Abstract
Background:
Every year, strokes take millions of lives and leave millions of individuals living with permanent disabilities. Recently more researchers embrace the concept of the neurovascular unit (NVU), which encompasses neurons, endothelial cells (ECs), pericytes, astrocyte, microglia, and the extracellular matrix. It has been well-documented that NVU emerged as a new paradigm for the exploration of mechanisms and therapies in ischemic stroke. To better understand the complex NVU and broaden therapeutic targets, we must probe the roles of multiple cell types in ischemic stroke. The aims of this paper are to introduce the biological characteristics of brain pericytes and the available evidence on the diverse functions and mechanisms involving the pericytes in the context of ischemic stroke.
Methods:
Research and online content related to the biological characteristics and pathophysiological roles of pericytes is review. The new research direction on the Pericytes in ischemic stroke, and the potential therapeutic targets are provided.
Results:
During the different stages of ischemic stroke, pericytes play different roles: 1) On the hyperacute phase of stroke, pericytes constriction and death may be a cause of the no-reflow phenomenon in brain capillaries; 2) During the acute phase, pericytes detach from microvessels and participate in inflammatory-immunological response, resulting in the BBB damage and brain edema. Pericytes also provide benefit for neuroprotection by protecting endothelium, stabilizing BBB and releasing neurotrophins; 3) Similarly, during the later recovery phase of stroke, pericytes also contribute to angiogenesis, neurogenesis, and thereby promote neurological recovery.
Conclusion:
This emphasis on the NVU concept has shifted the focus of ischemic stroke research from neuro-centric views to the complex interactions within NVU. With this new perspective, pericytes that are centrally positioned in the NVU have been widely studied in ischemic stroke. More work is needed to elucidate the beneficial and detrimental roles of brain pericytes in ischemic stroke that may serve as a basis for potential therapeutic targets.
Keywords: Pericytes, ischemic stroke, BBB, NVU, mechanisms, therapeutic targets
1. INTRODUCTION
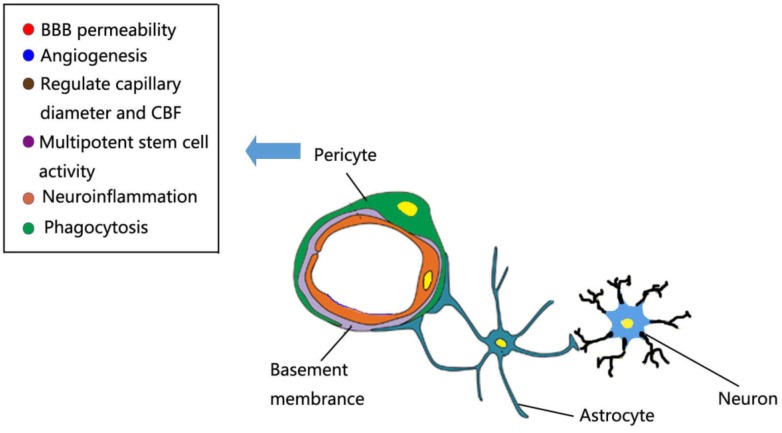
Every year, strokes take millions of lives and leave millions of individuals living with permanent disabilities [1, 2]. Seriously, it is a major public-health problem that puts a severe economic burden on the world [3, 4]. Ischemic stroke is the dominant subtype of stroke, characterized by the sudden cessation of cerebral blood flow (CBF) to an area of the brain, bringing about a homologous loss of neurologic function [5]. For decades, stroke researchers have devoted to interrogate the pathophysiology of ischemic stroke and have focused on how to salvage and protect agonal ischemic neurons. However, several promising approaches which have showed efficacy in experimental stroke models failed to be put into clinical practice [6, 7]. The translational failure of neuroprotective agents is largely attributed to the fact that stroke affects various cellular functions of the brain and disturbs multiple cellular signaling among different cell types, but most clinical trials using a single agent often aim at protecting neurons [6-8]. Therefore, recently more researchers embrace the concept of the neurovascular unit (NVU), which encompasses neurons, endothelial cells (ECs), pericytes, astrocyte, microglia, and the extracellular matrix [8, 9] (Fig. 1). It has been well-documented that NVU emerged as a new paradigm for the exploration of mechanisms and therapies in ischemic stroke. To better understand the complex NVU and broaden therapeutic targets, we must probe the roles of multiple cell types in ischemic stroke. In the present review, we go into more details concerning the perivascular cell in the NVU, which was called “pericytes”.
Fig. (1).
The multifunction role of brain pericytes at the neurovascular unit. Schematic representation showing a simplified NVU that encompasses neurons, endothelial cells (ECs), pericytes, astrocyte, microglia, and the basement membrane. Pericytes can regulate capillary diameter and CBF, BBB permeability, angiogenesis, phagocytosis, neuroinflammation and multipotent stem cell activity.
The perivascular cell was firstly described by Charles Rouget (1898) as “contractile elements” surrounding the ECs of small blood vessels. Furthermore, Zimmermann distinguished three subgroups of these cells and coined the term “pericytes” according to their morphology and location in 1923 [10, 11]. Over a century later, the biology and functions of pericytes in the CNS have been remarkably advanced via in vitro BBB models, brain slice preparations and pericytes-deficient transgenic mice [12]. It has been established that pericytes are not simply an important component of BBB, but also have a central position in the NVU among ECs, astrocyte and neurons. Pericytes receive, orchestrate and process signals from their neighboring cells to generate diverse neurovascular functions, including regulation of capillary hemodynamics, BBB permeability, clearance of toxic metabolites, angiogenesis and stem cell activity, all of which are necessary for normal brain homeostasis [12-14] (Fig. 1). Due to this versatile functionality, a growing number of studies focus on the roles of brain pericytes in ischemic stroke in recent years [15-18]. In this review article, we firstly introduce the biological characteristics of brain pericytes. Then we discuss in detail the available evidence on the diverse functions and mechanisms involving the pericytes in the context of ischemic stroke. In particular, we focus on four problem areas: (1) The current controversy about whether pericytes act as regulators of cerebral blood flow and culprit of no-reflow phenomenon; (2) The possible mechanisms of pericytes that affect BBB protection or damage; (3) The novel views that pericytes can involve in inflammation and immune response during stroke; (4) The signaling pathways that pericytes drive are angiogenesis and neurogenesis. Finally, we attempt to discuss epigenetic role of pericytes and explore the potential pericyte-related drug targets for future ischemic stroke therapies.
2. Biological characteristics of pericytes in the brain
2.1. Origin of Pericytes
Brain pericytes originate from several different progenitor cells. During early embryonic vascularization, origins of brain pericytes depend on the anatomical location of blood vessels. Quail-chick chimerization experiments have established that pericytes found in the forebrain are derived from neural crest cells, whereas those of mesodermal origin cover the vessels in the mid-brain, brainstem and spinal cord [19-21]. This idea was reinforced with recent observations that the neuroectoderm-derived progenitor cells give rise to pericytes in the anterior part of the brain, while the mesodermal origin of pericytes exists in the hindbrain vessels by utilizing live imaging and lineage tracing in zebrafish [22]. During later embryonic development and the early postnatal period, further increase of brain pericytes population rely on the proliferative expansion of pre-existing pericytes pools and this progress is also called “longitudinal recruitment” [12, 23]. In a rat model of stroke, circulating bone marrow-derived progenitor cells and subventricular zones (SVZ)-derived progenitor cells have been shown to contribute to adult brain pericyte populations [24-26]. Whereas critical insights have been obtained into the embryonic origin of brain pericytes, much less is known about how pericytes replenish and turnover in the adult and aging brain under physiological conditions.
2.2. Ultrastructure of Pericytes
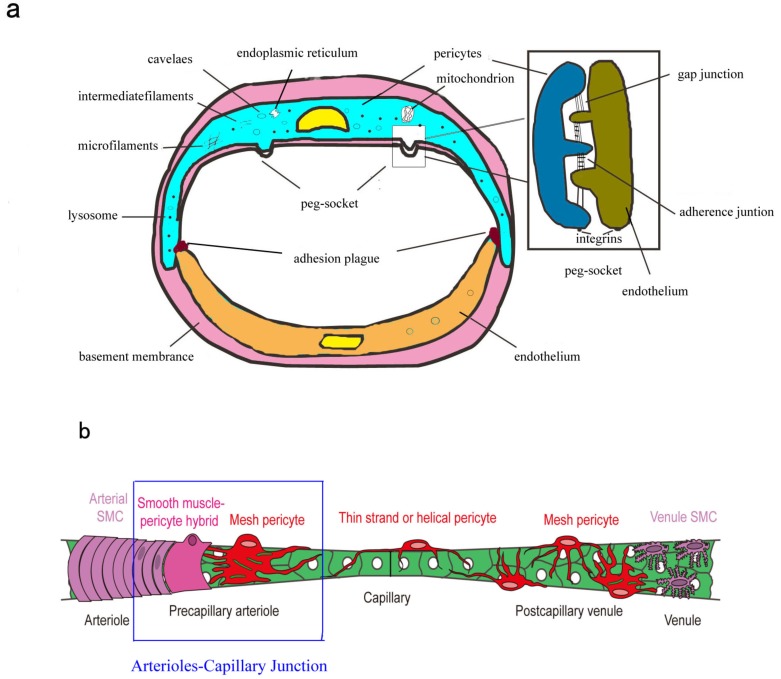
For decades, ultrastructure of brain pericytes has been well-established (Fig. 2a). Brain pericytes nucleus is relatively large, kidney-shaped and protrudes into tubal antrum [27]. The small amounts of perinuclear cytoplasm usually contain mitochondria, Golgi apparatus, endoplasmic reticulum, ribosomes and lysosomes [27]. Contractile microfilaments (containing actin, myosin and tropomyosin) and intermediate filaments are also observed in these cells [27, 28]. Moreover, numerous caveolae principally located in abluminal pericytes surface and single caveolae usually appear in the branches of pericytes (known as processes) [27]. Pericytes are embedded within the basement membrane (BM) surrounding ECs. In the areas lacking BM, pericytes and ECs form direct cell-to-cell contacts with each other by interdigitation, referred to as “peg-and-socket” contacts. These contacts include the connexin-43 hemichannels (CX-43) mediated gap junctions and N-cadherin based adherence junctions [29-31]. The other type of contacts, known as “adhesion plaques”, mediate the connection of the BM to the plasma membrane, and the underlying microfilament bundles of pericytes and ECs [27, 28]. The special connections of pericytes and ECs within the NVU are vital for proper CNS vascular homeostasis.
Fig. (2).
Brain pericytes anatomy. (a) Ultrastructural of brain pericytes. Brain pericytes nucleus is relatively large, kidney-shaped and protrudes into tubal antrum. The small amounts of perinuclear cytoplasm usually contain mitochondria, endoplasmic reticulum and lysosomes. Contractile microfilaments (containing actin, myosin and tropomyosin) and intermediate filaments are also observed in these cells. Numerous caveolae principally locate in abluminal pericytes surface and single caveolae usually appear in the processes of pericytes. In the areas lacking BM, pericytes and ECs form direct cell-to-cell contacts with each other by interdigitation, referred to as “peg-and-socket” contacts. These contacts include the connexin-43 hemichannels (CX-43) mediated gap junctions and N-cadherin based adherence junctions. The other type of contacts is “adhesion plaques”. (b) Topology and morphology of brain pericytes. Schematic showing the continuum of mural cell types along the cerebral microvessels. Brain arterioles wrapped by a continuous of vascular smooth muscle cells (vSMCs) further ramify and transit into smaller precapillary arterioles. The mural cells located in the point of transition show a mixed phenotype of vSMCs and pericytes, termed as “smooth muscle-pericytes hybrids”. Another type of pericytes at the precapillary arterioles, referred to as “mesh pericytes”, exhibit more interlocking mesh progresses. Pericytes in capillary beds have protruding cell bodies with thin strand or helical primary processes that run parallel to the long axis of mid-capillary tube. Mesh pericytes with many slender, branching processes become more prevalent on postcapillary venules. The boexs represent “arterioles-capillary junction”. (Reproduced from Hartmann et al. [32] with permission).
2.3. Topology and Morphology of Pericytes
Anatomically, brain pericytes are located directly on small blood vessels including capillaries, pre-capillary arterioles and post-capillary venules [12] (Fig. 2b). It has long been recognized that structure, morphology and distribution of pericytes vary along the arterial-venous axis, which has been identified by electron microscopy and recently by live confocal microscopy [27, 28, 32]. The topology and morphology of pericytes in the microvascular bed can be divided as follows:
2.3.1. Precapillary Pericytes
Brain arterioles wrapped by a continuous vascular smooth muscle cells (vSMCs) further ramify and transit into smaller precapillary arterioles. The mural cells located in the point of transition show a mixed phenotype of vSMCs and pericytes, as previously confirmed in ultrastructural studies, termed as “smooth muscle-pericytes hybrids” [32]. These cells are more elongated than vSMCs, encircled the entire vessel lumen, and also pose protruding ovoid soma similar to those of pericytes. Another type of pericytes at the precapillary arterioles, referred to as “mesh pericytes”, exhibit more interlocking mesh progress.
2.3.2. Capillary Pericytes
The capillary pericytes have protruding cell bodies with thin strands or helical primary processes that run parallel to the long axis of the mid-capillary tube. Small secondary processes extend perpendicularly from the primary processes.
2.3.3. Post-capillary Pericytes
Mesh pericytes with many slender, branching processes become more prevalent on post-capillary venules.
2.4. Identification of Pericytes
Several recent reviews have provided lists of reliable markers for pericytes [10, 11, 33]. But until now, the marker, completely stable and specific identification forebrain pericytes is lacking. This is due to several reasons: Firstly, these immuno-chemical markers are also expressed in other brain cells. For example, desmin, neuron-glial 2 (NG2) and α-smooth muscle actin (α-SMA) are mainly expressed by both pericytes and vSMCs [34-36], thus the identification of these two cell types must consider the vascular localization. Secondly, all of the markers presently used are dynamically expressed in pericytes, which may be up or down-regulated in different developmental stages, pathological processes, and even different culture conditions in vitro [10]. The last reason may be explained by the numerous lineages from which pericytes are derived and that pericytes themselves are multi-potent cells [37]. More recently, transgenic mice that base on several practicable markers have been developed, which provide great tools to better explore the fate and roles of brain pericytes [32]. In general, the relatively accurate identification of brain pericytes rely on a combination of at least two markers as well as the consideration of the topology, morphology and some specific circumstances. Table 1 provides lists of presently reliable and often-used brain pericytes markers as well as transgenic markers for murine studies.
Table 1.
Cell marker for brain pericytes.
| Pericytes Markers | Other Cells Stained in the Brain | Comments | Refs. |
|---|---|---|---|
| Cytosolic markers | |||
| α-SMA (alpha-smooth muscle actin) |
vSMCs | Contractile and cytosketetal proteins; expression in brain pericytes along the micro-vessels is controversial. | [36, 38] |
| RGS5 (regulator of G protein signaling 5) |
vSMCs | GTPase activating protein; involved inproliferation and recruitment of pericytes during angiogenesis. | [164] |
| Desmin | vSMCs | Cytosketetal proteins. | [35] |
| Membrane bound markers | |||
| PDGFR-β(plateletderived growth factor receptor-beta) |
Neurons; neuronal progenitors | A tyrosine kinase receptor involved in important function of pericytes; most frequently used and best characterized. | [165] |
| NG2 (neuron-glial 2) | vSMCs; neuronal progenitors, oligodendrocyte progenitors | Integral membrane chondroitin sulfate proteoglycan; broadly expressed in activated pericytes during development and in the adult under both physiological and pathological conditions. | [34] |
| CD13 (aminopeptidasesN) | vSMCs; endothelium | Type II membrane zinc-dependent metalloprotease. | [166] |
| Kir6.1 (potassium inwardly Rectifyingchannel) | vSMCs | ATP-sensitive potassium-channel; implicated in ion transport and intercellular signaling; highly expressed in brain pericytes. | [167] |
| Transgenic markers | |||
| NG2-DsRed | vSMCs; oligodendrocyte progenitors | BAC-transgene, reposited in the Jackson Laboratory, stock #008241. | [168] |
| RGS5-GFP NG2-creER-eGFP |
vSMC vSMCs |
GFP+ pericytes co-expressed PDGFR-β and CD13, but not α-SMA. Reposited in the Jackson Laboratory, stock #008533. |
[85] [48] |
| NG2/PDGFRβ-Cre-tdTomato | vSMCs | NG2-tdTomato line is inducible, whereas PDGFRβ-tdTomato line expressed tdTomato constitutively. | [32] |
3. Roles of pericytes in cerebral blood flow regulation during stroke
3.1. Pericytes are Contractile Cells
As mentioned above, contractile capabilities have been attributed to pericytes ever since the first descriptions by Rouget in the 1870s. Firstly, pericytes in the brain express actin and myosin filaments which exist as smooth muscle and non-smooth muscle isoforms [36, 38]. Other contraction-related proteins such as tropomyosin and desmin have also been detected in pericytes [39]. Secondly, a variety of vasoactive molecules, neurotransmitters and extracellular ions activate the respective receptors and ion channels expressed in pericytes, raising the intracellular calcium concentration and resulting in constriction and dilation of cultured brain pericytes [40, 41]. Finally, electrophysiological and pharmacological studies have showed that brain pericytes are electrically excitable cells, thus the interaction between Ca2+ signals and membrane potentials concertedly modulates the constriction of pericytes [42, 43]. But unfortunately, for the past decade, all of the above studies were indirect evidence that pericytes probably mediate the constriction of capillaries.
3.2. Pericytes as Regulators of Cerebral Blood Flow
What is the physiological relevance of brain pericytes contractility? To maintain neuronal function, the brain has invoked “neurovascular coupling” mechanisms to increase local blood flow to regions in which neurons are active, this process is termed functional hyperemia [44, 45]. It is widely accepted that VSMCs present at penetrating arterioles and/or pre-capillary arterioles regulate CBF by changing their tone in response to synaptic transmission and vasoactive substances [40]. As imaging techniques have evolved, this view has been challenged by the evidence that pericytes may regulate CBF at the capillary level. Bell et al. showed pericytes loss leads to diminishing in vivo CBF in response to functional hyperemia in pericytes-deficient mice [46]. Peppiatt et al. demonstrated that pericytes could constrict capillaries diameter in situ in cerebellar slices and retina preparations [41]. However, there is no blood flow, sheer stress and perfusion pressure in the blood vessels of brain slices, thus pericytes-mediated constriction of capillaries ex vivo cannot deduce that pericytes also participate in functional hyperemia.
More precisely, Fernandez-Klett and colleagues measured the capillary diameter by in vivo live imaging after application of a thromboxane A2 analog (U46619) inbicuculline-induced seizure model (a pathologic model of functional hyperemia), and in the cortical spreading depression model (CSD) [47]. Nevertheless, they failed to find capillaries dilatory response to functional hyperemia. On the contrary, Hall et al. described that capillaries dilate approximately 1 second before penetrating arterioles, which, may contribute up to 80% of the increase in blood flow evoked by neuronal activity, using electrical whisker pad stimulation, a more physiologic model of functional hyperemia [15]. Of note, Hall et al. did not propose explicitly which vessel segment of capillary could dilate in response to functional hyperemia. There may be two reasons for this contradictory conclusion: Firstly, both studies have a similar experimental set-up to observe brain capillaries in response to neuronal activity, but the functional hyperemia model was different; Secondly, brain pericytes in different vessel segments may have diverse functions in hemodynamic regulation.
Paradoxically, Hill et al. against the prevailing dogma that pericytes are involved in the regulation of cerebral blood flow, and put forward those arteriolar SMCs may be the key players in this basic process [48]. This may be because they regarded all contractile pericytes in the precapillary arterioles as SMCs, which leads to the opposite conclusion that “pericytes” cannot regulate vessel diameter. Therefore, the argument whether “these cells” in the precapillary arterioles is pericytes or SMCs due to the vague definition of “precapillary arterioles”. For a detailed discussion, the reader is referred to recent review articles [49]. In view of the above discussion, we can define the vague segments from pre-capillary arterioles to adjacent capillaries as “arterioles-capillary junction” (Fig. 2b). Pericytes residing at this junction appeared to a mixed phenotype, suggesting it possesses both characteristics of pericytes and SMCs [32]. More importantly, Hill et al. and Hall et al. found the cells at arterioles-capillary junction could dilate capillary, which is consistent with previous works [15, 48]. In general, these studies supported the idea that pericytes of arterioles-capillary junction may be the gatekeepers to control blood flow into the capillary beds.
3.3. Pericytes Contribute to Cerebral No-reflow Phenomenon
In the clinical settings, recanalization with thrombolysis is the best conceivable treatment for acute ischemic stroke but the narrow therapeutic window limits its efficacy [1]. In addition, there is also another obstacle to restrict recanalization therapies. After reopening of the occluded blood vessel, the microcirculatory flow fail to complete restoration, which is the so-called “no-reflow phenomenon” [50, 51]. Electron microscopic examination of the brain micro-vasculature in ischemic regions revealed capillary damage in the form of swollen ECs and astrocyte end-feet, intravascular platelets and fibrin, and leukocyte plugging [51, 52]. Experimental date of past decades and recent clinical evidence suggested these damage changes together compress the capillaries, resulting in the persistence of microcirculatory obstructions.
Although controversial, Yemisci et al. recently revealed that pericytes which controlled capillary diameter also has an unexpected role in ischemic stroke [16]. Pericytes remain contracted during ischemia despite successfully reopening of the main feeding artery, which may be a major cause of the no-reflow phenomenon by limiting oxygen/nutrients supply to the affected brain regions. Persistent pericytes contraction could be reversed by suppressing peroxynitrite formation and oxidative-nitrative stress, contributing to restoration of microcirculatory patency and the survival of ischemic tissue [16, 53]. More recently, Hall et al. demonstrated that rat brain ischemia indeed induces persistent pericytes contraction and subsequent pericytes death, which is consistent with a previous report shown that capillary pericytes rapidly die after MCAO in mice [15]. Interestingly, Hall et al. suggested that pericytes death in rigor mortis (the stiffness of death) lead to the long-lasting reduction of capillary blood flow. Moreover, the death may be evoked by excitotoxicity, but is not reduced by suppressing oxidative-nitrative stress [15, 54]. However, ischemia-induced coverage of pericytes in the vascular endothelium increases in young adult mice at 3 and 72 h, but without any change in aged mice, as shown by ultrastructural analysis of BBB in the peri-infarct zone [55]. It is suggested that pericytes in the aged brain may be more susceptible to ischemia. Above studies raise several questions that warrant further exploration: Does only some subtype of pericytes die during brain ischemia? Why are pericytes in the aged brain susceptible to ischemia-induced death?
Multiple signaling pathways may result in pericytes constriction and death after stroke. Brain pericytes constriction during ischemia is characterized by rising intracellular calcium concentration [15, 16, 42]. Brain ischemia leads to a lack of ATP molecules, which not only inhibit ion pumping but also hinders the separation between myosin and actin [15, 41]. Oxygen and nitrogen radicals formed in the microvasculature during ischemia/reperfusion also cause the persistent pericytes constriction that could be reversed by suppressing oxidative-nitrative stress [16]. Pericytes death may be partly evoked by excitotoxicity, because blocking ionotropic glutamate receptor or removing external Ca2+ significantly could reduce pericytes death [15]. Therefore, pharmacological agents that can prevent pericytes dysfunction or death may bring the thrombolytic and neuroprotective treatments to light.
4. Roles of pericytes in blood brain barrier function and stroke
4.1. Pericytes Contribute to BBB Formation and Maintenance
Studies over the past two decades have revealed that there is a pivotal role for pericytes in the formation and maintenance of the BBB. Early in embryogenesis, pericytes are recruited to the nascent vessels, induce formation of a functional BBB [56]. Furthermore, pericytes contribute to the BBB integrity by regulating tight junctions formation and transendothelial vesicle trafficking [56]. In addition, pericytes inhibit CNS immune cell infiltration and the expression of molecules that increase vascular permeability during the BBB development [56]. Corresponding studies in adult pericyte-deficient pdgfrβ+/- mice revealed that pericytes are vital to maintain the BBB properties, as the loss of brain pericytes could disrupt ECs tight junctions and finally result in increased non-specific paracellular transport [57]. Other works using young pericyte-deficient pdgfrβ+/- mice has also demonstrated, somewhat unexpectedly, that vessel permeability could be increased by enhanced tansendothelial vesicular transport (transcytosis) [46]. Age-dependent pericytes loss in pdgfrβ+/- mice leads to impair CNS function progressively through leakage and accumulation of several potentially neurotoxic and vasculotoxic substances [46], which may amplify neuronal functional and structural damage changes related to ischemic stroke and neurodegeneration disease.
4.2. Pericytes Contribute to BBB Protection in Stroke
Destruction of the BBB after ischemic stroke is an intractable event that conduces to further progress and enlargement of the injury. BBB dysfunction and damage not only promotes the evolution of neuroinflammation and brain edema, but also increases the risk of intracerebral hemorrhage of thrombolytic therapies [58]. This may limit the application of recombinant tissue plasminogen activator (rtPA) and results in a poor prognosis. Thus, BBB protection must remain an urgent priority for purpose of braking the downstream progression of brain parenchymal injury. As described above, brain pericytes contribute to the formation and maintenance of the BBB in developing an adult brain. Not surprisingly, pericytes also contribute to ECs survival and BBB protection after stroke [59]. In the ECs/pericytes co-culture model exposed to hypoxic condition, pericytes enhance tighter barrier function and protect endothelial monolayers from hypoxic injury [59]. During severe and prolonged oxygen deprivation, co-culture of ECs with pericytes is more effective than with astrocyte in preserving barrier function by maintaining TJs localization and protecting ECs survival [60]. Studies using the ultrastructural analysis of BBB in the peri-infarct zone found process area and vessel coverage of pericytes increased at 72 h after ischemia, suggesting a compensatory mechanism to limit BBB breakdown [55]. Similarly, pericytes in retinal micro- vascular can strengthen TJs of the blood-retinal barrier (BRB) by reversing occluding decrease under hypoxia [61]. In general, despite limited information, brain pericytes have appeared to protect ECs and stabilize BBB under hypoxic injury and ischemic stroke. Thus, BBB protection mechanisms of pericytes after ischemic insults remain poorly understood and warrants deeper exploration.
4.3. Pericytes Contribute to BBB Disruption in Stroke
Previous researches show that disassembly and reassembly of TJ protein complexes (TJs) are the major reasons for BBB breakdown after stroke [5]. However, recent works have demonstrated BBB disruption in response to ischemia is a complex, biphasic temporal profile by utilizing ultrastructural analysis, advanced imaging and transgenic animal models [62]. This profile contained early BBB hyperpermeability associated with upregulation of endothelial transcytosis (within 4-6 h after stroke), and followed by a delayed opening of the BBB mediating by dissolution of TJs (within 2-3 days after stroke) [56, 62]. In addition, pericytes have been shown to detach from brain microvessels within 2h after focal or global cerebral ischemia [63, 64]. This detachment indicates that ECs-pericytes signaling interactions and intercellular contacts are also disrupted, resulting in BBB breakdown. Pericytes promote BBB formation and maintenance by releasing inhibitory signals to reduce the number of caveolae and rate of endothelial transcytosis under physiology condition [46, 56]. This inhibitory signal may be removed after ischemic stroke, inducing increased caveolae and transcytosis in ECs [63, 65, 66]. In addition, the early detachment of pericytes also results in the loss of TJs contributing to BBB leakage through the paracellular pathway. Moreover, pericytes-derived MMPs, including MMP 2, 3 and 9, lead to secondary dissolution of TJs structure and function [67, 68]. Therefore, the detachment of pericytes and pericytes-derived factors promotes BBB disruption through both transcellular and paracellular pathways and contributes to stroke progression.
5. Pericytes involving in immune and inflammation response in ischemic stroke
Recent in vitro studies utilizing human and mouse brain pericytes have suggested that pericytes are implicated in immune and inflammation response at the NVU under physiological and pathophysiological conditions. Brain pericytes express receptors of damage-associated molecular patterns (DAMPs) such as toll-like receptor 4 (TLR4), recognizing and responding to micro-environmental cues [69, 70]. Soon after the onset of ischemic stroke, the injured brain tissues release various DAMPs, including high-mobility group box1 (HMGB1), adenosine triphosphate (ATP) and heat shock protein (HSP), initiating non-infectious immune responses [71-73]. Interestingly, HMGB1 binds to TLR4 on the human brain pericytes in vitro and induces the secretion of the proinflammatory cytokines and chemokines, including interleukin-6 (IL-6), IL-8, CXCL1, CXCL2, CXCL3, and CCL2 [69]. HSP also binds to TLR4 and stimulates the production of proinflammatory cytokines IL-6 in cultured pericytes [74]. These indicated brain pericytes may be a sensor in response to tissue damage, thereby involving in inflammatory response in ischemic stroke.
In addition, in vitro studies have also suggested multiple inflammatory conditions could induce human brain pericytes express proinflammatory cytokines, chemokines, or adhesion molecules. For example, tumor necrosis factor-alpha (TNF-α) and transforming growth factor beta 1 (TGFβ1) enhanced the expression of classical pro-inflammatory cytokines IL-6 in human brain pericytes [75, 76]. Tumor necrosis factor-alpha (TNF-α) and IL-1β stimulated brain pericytes to secrete chemokines IL-8 [77]. In response to interferon-gamma (IFN-γ), brain pericytes upregulated the expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [78]. Therefore, stroke-derived inflammatory cytokines also induce pericytes to release proinflammatory cytokines, chemokines and other inflammatory mediators, which could impair cells of the NVU and disrupt the BBB. However, it is also found that TGFβ1 attenuated the IL-1β-induced expression of CX3CL1, MCP-1 and VCAM-1 in pericytes and attenuated the phagocytic ability of pericytes by downregulating the expression of scavenger receptors CD36, CD47 and CD68 [76]. A single study reported that activation of CCAAT/ enhancer binding protein delta (C/EBPδ) in pericytes could downregulate IL-1β-induced production of ICAM-1 and monocyte chemoattractant protein-1 (MCP-1) [79]. Therefore, brain pericytes may adopt pro- or anti-inflammatory phenotype, according to inflammatory conditions during stroke, which requires further exploration. Overall, these works suggest that pericytes may be effectors as well as amplifiers of brain inflammatory processes during ischemic stroke.
Under CNS homeostasis conditions, pericytes express several macrophage makers, including Fc receptors (FcR), CD11b, ED2 and scavenger receptors [80, 81]. Pericytes can take up small and soluble molecules from the blood or brain parenchyma through interstitial fluid [82, 83]. Moreover, pericytes are capable of transporting substances by pinocytosis, phagocytosis and receptor-mediated endocytosis [83]. Thus, brain pericytes may be considered as another “resident macrophage” in the brain apart from microglia, involved in the first line of immunologic defense of the brain. Mounting evidence shows that pericytes have similar traits and overlapping markers with mesenchymal stem cells (MSCs) in vitro studies, suggesting brain pericytes have multipotent potential [10]. Unexpectedly, it has been reported that pericytes could be activated, proliferated and could migrate into the adjacent ischemic brain parenchyma, where they express microglia markers and acquire microglial phenotypes [84-86]. Therefore, pericytes may transform into microglia to phagocyte cell debris and involve in inflammation, immune during stroke. On the other hand, studies using transgenic pericytes-deficient mice have shown that leukocyte trafficking was enhanced in microvascular regions lacking pericytes coverage [56, 87, 88]. Thus, it is suggested that the detachment of pericytes may contribute leukocyte infiltration in ischemic stroke. Further studies are warranted to identify the more inflammatory role of pericytes in brain ischemia and provide an important new target for the development of future stroke anti-inflammatory therapies.
6. Roles of pericytes in neurorestoration after stroke
6.1. Pericytes and Angiogenesis after Stroke
Angiogenesis refers to the sprouting of new blood vessels from pre-existing ones and is often induced by hypoxia in physiological (e.g., development) and pathological (e.g., ischemic stroke) situations [89, 90]. Formation of new blood vessels would help to increase CBF and replenish oxygen/ nutrients supply to the affected brain tissue, thereby salvages agonal ischemic neurons and contributes to improve recovery of tissue-at-risk. The increased vascular density which is induced by angiogenesis in the peri-infract area was found at 3 days after the ischemic stroke in human and animal brain samples [91, 92]. Indeed, stroke patients with greater cerebral vascular density appear to have better recovery and longer survival than those with lower vascular density [93]. Angiogenesis in the brain is a step-wise process, which can be tightly regulated by a dynamic balance between pro-angiogenic and anti-angiogenic factors. Therefore, we must advance our basic understanding of the dynamic changes of post-stroke angiogenesis and develop practicable proangiogenic therapies.
The vascular angiogenesis remodeling after stroke includes three phases: (1) initiation of angiogenesis; (2) sprout formation, migration and stabilization; (3) maturation or termination [94, 95]. Pericytes may be involved in all of these three stages, which have recently become a growing interest research field and emerged as potential targets for post-stroke pro-angiogenic therapies. During the angiogenesis, the bidirectional ECs-pericytes signaling is crucial [12, 95]. Firstly, upon exposure to pro-angiogenic stimuli, pericytes express multiple MMPs (such as MMP-2, 3 and 9) to degrade the BM components, leading to detachment of pericytes and subsequent liberation of ECs [89, 96, 97]. In addition, increasing of vascular permeability leads to extravasation of plasma proteins, providing a provisional scaffold for vascular sprouting [98]. Then, pericytes secrete vascular endothelial growth factor-A (VEGF-A), which stimulates ECs to become motile and protrude filopodia, forming the tip cells [99, 100]. Following tip cells, stalk cells proliferate to support sprout elongation and form a vessel tube [101]. In this process, pericytes attach to ECs and regulate the deposition of extracellular matrix as well as the formation of inter-endothelial TJs, contributing to nascent lumen stabilization. At the same time, pericytes also secrete survival signals to protect ECs and maintain integrity of the immature vessel. Finally, tip cells anastomose with neighboring sprouts to build microvessels loops [102]. After this, pericytes convert to inhibit ECs proliferation and re-establish ECs quiescence in order to terminate angiogenesis. In addition, pericytes also secrete metalloproteinase 3 (TIMP3) to prevent possible proteolysis of matrix proteins of new formed vessels thereby promoting vessel maturation [103]. Here we conclude the major pericytes-associated signaling pathways driving angiogenesis after stroke.
6.1.1. VEGF-A/VEGFR2 Pathway
Pericytes (paracrine manner) and ECs (autocrine manner) secrete VEGF-A, the main component of the VEGF family, which activates the VEGF receptor-2 (VEGFR2) in ECs to trigger multiple downstream signals (such as PI3K/Akt and MEK/ERK pathways) that promote angiogenesis [104, 105]. VEGF-A/VEGFR2 pathway up-regulates DLL4 expression in tip cells which subsequently activates Notch in stalk cell. Activated Notch could down-regulate VEGFR-2 expression in stalk cell to induce a stable stalk cells identity [106]. During hypoxia, brain pericytes began to express VEGF within 24h, which was earlier than astrocyte [107]. Moreover, expression of VEGF and VEGFR-2 in microvessels within the peri-infarct region increased obviously 3h and 48h after stroke respectively, which is co-localized with neovas- cularization and continues up to 7 days [108]. Indeed, studies using laser scanning imaging technique to measure local cerebral blood flow, have demonstrated that VEGF/ VEGFR2-mediated signaling plays an important role in promoting post-ischemia neurovascular remodeling [109].
6.1.2. PDGF-BB/PDGFRβ Pathway
The platelet-derived growth factor B (PDGF-B) and its receptor (PDGFRβ) are critical for recruitment of brain pericytes into newly formed vessels during angiogenesis [110, 111]. In particular, PDGFRβ is mainly expressed on pericytes in the brain [111, 112]. ECs secrete PDGF-B as a disulfide-linked homodimer (PDGF-BB) [113, 114], which is confined in the extracellular matrix (ECM) or on the surface of the ECs, leading to localized retention of PDGF-BB that forms a concentration gradient [115]. The steep gradient is required for proper proliferation, migration and attachment of PDGFRβ-expressed pericytes in angiogenic context [56, 116, 117]. Inactivation of PDGF-BB/PDGFRβ signaling results in pericytes deficiency, microvascular reduction and micro-aneurysm formation [56, 111]. Analysis of human brain samples showed that PDGF-B and its receptor were expressed on microvessels following stroke [118]. PDGFRβ expression was significantly increased in microvessels, mainly on pericytes, in the infracted area 48h after MCAO [119].
6.1.3. TGF-β/TGFRβ2 Pathway
TGF-β is a bidirectional cytokine between ECs and pericytes which regulates cellular differentiation, attachment and maturation [30, 110, 120]. TGF-β is expressed in ECs and pericytes in a latent preform that can be activated by thrombospondin, proteases and integrins [110]. Once activated, TGF-β binds to its receptor TGFRβ2, which leads to recruitment and phosphorylation of activin-like kinase 1 (Alk1) or Alk5 [121]. Of note, Alk5 is expressed both on pericytes and ECs, but Alk1 only in ECs [122]. In pericytes and ECs, TGFRβ2/Alk5 initiates mothers against decapentaplegic homolog 2 (Smad2)/3/4 signaling that inhibits proliferation and promotes differentiation [121, 122]. Alk5-Smad2/3/4 pathway also enhances proper pericytes attachment to ECs via upregulation of the adhesion molecule N-cadherin and ECM proteins [123]. In ECs, activation of Alk1 induces Smad1/5/8 signaling that promotes ECs proliferation and migration [124, 125]. Accordingly, it is hypothesized that Alk1 singling may dominate in the early phase during angiogenesis, leading to cell proliferation and migration, whereas Alk5 singling may dominate later, resulting in cell differentiation and ECM proteins production, thereby promoting vessel maturation. TGF-β was significantly increased in microvessels in the ischemic areas, as shown by aser capture microdissection [126]. Upregulation of TGF-β mRNA has also been reported in the ischemic areas following stroke in human brain samples [127].
6.1.4. Notch Pathway
It has been well-documented that Notch signaling plays important role in angiogenesis [128]. In mammals, there are four Notch receptors (Notch1-4) and five ligands (DLL1, 3, and 4 and Jagged1-2), all of which are transmembrane proteins. Pericytes mainly express Notch 3, while ECs express Notch 1 and 4 receptors [14]. The ligand binds to its relevant receptors activating the proteolytic cleavages of receptors within the membrane and subsequently releasing the Notch intracellular domain (NICD). NICD translocates to the nucleus and binds to several transcription factors such as recombination signal binding protein Jκ (RBP-Jκ), resulting in downstream transcription of Notch-related genes [128]. As previously mentioned, VEGF and Notch signaling cooperate in an intercellular feedback manner to affect tip and stalk cells [89]. Recent studies have demonstrated that Notch signaling also play important roles in pericytes attachment and survival. For instance, the inhibition of Notch disrupts vessel stability and lead to pericytes detachment from the already formed vessel lumen [129]. Studies using Smad4 mutant mice have revealed that there is a mechanistic link between Notch and TGF-β/Smad4, which contributes to pericytes attachment to ECs via upregulating N-cadherin [30]. On the other hand, ECs induce the differentiation of mural cells (pericytes and vSMCs) by upregulating Notch protein [130]. In addition, Notch 3 has also been implicated in survival of mural cells [131, 132].
6.1.5. Ang-Tie2 Pathway
During angiogenesis, the angiopoietin (Ang)/Tie system is involved in blood vessel sprouting, maturation and maintenance. The Ang/Tie family in human contains three ligands (Ang-1, 2, 4) and two receptors (Tie 1, 2) [110]. Pericytes-derived Ang-1 binds to Tie2 on ECs to stimulate pericytes coverage and BCM proteins deposition, thus decreasing vascular permeability [10]. In addition, Ang-1/Tie2 forms a paracrine loop that promotes the survival of ECs and maintains ECs quiescence [103, 133]. Conversely, Ang-2 is expressed by angiogenic tip cell (autocrine manner) and acts as antagonistic ligands for Tie2. Ang-2/Tie2 activates the PI3K/Akt pathway to loosen the contacts between ECs and pericytes, resulting in vessel destabilization and pericytes detachment [103]. Furthermore, Ang-2 also binds to endothelial integrins to induce ECs migration and sprouting [134]. Both Ang-1 and Ang-2 was described to be increased up to even 28 days and their receptors also upregulated in capillaries in peri-infarct region following an ischemic injury [135, 136]. Thus, regulation of Ang1, 2/Tie2 signaling is vital to the maintenance and survival of nascent capillary tubes after stroke.
6.2. Pericytes and Neurogenesis after Stroke
Neurogenesis refers to the process of generating new neurons from neural stem cells (NSCs) and neural progenitor cells (NPCs) mainly residing in the subventricular zone (SVZ) and the subgranular zone (SGZ) [137, 138]. Neuro- genesis in the brain occurs throughout life, which is stimulated by physiological factors and pathological situations, such as ischemic stroke [139]. Mounting works in rodents have revealed that brain ischemia induced the proliferation of endogenous NSCs and these newly generated neurons could migrate into an ischemic brain and express mature neuron phenotype [140-143]. More importantly, analysis of human brain biopsies from stroke patients has demonstrated that there are newborn neurons in the ischemic penumbra [144, 145]. Stroke-induced compensatory neuro- genesis may replace the necrotic neurons and reconstruct the neural circuitry, suggesting it could contribute to post-stroke recovery and represent a target for stroke therapy.
Recent evidence support the views that stroke-induced neurogenesis and angiogenesis is highly coordinated and coupled [146]. For example, several angiogenic factors like VEGF, FGF and signaling pathways such as Notch, Ang-2 signaling are also crucial in ischemia-induced neurogenesis in the adult brain [139, 147]. In addition, newly formed microvessels are used as migratory conduits for neuroblasts to ischemic boundary zone, and provide neurotrophic support to newborn neurons [143, 148]. Thus, pericytes-related angiogenesis is involved in the formation of the vascular niche, which is required for neurogenesis after stroke. During hypoxia, pericytes secrete nerve growth factor (NGF) that contributes to neuronal survival [149]. In addition, pericytes also produce neurotrophin (NT)-3 that induce astrocyte to produce NGF under hypoxic conditions [149]. As previous mentioned, brain pericytes have multipotent potential [12]. Previous report also shows that brain pericytes may be an origin of NSCs [150]. Recently, a study reported that pericytes extracted from mouse brains and human brain acquired multipotential stem cell activity and differentiated into neural and vascular lineage cells following ischemic stroke [84]. In addition, pericytes can regulate inflammation that is also seen as a key factor influencing neurogenesis after ischemic injury [151]. Thus, brain pericytes with neurovasculogenic potential contribute to both angiogenesis and neurogenesis, which can be targeted to repair the injured CNS after stroke.
7. Epigenetic role of pericytes in ischemic stroke
MicroRNAs (miRNAs) are small non-coding RNAs of ~21 nucleotides, which act as negative regulators of target gene expression by binding to the 3’-untranslated regions (UTR) [152, 153]. They regulate diverse biological functions and processes, including growth, proliferation, differentiation, lineage determination, metabolism and apoptosis [152, 153]. Several recent reviews have revealed the role of miRNAs in stroke risk factors such as hypertension, atherosclerosis and atrial fibrillation [154-156]. The role of miRNAs in the component cells of NVU following ischemic stroke has been the subject of more recent investigations. For example, brain pericytes change the expression of miRNAs under hypoxic stress, which contain the upregulation of 27 miRNAs and the downregulation of 31 miRNAs [157]. In addition, pericytes expression of Let-7 miRNA is involved in pericytes differentiation in response to hypoxia [158]. Increased levels of MiR-145 in pericytes target the Friend leukemia virus integration 1 (Fil1) to block pericytes migration in response to a stable gradient of PDGF-BB using a microfluidic chemotaxis chamber [159]. Under conditions mimicking hyperglycemia and ischemia, pericytes could uptake ECs-derived miR-503, which reduce coverage of capillaries and increase capillary permeability [160]. On the other hand, stroke also significantly alters cerebral long non-coding RNAs (lncRNA) expression profiles [161]. Recent studies have demonstrated that lncRNA-myocardial infarction-associated transcript (MIAT) or lncRNA-metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) breakdown could ameliorate pericytes loss in diabetes mellitus–induced microvascular dysfunction [162, 163]. With miRNA and lncRNA research in pericytes still in its infancy, studies are required in this area to explore epigenetic regulation that provides a promising target in stroke therapeutics.
Conclusion and perspectives
This emphasis on the NVU concept has shifted the focus of ischemic stroke research from neuro-centric views to the complex interactions within NVU. With this new perspective, pericytes that are centrally positioned in the NVU have been widely studied in ischemic stroke. After a stroke, the persistent constriction and death of pericytes could contribute to no-reflow phenomenon during the hyperacute phase, and the inflammatory action and detachment of pericytes may aggravate the BBB damage during the acute phase. However, on the other hand, pericytes also provide beneficial roles in neuroprotection by protecting ECs, stabilizing BBB and releasing neurotrophins. Pericytes have potent neurorestorative effects after stroke by conducing to angiogenesis and neurogenesis. Therefore, the beneficial and detrimental effects of pericytes in brain injury and repair designate pericytes as a promising therapeutic target for ischemic stroke.
One of the effective approaches is to reveal how pericytes communicate with other NVU components (especially the neuron) as well as how they regulate each other’s functions, which could help to develop alternative approaches to protect the BBB and neuron during ischemic stroke. For example, discovering more ECs-derived or astrocytes-derived vasoactive substances that contract pericytes could boost to understand contractile mechanisms and inspire therapeutic strategy to restore incomplete microcirculatory reperfusion by reversing the persistent pericytes constriction. In addition, balancing the bidirectional effects (including anti-/pro-inflammatory effects of pericytes, anti-/pro-angiogenesis modulators released by pericytes) is very critical for any therapeutic strategies targeting at post-stroke repair. Recent research has highlighted the success in the effort to transplant MSCs into ischemic areas. And likewise, pericytes or pericytes progenitor cell transplantation may also promote tissue survival or NVU reconstruction. Therefore, future studies are warranted to evaluate the curative effect of pericytes or pericytes progenitor cell transplantation into ischemic brain areas. Last but not the least, we need to consider the age-related structural and functional changes of pericytes as important factors that promote the vulnerability to ischemic injury. However, according to this versatile functionality, a key question is whether pericytes are a heterogeneous cell population. Additionally, it is also confused how each pericytes subtype (e.g. contractile pericytes contributing to no-reflow phenomenon versus those determining BBB protection or damage) play its roles in ischemic stroke. Thus, we need to develop specific markers in different vessel segments and genetic models combined with RNA-seq and proteomic analyses, which will greatly help to promote the studies about the roles of pericyte subtypes in ischemic stroke.
ACKNOWLEDGEMENTS
This study is supported partially by the National Natural Science Foundation of China (31571039 to Ling-Qiang Zhu, 81571119 to Bo Hu and 81671147 to Huijuan Jin), Top-Notch Young Talents Program of China of 2014, Program of Outstanding Youth of Hubei Province, China (2014CFA017 to Ling-Qiang Zhu), Academic Frontier Youth Team of Huazhong University of Science and Technology to Ling-Qiang Zhu, National Key Research and Development Program of China (2016YFC1300600) and New Century Excellent Talents in University (NCET-10-0406) to Bo Hu.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Fisher M., Saver J.L. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 2015;14:758–767. doi: 10.1016/S1474-4422(15)00054-X. [https://doi.org/10. 1016/S1474-4422(15)00054-X]. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M., Demchuk A.M., Hill M.D. Endovascular therapy for ischemic stroke. N. Engl. J. Med. 2015;372:2366. doi: 10.1056/NEJMc1504715. [https://doi. org/10.1056/NEJMoa1414905]. [DOI] [PubMed] [Google Scholar]
- 3.Kassebaum N.J., Bernabe E., Dahiya M., Bhandari B. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J. Dent. Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [https:// doi.org/10.1177/0022034514552491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbyn Z. Statistics: a growing global burden. Nature. 2014;510:S2–S3. doi: 10.1038/510S2a. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz M.A., Lo E.H., Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rother J. Neuroprotection does not work! Stroke. 2008;39:523–524. doi: 10.1161/STROKEAHA.107.494799. [DOI] [PubMed] [Google Scholar]
- 7.Hussain M.S., Shuaib A. Research into neuroprotection must continue. But with a different approach. Stroke. 2008;39:521–522. doi: 10.1161/STROKEAHA.107.494781. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Zhang Z.G., Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol. Sci. 2012;33:415–422. doi: 10.1016/j.tips.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muoio V., Persson P.B., Sendeski M.M. The neurovascular unit - concept review. Acta Physiol. (Oxf.) 2014;210:790–798. doi: 10.1111/apha.12250. [https://doi.org/10.1111/apha.12250]. [DOI] [PubMed] [Google Scholar]
- 10.Armulik A., Genove G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Krueger M., Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58:1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 12.Winkler E.A., Bell R.D., Zlokovic B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–S15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney M.D., Ayyadurai S., Zlokovic B.V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall C.N., Reynell C., Gesslein B., Hamilton N.B. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yemisci M., Gursoy-Ozdemir Y., Vural A., Can A. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 17.Kamouchi M., Ago T., Kuroda J., Kitazono T. The possible roles of brain pericytes in brain ischemia and stroke. Cell. Mol. Neurobiol. 2012;32:159–165. doi: 10.1007/s10571-011-9747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalkara T., Gursoy-Ozdemir Y., Yemisci M. Brain micro- vascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [https://doi.org/10.1007/s00401-011-0847-6]. [DOI] [PubMed] [Google Scholar]
- 19.Etchevers H.C., Vincent C., Le Douarin N.M., Couly G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 20.Korn J., Christ B., Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J. Comp. Neurol. 2002;442:78–88. doi: 10.1002/cne.1423. [https://doi.org/10.1002/cne.1423]. [DOI] [PubMed] [Google Scholar]
- 21.Kurz H. Cell lineages and early patterns of embryonic CNS vascularization. Cell Adhes. Migr. 2009;3:205–210. doi: 10.4161/cam.3.2.7855. [https://doi. org/10.4161/cam.3.2.7855]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando K., Fukuhara S., Izumi N., Nakajima H. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development. 2016;143:1328–1339. doi: 10.1242/dev.132654. [https://doi.org/ 10.1242/dev.132654]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenzel D., Nye E., Nisancioglu M., Adams R.H. Peripheral mural cell recruitment requires cell-autonomous heparan sulfate. Blood. 2009;114:915–924. doi: 10.1182/blood-2008-10-186239. [https://doi.org/10.1182/blood-2008-10-186239]. [DOI] [PubMed] [Google Scholar]
- 24.Piquer-Gil M., Garcia-Verdugo J.M., Zipancic I., Sanchez M.J. Cell fusion contributes to pericyte formation after stroke. J. Cereb. Blood Flow Metab. 2009;29:480–485. doi: 10.1038/jcbfm.2008.150. [https://doi.org/10.1038/ jcbfm.2008.150]. [DOI] [PubMed] [Google Scholar]
- 25.Kokovay E., Li L., Cunningham L.A. Angiogenic recruitment of pericytes from bone marrow after stroke. J. Cereb. Blood Flow Metab. 2006;26:545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- 26.Sharma V., Ling T.W., Rewell S.S., Hare D.L. A novel population of alpha-smooth muscle actin-positive cells activated in a rat model of stroke: an analysis of the spatio-temporal distribution in response to ischemia. J. Cereb. Blood Flow Metab. 2012;32:2055–2065. doi: 10.1038/jcbfm.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz-Flores L., Gutierrez R., Madrid J.F., Varela H. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 28.Dore-Duffy P., Cleary K. Morphology and properties of pericytes. Methods Mol. Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt H., Wolburg H., Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Li F., Lan Y., Wang Y., Wang J. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev. Cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Bobbie M.W., Roy S., Trudeau K., Munger S.J. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest. Ophthalmol. Vis. Sci. 2010;51:3758–3763. doi: 10.1167/iovs.09-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartmann D.A., Underly R.G., Grant R.I., Watson A.N. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics. 2015;2:041402. doi: 10.1117/1.NPh.2.4.041402. [https://doi.org/10.1117/1.NPh.2.4.041402]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trost A., Lange S., Schroedl F., Bruckner D. Brain and retinal pericytes: Origin, function and role. Front. Cell. Neurosci. 2016;10:20. doi: 10.3389/fncel.2016.00020. [https://doi.org/10.3389/fncel.2016.00020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang F.J., You W.K., Bonaldo P., Seyfried T.N. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev. Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [https://doi. org/10.1016/j.ydbio.2010.06.023]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nehls V., Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993;99:1–12. doi: 10.1007/BF00268014. [https://doi.org/10.1007/BF00268014]. [DOI] [PubMed] [Google Scholar]
- 36.Bandopadhyay R., Orte C., Lawrenson J.G., Reid A.R. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J. Neurocytol. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- 37.da Silva Meirelles L., Bellagamba B.C., Camassola M., Nardi N.B. Mesenchymal stem cells and their relationship to pericytes. Front. Biosci. (Landmark Ed.) 2016;21:130–156. doi: 10.2741/4380. [https://doi.org/ 10.2741/4380]. [DOI] [PubMed] [Google Scholar]
- 38.Boado R.J., Pardridge W.M. Differential expression of alpha-actin mRNA and immunoreactive protein in brain microvascular pericytes and smooth muscle cells. J. Neurosci. Res. 1994;39:430–435. doi: 10.1002/jnr.490390410. [DOI] [PubMed] [Google Scholar]
- 39.Nehls V., Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J. Cell Biol. 1991;113:147–154. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attwell D., Buchan A.M., Charpak S., Lauritzen M. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peppiatt C.M., Howarth C., Mobbs P., Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamouchi M., Kitazono T., Ago T., Wakisaka M. Calcium influx pathways in rat CNS pericytes. Brain Res. Mol. Brain Res. 2004;126:114–120. doi: 10.1016/j.molbrainres.2004.03.008. [https://doi.org/10.1016/j.molbrainres.2004.03. 008]. [DOI] [PubMed] [Google Scholar]
- 43.Burdyga T., Borysova L. Calcium signalling in pericytes. J. Vasc. Res. 2014;51:190–199. doi: 10.1159/000362687. [https://doi.org/10.1159/000362687]. [DOI] [PubMed] [Google Scholar]
- 44.Lacoste B., Comin C.H., Ben-Zvi A., Kaeser P.S. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83:1117–1130. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girouard H., Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 1985;2006(100):328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 46.Bell R.D., Winkler E.A., Sagare A.P., Singh I. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Klett F., Offenhauser N., Dirnagl U., Priller J. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl. Acad. Sci. USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill R.A., Tong L., Yuan P., Murikinati S. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attwell D., Mishra A., Hall C.N., O’Farrell F.M. What is a pericyte? J. Cereb. Blood Flow Metab. 2016;36:451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalkara T., Arsava E.M. Can restoring incomplete micro-circulatory reperfusion improve stroke outcome after thrombolysis? J. Cereb. Blood Flow Metab. 2012;32:2091–2099. doi: 10.1038/jcbfm.2012.139. [https://doi. org/10.1038/jcbfm.2012.139]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rezkalla S.H., Kloner R.A. No-reflow phenomenon. Circulation. 2002;105:656–662. doi: 10.1161/hc0502.102867. [https://doi.org/10.1161/hc0502.102867]. [DOI] [PubMed] [Google Scholar]
- 52.Paljarvi L., Rehncrona S., Soderfeldt B., Olsson Y. Brain lactic acidosis and ischemic cell damage: quantitative ultrastructural changes in capillaries of rat cerebral cortex. Acta Neuropathol. 1983;60:232–240. doi: 10.1007/BF00691871. [https://doi.org/10.1007/BF00691871]. [DOI] [PubMed] [Google Scholar]
- 53.Vates G.E., Takano T., Zlokovic B., Nedergaard M. Pericyte constriction after stroke: the jury is still out. Nat. Med. 2010;16:959. doi: 10.1038/nm0910-959. [https://doi.org/10.1038/nm0910-959]. [DOI] [PubMed] [Google Scholar]
- 54.Greif D.M., Eichmann A. Vascular biology: Brain vessels squeezed to death. Nature. 2014;508:50–51. doi: 10.1038/nature13217. [DOI] [PubMed] [Google Scholar]
- 55.Nahirney P.C., Reeson P., Brown C.E. Ultrastructural analysis of blood-brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J. Cereb. Blood Flow Metab. 2016;36:413–425. doi: 10.1177/0271678X15608396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkler E.A., Sengillo J.D., Bell R.D., Wang J. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cereb. Blood Flow Metab. 2012;32:1841–1852. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y., Leak R.K., Keep R.F., Chen J. Translational stroke research on blood-brain barrier damage: Challenges, perspectives, and goals. Transl. Stroke Res. 2016;7:89–92. doi: 10.1007/s12975-016-0447-9. [https://doi.org/10.1007/ s12975-016-0447-9]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi K., Nakao S., Nakaoke R., Nakagawa S. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul. Pept. 2004;123:77–83. doi: 10.1016/j.regpep.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 60.Al Ahmad A., Gassmann M., Ogunshola O.O. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J. Cell. Physiol. 2009;218:612–622. doi: 10.1002/jcp.21638. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y.L., Hui Y.N., Guo B., Ma J.X. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye (Lond.) 2007;21:1501–1510. doi: 10.1038/sj.eye.6702716. [DOI] [PubMed] [Google Scholar]
- 62.Knowland D., Arac A., Sekiguchi K.J., Hsu M. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonul E., Duz B., Kahraman S., Kayali H. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc. Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 64.Duz B., Oztas E., Erginay T., Erdogan E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology. 2007;55:279–284. doi: 10.1016/j.cryobiol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 65.Dore-Duffy P., Owen C., Balabanov R., Murphy S. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc. Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 66.Cipolla M.J., Crete R., Vitullo L., Rix R.D. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front. Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- 67.Takata F., Dohgu S., Matsumoto J., Takahashi H. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J. Neuroinflammation. 2011;8:106. doi: 10.1186/1742-2094-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Machida T., Takata F., Matsumoto J., Takenoshita H. Brain pericytes are the most thrombin-sensitive matrix metalloproteinase-9-releasing cell type constituting the blood-brain barrier in vitro. Neurosci. Lett. 2015;599:109–114. doi: 10.1016/j.neulet.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 69.Guijarro-Munoz I., Compte M., Alvarez-Cienfuegos A. ; Alvarez-Vallina L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-kappaB signaling pathway and pro-inflammatory response in human pericytes. J. Biol. Chem. 2014;289:2457–2468. doi: 10.1074/jbc.M113.521161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edelman D.A., Jiang Y., Tyburski J.G., Wilson R.F. Lipopolysaccharide activation of pericyte’s Toll-like receptor-4 regulates co-culture permeability. Am. J. Surg. 2007;193:730–735. doi: 10.1016/j.amjsurg.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 71.Hayakawa K., Pham L.D., Arai K., Lo E.H. High-mobility group box 1: an amplifier of stem and progenitor cell activity after stroke. Acta Neurochir. Suppl. (Wien) 2013;118:31–38. doi: 10.1007/978-3-7091-1434-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.An C., Shi Y., Li P., Hu X. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog. Neurobiol. 2014;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shichita T., Ito M., Yoshimura A. Post-ischemic inflammation regulates neural damage and protection. Front. Cell. Neurosci. 2014;8:319. doi: 10.3389/fncel.2014.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilhelmus M.M., Boelens W.C., Kox M., Maat-Schieman M.L. Small heat shock proteins associated with cerebral amyloid angiopathy of hereditary cerebral hemorrhage with amyloidosis (Dutch type) induce interleukin-6 secretion. Neurobiol. Aging. 2009;30:229–240. doi: 10.1016/j.neurobiolaging.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Matsumoto J., Takata F., Machida T., Takahashi H. Tumor necrosis factor-alpha-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation. Neurosci. Lett. 2014;578:133–138. doi: 10.1016/j.neulet.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 76.Rustenhoven J., Aalderink M., Scotter E.L., Oldfield R.L. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J. Neuroinflammation. 2016;13:37. doi: 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pieper C., Pieloch P., Galla H.J. Pericytes support neutrophil transmigration via interleukin-8 across a porcine co-culture model of the blood-brain barrier. Brain Res. 2013;1524:1–11. doi: 10.1016/j.brainres.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 78.Balabanov R., Beaumont T., Dore-Duffy P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J. Neurosci. Res. 1999;55:578–587. doi: 10.1002/(SICI)1097-4547(19990301)55:5<578::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 79.Rustenhoven J., Scotter E.L., Jansson D., Kho D.T. An anti-inflammatory role for C/EBPdelta in human brain pericytes. Sci. Rep. 2015;5:12132. doi: 10.1038/srep12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balabanov R., Washington R., Wagnerova J., Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc. Res. 1996;52:127–142. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- 81.Mato M., Ookawara S., Sakamoto A., Aikawa E. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc. Natl. Acad. Sci. USA. 1996;93:3269–3274. doi: 10.1073/pnas.93.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bechmann I., Priller J., Kovac A., Bontert M. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur. J. Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 83.Thomas W.E. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res. Brain Res. Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 84.Nakagomi T., Kubo S., Nakano-Doi A., Sakuma R. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33:1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- 85.Ozen I., Deierborg T., Miharada K., Padel T. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128:381–396. doi: 10.1007/s00401-014-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakuma R., Kawahara M., Nakano-Doi A., Takahashi A. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J. Neuroinflammation. 2016;13:57. doi: 10.1186/s12974-016-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stark K., Eckart A., Haidari S., Tirniceriu A. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 88.Nourshargh S., Hordijk P.L., Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 89.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 90.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 91.Hayashi T., Noshita N., Sugawara T., Chan P.H. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J. Cereb. Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 92.Krupinski J., Kaluza J., Kumar P., Kumar S. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 93.Krupinski J., Kaluza J., Kumar P., Kumar S. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol. Pol. 1993;44:203–209. [PubMed] [Google Scholar]
- 94.Greenberg D.A. Cerebral angiogenesis: a realistic therapy for ischemic disease? Methods Mol. Biol. 2014;1135:21–24. doi: 10.1007/978-1-4939-0320-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stapor P.C., Sweat R.S., Dashti D.C., Betancourt A.M. Pericyte dynamics during angiogenesis: new insights from new identities. J. Vasc. Res. 2014;51:163–174. doi: 10.1159/000362276. [https://doi.org/10.1159/000362276]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Virgintino D., Girolamo F., Errede M., Capobianco C. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [https://doi.org/10.1007/s10456-006-9061-x]. [DOI] [PubMed] [Google Scholar]
- 97.Candelario-Jalil E., Yang Y., Rosenberg G.A. Diverse roles of matrix metalloproteinases and tissue inhibitors of metallo-proteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carmeliet P., Collen D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. N. Y. Acad. Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. [DOI] [PubMed] [Google Scholar]
- 99.Franco M., Roswall P., Cortez E., Hanahan D. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [https://doi.org/10.1182/blood-2011-01-331694]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jakobsson L., Franco C.A., Bentley K., Collins R.T. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [https://doi.org/10. 1038/ncb2103]. [DOI] [PubMed] [Google Scholar]
- 101.Eilken H.M., Adams R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [https://doi.org/10.1016/j.ceb.2010.08.010]. [DOI] [PubMed] [Google Scholar]
- 102.Jain R.K. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [https://doi.org/10.1038/nm0603-685]. [DOI] [PubMed] [Google Scholar]
- 103.Saharinen P., Eklund L., Miettinen J., Wirkkala R. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [https://doi.org/10.1038/ncb1715]. [DOI] [PubMed] [Google Scholar]
- 104.Beck H., Plate K.H. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [https://doi.org/10.1007/s00401-009-0483-6]. [DOI] [PubMed] [Google Scholar]
- 105.Greenberg D.A., Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [https://doi.org/10.1038/nature04481]. [DOI] [PubMed] [Google Scholar]
- 106.Phng L.K., Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev. Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [https://doi.org/10.1016/j. devcel.2009.01.015]. [DOI] [PubMed] [Google Scholar]
- 107.Dore-Duffy P., Wang X., Mehedi A., Kreipke C.W. Differential expression of capillary VEGF isoforms following traumatic brain injury. Neurol. Res. 2007;29:395–403. doi: 10.1179/016164107X204729. [DOI] [PubMed] [Google Scholar]
- 108.Marti H.J., Bernaudin M., Bellail A., Schoch H. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am. J. Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li W.L., Fraser J.L., Yu S.P., Zhu J. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp. Brain Res. 2011;214:503–513. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- 110.Gaengel K., Genove G., Armulik A., Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 111.Lindahl P., Johansson B.R., Leveen P., Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 112.Winkler E.A., Bell R.D., Zlokovic B.V. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol. Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bjarnegard M., Enge M., Norlin J., Gustafsdottir S. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 114.Enge M., Bjarnegard M., Gerhardt H., Gustafsson E. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Andrae J., Gallini R., Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Armulik A., Genove G., Mae M., Nisancioglu M.H. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 117.Lindblom P., Gerhardt H., Liebner S., Abramsson A. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krupinski J., Issa R., Bujny T., Slevin M. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- 119.Renner O., Tsimpas A., Kostin S., Valable S. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain Res. Mol. Brain Res. 2003;113:44–51. doi: 10.1016/s0169-328x(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 120.Walshe T.E., Saint-Geniez M., Maharaj A.S., Sekiyama E. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009;4:e5149. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sieczkiewicz G.J., Herman I.M. TGF-beta 1 signaling controls retinal pericyte contractile protein expression. Microvasc. Res. 2003;66:190–196. doi: 10.1016/s0026-2862(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 122.Van Geest R.J., Klaassen I., Vogels I.M., Van Noorden C.J. Differential TGF-{beta} signaling in retinal vascular cells: a role in diabetic retinopathy? Invest. Ophthalmol. Vis. Sci. 2010;51:1857–1865. doi: 10.1167/iovs.09-4181. [DOI] [PubMed] [Google Scholar]
- 123.Winkler E.A., Bell R.D., Zlokovic B.V. Lack of Smad or Notch leads to a fatal game of brain pericyte hopscotch. Dev. Cell. 2011;20:279–280. doi: 10.1016/j.devcel.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 124.Lebrin F., Deckers M., Bertolino P., Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc. Res. 2005;65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 125.Goumans M.J., Valdimarsdottir G., Itoh S., Rosendahl A. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haqqani A.S., Nesic M., Preston E., Baumann E. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. FASEB J. 2005;19:1809–1821. doi: 10.1096/fj.05-3793com. [DOI] [PubMed] [Google Scholar]
- 127.Krupinski J., Kumar P., Kumar S., Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- 128.Kume T. Ligand-dependent Notch signaling in vascular formation. Adv. Exp. Med. Biol. 2012;727:210–222. doi: 10.1007/978-1-4614-0899-4_16. [DOI] [PubMed] [Google Scholar]
- 129.Dimova I., Hlushchuk R., Makanya A., Styp-Rekowska B. Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis. 2013;16:921–937. doi: 10.1007/s10456-013-9366-5. [DOI] [PubMed] [Google Scholar]
- 130.Liu H., Kennard S., Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ. Res. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu H., Zhang W., Kennard S., Caldwell R.B. Notch3 is critical for proper angiogenesis and mural cell investment. Circ. Res. 2010;107:860–870. doi: 10.1161/CIRCRESAHA.110.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walshe T.E., Connell P., Cryan L., Ferguson G. Microvascular retinal endothelial and pericyte cell apoptosis in vitro: role of hedgehog and Notch signaling. Invest. Ophthalmol. Vis. Sci. 2011;52:4472–4483. doi: 10.1167/iovs.10-7061. [DOI] [PubMed] [Google Scholar]
- 133.Koh G.Y. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol. Med. 2013;19:31–39. doi: 10.1016/j.molmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 134.Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 135.Lin T.N., Wang C.K., Cheung W.M., Hsu C.Y. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2000;20:387–395. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Z., Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc. Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- 137.Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Emsley J.G., Mitchell B.D., Kempermann G., Macklis J.D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog. Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 139.Ruan L., Lau B.W., Wang J., Huang L. Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Prog. Neurobiol. 2014;115:116–137. doi: 10.1016/j.pneurobio.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 140.Jin K., Minami M., Lan J.Q., Mao X.O. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin K., Sun Y., Xie L., Peel A. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol. Cell. Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 142.Arvidsson A., Collin T., Kirik D., Kokaia Z. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 143.Yamashita T., Ninomiya M., Hernandez A.P., Garcia-Verdugo J.M. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jin K., Wang X., Xie L., Mao X.O. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakayama D., Matsuyama T., Ishibashi-Ueda H., Nakagomi T. Injury-induced neural stem/progenitor cells in post-stroke human cerebral cortex. Eur. J. Neurosci. 2010;31:90–98. doi: 10.1111/j.1460-9568.2009.07043.x. [DOI] [PubMed] [Google Scholar]
- 146.Ruan L., Wang B., ZhuGe Q., Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015;1623:166–173. doi: 10.1016/j.brainres.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu X.S., Zhang Z.G., Zhang R.L., Gregg S. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J. Cereb. Blood Flow Metab. 2007;27:564–574. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- 148.Palmer T.D., Willhoite A.R., Gage F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 149.Ishitsuka K., Ago T., Arimura K., Nakamura K. Neurotrophin production in brain pericytes during hypoxia: a role of pericytes for neuroprotection. Microvasc. Res. 2012;83:352–359. doi: 10.1016/j.mvr.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 150.Dore-Duffy P., Katychev A., Wang X., Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. 2006 doi: 10.1038/sj.jcbfm.9600272. https://doi.org/10 [DOI] [PubMed]
- 151.Wang B., Jin K. Current perspectives on the link between neuroinflammation and neurogenesis. Metab. Brain Dis. 2015;30:355–365. doi: 10.1007/s11011-014-9523-6. [https://doi.org/10.1007/s11011-014-9523-6]. [DOI] [PubMed] [Google Scholar]
- 152.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 153.Jonas S., Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [https://doi.org/10.1038/nrg3965]. [DOI] [PubMed] [Google Scholar]
- 154.Koutsis G., Siasos G., Spengos K. The emerging role of microRNA in stroke. Curr. Top. Med. Chem. 2013;13:1573–1588. doi: 10.2174/15680266113139990106. [https://doi.org/10.2174/15680266113139990106]. [DOI] [PubMed] [Google Scholar]
- 155.Ouyang Y.B., Stary C.M., Yang G.Y., Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr. Drug Targets. 2013;14:90–101. doi: 10.2174/138945013804806424. [https://doi.org/10.2174/138945013804806424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rink C., Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol. Genomics. 2011;43:521–528. doi: 10.1152/physiolgenomics.00158.2010. [https://doi.org/ 10.1152/physiolgenomics.00158.2010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Truettner J.S., Katyshev V., Esen-Bilgin N., Dietrich W.D. Hypoxia alters MicroRNA expression in rat cortical pericytes. MicroRNA. 2013;2:32–44. doi: 10.2174/2211536611302010005. [https://doi.org/10.2174/221153661130 2010005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Esen N., Vejalla A., Sharma R., Treuttner J.S. Hypoxia-Induced Let-7d Has a role in pericyte differentiation. Adv. Exp. Med. Biol. 2016;923:37–42. doi: 10.1007/978-3-319-38810-6_5. [DOI] [PubMed] [Google Scholar]
- 159.Larsson E., Fredlund Fuchs P., Heldin J., Barkefors I. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009;1:108. doi: 10.1186/gm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Caporali A., Meloni M., Nailor A., Mitic T. p75(NTR)-dependent activation of NF-kappaB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat. Commun. 2015;6:8024. doi: 10.1038/ncomms9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dharap A., Nakka V.P., Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43:2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu J.Y., Yao J., Li X.M., Song Y.C. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yan B., Yao J., Liu J.Y., Li X.M. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 164.Bondjers C., Kalen M., Hellstrom M., Scheidl S.J. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am. J. Pathol. 2003;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hutter-Schmid B., Humpel C. Platelet-derived Growth factor receptor-beta is differentially regulated in primary mouse pericytes and brain slices. Curr. Neurovasc. Res. 2016;13:127–134. doi: 10.2174/1567202613666160219120411. [DOI] [PubMed] [Google Scholar]
- 166.Kunz J., Krause D., Kremer M., Dermietzel R. The 140-kDa protein of blood-brain barrier-associated pericytes is identical to aminopeptidase N. J. Neurochem. 1994;62:2375–2386. doi: 10.1046/j.1471-4159.1994.62062375.x. [DOI] [PubMed] [Google Scholar]
- 167.Bondjers C., He L., Takemoto M., Norlin J. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J. 2006;20:1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 168.Zhu X., Bergles D.E., Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]