Abstract
Background:
Immunosuppressive drugs have been used in the treatment of multiple sclerosis (MS) for a long time. Today, orally available second generation immunosuppressive agents have been approved or are filed for licensing as MS therapeutics. Due to semi-selective targeting of cellular processes, these second-generation immunosuppressive compounds might rather be immunomodulatory. For example, Teriflunomide inhibits the de novo pyrimidine synthesis and thus only targets rapidly proliferating cells, including lymphocytes. It is used as first line disease modifying therapy (DMT) in relapsing-remitting MS (RRMS).
Methods:
Review of online content related to oral immunosuppressants in MS with an emphasis on Teriflunomide.
Results:
Teriflunomide and Cladribine are second-generation immunosuppressants that are efficient in the treatment of MS patients. For Teriflunomide, a daily dose of 14 mg reduces the annualized relapse rate (ARR) by more than 30% and disability progression by 30% compared to placebo. Cladribine reduces the ARR by about 50% compared to placebo but has not yet been licensed due to unresolved safety concerns. We also discuss the significance of older immunosuppressive compounds including Azathioprine, Mycophenolate mofetile, and Cyclophosphamide in current MS therapy.
Conclusion:
Teriflunomide has shown a favorable safety and efficacy profile in RRMS and is a therapeutic option for a distinct group of adult patients with RRMS.
Keywords: Disease modifying therapy, immunosuppression, leflunomide, multiple sclerosis, oral, teriflunomide
1. Introduction
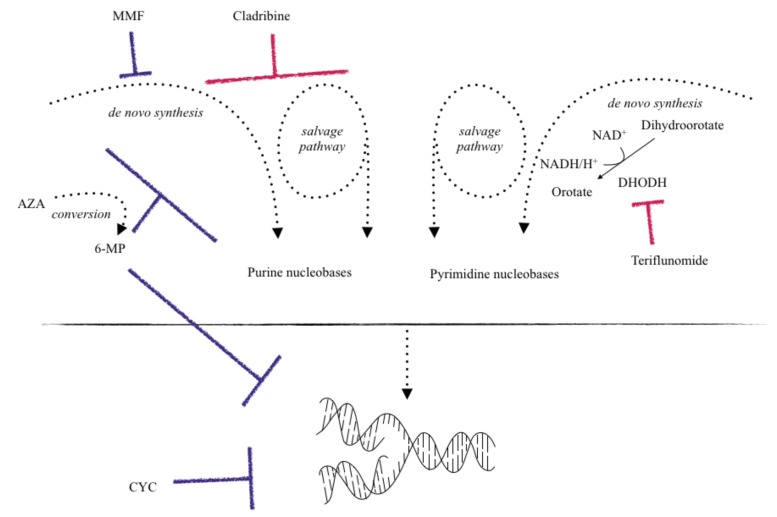
Cytostatic therapies inhibit cell division and thus proliferation-dependent immune responses in several ways: Azathioprine (AZA), a purine analogue, and Mycophenolate mofetil (MMF), by blocking the production of guanosine monophosphate, eventually lead to a depletion of purine nucleotides within cells. Cladribine is a synthetic deoxyadenosine analogue. It results in intracellular accumulation of cladribine-phosphates interfering with DNA synthesis and repair and thus leading to apoptosis and subsequently a reduction in T and B cell numbers. It is currently not yet clear why the lymphocytopenia in response to Cladribine is sustained over months, allowing for only one treatment cycle per year. Teriflunomide inhibits de novo pyrimidine synthesis via the inhibition of dihydroorotate dehydrogenase (DHODH). Apart from the inhibition of purine or pyrimidine synthesis, alkylating agents like cyclophosphamide (CYC) interfere with deoxyribonucleic acids (DNA), also resulting in decreased proliferation.
Teriflunomide is indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS) and is orally applied once daily. It reversibly inhibits the mitochondrial enzyme dihydroorotate dehydrogenase, leading to a decreased de novo pyrimidine synthesis. As a consequence, T- and B-cell activation, proliferation, and potentially immune responses towards autoantigens are reduced. Teriflunomide received approval in the USA in September 2012, and about one year later in Europe and Canada. It is the main active substance of Leflunomide, which has been applied in the treatment of rheumatoid arthritis (RA) since 1998 [1].
The drug is used at 7 mg or 14 mg per day in the USA, and at 14 mg daily dose in the EU. Both doses significantly reduce the annualized relapse rate (ARR) and other measures of disease activity in relapsing-remitting MS (RRMS) in a dose-dependent manner. The safety profile appears comparable between the two groups, although during clinical trials, adverse events occurred slightly less frequent in case of treatment with 7 mg daily. This review provides an overview on pharmaceutical and clinical effects of Teriflunomide in animal models and clinical trials and the role of other oral immunosuppressants in the treatment of MS.
2. Oral immunosuppressants in the treat- ment of MS
2.1. Azathioprine (AZA)
In Europe, AZA is approved as second line treatment of RRMS after the failure of beta interferons (IFNs) or in case of clinical stability during previous AZA treatment, but it is not approved for the treatment of MS in the USA. In the clinical routine, its usage is limited to very few circumstances, like in patients suffering from additional other autoimmune diseases. The antimetabolite AZA is the prodrug to 6-mercaptopurine (6-MP). 6-MP works by blockage of purine nucleotide synthesis. Other metabolites of AZA such as thioguanosine triphosphate and thioinosinic acid interfere with DNA replication as “false” substrates (Fig. 1).
Fig. (1).
Oral immunosuppressants and their mechanism of action. Among other mechanisms, AZA - after conversion to 6-mercapto-purine (6-MP) - works as an antimetabolite and suppresses the production of inosinic acid needed for purine synthesis. Additional antiproliferative effects result from incorporation of metabolites into DNA and RNA. MMF inhibits inosine monophosphate dehydrogenase required for de novo synthesis of guanosine nucleotides. CYC works by alkylation of guanine bases after conversion to its active metabolite, thus interfering with DNA replication and resulting in apoptosis of target cells. Cladribine is an adenosine analog leading to apoptosis after accumulation of toxic metabolites. Teriflunomide selectively inhibits DHODH required for de novo synthesis of pyrimidine nucleotides. Blue lines indicate inhibitory mechanisms of older substances; red lines indicate mechanism of inhibition by “novel” compounds.
After the development of 6-mercaptopurin (6-MP) in 1951 by Gertrude B. Elion and George H. Hitchings, who both later won the Nobel Prize for their discoveries of important principles in drug treatment, and the detection of a more favorable safety and efficacy profile of AZA as compared with 6-MP [2, 3], it has been applied in various indications. Most importantly, the steroid sparing purine analogue is used after renal homotransplantations, in hematologic malignancies, rheumatoid arthritis (RA) and inflammatory bowel disease, as well as other autoimmune diseases like myasthenia gravis.
Like Teriflunomide, AZA blocks only the de novo synthesis of nucleobases and its effect is therefore mostly limited to rapidly dividing lymphocytes. The so-called salvage pathway of nucleoside generation remains unaffected. Here, the cells recycle nucleosides from the degradation of DNA and RNA molecules. The salvage pathway is sufficient to meet the needs in nucleosides in resting lymphocytes or during homeostatic proliferation.
The first use in MS was reported after clinical trials in the early 1990s, but the usage of AZA in MS is significantly limited due to the lack of data from clinical trials meeting current quality standards [4, 5]. A Cochrane report summarized results from five trials containing data from more than 400 patients over 3 years. The report described ARR reductions of 20%, 23%, and 18% in the first, second, and the third year, respectively [6]. Data from only 87 patients were found to report on disease progression, and the relative risk reduction was estimated to be up to 42% during the three years of study time [6].
In a comparative trial between IFN-beta and AZA, AZA showed non-inferiority with regard to the ARR in 150 MS patients who were randomized to receive either IFN-beta or AZA [7]. Various imaging outcomes remained below the non-inferiority margin, indicating non-inferiority to IFN-beta by at least 73% efficacy. However, the number of adverse events was significantly higher during AZA treatment and particularly nausea and vomiting, as well as changes in blood count were more frequent with AZA [7]. Due to the low number of patients in this study, the data were insufficient to draw reliable conclusions. A comparison of the two drugs has also been performed in an indirect meta-analysis with regard to the relapse rate at 24 months, where again no inferiority was detected. On the contrary, AZA appeared to be more efficient than IFN-beta with a relative risk (RR) of 0.88 (95% CI: 0.78 to 1.08) [8]. Still, the study was based on information from patients with all types of MS, since no data on relapsing disease forms were available for AZA. Besides its potential in MS monotherapy, attempts to combine AZA with an established IFN treatment have been made on 15 patients [9]. The data suggested positive imaging effects and there were no serious adverse events, but 20% of the patients discontinued the therapy due to side effects. Over all, combinational therapy did not reach implementation into clinical practice and was not recommended in medical guidelines. Since the risk of malignancies increases with longterm treatment [10], a total of 10 years or life time dose of more than 600 g are not recommended. Generally, further large-scale trials would be needed to conclusively assess AZA in MS therapy in an evidence-based manner.
While not used in the clinical routine of MS therapy, AZA is a useful option in other inflammatory demyelinating conditions such as neuromyelitis optica (NMO). In NMO, AZA has shown positive effects on disease exacerbations in several smaller studies [11-13]. In 2011, Constanzi et al. published the follow-up results on 99 AZA-treated NMO patients with class IV evidence for effectiveness in relapse rate reduction. AZA showed a significant and dose-dependent ARR reduction from 2.20 to 0.52 relapses per year in NMO patients when applied with at least 2 mg/kg body weight/day [14].
2.2. Cyclophosphamide (CYC)
The nitrogen mustard prodrug CYC and its active metabolites like mainly 4-hydroxycyclophosphamide are alkylating antineoplastic agents and work by the attachment of an alkyl group to guanine bases of DNA (Fig. 1). Rapidly proliferating cells, benign or malignant, are affected by this mechanism and consequently undergo apoptosis. In this way, T and B-lymphocytes are reduced in proliferation, but also cytokine secretion, immunoglobulin production and finally, the activation of humoral and cell-mediated immunity is affected. An additional effect might be an induced shift from the autoimmunity associated Th1 helper subtypes towards Th2 helper cells, which potentially leads to a more favorable profile within the CD4+ T cell subset [15, 16].
CYC has been used for the treatment of MS since the 1970s [17, 18], and is currently only applied as third-line off label therapy in selected cases with fulminant disease course. In MS therapy, it has mostly been administered as an intravenous pulse at 800 mg/m2 of body surface area every 3 to 4 weeks over 1 year [4]. In some centers, intravenous “induction” regimens of CYC are applied with 350 mg/m2 per day on three consecutive days and again 3 x 350 mg/m2 one week later. After the induction regimen, maintenance doses of 600 mg/m2 are used every 6 weeks depending on a close monitoring of the absolute leukocyte count. Oral administration of CYC is also possible. Oral CYC is used in the treatment of certain malignant lymphomas, leukemias, neuroblastoma, retinoblastoma and second line in minimal change nephrotic syndrome in children. In 1991, the first trial was conducted to examine the effects of oral CYC with plasma exchange or intravenous CYC without plasma exchange on the disease course of 168 patients with progressive MS [19]. Randomized into three groups, patients received either daily oral CYC with dose adjustments aiming for a white blood count of 4-5 x 109/l plus prednisolone and weekly plasma exchange, or two intravenous CYC administrations up to 9 g total dose with prednisolone and no plasma exchange or oral placebo with sham plasma exchange. This study failed to detect any treatment advantages with regard to EDSS worsening.
While today, a therapeutic potential of intravenous CYC on the disease course of highly active MS is expected from clinical experience, especially at the beginning of the disease and in pediatric patients [20, 21], fairly little is known about oral CYC. Therefore, oral CYC is not used in today’s MS care.
2.3. Cladribine
Cladribine is licensed for the treatment of symptomatic hairy cell leukemia [22], and it can be used in chronic lymphocytic leukemia after insufficient response to treatment with alkylating antineoplastic agents. Being an adenosine analogue resistant to adenosine deaminase (ADA), it accumulates in immune cells and leads to the apoptosis of T and B lymphocytes (Fig. 1). First, the discovery of its immunosuppressive capacity dates back to the 1980s, when adenosine deaminase deficiency was described in patients with severe combined immunodeficiency (SCID) suffering from lymphocytopenia [23]. It was already understood, that SCID patients suffered from impaired lymphocyte growth and function, while other tissue functions were not compromised. This knowledge resulted in the production of drugs mimicking an ADA-deficient state in order to target lymphocytes, which could be useful in patients suffering from lymphoproliferative disorders. Later, the function of ADA was investigated in more detail, and it was discovered that ADA conducted the conversion of deoxyadenosine to deoxyinosine, changing a lymphotoxic substance into a non-toxic metabolite [24].
Sipe et al. first described the use of Cladribine in chronic progressive multiple sclerosis in a placebo-controlled trial in 1994. They found positive effects on disease progression and MRI parameters in a small matched cohort of 24 patients, each receiving either cladribine or placebo [25]. Later, the effect on relapse rates was assessed at 96 weeks in the CLARITY trial (Cladribine Tablets Treating Multiple Sclerosis Orally) comparing two doses of Cladribine (3.5 mg/kg and 5.25 mg/kg) to placebo [26]. Within the first 48 weeks, administration was conducted in a total of 4 cycles of either 0.875 mg/kg Cladribine, or 2 courses of placebo and 2 courses of cladribine, or 4 courses of placebo. During the second 48 weeks of the study period, patients received 2 more courses with either cladribine or placebo, leading to a cumulative cladribine dose of 0 mg (placebo), 3.5 mg/kg or 5.25 mg/kg. Cladribine was shown to reduce the ARR by more than 50% from 0.33 with placebo to 0.14 in the lower and 0.15 in the higher dose treated group. Cladribine also increased the number of patients who were free from relapses, and correspondingly increased the time to first relapse and the time to disability progression (defined as at least 3 months increase of at least 1 point in EDSS or an increase of at least 1.5 points if the baseline EDSS score was 0). Imaging results displayed a strong decrease in the mean number of Gd-enhancing lesions by 85.7 and 87.9%. As expected by its mechanism of action, lymphocytopenia was reported as one common adverse event and appeared in 21.6% with the lower dose of Cladribine and 31.5% in the higher dose treated group compared with 1.8% in the placebo-treated group and went along with an increase in serious adverse events with regard to infections and infestations. Laboratory testing revealed severe neutropenia in three patients receiving Cladribine, and severe thrombocytopenia and pancytopenia in one patient receiving the drug. Neoplasms, both benign and malignant, were dose-dependently reported in patients treated with Cladribine at 0.9 and 1.4%, while there were no cases in the placebo group and there was one patient with severe pancytopenia and lethal exacerbation of tuberculosis who received one treatment cycle in the higher dose group.
Cladribine has also been investigated with regard to the conversion of the first demyelinating event to clinically definite MS (CDMS) in a multicenter, double-blind, randomized phase 3 trial [27]. Treatment with Cladribine at 3.5 or 5.25 mg/kg resulted in a reduced risk of conversion to CDMS according to the Poser criteria with a hazard ratio (HR) of 0.33 and 0.38, respectively, in the 96-week double-blind period. Accordingly, the cumulative incidence of conversion to CDMS was 34% with placebo, 13% with Cladribine 3.5 mg/kg, and 15% with Cladribine 5.25 mg/kg. Cladribine was again very effective with regard to imaging parameters: it reduced the number of new or persisting T1 Gd-enhancing lesions by 89.3% in the 3.5 mg/kg group and 90.5% in the 5.25 mg/kg group. In all treated 616 patients, 412 of whom received cladribine at either 3.5 or 5.25 mg/kg bodyweight, only lymphocytopenia was reported at higher rates in the patients treated with the active substance. 5% and 2% of patients receiving Cladribine 5.25 and 3.5 mg/kg, respectively, developed lymphocytopenia as a severe treatment-emerging adverse event, and one patient displayed lymphocytopenia as a serious adverse event. This observation, alongside with the conclusion of a potential risk of adjacent malignancies and infections, ultimately led to early termination of the study in June 2011. Neither increased infections nor malignancies as a result of treatment with Cladribine were confirmed in a comparison of phase III trials of licensed MS drugs and the CLARITY trial in late 2015 [28].
Yet, due to safety concerns, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) did not approve Cladribine in 2011. As a consequence, Merck pulled back all marketing applications and withdrew it from the Russian and Australian market, where it had already been approved for the treatment of MS. Recently, based on the reanalysis of all data from clinical trials, Merck has announced to pursue resubmission of Cladribine for the treatment of RRMS for registration in Europe [29] and a review of the marketing authorization application has been accepted by the EMA recently [30]. Aside from possible adverse events, the significant reduction in CD4+, CD8+ and CD19+ lymphocytes with only partial recovery after several months [31, 32] make the substance an interesting drug candidate in MS therapy, perhaps also in the sense of an “induction” therapy.
2.4. Mycophelotate Mofetile (MMF)
MMF is licensed to prevent organ-rejection after transplantation since 1995, and it is used off-label for the treatment of other immune-mediated diseases like lupus-nephritis, and less frequently Behçet’s disease, pemphigus vulgaris, small vessel vasculitis or psoriasis. Like AZA, it operates via the inhibition of de novo synthesis of purine nucleobases. MMF and its active metabolite mycophenolic acid reveal immunosuppression via a selective, non-competitive and reversible inhibition of inosine-5'-monophosphate dehydro-genase, leading to a decreased de novo guanosine synthesis and subsequently less proliferation of T and B-lymphocytes (Fig. 1). Additional effects include a decreased IFN-gamma and interleukin (IL)-6 secretion [33, 34] and a decreased B cell proliferation and antibody production [35]. Good efficacy and tolerability in the majority of patients was suggested in a retrospective study of 79 patients with a 77% fraction of patients with a secondary progressive disease course [36]. Later, Frohman et al. compared daily MMF at a dose of 500 mg or later 100 mg twice daily, respectively, to once weekly intramuscular IFN beta-1a 30 µg in patients with relapsing-remitting MS (RRMS) in a randomized, blinded, parallel-group pilot trial [37]. They found no significant differences with regard to safety or efficacy outcomes between IFN-beta and the cost-effective MMF. Even a potential trend towards improved imaging parameters upon treatment with MMF was observed.
In a retrospective study in 344 patients, treatment with MMF led to a 68.1% reduction of the ARR from 1.11±0.08 in a control period before the initiation of treatment to 0.35±0.05 (95% CI) during 1 year of MMF therapy in a mixed study population of RRMS, secondary progressive MS (SPMS), primary-progressive (PPMS) and CIS patients. Adverse events, most frequently intestinal symptoms and asthenia, were reported in 11% of cases leading to discontinuation of the treatment in 7.5%. Lymphocytopenia led to discontinuation in half of the reported cases who finished the trial [38].
Its use as an add-on therapy to IFN beta was assessed in a one-year prospective, randomized, placebo-controlled, quadruple-blinded, phase II trial (TIME MS) [39] enrolling both RRMS and CIS patients. In the small group of 24 patients, adverse events were reported at similar rates and magnetic resonance imaging (MRI) as well as clinical outcomes were not significantly different between the IFN-beta only treated group and patients who were co-treated with MMF. However, a trend towards better stability favored combination therapy [39]. Another small trial with 26 patients assessing a combination therapy was conducted by Etemadifar et al. who also proposed a potential benefit if MMF was added to weekly i.m. IFN beta-1a. Treatment was well tolerated in both groups receiving either IFN beta-1a plus placebo or plus MMF, however, the study was not able to detect a significant difference with regard to new T2 lesions and disease progression [40]. Despite the very low sample size and short study duration of 1 year, this was the only placebo-controlled study found in a Cochrane meta-analysis on MMF in only RRMS patients with a study time of ≥ 6 months follow-up and was rated to be at a high risk of bias [41]. Thus, the lack of reliable data from randomized and placebo controlled trials precludes estimates of efficacy and safety of MMF in MS as mono- or combination therapy.
With regard to NMO, MMF is considered at least as efficient in preventing relapses and accumulation of disability as AZA [42, 43]. Multiple small and non-randomized studies have suggested that MMF is efficient in reducing ARR. Numbers vary between a reduction in ARR from 1.5 to 0.0 (Range 0.3-11.8 to 0.0-2.6) [44], 2.66 to 0.33 [45] and 1.28 to 0.09 [46]. As there is no approved drug for the treatment of NMO so far, MMF might be considered to treat patients NMO patients [47].
3. From Leflunomide to Teriflunomide
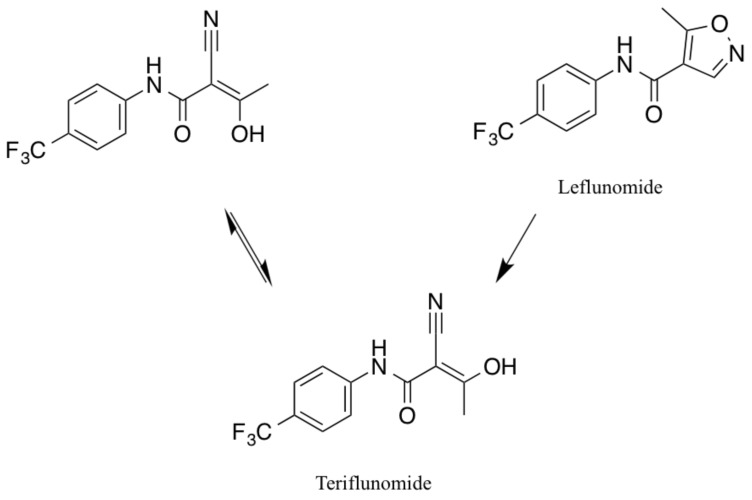
Leflunomide is an isoxazol derivate with immuno- suppressive capacities and it is used as disease modifying-antirheumatic drug in RA and psoriatic arthritis [48]. Its discovery dates back to 1978, when a potential immuno- suppressive effect of Leflunomide, which had initially been studied as an agricultural pesticide, was suspected [49]. It was then tested in an animal model of arthritis in Lewis rats and was able to prevent the onset of the disease when applied early after disease induction, or ease disease severity when applied later in the disease course [50]. Leflunomide has been used for the treatment of RA since 1998, but potential benefits in autoimmune diseases and organ transplantations were already discussed years earlier [51, 52]. The biotransformation from Leflunomide to its only active metabolite Teriflunomide occurs in vivo by the opening of its isoxazole ring [53]. The result is A771726 (Teriflunomide) in its two isoforms (Fig. 2). Subsequently, both drugs presumably function via the same main mechanism of action. Interference with pyrimidine synthesis and subsequently a reduced proliferation of activated lymphocytes was expected to bring along positive effects in MS and was investigated in its animal model experimental autoimmune encephalomyelitis (EAE) decades ago [52].
Fig. (2).
Chemical structure and conversion of Leflunomide to both isoforms of Teriflunomide.
Leflunomide is applied at a dose of 10-20 mg daily and while it is widely used in the above mentioned conditions of arthritis, it has also been discussed for other rheumatic diseases and immunologic conditions such as organ transplant rejections, polyoma BK virus nephropathy [54], systemic lupus erythematosus, and Wegener's granulomatosis [55].
Due to the molecular similarity and in vivo trans- formation of Leflunomide to Teriflunomide (Fig. 2), it is not surprising, that common side effects of both drugs are similar. Effects of Leflunomide include for instance an increase in blood pressure, leukocytopenia, paresthesia, headache, nausea, abdominal pain, increased hair loss and elevation of liver parameters. All of them have been discussed and reported during treatment with Teriflunomide as well. Very rare side-effects of Leflunomide include single cases of toxic epidermal necrolysis (TEN) [56, 57], two cases of pericarditis [58], and two cases of progressive multifocal leukoencephalopathy (PML) [59, 60] in approximately 2.1 million patient-years of use [61]. While there is one case of TEN during Teriflunomide treatment, pericarditis and PML have not yet been reported in patients receiving Teriflunomide.
The rate and extent of drug absorption have been investigated for Teriflunomide and no significant differences were found as compared to Leflunomide [62]. Considering a potentially equivalent safety profile and similar phar- macokinetics, patients under treatment with Teriflunomide might benefit from longterm-experience in the use of Leflunomide, which has been shown in a five-year follow-up study on Leflunomide in RA [63]. However, Leflunomide has been shown to function as an agonist at the aryl hydrocarbon receptor (AhR) [64], which is known to be involved in various immunologic processes as well as drug metabolism like for instance the biotransformation of Leflunomide to Teriflunomide. Teriflunomide in contrast to Leflunomide failed to signal via the AhR [65]. Thus, along with their distinct chemical structure both drugs seem to differ in several ways.
4. Clinical Pharmacology of Teriflunomide
Teriflunomide is a malononitrilamide and works as an inhibitor of DHODH, an enzyme required for de novo pyrimidine synthesis. Steady-state concentrations are reached after approximately 3 months, but this can be accelerated with a loading dose of 70 mg daily for 5 days. Following this procedure, steady-state serum levels of at least 39.1 µg/ml are achieved after 6 days. In the same study, Teriflunomide was still detectable at more than 30 µg/ml after drug withdrawal of 8 days [66]. The half-life is long with around 18-19 days, and serum-levels decrease below levels of 0.02 µg/ml after 8 months of treatment discontinuation if no rapid elimination procedure is performed. In individual cases, Teriflunomide has still been detectable up to 2 years after the last drug intake [67, 68]. One reason for long-lasting presence in plasma is the enterohepatic recirculation, since elimination occurs mainly through biliary excretion [69]. Oral bioavailability is close to 100%, and peak doses are reached about 1-4 hours after drug intake [61]. With more than 99%, Teriflunomide is extensively bound to plasma proteins.
Accelerated elimination is performed if patients plan to become pregnant or if fast drug elimination is clinically indicated, e.g. due to side effects. Oral cholestyramine is then applied at 8 g three times daily for 11 days, leading to a reduction of more than 98% of the plasma concentration [70]. Alternatively, activated charcoal can be applied at 50 g twice daily for 11 days.
5. Mode of action
The exact mechansim of action of Teriflunomide in MS is still not completely understood. However, the selective and reversible inhibition of DHODH, leading to an inhibition of de novo pyrimidine synthesis and consequently reduced B and T lymphocyte proliferation appears a key mechanism of action [71, 72].
DHODH is a mitochondrial enzyme which catalyses the oxidation from dihydroorotate to orotic acid, while NAD+ is reduced to NADH. Lymphocyte activation induces DHODH activation and subsequently increased synthesis of the pyrimidine molecules thymine and cytosine, enabling DNA replication and rapid proliferation. Relying on inhibition of de novo pyrimidine synthesis, Teriflunomide affects strongly proliferating T and B-lymphocytes. The function of resting and slower dividing T cells, using the salvage pathway and thus synthesizing nucleotides from intermediates from degradation of DNA and RNA without the use of DHODH, remains largely unaffected, which might potentially limit the effects of Teriflunomide to highly proliferative immune cells [73].
Other frequently dividing cells, like those in mucosal tissue, express less DHODH and are thus less dependent on de novo pyrimidine synthesis. In vitro, proliferation of T and B cells was limited according to the Teriflunomide concentration, but viability was not affected.
Effects of Teriflunomide on immune cell subsets in vivo include a reduction in the frequencies of T and B lympho- cytes. In 38 patients receiving Teriflunomide for 12 and 24 weeks, this effect was especially pronounced in Th1 cells and was accompanied by a relative increase in Tregs. (TERI-DYNAMIC, NCT01863888) [74]. The same study has also shown a reduced clonal diversity with regard to the CD4+ T cell repertoire, while ex vivo proliferation and cytokine secretion were not altered.
Other effects of Leflunomide were discovered in in vitro assays by Li et al. and comprise a reduced release of the proinflammatory cytokines IL-6, IL-8 and monocyte chemotactic protein-1 [75], while other cytokines were not affected. However, in line with a less inflammatory cytokine milieu, Leflunomide appeared to induce a shift from Th1 towards Th2 differentiation in vitro and enhanced the function of Th2 effector cells in vivo [76]. Since this effect could be abrogated by the addition of uridine triphosphate, which substitutes for the depletion of pyrimidines, it is most likely related to the inhibition of DHODH. Contrarily, the ability of Teriflunomide to potentially decrease intracellular protein aggregation as a typical component of neuro- degenerative polyglutamine (polyQ) diseases like spinobulbar muscular atrophy or Huntington's disease appeared independent of its effects on pyrimidine synthesis in vitro [77]. Underlying mechanisms how Teriflunomide inhibited aggregation and decreased aggregate size of polyQ aggregates in this study are not clear, but the authors suggested that Teriflunomide prevented the incorporation of new polyQ into existing aggregates rather than disintegrating already formed aggregates. There have been multiple potential additional effects of Leflunomide/Teriflunomide, such as an inhibition of protein tyrosine kinases [78, 79] resulting in decreased IgG1 secretion of stimulated B cells via a reduction of JAK3 and STAT6, or an inhibition of the mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) pathways [80, 81], an inhibition of cyclooxygenase-2 [82], interference with the kynurenine pathway [83], and decreased T cell receptor (TCR)/CD3-mediated calcium mobilization during formation of the immunological synapse [84]. However, all these effects were observed in vitro at much higher concentrations than those required to block DHODH.
6. LEFLUNOMIDE AND Teriflunomide in animal models of MS
Dark agouti (DA) rats have been used for the induction of a relapsing-remitting disease course in EAE and provided information on therapeutic effects of Teriflunomide. Already in 1997, a DHODH dependency was shown by a reversal of anti-proliferative Leflunomide effects after intraperitoneal injection of uridine 4 and 24 hours after drug treatment [85]. Animal models have shown a decreased IFN-gamma production along with an increase in IL-10 secretion and clinically improved disease course in EAE upon treatment with Leflunomide [86].
Rats treated with Teriflunomide exhibited less spinal cord infiltration of T cells, NK cells and macrophages [87]. In line with this, reduced inflammation, demyelination, and axonal loss in histopathological analyses have been found with Teriflunomide as well as a delayed onset of EAE and decreased disease severity when rodents had received oral Teriflunomide at a dose of 3 and 10 mg/kg. When administered at the onset of disease or at disease remission, Teriflunomide still led to better maximal and cumulative disease scores [88]. Other measures of demyelination, like decrease in latency of motor-evoked potentials induced by transcranial magnetic stimulation, were as well improved with Teriflunomide in DA rats [89].
Flow cytometric analysis revealed, that C57BL/6 mice treated with Teriflunomide hold less antigen-presenting cells in their Peyer's patches, while the fraction of CD39+ Foxp3+ regulatory T cells increased. Furthermore, adoptive transfer of CD39+ T cells isolated from the gut-associated lymphoid tissue (GALT) from Teriflunomide-treated mice reduced EAE severity both if administered before disease induction or at onset of EAE compared with adoptive transfer of CD39+ T cells isolated from vehicle-treated animals. Thus, Teriflunomide might alter both the frequency and the potency of regulatory GALT derived T cells in mice [90].
7. Clinical effects of Teriflunomide in MS
7.1. Phase 2 Trials
The efficacy and safety of Teriflunomide was first assessed starting April 2001 with a randomized, placebo-controlled and double blind phase 2 clinical trial (Table 1) [91]. 179 patients, out of those 157 patients with RRMS and 22 patients with SPMS with relapses, received placebo, Teriflunomide 7 mg, or Teriflunomide 14 mg daily (with twice the dose during the first week) for 36 weeks. The number of combined unique (CU) active lesions showed a decrease from an average of 2.68 to 1.04 and 1.06 for Teriflunomide 7 mg and 14 mg, corresponding to a relative reduction of 61.1% and 61.3%, respectively. Also secondary imaging endpoints like the median number of T1 Gd-enhancing lesions, new enlarging T2 lesions or the T2 lesion volume showed significant benefits from Teriflunomide treatment. The ARR was by trend reduced from 0.81 (SD ± 1.22) to 0.58 (SD ± 0.85) and 0.55 (SD ± 1.12), which was, however, not significant. Besides clinical and MRI outcomes, there was an excellent compliance noted, with more than 98% of correct intake of the study medication.
Table 1.
Clinical phase 2 and 3 trials on Teriflunomide in MS.
| Study Name | Design/ Study Phase | Arms | Patients Enrolled | Treatment Duration | Primary Endpoint and Result | Key Secondary Endpoints and Results | Time of Patient Assignment |
ClinicalTrials. gov
Identifier |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 2 “proof of concept” [91] | Multicenter, randomized, double-blind, placebo-controlled phase 2 trial | 1:1:1 Teriflunomide 7 mg, Teriflunomide 14 mg, Placebo |
179 RMS patients: 157 RRMS, 22 SPMS with relapses | 36 weeks | Reduction of 61.3% (7 mg) and 61.1% (14 mg) of CU active lesions per MRI scan | Clinical outcomes - By trend fewer relapses - Fewer patients with disability increase in the 14 mg/d group - By trend ARR reduction (n.s.) Imaging parameters - Fewer Gd-enhancing lesions - Fewer new or enlarging T2 lesions - Fewer new T2 lesions |
04/2001 – 03/2003 | NCT01487096 | |||
| Long-term-follow up on phase 2 study [92] | Multicenter, randomized, open-label phase 2 extension of NCT01487096 [88] | Teriflunomide 7 mg, Teriflunomide 14 mg |
147 RMS patients who had completed NCT01487096 | Up to 8.5 years; 372 week evaluation period |
Safety assessment, serious TEAE occurred at 35.5% (7 mg) and 28.8% (14 mg) | Clinical outcomes - ARR remained lowered throughout entire study period - Minimal disability progression throughout entire study period Imaging parameters - Less reduction in cerebral volume with 14 mg - Smaller increase in T2 lesion volume with 14 mg |
03/2003 | NCT00228163 | |||
| TEMSO [93] | Multicenter, randomized, double-blind, placebo-controlled phase 3 trial | 1:1:1 Teriflunomide 7 mg, Teriflunomide 14 mg, Placebo |
1088 RMS patients with or without progression | 108 weeks | ARR RR of 31.2% (7 mg) and 31.5% (14 mg) | Clinical outcomes - Number of patients without relapses at 108 weeks is improved with a HR of 0.76 (7 mg) and 0.72 (14 mg) - HR of sustained disability progression is reduced by 23.7% (7 mg) and 29.8% (14 mg) - HR reduction of sustained disability progression of 23.7% (7 mg, n.s.) and 29.8% (14 mg) Imaging parameters - RR of total lesion volume change from baseline of 39.4% (7 mg) and 67.4% (14 mg) - RR of Gd-enhancing T1 lesions of 57% (7 mg) and 85% (14 mg) |
09/2004 – 03/2008 | NCT00134563 | |||
| Long-term safety and efficacy, 9 year follow-up of TEMSO [96] | Randomized, dose-blind, controlled phase 3 trial | Teriflunomide 7 mg, Teriflunomide 14 mg; Original TEMSO dose was maintained, plus 1:1 randomization of patients previously receiving placebo |
742 RMS patients | Up to 9 years, median 190 weeks | Adverse events were comparable to those in TEMSO (Class III evidence) | - Drop of ARR in patients who were switched from placebo to Teriflunomide - Stable ARR in patients during treatment - 63% of patients initially randomized in the TEMSO study remained on treatment - Probability of 12-week disability progression stable ≤ 0.48 - No Gd-enhancing lesions in >80% of patients throughout the study |
Core: 09/2004 – 03/2008 (TEMSO), Extension: 03/2008 – 06/2013 |
NCT00803049 | |||
| Study Name | Design/ Study Phase | Arms | Patients Enrolled | Treatment Duration | Primary Endpoint and Result | Key Secondary Endpoints and Results | Time of Patient Assignment |
ClinicalTrials. gov Identifier |
|||
| TOWER [97] | Multicenter, randomized, double-blind, placebo-controlled phase 3 trial | 1:1:1 Teriflunomide 7 mg, Teriflunomide 14 mg, Placebo |
1169 RRMS patients | 48 weeks | ARR RR of 22.3% (7 mg) and 36.3% (14 mg) |
Clinical outcomes - Time to sustained disability is improved significantly only with 14 mg (HR 0.68) - Proportion free from relapse at 48 weeks is reduced in both doses (HR 0.70 for 7 mg and HR 0.63 for 14 mg) - Proportion free from sustained accumulation of disability at 108 weeks is not significantly improved with 78.9% (7 mg) and 84.2% (14 mg) vs. 80.3% (placebo) |
09/2008 – 02/2011 | NCT00751881 | |||
| TOPIC [98] | Multicenter, randomized, double-blind, placebo-controlled, phase 3 trial | 1:1:1 Teriflunomide 7 mg, Teriflunomide 14 mg, Placebo |
618 CIS patients | Up to 108 weeks | Time to relapse and conversion to clinically definite MS with a RR of 37.2% (7mg) and 42.6% (14 mg) | Clinical and imaging Parameters - RR of time to relapse or MRI lesion of 31.4% (7 mg) and 34.9% (14 mg) Clinical outcomes - RR in ARR of 33.1% (7 mg, n.s.) and 31.9% (14 mg, n.s.) - No significant RR in disability progression over 12 weeks (2.2% for 7 mg, n.s. and 29.9% for 14 mg) Imaging Parameters RR of number od Gd-enhancing T1 lesions of 21.4% (7 mg, n.s.) and 58.5% (14 mg) |
02/2008 –08/2012 | NCT00622700 | |||
| TENERE [99] | Multicenter, randomized, rater-blinded phase 3 trial | 1:1:1 Teriflunomide 7 mg, Teriflunomide 14 mg, IFN β-1a 44 µg three times per week s.c. |
324 RMS patients with or without progression | Variable, 48 weeks after randomization of the last patient, median exposure >60 weeks | No difference in time to failure, defined as first relapse or permanent treatment discontinuation | Clinical outcomes - Adjusted ARR was n.s. between IFN β (0.22) and 14 mg (0.26), but higher with 7 mg (0.41, p versus IFN β 0.03) - Treatment discontinuation was highest with IFN β (24%), 6.4% with 7 mg and 13.5% with 14 mg Patient satisfaction - Increased TSQM with both doses of Teriflunomide - Less fatigue in FIS score with Teriflunomide 7 mg |
04/2009 – 09/2011 | NCT00883337 | |||
| Teriflunomide as add on to IFN β [102] | Multicenter, randomized, placebo- controlled, double-blind phase 2 trial | 1:1:1 IFN β + placebo, IFN β + Teriflunomide 7 mg, IFN β + Teriflunomide 14 mg |
118 RMS patients with or without progression | 24 weeks plus 24 weeks extension | Class II evidence that Teriflunomide added to IFN β is safe Same frequency of treatment discontinuation due to TEAEs among all three groups |
TEAE - Slightly higher frequency of TEAE in combination therapy - Same frequency of TEAE leading to discontinuation - Infections and hematologic disorders occurred more often in dual treated groups Clinical outcomes - By trend lower ARR with of 32.6% (IFN β + 7 mg, n.s.) and 57.9% (IFN β + 14 mg, n.s.) compared with IFN β + placebo Imaging parameters - RR of Gd-enhancing T1 lesions of 84.6% (IFN β + 7 mg) and 82.8% (IFN β + 14 mg) |
10/2007 – 04/2010 |
NCT00489489 (24 week) NCT00811395 (24 week extension) |
|||
| Study Name | Design/ Study Phase | Arms | Patients Enrolled | Treatment Duration | Primary Endpoint and Result | Key Secondary Endpoints and Results | Time of Patient Assignment |
ClinicalTrials. gov Identifier |
|||
| Teriflunomide as add on to GA [101] | Multicenter, randomized, placebo-controlled, double-blind phase 2 trial | 1:1:1 GA + placebo, GA + Teriflunomide 7 mg, GA + Teriflunomide 14 mg |
123 RMS patients with or without progression | 24 weeks | Acceptable safety profile | TEAE - 2 TEAE leading to discontinuation with GA + placebo vs. 3 and 5 with GA + 7 mg and 14 mg Imaging parameters - RR of Gd-enhancing T1 lesions of 64% (GA + 7 mg) and 47% (14 mg, n.s.) |
04/2007 – 12/2008 | NCT00475865 | |||
| Long Term safety of Teriflunomide when added to IFN β or GA [104] | Multicenter, randomized, placebo-controlled, double-blind phase 2 trial | 1:1:1:1:1:1 IFN β + placebo, IFN β + Teriflunomide 7 mg, IFN β + Teriflunomide 14 mg, GA + placebo, GA + Teriflunomide 7 mg, GA+ Teriflunomide 14 mg |
182 RMS patients who had completed NCT00489489 or NCT00475865 and wished to continue |
48 weeks | TEAE similar among all groups Safety profile as expected by known side effects of monotherapies |
Clinical outcomes - ARR was highest with GA + 14 mg (0.497) - All other groups showed by trend ARR reductions in combination therapies (ARR IFN β + placebo: 0.343, + 7 mg: 0.231, + 14 mg: 0.144, GA + placebo: 0.420, + 7 mg: 0.262) - No advantage with regard to disability progression in combination therapies Imaging parameters - By trend reduced number of Gd-enhancing T1 lesions in combination therapies |
04/2007 – 04/2010 | NCT00811395 | |||
| TERIVA [105] | Multicenter, non-randomized, open-label phase 2 trial | 1:1:1 IFN β, Teriflunomide 7 mg, Teriflunomide 14 mg |
128 RMS patients from NCT00228163 and NCT00803049 and IFN β receiving patients | 28 days | Sufficient anti-H1N1 and B strain-antibodies in all groups H2N3 seroprotection achieved by 76.9% (14 mg) and ≥90% (7 mg or IFN β) |
- By trend lower percentage of patients with ≥ 4 fold in AB titer increase in Teriflunomide-treated groups By trend lower pre vs. post vaccination ratios in geometric mean titers in Teriflunomide-treated groups |
09/2011 – 01/2012 | NCT01403376 | |||
Abbreviations: Annualized relapse rate (ARR), risk reduction (RR), relative risk reduction (RRR), Combined unique (CU), Treatment Satisfaction Questionnaire for Medication (TSQM), Hazard ratio (HR), Fatigue impact scale (FIS), Treatment-emergent adverse events (TEAE), glatiramer acetate (GA).
With regard to long-term safety there is experience from an open-label long term extension study of a phase 2 trial [91, 92]. Clinical activity in the 147 patients entering the follow up study was generally similar to the initial trial over a follow-up of up to 8.5 years.
7.2. TEMSO
The randomized, double-blind, placebo-controlled TEMSO study (Teriflunomide Multiple Sclerosis Oral) (Table 1) [93] was the first phase 3 study to assess the safety and efficacy of Teriflunomide in adult patients with MS. Inclusion criteria contained the diagnosis of MS according to the McDonald criteria, an EDSS score of 5.5 or less and disease activity proven by either one or more relapses within the past year or two or more relapses within the last two years, but no relapse during the past 60 days before enrollment. All together 1088 patients were enrolled into one of the three study arms and subsequently treated with placebo, Teriflunomide 7 mg or Teriflunomide 14 mg per day for 108 weeks.
Teriflunomide reduced the ARR (primary endpoint), from 0.54 relapses in the placebo group to 0.37 relapses ([95% CI 0.32–0.43] for 7 mg daily and [95% CI 0.31–0.44] for 14 mg daily), leading to an ARR reduction of 31.2% and 31.5% when applied at 7 mg and 14 mg, respectively. Disability progression with an increase in EDSS of at least 1 point (or 0.5 points in patients with an EDSS > 5.5) over at least 12 weeks was defined as key secondary endpoint, and was 27.3% with placebo compared to 21.7% in the 7 mg Teriflunomide group and 20.2% in the 14 mg Teriflunomide group. Accordingly, disability progression was reduced by 23.7% and 29.8% by Teriflunomide 7 mg and 14 mg. The proportion of patients without relapses during the 108 weeks of the study was bigger in the treated groups (Hazard ratio vs. placebo was 0.76 and 0.72). Patients reported no significant improvement on a fatigue impact scale (FIS). Taken together, treatment with Teriflunomide led to a modest reduction of clinical disease activity comparable with effects from injectable disease modifying therapies.
Teriflunomide also improved several measures of disease activity in MRI in the TEMSO study. Patients receiving Teriflunomide at 7 mg and 14 mg daily displayed a reduced change in total lesion volume over the study period. While the lower dose reduced the increase in lesion volume compared to baseline by 39.4%, the 14 mg daily dosage achieved a 67.4% reduction in change of lesion volume compared to placebo. The number of Gd-enhancing lesions per T1-weighted scan was 0.57 and 0.26 in Teriflunomide 7 mg and 14 mg groups, respectively, compared to 1.33 in the placebo group. Accordingly, the relative risk was 0.43 and 0.2. There were also fewer unique active lesions per scan. Solely changes in brain atrophy compared to baseline were not significantly different among the groups. Beneficial effects of Teriflunomide on MRI measures were additionally seen in a dose dependent manner for the proportion of patients free from Gd-enhancing lesions, the increase in T1-lesion volume from baseline to week 72 and changes in the Z4 score, a composite MRI score taking into account various parameters of disease burden and activity [94] in the same study population [95].
Results obtained in the TEMSO study were verified in a long-term extension study [96]. 742 patients (68% of the initially randomized patients and 93% of patients completing the TEMSO trial) entered the extension phase and were
followed up for a maximum of 325 weeks. Teriflunomide showed the same side-effects as expected from the core study and maintained stable efficacy with regard to clinical and imaging parameters (Table 1).
7.3. TOWER
The randomized, double-blind, placebo-controlled (TOWER) study (Teriflunomide Oral in people With relapsing multiplE scleRosis) (Table 1) [97] was an additional phase 3 study aiming to provide further information on safety and efficacy of Teriflunomide in adult patients with RRMS at once daily oral application of 7 or 14 mg. Inclusion criteria contained the diagnosis of RRMS with an EDSS of up to 5.5 with activity proven by at least one relapse during the last year or 2 relapses during the past 2 years. Taken together, 1169 patients were enrolled with at least 370 patients in each of the three groups treated with placebo, Teriflunomide 7 mg, or Teriflunomide 14 mg. Teriflunomide was applied over a variable period of time and at least 48 weeks.
The study showed with its primary endpoint a significant ARR reduction (presented as the number of relapses per patient-year) of 22.3% and 36.3% for Teriflunomide 7 mg and 14 mg, respectively. The ARR in the placebo group was adjusted to 0.5. The time to sustained accumulation of disability was measured as an increase in the EDSS of at least 1 point for at least 12 weeks and served as major secondary endpoint. Only the 14 mg group achieved a significant increase in time to disability progression (hazard ratio 0.68 [95% CI 0.47-1.00] log rank p=0.0442). No significant difference was seen with the 7 mg dosage (p=0.7620). Significant changes in the Short Form-36 (SF-36) mental health summary score were only observed in the 14 mg Teriflunomide group when measured from baseline to the last patient visit. There was no significant difference from baseline to week 48, and no differences were detected for the SF-36 physical health summary score for any dosage and any duration. In contrast to the TEMSO study, there was a detectable improvement of fatigue impact scale from baseline to last visit in the 14 mg Teriflunomide group.
Other secondary endpoints contained the time to first relapse, which was significantly prolonged with a hazard ratio of 0.7 and 0.63 for Teriflunomide 7 mg and 14 mg, respectively. There was a therapeutic effect of Teriflunomide 14 mg on change in EDSS score from baseline to week 48 (p=0.0429).
Taken together, both doses of Teriflunomide had an effect on certain parameters of disease activity, but the 14 mg daily dose presented stronger clinical effects particularly on relapses and disease progression than Teriflunomide 7 mg daily. There were no MRI endpoints defined in the TOWER study.
7.4. TOPIC
The use of Teriflunomide in patients with CIS, i.e. a first clinical sign suggestive of MS, was investigated in the randomized, double blind, placebo-controlled TOPIC study (TeriflunOmide vs. Placebo In patients with first Clinical symptom of MS) (Table 1) [98]. 618 patients were randomly assigned and received either placebo, Teriflunomide at 7 mg or Teriflunomide at 14 mg once daily. As expected, there was a positive clinical effect with regard to the primary endpoint of time to the first relapse and with that conversion to clinically definite MS. 28% of patients in the placebo cohort experienced a relapse during the time of study treatment, while this occurred only in 18% of patients treated with Teriflunomide 14 mg, correlating with a risk reduction of 42.6%. Teriflunomide 7 mg reduced the risk to a slightly lesser extent by 37.2%. Key secondary endpoint was the time to first relapse or the detection of new Gd-enhancing T1 or new T2 lesions in MRI as an indicator of either clinical or radiological disease activity. 76% of patients treated with placebo presented either with a relapse or new lesion in MRI, while under therapy with Teriflunomide this proportion was reduced to 64% (14 mg) and 62% (7 mg). Consequently, there was a 34.9% and 31.4% reduction with Teriflunomide 14 mg or 7 mg, respectively. The ARR among patients with placebo was 0.284 and decreased to 0.194 with 14 mg Teriflunomide or 0.190 with 7 mg. However, this relative risk reduction of 31.9% and 33.1% was not statistically significant. There were no significant differences with regard to volume of T2 lesions or brain atrophy. Taken together, Teriflunomide has proven efficacy for patients with clinically isolated syndrome. However, only 74% of patients completed the total 108 weeks of study medication, and only 44% among the Teriflunomide 14 mg treated patients completed the whole study duration. The median duration of study treatment was 90 weeks with Teriflunomide 14 mg.
7.5. Head-to-head Trials
Oral Teriflunomide was compared with IFN beta-1a s.c. in RRMS in a head-to-head trial. TENERE (Teriflunomide and Rebif®) was a phase 3 clinical trial (Table 1) [96] with the time to treatment failure as composite primary endpoint. Time to failure was defined as the time to the first confirmed relapse or the discontinuation of study treatment, whichever occurred first. According to the study, there were no differences found between the group receiving 14 mg Teriflunomide daily and IFN beta-1a 44 µg three times per week with treatment failure rates of 37% and 36% at week 48, respectively. Noteworthy, the fewest relapses were documented in the IFN beta-1a group with a total of 16 compared to 26 and 46 (Teriflunomide 14 mg and 7 mg), while treatment discontinuation was higher with IFN beta-1a. The ARR presented with no significant difference between both groups (0.22 with IFN beta-1a versus 0.26 with Teriflunomide, p=0.6), while the group who had received only 7 mg Teriflunomide daily showed an ARR of 0.41 which was significantly higher compared with IFN beta-1a (p=0.03). At the same time, the 7 mg Teriflunomide group experienced less frequent fatigue than both other groups. The Treatment Satisfaction Questionnaire for Medication (TSQM) was better in both groups who had received oral treatment with Teriflunomide. However, a Cochrane analysis rated the quality of evidence in this study to be very low [100].
7.6. Teriflunomide in Combination Therapies
A comparison between the treatment of Teriflunomide 7 mg or 14 mg as add-on therapy to glatiramer acetate and glatiramer acetate monotherapy revealed an acceptable safety profile in 123 RMS patients over 24 weeks (Table 1) [101]. MRI findings showed an enhanced reduction of Gd-enhancing T1 lesions with a relative risk reduction of 64% in patients co-treated with 7 mg Teriflunomide (p=0.031), while the group co-treated with Teriflunomide 14 mg did not reach significance with a reduction of 47% as compared with glatiramer acetate monotherapy (p=0.193). This might have been due to a higher MRI activity at baseline in the latter group [67]. At the same time, the T1 Gd-enhancing lesion volume was significantly reduced with Teriflunomide 14 mg (RRR 73%, p=0.038) but not Teriflunomide 7 mg (RRR 40%, p=0.134) [101].
Freedman et al. performed a phase 2 trial on Teriflunomide as add-on therapy to IFN-beta (Table 1) [102], in which patients were randomly assigned to receive either placebo, Teriflunomide 7 mg or Teriflunomide 14 mg daily in addition to their unchanged medication with IFN-beta 1a s.c. or i.m. or IFN-beta 1b s.c. The frequency of treatment emergent adverse events (TEAE) and serious TEAEs was slightly higher with combined Teriflunomide treatment, although the frequency of study discontinuations due to TEAE was equal in all groups and there were no deaths in any group. Most commonly reported adverse events included known Teriflunomide-associated side effects like ALT increase, headache, neutropenia, nasopharyngitis and fatigue. With regard to clinical efficacy there was a trend towards a decreased estimated ARR with a relative relapse reduction of 57.9% (Teriflunomide 14 mg added to IFN-beta) and 32.6% (Teriflunomide 7 mg added to IFN-beta) compared to IFN-beta treatment alone. Nonetheless, these potential differences did not reach significance (p=0.4355 and p=0.1005). MRI activity, as measured by the number of Gd-enhancing lesions per scan, was significantly reduced by more than 80% in dual treated groups. A post hoc analysis demonstrated a stronger therapeutic effect in patients with higher disease activity. Taken together, the authors concluded an acceptable safety profile and potential beneficial effects from combined IFN-beta and Teriflunomide treatment for distinct MS patients [102]. Therefore a longer phase 3 trial (TERACLES, NCT01252355) was initiated, but was discontinued in December 2012 due to recruitment difficulties [103].
Patients from both above mentioned studies [101, 102] could continue their treatment entering an extension phase 2 trial with direct comparison between IFN-beta or GA mono- therapy and IFN-beta or GA plus Teriflunomide 7 mg or 14 mg (NCT00475865). 166 of the 182 patients completed 48 weeks and again good safety was demonstrated as primary endpoint. The ARR was changed from 0.343 or 0.420 with IFN-beta or GA monotherapy, respectively, to 0.231 and 0.144 with Teriflunomide 7 mg and 14 mg added to IFN-beta and 0.262 with Teriflunomide 7 mg added to GA. In the 14 mg Teriflunomide plus GA group, the ARR was noteworthy highest among all groups with 0.497. The same was reflected by another secondary endpoint, the fraction of patients with sustained disease progression, which was 0 for IFN-beta monotherapy, but 3/37 and 2/38 patients presented disease progression when Teriflunomide was concomitantly added, and it changed from 4/41 in the group receiving GA monotherapy to 1/42 in patients co-treated with Teriflunomide 7 mg and 4/40 in patients receiving Teriflunomide 14 mg as add-on therapy (Table 1) [104]. Although the number of Gd-enhancing lesions was by trend improved with combination therapy, findings in this study do not provide evidence that combination therapy of IFN-beta or particularly GA with Teriflunomide has significant advantages compared to monotherapy.
7.7. Teriflunomide and Vaccination
The Teriflunomide and Vaccination Trial (TERIVA) (Table 1) [105] investigated immune reactions to seasonal influenza vaccination of MS patients on treatment with Teriflunomide. Patients were immunized with a single IM or intradermal administration of the 2011/2012 inactivated seasonal influenza vaccines containing H1N1, H3N2 and Influenza B, namely the split-virus vaccine Mutagrip® and Vaxigrip®. Strain-specific anti-influenza antibodies were detected 28 days post immunization by hemagglutination inhibition assay. More than 90% of patients from both Teriflunomide-treated groups achieved sufficient antibody titers against H1N1 and influenza B after vaccination, which is comparable to patients under treatment with IFN-beta [106]. Seroprotection rates against H2N3 were lower, though still met the European criterion for efficacy of an influenza vaccine. They were reached by 76.9% of patients receiving 14 mg Teriflunomide and were present in ≥90% of patients in both other groups (Teriflunomide 7 mg and IFN-beta). Secondary endpoints comprised the percentage of patients developing a 4 or more fold increase in antibody titers and the ratio of pre and post vaccination geometric mean titers, and showed by trend lower responses in Teriflunomide-treated patients compared with IFN-beta. There were no serious TEAEs or relapses reported and vaccination was generally well tolerated among all groups. In another study, 46 patients received either rabies vaccination during 30 days of Teriflunomide- or placebo-treatment [66]. Although rabies antibody titers were lower in Teriflunomide-treated patients, both groups developed sufficient titers above 0.5 IU/ml, indicating a satisfactory response to vaccination. Furthermore, responses to recall antigens, as measured by a delayed-type hypersensitivity to Candida albicans, Trychophyton and Tuberculin, did not significantly change upon Teriflunomide treatment [66]. Taken together, sufficient seroprotection was achieved by the majority of patients treated with Teriflunomide after vaccination and the response to recall antigens does not appear to be altered during Teriflunomide therapy. Still, vaccination with live vaccines is not recommended for patients under Teriflunomide treatment.
7.8. Current Trials
With regard to approval in different groups of MS patients, there is one phase 3 trial verifying the efficacy and safety of Teriflunomide in pediatric patients with RMS (TERIKIDS, NCT02201108) and a phase 1 trial addressing the safety and tolerability and pharmacokinetics of ASLAN003 in elderly healthy volunteers (NCT02342652). Also safety and effectiveness of Teriflunomide treatment in patients at risk for PML after termination of Natalizumab is currently being investigated within a phase IV trial (NCT01970410).
8. Safety and tolerability of Teriflunomide
The most common adverse events were considered mild to moderate and presented as increases in alanine amino- transferase (ALT) levels, neutropenia, hair thinning, diarrhea, hypertension, paresthesia and upper respiratory tract infection. Although not considered as severe adverse event, hair thinning appeared in frequencies of up to 13% in both TEMSO and TOWER trials and led to discontinuation of treatment in 6 patients (2%) in the 14 mg group in the TOWER study and in five cases (1.4%) in the TEMSO trial. Although hair thinning usually resolved after 6 months, and there were no cases of complete hair loss [107], this side effect might still influence a patient's adherence to treatment. FDA warnings and precautions exist for hepatotoxicity with ALT increases of 6% of treated patients versus 3.8% in the groups treated with placebo, immunosuppressive effects and subsequent infections with white blood cell decrease, peripheral neuropathy and increased blood pressure [108]. Peripheral neuropathy was recorded in 1.2% and 1.9% of patients during the TEMSO trial and was more frequent in the 14 mg group than in the 7 mg group. Peripheral neuropathy was considered mild to moderate. It occurred at similar frequencies in the TOWER study again and led to discontinuation of treatment in five cases (0.64%) in both treatment groups [97]. Mononeuropathies such as carpal tunnel syndrome were observed in both groups.
In the TEMSO and TOWER study, there was slightly more study discontinuation due to adverse events in the Teriflunomide-treated groups. One case of intestinal tuberculosis occurred in the Teriflunomide 14 mg group and was considered an opportunistic infection. During therapy with Teriflunomide, one patient died from sudden cardiac disorder [92]. However, the patient had a history of an SAE with respiratory failure associated with pneumonia and tachycardia 2 years before and was treated with multiple drugs at the time of her death, so contributory effects of co-medication and underlying medical condition appeared possible.
Other reported adverse events such as back pain, urinary tract infection or life-threatening adverse events occurred at the same prevalence among the three groups. Mild infections such as nasopharyngitis, cystitis, urinary tract infections and oral herpes, as well as fatigue and sensory disturbances have been associated with Teriflunomide treatment [92].
There is extensive longterm experience with Leflunomide with approximately 2.1 million patient-years since 1991, and common adverse events in Teriflunomide-treated patients are so far similar to those in Leflunomide-treated patients. A rare adverse event, the life-threatening toxic epidermal necrolysis has recently been reported in one case report of a RRMS patient who had been started on Teriflunomide therapy for about 3 weeks [109]. Two cases of PML have been reported during monotherapy with Leflunomide [59, 110], even though both had received immunosuppressive treatment with AZA or Methotrexate prior to their medication with Leflunomide. Until today, there has been no report of PML during more than 40.000 patient-years with Teriflunomide in MS. Despite the long-term experience with Leflunomide, the recognition of potentially different or extremely rare adverse events under Teriflunomide therapy will require more experience.
8.1. Teriflunomide in Pregnancy
Teriflunomide is classified as pregnancy category X, therefore expected benefits from treatment do not outweigh drug-associated risks and the use in pregnant women is contraindicated [111]. Women with childbearing potential must be informed about the necessity of an effective contraception, also beyond the exact time of medication use.
Testing in animals suggested an association with pregnancy loss in rats and rabbits treated with equivalent doses of Teriflunomide, as well as increased teratogenicity and decreased survival of newborn rodents (summary of product characteristics). There was so far no evidence of increased rates of spontaneous abortion, decreased birth weight or congenital malformation in human trials [112]. Plasma levels of less than 0.02 mg/L are expected to have no teratogenic impact.
Pregnancy or breastfeeding were exclusion criteria in the TEMSO and TOWER studies. However, taken together both studies, 25 female patients became pregnant in both the placebo and the Teriflunomide group and 16 of those decided to have an induced abortion. There were 4 spontaneous abortions, one of them in the placebo group and 3 in the higher dose Teriflunomide group. The remaining 5 pregnancies resulted in healthy babies; out of those one mother had been treated with placebo, two had been treated with Teriflunomide 7 mg and two had been treated with the 14 mg dose. All pregnancy reports resulted in study discontinuation and a rapid elimination procedure over 11 days. Kieseier et al. performed a retrospective analysis of the global pharmacovigilance project and analyzed 83 reported pregnancies, out of which 70 pregnancies occurred in women with documented Teriflunomide intake. There were 26 live births with healthy newborns and 13 spontaneous abortions, which implies no increased risk of spontaneous abortion [113]. Partners of Teriflunomide-treated male patients became pregnant in 19 cases and as with treated women, rates of spontaneous abortion were not higher as expected in a general population. Also there were no structural or functional invalidities documented [113].
Start of immunotherapy with Teriflunomide is recommended only after a pregnancy has been ruled out by a negative pregnancy test and as a measure of precaution women are encouraged to ensure a safe contraception while receiving the drug and 2 months after a rapid elimination procedure or rather after the Teriflunomide serum concentration has been measured to be less than 0.02 mg/L on two time points. With regard to male patients, there are contradictory recommendations: while in the USA, men undergo an accelerated elimination procedures before fathering a child, therapy can be continued regardless of family planning of male patients in Europe.
8.2. Usage after other Disease Modifying Therapies (DMT)
Teriflunomide is currently not recommended in combination with other DMT, but switching of treatment from other DMT to Teriflunomide might be considered in specific cases. Below, we give recommendations for laboratory testing and therapeutic pauses when changing treatment from distinct other MS drugs to Teriflunomide in accordance with German MS treatment guidelines.
A drug holiday is not needed for patients who had received beta IFNs or GA, if laboratory findings are normal. If patients had been treated with dimethyl fumarate, a wash-out period of at least 2 months is recommended, and a differential blood count is required to be within normal limits again.
In case of previous treatment with Fingolimod we recommend a pause of at least 6 weeks, before treatment with Teriflunomide should be initiated. As for most previous therapies, a differential blood count is required to rule out a potential overlap of both therapies. Natalizumab needs to be stopped at least 6-8 weeks ahead of starting Teriflunomide therapy, if this is feasible with regard to disease activity. In any case an MRI of the brain should be performed to exclude atypical lesions suggestive of PML in patients who were treated with Natalizumab. In patients with positive JCV serology who have been treated with Natalizumab for more than 20 months, a CSF analysis for JCV may be considered to rule out subclinical PML.
Previous treatment with Mitoxantrone requires a discontinuation of at least 3 months before Teriflunomide is started, and peripheral immunocompetence should be assessed to be normal again at the time of initiation of Teriflunomide. Longest intervals of at least 6 months are recommended when treatment is changed from Alemtuzumab or Rituximab and a broad laboratory testing including differential blood count and flow cytometric analysis of T- and B cells is necessary for the evaluation of the immune status of the patient.
In all cases, it is recommended to perform an MRI when changing the therapy or starting treatment with Teriflunomide. Contraindications should be ruled out prior to treatment initiation. Laboratory testing must include blood count, differential blood count, liver and pancreatic parameters, namely aspartate aminotransferase (AST), ALT, gamma-glutamyl transpeptidase (gamma-GT), total bilirubin, lipase, amylase, and total protein and creatinine. If laboratory testing reveals abnormalities, an abdominal ultrasound should be performed. Ruling out current infections with hepatitis B and C and HIV is recommended. If an infection with tuberculosis appears possible, it should be excluded beforehand, potentially also with the conduction of a chest x-ray. Also the documentation of systolic and diastolic blood pressure is necessary. After all diagnostic measures, written informed consent of the patient must be given.
8.3. Contraindications and Patient Surveillance During Treatment
Teriflunomide is contraindicated in patients suffering from severe hepatic impairment. Also, treatment may not be started during acute or chronic infections, pregnancies and in patients receiving Leflunomide concomitantly. Patients with a hypersensitivity reaction against Teriflunomide or Leflunomide should not receive the drug again. Due to potential teratogenic effects, patients must ensure proper contraception while treated with Teriflunomide.
During treatment, laboratory testings must be performed with regularity. We recommend a blood count and differential blood count every 8 weeks during the first 6 months of treatment, succeeded by intervals of every three months during treatment. A lymphocytopenia of less than 200/µl must lead to treatment discontinuations.
With intervals of every second week during the first 6 months of treatment, liver damage should be ruled out by measuring ALT, AST and gamma-GT. After 6 months of good tolerance of the drug, intervals can be stretched to every second month, as long as values remain within normal ranges. A repeated increase of liver transaminases of more than 3 times upper limit of normal (ULN) requires discontinuation of Teriflunomide treatment. If pancreatic or pulmonary impairment is suspected, further diagnostic measures by a specialized gastroenterologist or pulmonologist, respectively, are required. Discontinuation of Teriflunomide should also be considered when patients develop peripheral neuropathy, acute renal failure, or severe skin reactions.
Due to the potential risk of increased blood pressure during treatment with Teriflunomide, we recommend blood pressure measurements on a regular basis. Disease activity should be monitored by yearly MRI scans. Due to recent reports on cerebral deposits of Gadolinium-based contrast agents (GBCA) with unclear clinical impact [114, 115], the administration of GBCA in these scans should be individually evaluated by the treating physicians until national guidelines are available.
Conclusions and Outlook
Teriflunomide has a favorable safety profile and clinical efficacy with respect to relapse rates, imaging outcomes and accumulation of disability in RRMS. It is only approved for the treatment of adult patients with RRMS but not CIS or progressive forms of MS. Although potential benefits from combination therapies have been discussed [116, 117], the use of Teriflunomide is only recommended in monotherapy. So far, the side effects appeared similar to those in Leflunomide treatment. They included most importantly increased liver enzymes, hair thinning, peripheral neuropathy and potential teratogenicity. Although physicians have extensive experience with its functional prodrug Leflunomide, slight differences between the two substances or the two target patient populations (rheumatoid arthritis vs MS), potentially due to different underlying pathological conditions, cannot be completely ruled out. For this reason frequent laboratory assessments especially at the beginning of Teriflunomide therapy are necessary.
Potential teratogenic effect of Teriflunomide were assumed from experience in animal studies but have not been verified so far by an increase in the abortion or disability rates in humans. However, Teriflunomide is not recommended as treatment for patients planning to conceive a child. This measure of precaution potentially leads to some hesitation when choosing between different treatment options in young and particularly female MS patients. At the same time, it appears important to mention the possibility of a rapid elimination procedure. After two months following the rapid elimination procedure, it is likely safe for women to conceive a child, in particular when a nonhazardous serum level has been assured.
It appears likely, that oral therapies will gain more importance in future treatment decisions in MS, not only but importantly due to the more convenient route of application. Treatment with Teriflunomide in particular might become more frequently used with a more comprehensive knowledge regarding adverse events and long-term experience and once the rapid elimination procedure has been broadly recognized.
Cladribine is a potential future therapeutic option as it has lately shown good efficacy in reducing disease activity in MS patients. The administration in repeated cycles followed by sustained lymphocyte decline may qualify Cladribine as an “induction therapy” for patients with RRMS. Its risk-benefit profile will likely be re-evaluated by the respective agencies. Possible benefits from combination therapies will be one future issue in MS therapy, however, particularly with immunosuppressive compounds, this requires a thorough analysis and debate on risks and benefits.
Taken together, Teriflunomide is a good therapeutic option for a certain group of adult patients with RRMS, not only if contraindications against other DMT are present. Its oral administration and the lack of flu-like symptoms are particularly attractive and might lead to an increased compliance rate.
ACKNOWLEDGEMENT
Declared none.
LIST OF ABBREVIATIONS
- ARR
Annualized relapse rate
- CIS
Clinically isolated syndrome
- DMT
Disease modifying therapy
- EDSS
Expanded disability status scale
- MS
Multiple Sclerosis
- RRMS
Relapsing-remitting multiple sclerosis
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Anonymous Leflunomide approved for rheumatoid arthritis; other drugs nearing approval. Am. J. Health Syst. Pharm. 1998;55(21):2225–2226. doi: 10.1093/ajhp/55.21.2225. [DOI] [PubMed] [Google Scholar]
- 2.Elion G.B. The pharmacology of azathioprine. Ann. N. Y. Acad Sci. 1993;685:401–407. doi: 10.1111/j.1749-6632.1993.tb35897.x. https://doi.org/10.1111/j.1749-6632.1993. tb35897.x. [DOI] [PubMed] [Google Scholar]
- 3.Maltzman J., Koretzky G.A. Azathioprine: old drug, new actions. J. Clin. Invest. 2003;111(8):1221–1230. doi: 10.1172/JCI200318384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuda D.T. Immunosuppressive treatments in multiple sclerosis. Handbook of Clinical Neurology. Elsevier; 2014. pp. 503–511. [DOI] [PubMed] [Google Scholar]
- 5.Stankiewicz J.M., Kolb H., Karni A., Weiner H.L. Role of immunosuppressive therapy for the treatment of multiple sclerosis. Neurotherapeutics. 2013;10(1):77–88. doi: 10.1007/s13311-012-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casetta I., Iuliano G., Filippini G. Azathioprine for multiple sclerosis. Cochrane Libr. 2007;4:1–3. doi: 10.1002/14651858.CD003982.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massacesi L., Tramacere I., Amoroso S., Battaglia M.A., Benedetti M.D., Filippini G., La Mantia L., Repice A., Solari A., Tedeschi G., Milanese C. Azathioprine versus beta interferons for relapsing-remitting multiple sclerosis: A multicentre randomized non-inferiority Trial. 2014. [DOI] [PMC free article] [PubMed]
- 8.Messori A., Fadda V., Maratea D., Trippoli S. Indirect meta-analytical comparison of azathioprine and of beta interferon effectiveness in all forms of multiple sclerosis pooled together. 2014. [DOI] [PubMed]
- 9.Pulicken M., Bash C.N., Costello K., Said A., Cuffari C., Wilterdink J.L., Rogg J.M., Mills P., Calabresi P.A. Optimization of the safety and efficacy of interferon beta 1b and azathioprine combination therapy in multiple sclerosis. Mult. Scler. 2005;11:169–174. doi: 10.1191/1352458505ms1141oa. [DOI] [PubMed] [Google Scholar]
- 10.Confavreux C., Saddier P., Grimaud J., Moreau T., Adeleine P., Aimard G. Risk of cancer from azathioprine therapy in multiple sclerosis: A case-control study. Neurology. 1996;46(6):1607–1612. doi: 10.1212/wnl.46.6.1607. [DOI] [PubMed] [Google Scholar]
- 11.McKeon A., Lennon V.A., Lotze T., Tenenbaum S., Ness J.M., Rensel M., Kuntz N.L., Fryer J.P., Homburger H., Hunter J., Weinshenker B.G., Krecke K., Lucchinetti C.F., Pittock S.J. CNS aquaporin-4 autoimmunity in children. 2008. [DOI] [PubMed]
- 12.Mandler R.N., Ahmed W., Dencoff J.E. Devic’s neuromyelitis optica: A prospective study of seven patients treated with prednisone and azathioprine. Neurology. 1998;51(4):1219–1220. doi: 10.1212/WNL.51.4.1219. [DOI] [PubMed] [Google Scholar]
- 13.Bichuetti D.B., Lobato, de Oliveira E.M., Oliveira D.M., Amorin de Souza N., Gabbai A.A. Neuromyelitis optica treatment: analysis of 36 patients. Arch. Neurol. 2010;67(9):1131–1136. doi: 10.1001/archneurol.2010.203. [DOI] [PubMed] [Google Scholar]
- 14.Costanzi C., Matiello M., Lucchinetti C.F., Weinshenker B.G., Pittock S.J., Mandrekar J., Thapa P., McKeon A. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77(7):659–666. doi: 10.1212/WNL.0b013e31822a2780. [DOI] [PubMed] [Google Scholar]
- 15.Balashov K.E., Smith D.R., Khoury S.J., Hafler D.A., Weiner H.L. Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc. Natl. Acad. Sci. USA. 1997;94(2):599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karni A., Balashov K., Hancock W.W., Bharanidharan P., Abraham M., Khoury S.J., Weiner H.L. Cyclophosphamide modulates CD4+ T cells into a T helper type 2 phenotype and reverses increased IFN-gamma production of CD8+ T cells in secondary progressive multiple sclerosis. J. Neuroimmunol. 2004;146(1-2):189–198. doi: 10.1016/j.jneuroim.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Hommes O.R., Prick J.J., Lamers K.J. Treatment of the chronic progressive form of multiple sclerosis with a combination of cyclophosphamide and prednisone. Clin. Neurol. Neurosurg. 1975;78(1):59–72. doi: 10.1016/S0303-8467(75)80007-2. [DOI] [PubMed] [Google Scholar]
- 18.Gonsette R.E., Demonty L., Delmotte P. Intensive immuno- suppression with cyclophosphamide in multiple sclerosis. Follow up of 110 patients for 2-6 years. J. Neurol. 1977;214(3):173–181. doi: 10.1007/BF00316148. [DOI] [PubMed] [Google Scholar]
- 19.GROUP TCCMSS The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Elsevier. Lancet. 1991;337(8739):441–446. [PubMed] [Google Scholar]
- 20.Makhani N., Gorman M.P., Branson H.M., Stazzone L., Banwell B.L., Chitnis T. Cyclophosphamide therapy in pediatric multiple sclerosis. Neurology. 2009;72(24):2064–2065. doi: 10.1212/WNL.0b013e3181a8164c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevan C., Gelfand J.M. Therapeutic management of severe relapses in multiple sclerosis. Curr. Treat Options Neurol. Springer USA. 2015;17(4):17–14. doi: 10.1007/s11940-015-0345-6. [DOI] [PubMed] [Google Scholar]
- 22.Else M., Dearden C.E., Matutes E., Garcia-Talavera J., Rohatiner A.Z., Johnson S.A., O’Connor N.T., Haynes A., Osuji N., Forconi F., Lauria F., Catovsky D. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br. J. Haematol. 2009;145(6):733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 23.Giblett E.R., Anderson J.E., Cohen F., Pollara B., Meuwissen H.J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2(7786):1067–1069. doi: 10.1016/S0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- 24.Freyer C.W., Gupta N., Wetzler M., Wang E.S. Revisiting the role of cladribine in acute myeloid leukemia: An improvement on past accomplishments or more old news? Am. J. Hematol. 2014;90(1):62–72. doi: 10.1002/ajh.23862. [DOI] [PubMed] [Google Scholar]
- 25.Sipe J.C., Romine J.S., Koziol J.A., McMillan R., Zyroff J., Beutler E. Cladribine in treatment of chronic progressive multiple sclerosis. Lancet. 1994;344(8914):9–13. doi: 10.1016/S0140-6736(94)91046-4. [DOI] [PubMed] [Google Scholar]
- 26.Giovannoni G., Comi G., Cook S., Rammohan K., Rieckmann P., Soelberg S.P., Vermersch P., Chang P., Hamlett A., Musch B., Greenberg S.J. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010;362(5):416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 27.Leist T.P., Comi G., Cree B.A., Coyle P.K., Freedman M.S., Hartung H-P., Vermersch P., Casset-Semanaz F., Scaramozza M. oral cladribine for early MS (ORACLE MS) Study Group. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Expert Opin. Pharmacother. 2013;14(1):123–136. doi: 10.1016/S1474-4422(14)70005-5. [DOI] [PubMed] [Google Scholar]
- 28.Pakpoor J., Disanto G., Altmann D.R., Pavitt S., Turner B.P., Marta M., Juliusson G., Baker D., Chataway J., Schmierer K. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol. Neuroimmunol. Neuroinflamm. 2015;2(6):e158. doi: 10.1212/NXI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anonymous. Merck Intends to Submit Cladribine Tablets to Treat Multiple Sclerosis for Registration in Europe. http: //www.merckgroup.com/en/media/extNewsDetail.html?newsId=1C
- 30.Anonymous. Merck Receives European Medicines Agency Acceptance for Review of Marketing Authorization Application for Cladribine Tablets. http://www.presseportal.de/pm/ 6873/3380617
- 31.Selby R., Brandwein J., O’Connor P. Safety and tolerability of subcutaneous cladribine therapy in progressive multiple sclerosis. Can. J. Neurol. Sci. 1998;25(4):295–299. doi: 10.1017/S0317167100034302. [DOI] [PubMed] [Google Scholar]
- 32.Stelmasiak Z., Solski J., Nowicki J., Jakubowska B., Ryba M., Grieb P. Effect of parenteral cladribine on relapse rates in patients with relapsing forms of multiple sclerosis: results of a 2-year, double-blind, placebo-controlled, crossover study. Mult. Scler. 2009;15(6):767–770. doi: 10.1177/1352458509103610. [DOI] [PubMed] [Google Scholar]
- 33.Stosic-Grujicic S., Maksimovic-Ivanic D., Miljkovic D., Trajkovic V., Lukic M., Mostarica S.M. Inhibition of autoimmune diabetes by mycophenolate mofetil is associated with down-regulation of TH1 cytokine-induced apoptosis in the target tissue. Transplant. Proc. 2002;34(7):2955–2957. doi: 10.1016/S0041-1345(02)03502-9. [DOI] [PubMed] [Google Scholar]
- 34.Miljkovic D., Samardzic T., Drakulic D., Stosic-Grujicic S., Trajkovic V. Immunosuppressants leflunomide and mycophenolic acid inhibit fibroblast IL-6 production by distinct mechanisms. Cytokine. 2002;19(4):181–186. doi: 10.1006/cyto.2002.0885. [DOI] [PubMed] [Google Scholar]
- 35.Haneda M., Owaki M., Kuzuya T., Iwasaki K., Miwa Y., Kobayashi T. Comparative analysis of drug action on B-cell proliferation and differentiation for mycophenolic acid, everolimus, and prednisolone. Transplantation. 2014;97(4):405–412. doi: 10.1097/01.tp.0000441826.70687.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frohman E.M., Brannon K., Racke M.K., Hawker K. Mycophenolate mofetil in multiple sclerosis. Clin. Neuropharmacol. 2004;27(2):80–83. doi: 10.1097/00002826-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Frohman E.M., Cutter G., Remington G., Gao H., Rossman H., Weinstock-Guttman B., Durfee J.E., Conger A., Carl E., Treadaway K., Lindzen E., Salter A., Frohman T.C., Shah A., Bates A., Cox J.L., Dwyer M.G., Stuve O., Greenberg B.M., Racke M.K., Zivadinov R. A randomized, blinded, parallel-group, pilot trial of mycophenolate mofetil (CellCept) compared with interferon beta-1a (Avonex) in patients with relapsing-remitting multiple sclerosis. Ther. Adv. Neurol. Disord. SAGE Publications. 2010;3(1):15–28. doi: 10.1177/1756285609353354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel L., Vukusic S., De Seze J., Ducray F., Ongagna J.C., Lefrère F., Jacq-Foucher M., Confavreux C., Wiertlewski S., Laplaud D.A. Mycophenolate mofetil in multiple sclerosis: a multicentre retrospective study on 344 patients. 2014 doi: 10.1136/jnnp-2013-305298. https://doi.org/10 [DOI] [PubMed]
- 39.Remington G.M., Treadaway K., Frohman T., Salter A., Stuve O., Racke M.K., Hawker K., Agosta F., Sormani M.P., Filippi M., Frohman E.M. A one-year prospective, randomized, placebocontrolled, quadruple-blinded, phase II safety pilot trial of combination therapy with interferon beta-1a and mycophenolate mofetil in early relapsing--remitting multiple sclerosis (TIME MS). Ther. Adv. Neurol. Disord. 2010;3(1):3–13. doi: 10.1177/1756285609355851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etemadifar M., Kazemim M., Chitsaz A., Hekmatnia A., Tayari N., Ghazavi A., Maghzi A.H. Mycophenolate mofetil in combination with interferon beta-1a in the treatment of relapsing-remitting multiple sclerosis: A preliminary study. J. Res. Med. Sci. 2011;16(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao Y., Huang J., Luo H., Wang J. Mycophenolate mofetil for relapsing-remitting multiple sclerosis. Cochrane Libr. 2014;2:1–16. doi: 10.1002/14651858.cd010242.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H., Qiu W., Zhang Q., Wang J., Shi Z., Liu J., Lian Z., Feng H., Miao X., Zhou H. Comparisons of the efficacy and tolerability of mycophenolate mofetil and azathioprine as treatments for neuromyelitis optica and neuromyelitis optica spectrum disorder. Eur. J. Neurol. Wiley Online Library. 2016;24:219–226. doi: 10.1111/ene.13186. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y., Wang Q., Ren H-T., Qiao L., Zhang Y., Fei Y-Y., Zhao Y., Cui L-Y. Comparison of efficacy and tolerability of azathioprine, mycophenolate mofetil, and cyclophosphamide among patients with neuromyelitis optica spectrum disorder: A prospective cohort study. J. Neurol. Sci. 2016;370:224–228. doi: 10.1016/j.jns.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 44.Huh S-Y., Kim S-H., Hyun J-W., Joung A-R., Park M.S., Kim B-J., Kim H.J. Mycophenolate mofetil in the treatment of neuromyelitis optica spectrum disorder. JAMA Neurol. 2014;71(11):1372–1378. doi: 10.1001/jamaneurol.2014.2057. [DOI] [PubMed] [Google Scholar]
- 45.Mealy M.A., Wingerchuk D.M., Palace J., Greenberg B.M., Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica. JAMA Neurol. 2014;71(3):324. doi: 10.1001/jamaneurol.2013.5699. [DOI] [PubMed] [Google Scholar]
- 46.Jacob A., Matiello M., Weinshenker B.G., Wingerchuk D.M., Lucchinetti C., Shuster E., Carter J., Keegan B.M., Kantarci O.H., Pittock S.J. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch. Neurol. 2009;66(9):1128–1133. doi: 10.1001/archneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 47.Wingerchuk D.M., Weinshenker B.G. Neuromyelitis optica. Curr. Treat. Options Neurol. 2008;10(1):55–66. doi: 10.1007/s11940-008-0007-z. [DOI] [PubMed] [Google Scholar]
- 48.Leflunomide summary of product characteristics. http: //www.ema.europa.eu/docs/en_GB/document_library/EPAR_-
- 49.Allison A.C. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology. 2000;47(2-3):63–83. doi: 10.1016/S0162-3109(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 50.Bartlett R.R., Schleyerbach R. Immunopharmacological profile of a novel isoxazol derivative, HWA 486, with potential antirheumatic activity--I. Disease modifying action on adjuvant arthritis of the rat. Int. J. Immunopharmacol. 1985;7(1):7–18. doi: 10.1016/0192-0561(85)90003-7. https://doi.org/10 [DOI] [PubMed] [Google Scholar]
- 51.Bartlett R.R., Dimitrijevic M., Mattar T., Zielinski T., Germann T., Rüde E., Thoenes G.H., Küchle C.C., Schorlemmer H.U., Bremer E., Finnegan A., Schleyerbach R. Leflunomide (HWA 486), a novel immunomodulating compound for the treatment of autoimmune disorders and reactions leading to transplantation rejection. Inflamm Res. Birkhäuser-Verlag. 1991;32(1-2):10–21. doi: 10.1007/BF01983301. [DOI] [PubMed] [Google Scholar]
- 52.Bartlett R., Anagnostopulos H., Zielinski T., Mattar T., Schleyerbach R. Effects of leflunomide on immune responses and models of inflammation. Springer Semin. Immunopathol. 1993;14(4):381–394. doi: 10.1007/BF00192310. [DOI] [PubMed] [Google Scholar]
- 53.Rozman B. Clinical pharmacokinetics of leflunomide. Clin. Pharmacokinet. 2002;41(6):421–430. doi: 10.2165/00003088-200241060-00003. [DOI] [PubMed] [Google Scholar]
- 54.Blanckaert K., De Vriese A.S. Current recommendations for diagnosis and management of polyoma BK virus nephropathy in renal transplant recipients. Nephrol. Dial. Transplant. 2006;21(12):3364–3367. doi: 10.1093/ndt/gfl404. [DOI] [PubMed] [Google Scholar]
- 55.Sanders S., Harisdangkul V. Leflunomide for the treatment of rheumatoid arthritis and autoimmunity. Am. J. Med. Sci. 2002;323(4):190–193. doi: 10.1097/00000441-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Hassikou H., Haouri E.M., Tabache F., Baaj M., Safi S., Hadri L. Leflunomide-induced toxic epidermal necrolysis in a patient with rheumatoid arthritis. Joint Bone Spine. 2008;75(5):597–599. doi: 10.1016/j.jbspin.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Teraki Y., Hitomi K., Sato Y., Hamamatsu Y., Izaki S. Leflunomide-induced toxic epidermal necrolysis. Int. J. Dermatol. 2006;45(11):1370–1371. doi: 10.1111/j.1365-4632.2006.03003.x. https://doi.org/10.1111/j.1365-4632.2006.03003.x [DOI] [PubMed] [Google Scholar]
- 58.Vandenbos F., Figueredo M., Tarhini A., Ribière J. Pleuro-péricardite développée au décours d’un traitement par léflunomide. Rev. Pneumol. Clin. 2015;71(1):57–59. doi: 10.1016/j.pneumo.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Rahmlow M., Shuster E.A., Dominik J., Deen H.G., Dickson D.W., Aksamit A.J., Robles H.A., Freeman W.D. Leflunomide-Associated Progressive Multifocal Leukoencephalopathy. Arch. Neurol. Am. Med. Assoc. 2008;65(11):1538–1539. doi: 10.1001/archneur.65.11.1538. [DOI] [PubMed] [Google Scholar]
- 60.Warnatz K., Peter H., Schumacher M., Wiese L., Prasse A., Petschner F., Vaith P., Volk B., Weiner S.M. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann. Rheum. Dis. 2003;62(1):50. doi: 10.1136/ard.62.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Connor P. An update of teriflunomide for treatment of multiple sclerosis. TCRM. 2013;9:177–190. doi: 10.2147/TCRM.S30947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agarwal S., Das A., Ghosh D., Sarkar A., Chattaraj T., Pal T. Comparative bioequivalence study of leflunomide tablets in indian healthy volunteers. Arzneimittelforschung. 2012;62(03):145–148. doi: 10.1055/s-0031-1298024. [DOI] [PubMed] [Google Scholar]
- 63.Kalden J.R., Schattenkirchner M.S., Rensen H., Emery P., Deighton C., Rozman B., Breedveld F. The efficacy and safety of leflunomide in patients with active rheumatoid arthritis: A five-year followup study. Arthritis Rheum. 2003;48(6):1513–1520. doi: 10.1002/art.11015. [DOI] [PubMed] [Google Scholar]
- 64.Hu W., Sorrentino C., Denison M.S., Kolaja K., Fielden M.R. Induction of Cyp1a1 is a nonspecific biomarker of Aryl Hydrocarbon receptor zctivation: results of large scale screening of Phar-maceuticals and toxicants in vivo and in vitro. Mol. Pharmacol. 2007;71(6):1475–1486. doi: 10.1124/mol.106.032748. https://doi.org/10.1124/mol.106.032748 [DOI] [PubMed] [Google Scholar]
- 65.O'Donnell E.F., Saili K.S., Koch D.C., Kopparapu P.R., Farrer D., Bisson W.H., Mathew L.K., Sengupta S., Kerkvliet N.I., Tanguay R.L., Kolluri S.K. The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PLoS. One, 2010;5(10):pii: e13128.. doi: 10.1371/journal.pone.0013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bar-Or A., Wiendl H., Miller B., Benamor M., Truffinet P., Church M., Menguy-Vacheron F. Randomized study of teriflunomide effects on immune responses to neoantigen and recall antigens. Neurol. Neuroimmunol. Neuroinflamm. 2015;15(2):1019–1027. doi: 10.1212/nxi.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freedman M.S. Teriflunomide in relapsing multiple sclerosis: therapeutic utility. Ther. Adv. Chronic Dis. 2013;4(5):192–205. doi: 10.1177/2040622313492810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anonymous Teriflunomide prescribing information. http: //products.sanofi.us/aubagio/aubagio.pdf
- 69.Wiese M.D., Rowland A., Polasek T.M., Sorich M.J., O'Doherty C. Pharmacokinetic evaluation of teriflunomide for the treatment of multiple sclerosis. Expert Opin. Drug Metab. Toxicol. 2013;9(8):1025–1035. doi: 10.1517/17425255.2013.800483. [DOI] [PubMed] [Google Scholar]
- 70. A guide to the accelerated elimination procedure for Aubagio (Teriflunomide) Availabe at: http: //www.aubagio.com/media/ pdf/Guide_to_Accelerated_Elimination_Procedure_for_AUBAGIO(R)(teriflunomide).pdf
- 71.Cherwinski H.M., Byars N., Ballaron S.J., Nakano G.M., Young J.M., Ransom J.T. Leflunomide interferes with pyrimidine nucleotide biosynthesis. Inflamm. Res. 1995;44(8):317–322. doi: 10.1007/BF01796261. [DOI] [PubMed] [Google Scholar]
- 72.Rückemann K., Fairbanks L.D., Carrey E.A., Hawrylowicz C.M., Richards D.F., Kirschbaum B., Simmonds H.A. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J. Biol. Chem. 1998;273(34):21682–21691. doi: 10.1074/jbc.273.34.21682. [DOI] [PubMed] [Google Scholar]
- 73.Garnock-Jones K.P. Teriflunomide: a review of its use in relapsing multiple sclerosis. CNS Drugs. 2013;27(12):1103–1123. doi: 10.1007/s40263-013-0118-2. [DOI] [PubMed] [Google Scholar]
- 74.Wiendl H., Gross C., Lindner M., Eschborn M., Weisser L., Posevitz-Fejfar A., Schulte-Mecklenbeck A., Van Wijmeersch B., Hupperts R., Brette S., Turner T., Bar-Or A., Klotz L. TERI-DYNAMIC: exploring the impact of Teriflunomide on immune cell Population size, receptor repertoire, and function in patients with RRMS (P5.282). Neurology. 2016;86(16) Suppl. [Google Scholar]
- 75.Li L., Liu J., Delohery T., Zhang D., Arendt C., Jones C. The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells. J. Neuroimmunol. 2013;265(1-2):82–90. doi: 10.1016/j.jneuroim.2013.10.003. https://doi.org/10.1016/j.jneuroim.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Dimitrova P., Skapenko A., Herrmann M.L., Schleyerbach R., Kalden J.R., Schulze-Koops H. Restriction of de novo pyrimidine biosynthesis inhibits Th1 cell activation and promotes Th2 cell differentiation. J. Immunol. 2002;29(169):3392–3399. doi: 10.4049/jimmunol.169.6.3392. [DOI] [PubMed] [Google Scholar]
- 77.Fuentealba R.A., Marasa J., Diamond M.I., Piwnica-Worms D, Weihl C.C. An aggregation sensing reporter identifies leflunomide and teriflunomide as polyglutamine aggregate inhibitors. Hum. Mol. Genet. 2012;21(3):664–80. doi: 10.1093/hmg/ddr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X., Shen J., Mall J.W., Myers J.A., Huang W., Blinder L., Saclarides T.J., Williams J.W., Chong A.S. In vitro and in vivo antitumor activity of a novel immunomodulatory drug, leflunomide: mechanisms of action. Biochem. Pharmacol. 1999;58(9):1405–1413. doi: 10.1016/S0006-2952(99)00228-2. [DOI] [PubMed] [Google Scholar]
- 79.González-Alvaro I., Ortiz A.M., Domínguez-Jiménez C., Aragón-Bodi A., Díaz Sánchez B., Sánchez-Madrid F. Inhibition of tumour necrosis factor and IL-17 production by leflunomide involves the JAK/STAT pathway. Ann. Rheum. Dis. 2009;68(10):1644–1650. doi: 10.1136/ard.2008.096743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manna S.K., Aggarwal B.B. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression. J. Immunol. 1999;162(4):2095–2102. [PubMed] [Google Scholar]
- 81.Nicoletti F., Di Nuzzo L., Orlando R., Nasca C. Molecular pharmacodynamics of new oral drugs used in the treatment of multiple sclerosis. DDDT. 2014;8:555–568. doi: 10.2147/DDDT.S52428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamilton L.C., Vojnovic I., Warner T.D. the active metabolite of leflunomide, directly inhibits the activity of cyclooxygenase- 2 in vitro and in vivo in a substrate-sensitive manner. Br. J. Pharmacol. 1999;127(7):1589–1596. doi: 10.1038/sj.bjp.0702708. https://doi.org/10.1038/sj.bjp.0702708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y., Meininger V., Guillemin G. Recent advances in the treatment of amyotrophic lateral sclerosis. Emphasis on kynurenine pathway inhibitors. Cent. Nerv. Syst. Agents Med. Chem. 2009;9(1):32–39. doi: 10.2174/187152409787601941. [DOI] [PubMed] [Google Scholar]
- 84.Zeyda M., Poglitsch M., Geyeregger R., Smolen J.S., Zlabinger G.J., Hörl W.H., Waldhäusl W., Stulnig T.M., Säemann M.D. Disruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formation. Arthritis Rheum. 2005;52(9):2730–2739. doi: 10.1002/art.21255. [DOI] [PubMed] [Google Scholar]
- 85.Silva H.T., Cao W., Shorthouse R.A., Löffler M., Morris R.E. In vitro and in vivo effects of leflunomide, brequinar, and cyclosporine on pyrimidine biosynthesis. Transplant. Proc. 1997;29(1-2):1292–1293. doi: 10.1016/S0041-1345(96)00523-4. [DOI] [PubMed] [Google Scholar]
- 86.Korn T., Magnus T., Toyka K., Jung S. Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide—mechanisms independent of pyrimidine depletion. J. Leukoc. Biol. 2004;76(5):950–960. doi: 10.1189/jlb.0504308. https://doi.org/10.1189/jlb.0504308 [DOI] [PubMed] [Google Scholar]
- 87.Ringheim G.E., Lee L., Laws-Ricker L., Delohery T., Liu L., Zhang D., Colletti N., Soos T.J., Schroeder K., Fanelli B., Tian N., Arendt C.W., Iglesias-Bregna D., Petty M., Ji Z., Qian G., Gaur R., Weinstock D., Cavallo J., Telsinskas J., McMonagle-Strucko K. Teriflunomide attenuates immunopathological changes in the dark Agouti Rat model of experimental autoimmune encephalomyelitis. Front. Neurol. Frontiers. 2013;4:169. doi: 10.3389/fneur.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merrill J.E., Hanak S., Pu S-F., Liang J., Dang C., Iglesias-Bregna D., Harvey B., Zhu B., McMonagle-Strucko K. Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. J. Neurol. 2009;256(1):89–103. doi: 10.1007/s00415-009-0075-3. [DOI] [PubMed] [Google Scholar]
- 89.Iglesias-Bregna D., Hanak S., Ji Z., Petty M., Liu L., Zhang D., McMonagle-Strucko K. Effects of prophylactic and therapeutic Teriflunomide in transcranial magnetic stimulation–Induced Motor-Evoked potentials in the Dark Agouti rat model of experimental autoimmune Encephalomyelitis. J. Pharmacol. Exp. Ther. 2013;1:203–211. doi: 10.1124/jpet.113.205146. [DOI] [PubMed] [Google Scholar]
- 90.Ochoa-Reparaz J., Colpitts S.L., Kircher C., Kasper E.J., Telesford K.M., Begum-Haque S., Pant A., Kasper L.H. Induction of gut regulatory CD39 +T cells by teriflunomide protects against EAE. Neurol. Neuroimmunol. Neuroinflamm. 2016;3(6):e291. doi: 10.1212/NXI.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Connor P.W., Li D., Freedman M.S., Bar-Or A., Rice G.P., Confavreux C., Paty D.W., Stewart J.A., Scheyer R., Group O.B., University of British Columbia MS/MRI Research Group A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66:894–900. doi: 10.1212/01.wnl.0000203121.04509.31. [DOI] [PubMed] [Google Scholar]
- 92.Confavreux C., Li D.K., Freedman M.S., Truffinet P., Benzerdjeb H., Wang D., Bar-Or A., Traboulsee A.L., Reiman L.E., O’Connor P.W. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult. Scler. 2012;9:1278–1289. doi: 10.1177/1352458512436594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Connor P., Wolinsky J.S., Confavreux C., Comi G., Kappos L., Olsson T.P., Benzerdjeb H., Truffinet P., Wang L., Miller A., Freedman M.S. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N. Engl. J. Med. 2011;365(14):1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 94.Poonawalla A.H., Datta S., Juneja V., Nelson F., Wolinsky J.S., Cutter G., Narayana P.A. Composite MRI scores improve correlation with EDSS in multiple sclerosis. Mult. Scler. 2010;16(9):1117–1125. doi: 10.1177/1352458510374892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolinsky J.S., Narayana P.A., Nelson F., Datta S., O’Connor P., Confavreux C., Comi G., Kappos L., Olsson T.P., Truffinet P., Wang L., Miller A., Freedman M.S. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult. Scler. J. 2013;19(10):1310–1319. doi: 10.1177/1352458513475723. [DOI] [PubMed] [Google Scholar]
- 96.O'Connor P., Comi G., Freedman M.S., Miller A.E. ; Kappos L., Bouchard J-P., Lebrun-Frenay C., Mares J., Benamor M., Thangavelu K., Liang J., Truffinet P., Lawson V.J., Wolinsky J.S. Long-term safety and efficacy of teriflunomide. Neurology. 2016;86(10):920–930. doi: 10.1212/WNL.0000000000002441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Confavreux C., O’Connor P., Comi G., Freedman M.S., Miller A.E., Olsson T., Wolinsky J.S., Bagulho T., Delhay J-L., Dukovic D., Truffinet P., Kappos L. Group, FTTT Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(10):977–986. doi: 10.1016/S1474-4422(14)70191-7. [DOI] [PubMed] [Google Scholar]
- 98.Miller A.E., Wolinsky J.S., Kappos L., Comi G., Freedman M.S., Olsson T.P., Bauer D., Benamor M., Truffinet P., O'Connor P.W. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol., 2014;13(10):977–986. doi: 10.1016/S1474-4422(14)70191-7. [DOI] [PubMed] [Google Scholar]
- 99.Vermersch P., Czlonkowska A., Grimaldi L.M., Confavreux C., Comi G., Kappos L., Olsson T.P., Benamor M., Bauer D., Truffinet P., Church M., Miller A.E., Wolinsky J.S., Freedman M.S., O’Connor P. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult. Scler. 2014;20:705–716. doi: 10.1177/1352458513507821. [DOI] [PubMed] [Google Scholar]
- 100.He D., Zhang C., Zhao X., Zhang Y., Dai Q., Li Y., Chu L. Teriflunomide for multiple sclerosis. Cochrane Libr. 2016;3:1–52. doi: 10.1002/14651858.cd009882.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Freedman M.S., Wolinsky J.S., Wamil B., Confavreux C., Comi G., Kappos L., Olsson T.P., Miller A., Benzerdjeb H., Li H., O’Connor P.W. Oral Teriflunomide plus glatiramer acetate in relapsing multiple sclerosis. J. Int. MS Care. 2011;13(Suppl. 3):9. [Abstract P17]. [Google Scholar]
- 102.Freedman M.S., Wolinsky J.S., Wamil B., Confavreux C., Comi G., Kappos L., Olsson T.P., Miller A., Benzerdjeb H., Li H., Simonson C., O’Connor P.W., Gazda S., Huffman C., Kantor D., Stein M., Hendin B., Grand’Maison F., Kremenchutzky M., Marrie R.A., Jacques F., Mäurer M., Sigel K.O., Elias W-G., Ziemssen T., Tiel-Wilck K., Lensch E., Capra R., Pozzilli C., Saiz A., Arbizu T., Montalban X., Rodríguez-Antigüedad A., Fernandez O., Merino A.G., Arroyo R., Olascoaga J., Meca J., Narayana P.A., Nelson F., Vainrub I., Datta S., He R., Gates B., Ton K. Teriflunomide added to interferon-β in relapsing multiple sclerosis. Neurology. 2012;78:1877–1885. doi: 10.1212/WNL.0b013e318258f7d4. [DOI] [PubMed] [Google Scholar]
- 103.Oh J., O’Connor P.W. Teriflunomide in the treatment of multiple sclerosis: current evidence and future prospects. 2014 doi: 10.1177/1756285614546855. https://doi.org/10 [DOI] [PMC free article] [PubMed]
- 104.Long Term Safety of Teriflunomide When Added to Interferon-Beta or Glatiramer Acetate in Patients With Multiple Sclerosis. Availabe at. https: //clinicaltrials.gov/ct2/show/study/ NCT00811395? term=NCT00475865&rank=2§=X01256
- 105.Bar-Or A., Freedman M.S., Kremenchutzky M., Menguy-Vacheron F., Bauer D., Jodl S., Truffinet P., Benamor M., Chambers S., O'Connor P.W. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. 2013. [DOI] [PMC free article] [PubMed]
- 106.Schwid S.R., Decker M.D., Lopez-Bresnahan M. Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. 2005. [DOI] [PubMed]
- 107.Comi G., Freedman M.S., Kappos L., Olsson T.P., Miller A.E., Wolinsky J.S., O’Connor P.W., Benamor M., Dukovic D., Truffinet P., Leist T.P. Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions. Multiple Sclerosis and Related Disorders. Elsevier. 2016;5(c):97–104. doi: 10.1016/j.msard.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 108.MedWatch The FDA Safety Information and Adverse Event Reporting Program. http: //www.fda.gov/Safety/ MedWatch/SafetyInformation/ucm423101.htm
- 109.Gerschenfeld G., Servy A., Valeyrie-Allanore L., de Prost N., Cecchini J. Fatal toxic epidermal necrolysis in a patient on teriflunomide treatment for relapsing multiple sclerosis. Mult. Scler. 2015;21(11):1476–1477. doi: 10.1177/1352458515596601. [DOI] [PubMed] [Google Scholar]
- 110.Warnatz K., Peter H., Schumacher M., Wiese L., Prasse A., Petschner F., Vaith P., Volk B., Weiner S.M. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann. Rheum. Dis. BMJ Group, 2003;62(1):50–57. doi: 10.1136/ard.62.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu E., Wang W.B., Guimond C., Synnes A., Sadovnick A.D., Dahlgren L., Traboulsee A., Tremlett H. Safety of disease-modifying drugs for multiple sclerosis in pregnancy: current challenges and future considerations for effective pharmacovigilance. Expert Rev. Neurother. 2013;3:251–261. doi: 10.1586/ern.13.12. [DOI] [PubMed] [Google Scholar]
- 112.Stüve O., Benamor M., Benzerdjeb H., Kieseier B.C. Outcomes from the Teriflunomide Clinical Development Program: Retrospective Analysis of a Global Pharmacovigilance Database(P06.190); Neurology; 2012. 78: Meeting Abstracts. 2015 Oct 19;1-1. [Google Scholar]
- 113.Kieseier B.C., Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol. Ther. 2014;3(2):133–138. doi: 10.1007/s40120-014-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McDonald R.J., McDonald J.S., Kallmes D.F., Jentoft M.E., Murray D.L., Thielen K.R., Williamson E.E., Eckel L.J. Intracranial gadolinium deposition after Contrast-enhanced MR Imaging. 2015 doi: 10.1148/radiol.15150025. https://doi.org/10 [DOI] [PubMed]
- 115.Kanda T., Fukusato T., Matsuda M., Toyoda K., Oba H., Kotoku J., Haruyama T., Kitajima K., Furui S. Gadoliniumbased contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology, 2015;276(1):228–232. doi: 10.1148/radiol.2015142690. [DOI] [PubMed] [Google Scholar]
- 116.Conway D., Cohen J.A. Combination therapy in multiple sclerosis. 2010 doi: 10.1016/S1474-4422(10)70007-7. https://doi.org/10 [DOI] [PubMed]
- 117.Milo R., Panitch H. Combination therapy in multiple sclerosis. J. Neuroimmunol. 2011;231(1-2):23–31. doi: 10.1016/j.jneuroim.2010.10.021. [DOI] [PubMed] [Google Scholar]