G. Concentration dependent actions of glucocorticoids on neuronal viability and survival. Dose Response. 2006;4(1):38–54. doi: 10.2203/dose-response.004.01.004.Abraham. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anacker C., Zunszain P.A., Carvalho L.A., Pariante C.M. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36(3):415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker E., Mittal V., Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 113.Xing Y., Hou J., Meng Q., Yang M., Kurihara H., Tian J. Novel antidepressant candidate RO-05 modulated glucocorticoid receptors activation and FKBP5 expression in chronic mild stress model in rats. Neuroscience. 2015;290:255–265. doi: 10.1016/j.neuroscience.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 114.Lavicky J., Dunn A.J. Corticotropin-releasing factor stimulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis. J. Neurochem. 1993;60(2):602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- 115.Heinrichs S.C., Koob G.F. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 2004;311(2):427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 116.Ishitobi Y., Nakayama S., Yamaguchi K., Kanehisa M., Higuma H., Maruyama Y., Ninomiya T., Okamoto S., Tanaka Y., Tsuru J., Hanada H., Isogawa K., Akiyoshi J. Association of CRHR1 and CRHR2 with major depressive disorder and panic disorder in a Japanese population. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2012;159B(4):429–436. doi: 10.1002/ajmg.b.32046. [DOI] [PubMed] [Google Scholar]
- 117.Baker D.G., West S.A., Nicholson W.E., Ekhator N.N., Kasckow J.W., Hill K.K., Bruce A.B., Orth D.N., Geracioti T.D., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1999;156(4):585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 118.Dunlop B.W., Rothbaum B.O., Binder E.B., Duncan E., Harvey P.D., Jovanovic T., Kelley M.E., Kinkead B., Kutner M., Iosifescu D.V., Mathew S.J., Neylan T.C., Kilts C.D., Nemeroff C.B., Mayberg H.S. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240. [https://doi.org/10.1186/1745-6215-15-240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Strawn J.R., Geracioti T.D., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress. Anxiety. 2008;25(3):260–271. doi: 10.1002/da.20292. [https://doi.org/10.1002/da.20292]. [DOI] [PubMed] [Google Scholar]
- 120.Zandi P.P., Zollner S., Avramopoulos D., Willour V.L., Chen Y., Qin Z.S., Burmeister M., Miao K., Gopalakrishnan S., McEachin R., Potash J.B., Depaulo J.R., Jr, McInnis M.G. Family-based SNP association study on 8q24 in bipolar disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008;147B(5):612–618. doi: 10.1002/ajmg.b.30651. [https://doi.org/10.1002/ajmg.b.30651]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Procopio D.O., Saba L.M., Walter H., Lesch O., Skala K., Schlaff G., Vanderlinden L., Clapp P., Hoffman P.L., Tabakoff B. Genetic markers of comorbid depression and alcoholism in women. Alcohol. Clin. Exp. Res. 2013;37(6):896–904. doi: 10.1111/acer.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alberini C.M., Milekic M.H., Tronel S. Mechanisms of memory stabilization and de-stabilization. Cell. Mol. Life Sci. 2006;63(9):999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Neame M., Aragon O., Fernandes R.M., Sinha I. Salbutamol or aminophylline for acute severe asthma: how to choose which one, when and why? Arch. Dis. Child. Educ. Pract. Ed. 2015;100(4):215–222. doi: 10.1136/archdischild-2014-306186. [DOI] [PubMed] [Google Scholar]
- 124.Reihsaus E., Innis M., MacIntyre N., Liggett S.B. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am. J. Respir. Cell Mol. Biol. 1993;8(3):334–339. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi I., Sledjeski E., Fallon W., Jr, Spoonster E., Riccio D., Delahanty D. Effects of early albuterol (salbutamol) administration on the development of posttraumatic stress symptoms. Psychiatry Res. 2011;185(1-2):296–298. doi: 10.1016/j.psychres.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou D., Lambert S., Malen P.L., Carpenter S., Boland L.M., Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998;143(5):1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leussis M.P., Madison J.M., Petryshen T.L. Ankyrin 3: genetic association with bipolar disorder and relevance to disease pathophysiology. Biol. Mood Anxiety Disord. 2012;2(1):18. doi: 10.1186/2045-5380-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang C., Cai J., Zhang J., Li Z., Guo Z., Zhang X., Lu W., Zhang Y., Yuan A., Yu S., Fang Y. Genetic modulation of working memory deficits by ankyrin 3 gene in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;50:110–115. doi: 10.1016/j.pnpbp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 129.White F., Nicoll J.A., Roses A.D., Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol. Dis. 2001;8(4):611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- 130.Ghebranious N., Ivacic L., Mallum J., Dokken C. Detection of ApoE E2, E3 and E4 alleles using MALDI-TOF mass spectrometry and the homogeneous mass-extend technology. Nucleic Acids Res. 2005;33(17):e149. doi: 10.1093/nar/gni155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jun G., Vardarajan B.N., Buros J., Yu C.E., Hawk M.V., Dombroski B.A., Crane P.K., Larson E.B., Mayeux R., Haines J.L., Lunetta K.L., Pericak-Vance M.A., Schellenberg G.D., Farrer L.A. Alzheimer’s Disease Genetics, C.; Comprehensive search for Alzheimer disease susceptibility loci in the APOE region. Arch. Neurol. 2012;69(10):1270–1279. doi: 10.1001/archneurol.2012.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lohse P., Mann W.A., Stein E.A., Brewer H.B., Jr Apolipoprotein E-4Philadelphia (Glu13----Lys,Arg145----Cys). Homozygosity for two rare point mutations in the apolipoprotein E gene combined with severe type III hyperlipoproteinemia. J. Biol. Chem. 1991;266(16):10479–10484. [PubMed] [Google Scholar]
- 133.Cramer P.E., Cirrito J.R., Wesson D.W., Lee C.Y., Karlo J.C., Zinn A.E., Casali B.T., Restivo J.L., Goebel W.D., James M.J., Brunden K.R., Wilson D.A., Landreth G.E. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yehuda R., Southwick S., Giller E.L., Ma X., Mason J.W. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. J. Nerv. Ment. Dis. 1992;180(5):321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 135.Weinshilboum R.M. Human biochemical genetics of plasma dopamine-beta-hydroxylase and erythrocyte catechol-o-methyltransferase. Hum. Genet. Suppl. 1978;(1):101–112. doi: 10.1007/978-3-642-67179-1_15. [DOI] [PubMed] [Google Scholar]
- 136.Tang Y.L., Li W., Mercer K., Bradley B., Gillespie C.F., Bonsall R., Ressler K.J., Cubells J.F. Genotype-controlled analysis of serum dopamine beta-hydroxylase activity in civilian post-traumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(8):1396–1401. doi: 10.1016/j.pnpbp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.U.S. National Institutes of Health. Pharmacogenetic clinical trial of nepicastat for post traumatic stress disorder (PTSD) 2015 https:// www.clinicaltrials.gov/ct2/show/NCT00641511
- 138.Graham D.P., Nielsen D.A., Kosten T.R., Davis L.L., Hamner M.B., Makotkine I., Yehuda R. Examining the utility of using genotype and functional biology in a clinical pharmacology trial: pilot testing dopamine beta-hydroxylase, norepinephrine, and post-traumatic stress disorder. Psychiatr. Genet. 2014;24(4):181–182. doi: 10.1097/YPG.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin L., Long L.K., Hatch M.M., Hoffman D.A. DPP6 domains responsible for its localization and function. J. Biol. Chem. 2014;289(46):32153–32165. doi: 10.1074/jbc.M114.578070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weizmann Institute of Science. Gene Cards. 2015 http://www.genecards.org/cgi-bin/carddisp. pl?gene=DPP6&search=821f43ca07f64603ab733f04bf7adbde
- 141.Brambilla P., Esposito F., Lindstrom E., Sorosina M., Giacalone G., Clarelli F., Rodegher M., Colombo B., Moiola L., Ghezzi A., Capra R., Collimedaglia L., Coniglio G., Celius E.G., Galimberti D., Sorensen P.S., Martinelli V., Oturai A.B., Harbo H.F., Hillert J., Comi G., Martinelli-Boneschi F. Association between DPP6 polymorphism and the risk of progressive multiple sclerosis in Northern and Southern Europeans. Neurosci. Lett. 2012;530(2):155–160. doi: 10.1016/j.neulet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 142.Marshall C.R., Noor A., Vincent J.B., Lionel A.C., Feuk L., Skaug J., Shago M., Moessner R., Pinto D., Ren Y., Thiruvahindrapduram B., Fiebig A., Schreiber S., Friedman J., Ketelaars C.E., Vos Y.J., Ficicioglu C., Kirkpatrick S., Nicolson R., Sloman L., Summers A., Gibbons C.A., Teebi A., Chitayat D., Weksberg R., Thompson A., Vardy C., Crosbie V., Luscombe S., Baatjes R., Zwaigenbaum L., Roberts W., Fernandez B., Szatmari P., Scherer S.W. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008;82(2):477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Es M.A., van Vught P.W., Blauw H.M., Franke L., Saris C.G., Van den Bosch L., de Jong S.W., de Jong V., Baas F., van’t Slot R., Lemmens R., Schelhaas H.J., Birve A., Sleegers K., Van Broeckhoven C., Schymick J.C., Traynor B.J., Wokke J.H., Wijmenga C., Robberecht W., Andersen P.M., Veldink J.H., Ophoff R.A., van den Berg L.H. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 2008;40(1):29–31. doi: 10.1038/ng.2007.52. [DOI] [PubMed] [Google Scholar]
- 144.Weizmann Institute of Science. Gene Cards. 2015 http://www.genecards.org/cgi-bin/carddisp. pl?gene=SLC1A1&search=d8a9f1b88a9c6549ddd330e8e2e76869
- 145.Veltman G., Bahrs G. Arztl. Wochensch. 1955;10(43-44):1000–1006. [Further study of the effect of isonicotinic acid hydrazide on the pituitary-adrenocortical system]. [PubMed] [Google Scholar]
- 146.Gegelashvili G., Bjerrum O.J. High-affinity glutamate transporters in chronic pain: an emerging therapeutic target. J. Neurochem. 2014;131(6):712–730. doi: 10.1111/jnc.12957. [DOI] [PubMed] [Google Scholar]
- 147.Dallaspezia S., Mazza M., Lorenzi C., Benedetti F., Smeraldi E. A single nucleotide polymorphism in SLC1A1 gene is associated with age of onset of obsessive-compulsive disorder. Eur. Psychiatry. 2014;29(5):301–303. doi: 10.1016/j.eurpsy.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 148.Wu H., Wang X., Yu S., Wang D., Chen J., Jiang K., Zhu L., Xiao Z., Fralick D. Association of the candidate gene SLC1A1 and obsessive-compulsive disorder in Han Chinese population. Psychiatry Res. 2013;209(3):737–739. doi: 10.1016/j.psychres.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 149.Battista M.A., Hierholzer R., Khouzam H.R., Barlow A., O’Toole S. Pilot trial of memantine in the treatment of posttraumatic stress disorder. Psychiatry. 2007;70(2):167–174. doi: 10.1521/psyc.2007.70.2.167. [DOI] [PubMed] [Google Scholar]
- 150.McGhee L.L., Maani C.V., Garza T.H., Gaylord K.M., Black I.H. The correlation between ketamine and posttraumatic stress disorder in burned service members. J. Trauma. 2008. [DOI] [PubMed]
- 151.Feder A., Parides M.K., Murrough J.W., Perez A.M. ; Morgan J.E., Saxena S., Kirkwood K., Aan Het Rot M., Lapidus K.A., Wan L.B., Iosifescu D., Charney D.S. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(6):681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 152.Erichsen M.N., Hansen J., Ruiz J.A., Demmer C.S., Abrahamsen B., Bastlund J.F., Bundgaard C., Jensen A.A., Bunch L. Probing for improved potency and in vivo bioavailability of excitatory amino acid transporter subtype 1 inhibitors UCPH-101 and UCPH-102: design, synthesis and pharmacological evaluation of substituted 7-biphenyl analogs. Neurochem. Res. 2014;39(10):1964–1979. doi: 10.1007/s11064-014-1264-8. [DOI] [PubMed] [Google Scholar]
- 153.Moreira F.A., Kaiser N., Monory K., Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54(1):141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 154.Weizmann Institute of Science. Human Gene Database. 2015 http://www.genecards.org/cgi-bin/carddisp.pl?gene=FAAH&
- 155.Buhler K.M., Huertas E., Echeverry-Alzate V., Gine E., Molto E., Montoliu L., Lopez-Moreno J.A. Risky alcohol consumption in young people is associated with the fatty acid amide hydrolase gene polymorphism C385A and affective rating of drug pictures. Mol. Genet. Genomics. 2014;289(3):279–289. doi: 10.1007/s00438-013-0809-x. [DOI] [PubMed] [Google Scholar]
- 156.Varvel S.A., Wise L.E., Niyuhire F., Cravatt B.F., Lichtman A.H. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32(5):1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- 157.Weizmann Institute of Science. Human Gene Database. http://www.genecards.org/cgi-bin/carddisp.pl?gene=
- 158.Daskalakis N.P., Binder E.B. Schizophrenia in the spectrum of gene-stress interactions: The FKBP5 example. Schizophr. Bull. 2015;41(2):323–329. doi: 10.1093/schbul/sbu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B., Schwartz A.C., Cubells J.F., Ressler K.J. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xie P., Kranzler H.R., Poling J., Stein M.B., Anton R.F., Farrer L.A., Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mitjans M., Catalan R., Vazquez M., Gonzalez-Rodriguez A., Penades R., Pons A., Massana G., Munro J., Arranz M.J., Arias B. Hypothalamic-pituitary-adrenal system, neurotrophic factors and clozapine response: association with FKBP5 and NTRK2 genes. Pharmacogenet. Genomics. 2015;25(5):274–277. doi: 10.1097/FPC.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 162.Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.GABA and benzodiazepine receptor subtypes. Molecular biology, pharmacology, and clinical aspects. Proceedings of the 6th Capo Boi Conference on Neuroscience. Villasimius, Italy, June 1989. Adv. Biochem. Psychopharmacol. 1990;46:1–239. [PubMed] [Google Scholar]
- 164.Warrier V., Baron-Cohen S., Chakrabarti B. Genetic variation in GABRB3 is associated with Asperger syndrome and multiple endophenotypes relevant to autism. Mol. Autism. 2013;4(1):48. doi: 10.1186/2040-2392-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith K.S., Meloni E.G., Myers K.M., Van’t Veer A., Carlezon W.A., Jr, Rudolph U. Reduction of fear-potentiated startle by benzodiazepines in C57BL/6J mice. Psychopharmacology (Berl.) 2011;213(4):697–706. doi: 10.1007/s00213-010-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gao X., Scott W.K., Wang G., Mayhew G., Li Y.J., Vance J.M., Martin E.R. Gene-gene interaction between FGF20 and MAOB in Parkinson disease. Ann. Hum. Genet. 2008;72(Pt 2):157–162. doi: 10.1111/j.1469-1809.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 167.Schulte B., Goerke C., Weyrich P., Grobner S., Bahrs C., Wolz C., Autenrieth I.B., Borgmann S. Clonal spread of meropenem-resistant Acinetobacter baumannii strains in hospitals in the Mediterranean region and transmission to South-west Germany. J. Hosp. Infect. 2005;61(4):356–357. doi: 10.1016/j.jhin.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 168.Naoi M., Maruyama W. Functional mechanism of neuroprotection by inhibitors of type B monoamine oxidase in Parkinson’s disease. Expert Rev. Neurother. 2009;9(8):1233–1250. doi: 10.1586/ern.09.68. [DOI] [PubMed] [Google Scholar]
- 169.Snyder S.H., Jaffrey S.R., Zakhary R. Nitric oxide and carbon monoxide: parallel roles as neural messengers. Brain Res. Brain Res. Rev. 1998;26(2-3):167–175. doi: 10.1016/s0165-0173(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 170.Sapolsky R.M. Atrophy of the hippocampus in posttraumatic stress disorder: how and when? Hippocampus. 2001;11(2):90–91. doi: 10.1002/hipo.1026. [DOI] [PubMed] [Google Scholar]
- 171.Harvey B.H., Oosthuizen F., Brand L., Wegener G., Stein D.J. Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology (Berl.) 2004;175(4):494–502. doi: 10.1007/s00213-004-1836-4. [DOI] [PubMed] [Google Scholar]
- 172.Elzinga B.M., Bremner J.D. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord. 2002;70(1):1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Akyol O., Zoroglu S.S., Armutcu F., Sahin S., Gurel A. Nitric oxide as a physiopathological factor in neuropsychiatric disorders. In Vivo. 2004;18(3):377–390. [PubMed] [Google Scholar]
- 174.Harkin A.J., Bruce K.H., Craft B., Paul I.A. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 1. Acute treatments are active in the forced swim test. Eur. J. Pharmacol. 1999;372(3):207–213. doi: 10.1016/s0014-2999(99)00191-0. [DOI] [PubMed] [Google Scholar]
- 175.Vitecek J., Lojek A., Valacchi G., Kubala L. Arginine-based inhibitors of nitric oxide synthase: therapeutic potential and challenges. Mediators Inflamm. 2012 doi: 10.1155/2012/318087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rodrigues S.M., Schafe G.E., LeDoux J.E. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44(1):75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 177.de Quervain D.J., Kolassa I.T., Ackermann S., Aerni A., Boesiger P., Demougin P., Elbert T., Ertl V., Gschwind L., Hadziselimovic N., Hanser E., Heck A., Hieber P., Huynh K.D., Klarhofer M., Luechinger R., Rasch B., Scheffler K., Spalek K., Stippich C., Vogler C., Vukojevic V., Stetak A., Papassotiropoulos A. PKCalpha is genetically linked to memory capacity in healthy subjects and to risk for posttraumatic stress disorder in genocide survivors. Proc. Natl. Acad. Sci. USA. 2012;109(22):8746–8751. doi: 10.1073/pnas.1200857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Welin M., Egeblad L., Johansson A., Stenmark P., Wang L., Flodin S., Nyman T., Tresaugues L., Kotenyova T., Johansson I., Eriksson S., Eklund H., Nordlund P. Structural and functional studies of the human phosphoribosyltransferase domain containing protein 1. FEBS J. 2010;277(23):4920–4930. doi: 10.1111/j.1742-4658.2010.07897.x. [DOI] [PubMed] [Google Scholar]
- 179.Suzuki E., Imoto I., Pimkhaokham A., Nakagawa T., Kamata N., Kozaki K.I., Amagasa T., Inazawa J. PRTFDC1, a possible tumor-suppressor gene, is frequently silenced in oral squamous-cell carcinomas by aberrant promoter hypermethylation. Oncogene. 2007;26(57):7921–7932. doi: 10.1038/sj.onc.1210589. [DOI] [PubMed] [Google Scholar]
- 180.Comuzzie A.G., Cole S.A., Laston S.L., Voruganti V.S., Haack K., Gibbs R.A., Butte N.F. Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population. PLoS One. 2012;7(12):e51954. doi: 10.1371/journal.pone.0051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Weizmann Institute of Science. Gene Cards. 2015 http://www.genecards.org/cgi-bin/carddisp.pl? gene=PRTFDC1&search=d17d994893da8aca6c75a6cbd22373c9
- 182.Miller M.W., Wolf E.J., Logue M.W., Baldwin C.T. The retinoid-related orphan receptor alpha (RORA) gene and fear-related psychopathology. J. Affect. Disord. 2013;151(2):702–708. doi: 10.1016/j.jad.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Amstadter A.B., Sumner J.A., Acierno R., Ruggiero K.J., Koenen K.C., Kilpatrick D.G., Galea S., Gelernter J. Support for association of RORA variant and post traumatic stress symptoms in a population-based study of hurricane exposed adults. Mol. Psychiatry. 2013;18(11):1148–1149. doi: 10.1038/mp.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Terracciano A., Tanaka T., Sutin A.R., Sanna S., Deiana B., Lai S., Uda M., Schlessinger D., Abecasis G.R., Ferrucci L., Costa P.T., Jr Genome-wide association scan of trait depression. Biol. Psychiatry. 2010;68(9):811–817. doi: 10.1016/j.biopsych.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Solt L.A., Burris T.P. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol. Metab. 2012;23(12):619–627. doi: 10.1016/j.tem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.U.S. Department of Veterans Affairs. Million Veteran Program (MVP) 2015 http://www.research.va.gov/MVP/
- 187.Ogino S., Galon J., Fuchs C.S., Dranoff G. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat. Rev. Clin. Oncol. 2011;8(12):711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dedert E.A., Elbogen E.B., Hauser M.A., Hertzberg J.S., Wilson S.M., Dennis M.F., Calhoun P.S., Kirby A.C., Beckham J.C. Consumer perspectives on genetic testing for psychiatric disorders: the attitudes of veterans with posttraumatic stress disorder and their families. Genet. Test. Mol. Biomarkers. 2012;16(9):1122–1129. doi: 10.1089/gtmb.2012.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yehuda R., Neylan T.C., Flory J.D., McFarlane A.C. The use of biomarkers in the military: from theory to practice. Psychoneuroendocrinology. 2013;38(9):1912–1922. doi: 10.1016/j.psyneuen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 190.Kalinichev M., Rouillier M., Girard F., Royer-Urios I., Bournique B., Finn T., Charvin D., Campo B., Le Poul E., Mutel V., Poli S., Neale S.A., Salt T.E., Lutjens R. ADX71743, a potent and selective negative allosteric modulator of metabotropic glutamate receptor 7: in vitro and in vivo characterization. J. Pharmacol. Exp. Ther. 2013;344(3):624–636. doi: 10.1124/jpet.112.200915. [DOI] [PubMed] [Google Scholar]
- 191.Norberg M.M., Krystal J.H., Tolin D.F. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol. Psychiatry. 2008;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 192.Attari A., Rajabi F., Maracy M.R. D-cycloserine for treatment of numbing and avoidance in chronic post traumatic stress disorder: A randomized, double blind, clinical trial. J. Res. Med. Sci. 2014;19(7):592–598. [PMC free article] [PubMed] [Google Scholar]