Abstract
Background:
Growing body of evidence suggests that the pathogenesis of Alzheimer’s disease (AD), a progressing neurodegenerative condition, is not limited to the neuronal compartment, but also involves various immunological mechanisms. Insoluble Aβ aggregates in the brain can induce the activation of microglia, resulting in the synthesis of proinflammatory mediators, which further can stimulate astrocytic expression of YKL-40. Therefore, the aim of the current review is to present up-to-date data about the role of YKL-40 as a biomarker of AD as well as the possibility of therapeutic strategies targeting neuroinflammation.
Objective/Methods:
We searched PubMed articles for the terms “YKL-40”, “neurodegeneration”, “neuroinflammation” and “Alzheimer’s disease”, and included papers focusing on this review’s scope.
Results:
Recent studies indicate that CSF concentrations of YKL-40 were significantly higher in AD patients than in cognitively normal individuals and correlated with dementia biomarkers, such as tau proteins and amyloid beta. Determination of YKL-40 CSF concentration may be also helpful in differentiation between types of dementia and in the distinction of patients in the stable phase of MCI from those who progressed to dementia. Moreover, significantly increased levels of YKL-40 mRNA were found in AD brains in comparison with non-demented controls. Additionally, it was suggested that anti-inflammatory treatment might relief the symptoms of AD and slow its progression.
Conclusion:
Based on the recent knowledge, YKL-40 might be useful as a possible biomarker in the diagnosis and prognosis of AD. Modulation of risk factors and targeting of immune mechanisms, including systemic inflammation could lead to future preventive or therapeutic strategies for AD.
Keywords: YKL-40, Alzheimer’s disease, neurodegeneration, biomarkers, neuroinflammation, dementia
1. Introduction
1.2. Alzheimer’s Disease (AD)
Alzheimer’s disease (AD) is a devastating, continuous neurodegenerative disorder leading to neuronal loss and dysfunction of these cells. AD is the most frequent cause of dementia which constitutes 60%-70% of all dementia cases [1-3]. According to the World Alzheimer Report 2015, currently AD affects over 46 million people worldwide, which makes it one of the main health-care problem nowadays and the sixth-leading cause of death in the United States [4]. This number is expected to nearly double in the next 20 years, reaching almost 75 million in 2030 and over 130 million in 2050 [5]. AD is histopathologically characterized by the accumulation of intracellular neurofibrillary tangles (NFTs) consisted of many forms of phosphorylated Tau proteins (including pTau181) or truncated tau, localized mainly in neurons [6] and extracellular amyloid plaques consisted of amyloid beta (Aβ) throughout the brain [2]. It is estimated that all of these changes may start even 10-20 years before the onset of cognitive decline [7]. The development of pathological changes in the brains of AD patients could be examined convincingly only by the post-mortem identification or autopsy [8].
As reported by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR), the National Institute of Neurological Disorders and Stroke–Alzheimer Disease and Related Disorders (NINCDS–ADRDA) working group [9] and the 2011 recommendations from National Institute on Aging and the Alzheimer’s Association (NIA-AA), AD is perceived as a disease continuum [10] and is considered to include three basic phases: preclinical (asymptomatic), mild cognitive impairment (MCI) and dementia due to AD [10-12]. Researchers assessed that progression rate from MCI to AD is about 10-20% yearly [13]. The main risk factors of AD are increasing age, low level of education [14] and vascular factors including smoking, obesity and diabetes [15]. Genetic changes also appear to have a significant impact on the risk of developing AD, with special attention to the presence of apolipoprotein E (APOE) ε4 genotype. In comparison to subjects without ε4 allele, the elevated risk for AD tends to be almost three-fold higher in people with one ε4 allele and twelve-fold higher in those who inherited two ε4 allele [16]. Moreover, the development of AD seems to occur in ε4 form carriers at younger age than in those with the presence of ε2 or ε3 allele of the APOE gene [17].
2. Neuroimmflamation in AD
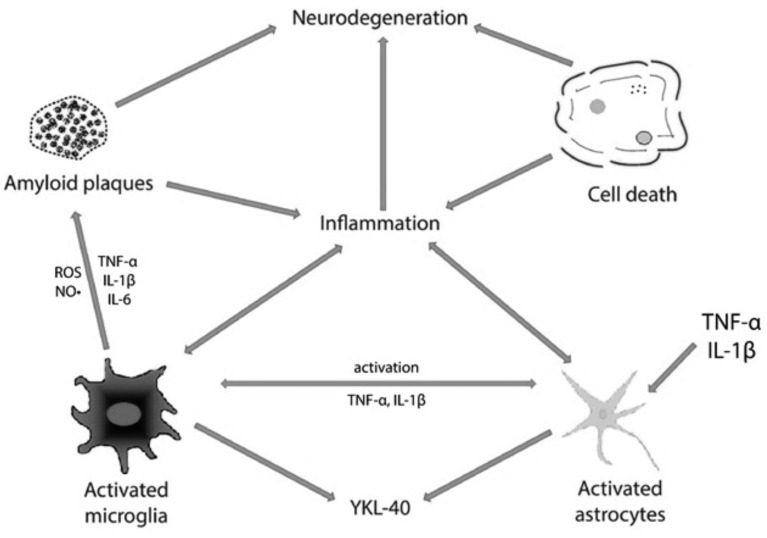
A growing body of evidence obtained from recent studies has proposed that the pathological process of AD is not only limited to the neuronal tissue, but also combines with immunological reactions in the brain [18] (Fig. 1). It is already known that brains of AD patients and other neurodegenerative diseases (NDs) are characterized by chronic inflammation [19, 20]. In AD brains, neuronal death and dysfunction together with the presence of insoluble Aβ deposits and NFTs, can trigger the process of inflammation [19], which stays in close connection with AD pathology and cognitive impairment [18]. Furthermore, the pathological aggregates of insoluble Aβ are recognized as foreign material and may cause the activation of the inflammatory reactions [21].
Fig. (1).
The role of YKL-40 in AD-associated neuroinflammation.
Cell mediators of inflammation in the AD brains are microglia and astrocytes, which are involved primarily in the inflammatory mechanisms ongoing in the brain [22]. Microglial cells are intensively activated cells [22], which gather during a chemotactic reaction within the senile plaques in the neocortex of AD patients. This phenomenon can be observed already in the early phase of the disease [23-25]. When activated by Aβ, these cells become the origin of inflammatory mediators, such as components of the complement system, inflammatory interleukins IL-1β and IL-6, tumor necrosis factor (TNFα), chemokines, macrophage inflammatory protein 1 (MIP-1), membrane-bound channel-activating serine protease 1 (mCAP-1), as well as free radicals [24, 26]. Moreover, in patients with AD, there is a significant cell loss in the locus coeruleus, a brain region responsible for the production of noradrenaline, which is also the endogenous anti-inflammatory agent [27]. In mice, this neurotransmitter stimulates microglial cells to suppress the production of cytokines induced by Aβ and to Aβ phagocytosis [27]. Various CSF inflammatory biomarkers [28], including soluble CD14, monocyte chemoattractant protein 1 (MCP-1), matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [29] have been associated with microglial activation in neurological diseases.
It was shown that astrocytes act as immune sensing cells in the brain [30], and may also be implicated in the response to infection, injury and inflammation [31]. Astrocytes support neural transmission and improve the removal of nonessential synapses by trimming useless connections with the help of microglia [32]. Additionally, they are involved in the response to Aβ peptides and localize within close proximity to senile plaques [33]. Activated astrocytes may also release a variety of pro-inflammatory compounds, including interleukins, complement components, thromboxanes, coagulation factors, prostaglandins, leukotrienes, proteases and protease inhibitors [34-36]. Moreover, it has been demonstrated that neurons can also produce complement components, C-reactive protein (CRP), prostaglandins and cytokines (IL-1, IL-6, TNF-α) [19, 25].
The important role of neuroinflammation is supported by findings that genes for immune receptors, such as TREM2, a gene for triggering receptor expressed on myeloid cells 2 protein (TREM2), or CD33, encoding a transmembrane receptor CD33, expressed on cells of myeloid lineage, are associated with AD. The inflammatory response mediated by monocytes/macrophages can be stimulated through a variety of receptors, including TREM2 receptor. It was shown that homozygotic mutation in TREM2 gene is associated with significantly increased risk of AD with early onset [37]. Furthermore, mutant rs3865444C risk allele of CD33 was associated with altered monocyte function and amyloid biology. CD33 locus is one of the nine whole-genome loci associated with AD susceptibility. This implicates the immune system in AD predisposition. Moreover, the presence of this mutation was associated with increased cell surface expression of CD33 in the monocytes, increased numbers of activated human microglia and decreased internalization of Aβ1-42 peptide, accumulation of both neuritic amyloid plaques and fibrillar amyloid on in vivo imaging [38, 39].
Systemic inflammation is also believed as a factor influencing neuroinflammatory processes of the brain and then promoting AD progression [40]. Animal studies shown that the local inflammatory response to central and systemic endotoxins leads to increased neuronal death during chronic neurodegeneration [41]. The results of clinical studies of Alzheimer’s disease revealed that cognitive decline was enhanced by acute and chronic systemic inflammatory diseases [42, 43]. Although priming of microglia is likely to
result from peripheral immune reaction, it might also be the response to chronic cerebrovascular dysregulation and microinfarcts within brain [44]. Moreover, these reactions are exaggerated in the ageing brain.
3. Biomarkers of Alzheimer’s disease
Various hypotheses trying to explain the etiology of AD also highlight many biochemical indicators of the disease. It is believed that the sooner we are able to establish diagnosis of AD, the more benefits we will receive from the applied therapy (even if the current opportunities in this field are limited). The reliable diagnosis of AD is based on the detection of the presence of amyloid plaques and NFTs composed of pathological deposits of Tau protein in the neuronal tissue [45, 46]. The distribution of these deposits in the brain correlates with a stage of the disease [47]. However, the histopathological examination is not possible in patients’ lifetime.
The diagnosis of living AD patients relies on the neuropsychological tests, imaging studies and biomarkers. There is sufficient evidence of the value of both AD CSF biomarkers as AD pathological correlates and the same with amyloid PET. Currently, AD biomarkers include Tau protein and its phosphorylated form pTau181 (indicators of neuronal injury), Aβ peptides (40 and 42) and the coefficient ratio Aβ42/40, the markers of amyloid precursor protein (APP) pathway, which are identified in the CSF [48-51], a biological material that requires invasive procedures to procure. Hence, looking for specific markers of AD, which could be determined in much more easily available biological materials, such as serum or plasma is urgent challenge in the diagnosis of this disease.
Although it is known that inflammation plays a significant role in AD pathogenesis [52], there is currently no inflammatory biomarker identified in body fluids as validated diagnostic method which would be useful to detect and/or monitor the course of the disease. Therefore, the modern and future research direction will focus also on the determination of inflammatory biomarkers in various body fluids, such as CSF and blood of AD patients [18]. Increasing knowledge about the neuroinflammation and regulation of the immunity mechanisms can pave the way towards the establishment of new therapies that may be useful to halt AD development or at least delay the onset of the cognitive decline [18].
In the last three decades, the concentrations of CSF inflammatory mediators in patients with AD and mild cognitive impairment (MCI) were determined [for review see: 53]. The reports suggest the possible role of elevated CSF levels of pro-inflammatory cytokines as risk factors for conversion of MCI to AD or as markers of the disease progression [54, 55]. In order to acquire the best possible explanation of the role of cytokines in AD, there is a growing need to obtain a satisfactory level of the method setting and patients’ characteristics, along with the use of longitudinal studies [18].
4. YKL-40- GENERAL CHARACTERISTICS
YKL-40, recognized as chitinase 3-like protein 1 (CHI3L1) or human cartilage glycoprotein 39 (HC-gp39) is a chitin-binding lectin which belongs to the glycosyl hydrolase family 18 [56,57]. The name of YKL-40 was established based on its structure which consists of three N-terminal aminoacids: tyrosine (Y), lysine (K) and leucine (L) and the molecular mass of the protein is 40 kDa [58]. The amino acid sequence of human YKL-40 cDNA was described by Hakala et al. [59]. This protein consists of one polypeptide chain which includes 383 amino acids [59]. The construction of YKL-40 is based on two globular domains. The first one is a large domain composed of a (β/α)8 structure with the presence of a triose-phosphate isomerase (TIM) barrel fold, and the second one represents a small α/β domain consisted of five antiparallel β-strands and one α-helix that is settled in the loop between strand β7 and helix α7 of the TIM barrel. It results in complex grooved-shaped construction of YKL-40 molecule [58]. Isoelectric point of YKL-40 is approximately 7.6 [60].
CHI3L1, the human gene responsible for encoding YKL-40, was discovered in 1997 [61]. This gene is located in chromosome 1q31-q32 [56]. It has been shown that CHI3L1 is composed of 10 exons with approximately 8 kilobases length of DNA data. There are two important mutations in the human YKL-40 gene. The first one involves the catalytic glutamic acid to leucine (L, residue 140) and the second is a mutation of the catalytic aspartic acid to alanine (A, residue 138). Presence of these mutations leads to deactivation of YKL-40 hydrolytic properties [62].
The expression of YKL-40 mRNA in vitro is highly intense in the course of human macrophage differentiation, especially in the final phase of this process. Furthermore, in vivo studies reported that expression of YKL-40 mRNA and protein is present in a various inflammation infiltrates and is involved in remodeling of extracellular matrix (ECM) [58]. YKL-40 protein is expressed by several types of cells including macrophages, chondrocytes, neutrophils and synovial fibroblasts [63]. Interestingly, there is no protein expression of YKL-40 in monocytes [56]. It has been indicated, that proinflammatory cytokines TNF-α and IL-1β induce the synthesis of YKL-40 in macrophages and chondro- cytes, especially in peripheral inflammatory conditions, such as arthritis and asthma [64, 65].
The mechanisms of the regulation of YKL-40 expression were also assessed in astrocytes in vitro [57]. It was shown that abundant expression of this protein was present in astrocytes in neuroinflammatory conditions as well as in cultured macrophages. In macrophages, the YKL-40 transcription was induced by classical activation pathway (M1) and inhibited by alternative activation (M2), whereas transcription of this protein in microglia in vitro was minimally changed by M1 or M2 activation [57]. Moreover, production of YKL-40 by macrophages increased as a function of time in in vitro culture [57]. The transcription of YKL-40 in astrocytes was induced by cytokines released from macrophages, resulting in morphological changes of astrocytes and their altered motility.
The physiological role of YKL-40 and its specific cell surface receptor are not known at this time. Although the protein is highly conserved in mammals, a consensus regarding its role in human pathologies is currently lacking. This protein was hypothesized to be involved in tissue remodeling during inflammation. In particular, it was indicated as a factor preventing the damage of extracellular matrix in response to proinflammatory cytokines, even though its biological function remains speculative. However, the physiological role of YKL-40 in brain tissue still remains unknown [52].
5. The role of YKL-40 in AD
In AD brains, the neuritic plaques consisting of fibrous deposits of the Aβ fragments of the amyloid precursor protein (APP) are surrounded by microglia. These cells play a role as the components of the immune response in the brain and express various pro-inflammatory cytokines at mRNA and protein level. Significantly increased expression of mRNA for chitinase-3 like 3 (CHI3L3), a mouse homologue of YKL-40, was found in brains of mice models of AD when compared to age-matched controls [66]. Similarly, in human brain samples, obtained in autopsy from individuals with pathologically confirmed AD, the levels of mRNAs for YKL-40 and chitinase-3 like 2 (CHI3L2), as well as mRNA for TNF-α, and were significantly increased in comparison with non-demented controls [66].
Despite the fact that there is no explanation which factors influence the expression of YKL-40 protein in the pathogenesis of AD, and how elevated expression level of YKL-40 can affect the process of the disease [52], it has been suggested, that YKL-40 might be a candidate for a biomarker of some chronic neuropathologies, including AD, which have an inflammatory background [67]. It can be assumed that astrocytic expression of YKL-40 might be activated by TNF-α and IL-1β, since it is known that these proinflammatory cytokines are involved in the process of neuroinflammation in AD pathology. Bearing in mind that these cytokines have an ability to pass the blood brain barrier (BBB), it can be also suspected that concentrations of YKL-40 in body fluids, such as CSF and plasma may be influenced by inflammatory processes ongoing in central nervous system or peripheral inflammation [52].
There are only few studies concerning concentrations of YKL-40 in cerebrospinal fluid of patients with full symptomatic AD and predementia stages as well as in other types of dementia (Table 1). Recent studies have suggested the potential value of determination of YKL-40 CSF levels in the diagnosis of AD. In the paper by Rosén et al. [68], in AD patients CSF concentrations of YKL-40 were significantly elevated in comparison to cognitively normal individuals (77% increase), with the area under the curve (AUC) = 0.88. However, no correlations between the levels of YKL-40 and patients’ age, mini-mental state examination (MMSE) or AD biomarkers have been found [68].
Table 1.
A comparison of YKL-40 concentrations in CSF (all using ELISA method).
| Refs. | Group Tested |
Concentration
(Median*/mean**) [ng/mL] |
|
|---|---|---|---|
| Rosén et al.; 2014 [60] | 25 AD | 199.211 * | |
| 25 H | 112.631 * | ||
| Antonell et al.; 2014 [61] | 18 preAD | 330.0 ** | |
| 22 prodAD | 364.1 ** | ||
| 43 H | 260.5 ** | ||
| Kester et al.; 2015 [62] | 65 AD | 288.0 ** | |
| 61 MCI | 304.0 ** | ||
| 37 H | 231.0 ** | ||
| Sutphen et al.; 2015 [63] | 169 H | 61 APOE ε4 carriers (early age: 45-54 yrs; mid age: 55-64 yrs; late age: 65-74 yrs): 19 early (2 with genotype ε2/ε4, 14 with genotype ε3/ε4, 3 with ε4/ε4) 17 mid (2 with genotype ε2/ε4, 12 with genotype ε3/ε4, 3 with ε4/ε4) 25 late (2 with genotype ε2/ε4, 20 with genotype ε3/ε4, 3 with ε4/ε4) |
early: 188.4 ** |
| mid: 240.6 ** | |||
| late: 281.5 ** | |||
| 108 APOE ε4 non-carriers (early age: 45-54 yrs; mid age: 55-64 yrs; late age: 65-74 yrs): 26 early (3 with genotype ε2/ε3, 23 with ε3/ε3) 44 mid (1 with genotype ε2/ε2, 8 with genotype ε2/ε3, 35 with ε3/ε3) 38 late (1 with genotype ε2/ε2, 6 with genotype ε2/ε3, 31 with ε3/ε3) |
early: 180.3 ** | ||
| mid: 231.3 ** | |||
| late: 301.1 ** | |||
| Alcolea et al.; 2015 [64] | 27 aMCI | 247.07 ** | |
| 80 CN | 200.37 ** | ||
| Racine et al.; 2016 [65] | 108 AD | 139.47 ** | |
| Alcolea et al.; 2015 [66] | 266 H | 196.77 * | |
| Gispert et al.; 2016 [75] | 15 AD | 333.47 ** | |
| 28 MCI due to AD | 427.71 ** | ||
| 20 preAD | 320.57 ** | ||
| 53 H | 283.86 ** | ||
| Olsson et al.; 2013 [67] | 96 AD | 241.582 ** | |
| 81 sMCI | 171.687 ** | ||
| 65 H | 194.622 ** | ||
| Bonneh-Barkay et al.; 2010 [81] | 10 AD | 212.0 * | |
| 12 H at younger age (<40 yrs) | 109.0 * | ||
| 7 H at older age (60-70 yrs) | 218.0 * | ||
Increased concentrations of YKL-40 were observed not only in fully developed AD, but also in early stages of this disease. In the study of Antonell et al. the CSF concentrations of YKL-40 were significantly increased already in prodromal phase of AD when compared to cognitively normal controls [69]. Interestingly, the authors demonstrated a significant correlation between CSF YKL-40 and levels of Tau and pTau181, as well as with MMSE, opposite to the results of above mentioned study of Rosén et al. [68]. Similar observations were made in patients with preclinical AD [52], where CSF concentrations of YKL-40 were increased in very mild and mild dementia subjects in comparison with cognitively normal individuals. Moreover, the authors postulated that in evaluation of the risk of future cognitive decline, the prognostic value of ratio of YKL-40 to Aβ1-42 is similar to the ratio of Tau to Aβ1-42 [52]. The same authors revealed that astrocytes in close vicinity of amyloid plaques were immunoreactive to YKL-40, what confirms involvement of this protein in the neuroinflammatory response to Aβ deposition [52]. Results of more recent study of Kester et al. [70], concerning prognostic value of YKL-40 in AD are in line with these observations. CSF concentrations of YKL-40 in both AD and MCI patients were higher than in individuals without cognitive impairment. Additionally, baseline CSF levels of this biomarker were elevated in MCI patients who further progressed to AD in comparison with those MCI subjects who were clinically stable. Increased levels of YKL-40 predicted progression from MCI to symptomatic AD and other types of dementia as measured by annual assessment of MMSE within follow-up [70].
CSF concentrations of YKL-40 were also determined in middle-aged cognitively normal subjects with Clinical Dementia Rating (CDR) of 0 and compared with concentrations of AD biomarkers, such as Aβ1-42, Aβ1-40, Tau and pTau181 as well as with amyloid imaging in serial examinations within 6-year mean period of follow-up [71]. Additionally, APOΕ ε4 genotype was assessed in participants of the study to evaluate possible risk for further development of AD pathology. CSF levels of YKL-40 were significantly higher in older subject compared with younger participants of the study, both in ε4 group (180.3 ng/mL in early age, 231.3 ng/mL in mid age and 301.1 ng/mL in late age group) and in the ε4 non-carriers (188.4 ng/mL in early age, 240.6 ng/mL in mid age and 281.5 ng/mL in late age group). Within the same age group, the concentrations of YKL-40 did not differ significantly between APOE groups, i.e., ε4 carriers and non-carriers. Moreover, concentration of this protein significantly increased within individuals over time, regardless age interval. The rate of the increase in YKL-40 concentration in the ε4 carriers was significantly higher in comparison with ε4 non-carriers. Elevation of YKL-40 CSF levels in all age intervals observed might be explained by the fact that neuroinflammation is a process ongoing normally with aging. However, the highest increases observed in ε4 carriers suggest that this process may be further intensified in the presence of neuronal damage and amyloid accumulation [71].
YKL-40 CSF levels in cognitively normal individuals were described by Alcolea et al. [72], who assessed it across the preclinical stages of the NIA-AA classification: stage 0, 1, 2, 3, and in patients with suspected non-Alzheimer pathology (SNAP). All participants had a Mini-Mental State Examination (MMSE) score at least 24 points and normal memory performance. The participants with preclinical stages of AD and those with SNAP showed different profiles of YKL-40 concentration in CSF. Significantly increased levels of YKL-40 were observed in cognitively normal patients with stages 2 and 3, as well as in patients with SNAP when compared to those in stages 0 and 1 [64]. Independently on APOE status, CSF levels of YKL-40 correlated positively with patients’ age, similarly to concentrations of Tau and pTau181 in CSF, and with Tau regardless Aβ1-42 concentrations in CSF [72]. These observations suggest that the development of AD may be connected with the inflammatory response in the brains of ageing patients.
ELISA-enzyme-linked immunosorbent assay; APOE-apolipoprotein E; AD-Alzheimer’s disease; MCI-mild cognitive impairment; H-healthy individuals; CN-cognitively normal subjects; aMCI-amnestic mild cognitive impairment; sMCI-stable mild cognitive impairment; preAD-preclinical Alzheimer’s disease; prodAD-prodromal Alzheimer’s disease
All above mentioned studies [52, 69, 72] have consistently found elevated CSF levels of YKL-40 in patients with AD and revealed a correlation between YKL-40 and markers of neurodegeneration, such as Tau and pTau181, even in preclinical stages of AD. This may give an assumption that neuroinflammation can emerge through a non-amyloid-related pathway. On the other hand, it is known that insoluble aggregates of Aβ may induce the inflammatory reactions and activation of microglia, resulting in production of proinflammatory mediators. Therefore, the relationship between YKL-40 and amyloid-related pathway in the development of AD was assessed in the paper of Alcolea et al. [72]. They divided cognitively normal patients according to Aβ1-42 levels, as above and below the cut-off point (550 pg/mL). The directionality of the correlation between YKL-40 and Aβ1-42 differed between participants: significant negative correlation was found in patients who had lower levels of Aβ1-42, whereas positive correlation between YKL-40 and Aβ1-42 was observed in patients with concentration of Aβ1-42 higher than 550 pg/mL [72]. This finding could explain some discrepancies found across studies concerning relationship of AD biomarkers with other biochemical CSF parameters, although mechanisms underlying this correlation in the absence of the pathologic process of AD require further investigation of amyloid deposition pathways, targeting not only basic AD biomarkers (Aβ1-42 and Aβ1-42 /Aβ1-40 ratio), but also amyloid precursor proteins or, perhaps, other isoforms of Aβ.
What is more, the connections between YKL-40 in CSF and amyloid pathology in cognitively healthy adults with increased risk for sporadic AD were recently assessed in the study of Racine et al. [73]. They hypothesized that CSF biomarkers of various pathological phenomena ongoing in the development of AD, such as amyloid plaques (lower Aβ1-42), NFTs (elevated pTau181), axonal injury (increased Tau), and microglial activation/inflammation (high levels of YKL-40) should occur with larger amyloid deposition in neuroimaging at baseline and in longitudinal observation within 2 years. Indeed, in the initial assessment, Pittsburgh compound B (PiB) binding in AD-vulnerable regions was significantly associated with ratio of CSF YKL-40 to Aβ1-42 as well as other AD biomarkers to Aβ1-42. The authors indicated the possible use of YKL-40/Aβ1-42 ratio as a predictor of amyloid burden in PiB imaging at the baseline, although not in longitudinal observation [73].
The possible connection of CSF levels of YKL-40 with cortical atrophy, as a result of neuronal damage at early stage of AD, has been pointed out in another latest report from Alcolea et al. [74]. It seems that YKL-40 CSF concentrations may be linked to the AD pathology, especially to the cortical thickness (CTh) in some brain regions. In patients with amnestic MCI (aMCI), CSF levels of YKL-40 were not only increased when compared to cognitively normal subjects, but also significant negative correlations were found between CTh, especially in middle and inferior temporal areas, and CSF levels of YKL-40, Tau, and pTau181 [74]. These observations were confirmed in subgroup of aMCI patients with low levels of Aβ1-42. Additionally, significant correlation between CSF levels of YKL-40 and concentrations of Tau and pTau181 but not Aβ1-42 has been demonstrated, similar to described previously results of Craig-Schapiro [52]. These findings suggest that YKL-40 CSF levels are related to the Tau-connected neurodegeneration [74].
Another interesting observation has been made by Gispert et al. [75], who revealed that CSF concentrations of YKL-40 and pTau181 in patients with early AD may be related to different cerebral morphometric patterns, such as gray matter (GM) volume. The authors evaluated connections between specific cerebral structures and CSF levels of YKL-40 across the early stages of AD, from normal through preclinical AD to mild dementia. They demonstrated that age-corrected YKL-40 levels were significantly increased in MCI patients in comparison with the rest of groups tested, i.e. normal controls and in subjects with preclinical AD or mild AD dementia patients. Moreover, no significant association was found with Aβ1-42, but a significant regression was found with pTau181 that was better modeled by a quadratic function. In patients with MCI due to AD and in AD group, referred to as “early AD”, GM volume of certain cerebral structures was associated with age-corrected YKL-40 levels in inverse u-shaped nonlinear manner [75]. What is most interesting, CSF levels of YKL-40 related to a cerebral structures distinct from those affected with the progressive neurodegenerative atrophy associated with increasing CSF p-tau values. This suggests that neuroinflammatory and neurodegenerative processes exist concurrently already at the early stages of cognitive impairment due to AD [75].
Determination of YKL-40 levels in CSF may be helpful not only in diagnosis of AD patient, but also in differentiation between various types of dementia. Olsson et al. [76] demonstrated that CSF levels of YKL-40 were significantly elevated in AD patients in comparison with cognitively healthy elderly controls. Additionally, a significant positive correlation was found between YKL-40 levels in CSF and Tau protein in AD patients. Interestingly, there were also higher concentrations of YKL-40 in CSF of MCI patients with an AD-indicative profile of biomarkers than in those with stable form of MCI. Moreover, concentrations of YKL-40 were increased in CSF of patients with MCI who progressed to vascular dementia (VaD) when compared to subjects with stable MCI within over 5-years observation period. It suggests diagnostic usefulness of CSF levels of YKL-40 in AD and for the distinction between stable phase of MCI and patients who progressed to VaD and AD [76].
6. Role of YKL-40 in other neurological diseases
YKL-40, as a biomarker of inflammation and activation of microglia within central nervous system, was also assessed in variety of neurological disorders occurring with neuroinflammatory process in reaction to pathological deposits, leading to neurodegeneration. Generally, NDs are characterized with similar changes on a subcellular level, including genetic mutations, atypical protein deposits and cell death [77, 78]. These atypical proteins include huntingtin, alpha-synuclein, tau protein, Aβ and many other proteins. Intracellular aggregation of misfolded toxic proteins and products of their degradation in cell structures are the histopathological changes seen not only in AD, but also observed in Huntington’s (HD), and Parkinson’s diseases (PD), amyotrophic lateral sclerosis (ALS) and dementia with Lewy bodies (DLB).
Increased concentrations of YKL-40 in CSF were observed in HD, which is a neurodegenerative disorder caused by a mutation in huntingtin gene (also called HD), where excessive (more than 36) repeats in codon CAG (cytosine-adenine-guanine) result in formation of an unstable, mutated Huntingtin protein (mHtt). HD affects muscle coordination and leads to mental decline as well. Moreover, neuroinflammation in HD is a well-known process, which is probably an early event in the pathology of this disease. In development of HD, the activation of immune system in brain tissue may occur even 15 years before onset of symptomatic disease [79]. CSF levels of YKL-40 were 17% higher in full-symptomatic carriers of HD gene in comparison with premanifest HD gene-expansion carriers and 18% higher than in control group, whereas no difference between presymptomatic HD gene carriers and healthy subjects was found [80]. It has not been elucidated whether YKL-40, a marker for glia activation, plays a direct role in the pathology of HD, although their results are in line with earlier studies on increased glia activation [79]. It suggests that YKL-40 might play a role in pathology of HD, although it remains unclear whether elevated YKL-40 is a part of the disease-specific pathology, nonspecific response to inflammation or ongoing neuro- degeneration processes.
PD is a long term disorder of the central nervous system that affects the motor neuron system and cognitive functioning. Pathological changes include accumulation of alpha-synuclein, which results in forming insoluble fibrils, a primary structural component of Lewy bodies in neuronal tissue [81]. Therefore, PD is described as synucleinopathy [82, 83]. Atypical parkinsonian disorders (P+ diseases), such as progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atrophy (MSA) are also connected with pathological protein deposits in the brain. These P+ diseases currently are divided to synucleinopathies (DLB, MSA) or tauopathies (PSP, CBD), concerned with pathological tau deposits. CSF concentrations of YKL-40 were assessed in subjects suffering from PD and in patients with various P+ diseases [84]. Interestingly, YKL-40 was significantly lower in patients with PD and P+ than in healthy controls. Moreover, YKL-40 levels were decreased in patients with synucleinopathies when compared to tauopathies, what suggests that glial activation is reduced in the brains of PD patients and other synucleinopathies in comparison with patients who have tauopathies or healthy controls [84].
As it was stated above, neuroinflammation is connected with cognitive impairment. Therefore, YKL-40 was also pointed out as a possible biomarker in other neurological diseases ongoing with cognitive decline. Bipolar disorder (BD) is one of such diseases, where cognitive loss is a common symptom and a key predictor of patients’ functioning. On the other hand, it was indicated that chronic neuroinflammation may be present in BD [85]. Increased levels of microglia markers in frontal cortex were found in post-mortem examination of patients with BD [86], what suggests that microglia play a role in the pathophysiology of this disease. Moreover, elevated CSF levels of YKL-40 were found in euthymic patients with BD in comparison with healthy controls, independent on age, gender, smoking status, body mass index, functioning of blood-brain barrier, and acute-phase serum proteins [87]. In the study of Rolstad et al., CSF levels of YKL-40 were also assessed in mood-stabilized patients with various types of BD associated with cognitive impairment [88]. The linear regression analysis revealed that YKL-40 significantly accounted for the variance in executive functioning, but not in cognitively healthy control subjects, what confirms the hypothesis that inflammatory processes within brain tissue are involved in the pathophysiology of BD [88].
Since YKL-40 is up-regulated in a variety of inflammatory conditions, this protein was also assessed in multiple sclerosis (MS), which is also characterized with inflammatory response [67]. It was shown that expression of YKL-40 was mainly associated with reactive astrocytes and was more pronounced in regions of inflammatory cells in MS [89]. Moreover, significant elevation of YKL-40 concentrations was observed in the CSF of MS patients [89]. When evaluated during various phases of MS, the concentrations of YKL-40 in CSF were increased during relapse, remission and secondary progression phase when compared with healthy subjects [90]. Additionally, CSF levels of this protein decreased after immunosuppressive treatment of MS patients [90], what suggests possible use of YKL-40 as a pharmacodynamic marker in this disease.
7. Possibility of therapeutic strategies targeting neuroinflammation and YKL-40 in AD
Up to this point, there are no efficient and accessible treatments to prevent the beginning and/or the progression of AD [6, 8]. Although for the last twenty years a lot of attempts have been done to establish an effective disease-modifying treatment and to delay the development of AD, they are still insufficient. Most of AD medications used are nootropic, procognitive drugs, that could to some extent improve the cognition and memory in dementia affected patients. These drugs belong to different groups, including agonists of N-methyl-D-aspartate (NDMA) receptor, reversible inhibitors of cholinesterase, cerebral blood flow improving treatment and psychotropic drugs. Agonists of NMDA receptor, such as memantine, exhibit a protective effect on the neuronal cells and also enhance the cognition in AD [91]. Reversible inhibitors of acetylcholinesterase (AChE), for instance donepezil or rivastigmine, can improve memory and stabilize patient’s behavior [92]. The drugs used to improve cerebral blood flow can also influence the brain nutrition and its functioning, which may have a positive impact on mental processes and daily activity. Additionally, a supplementation of antioxidants such as vitamin C, E, and coenzyme Q it also recommended. Unfortunately, no new drugs have been licensed for AD since memantine in 2002.
The hypothesis that neuroinflammation is closely linked with a variety of neurodegenerative diseases, including AD, implies the probability that novel anti-inflammatory therapies could reverse the consequences of neurodegeneration. Therefore, the use of non-steroidal anti-inflammatory drugs (NSAIDs) or glucocorticoids is expected to decrease the risk of developing the disease.
NSAIDs act by blocking the conversion of prostaglandin H2 into other prostaglandins (PGs) and thromboxane (TX). By inhibition of cyclooxygenase activity, NSAIDs could block inflammation underlying AD pathogenesis at early stages. The positive influence of indometacin administration on the results of psychometric tests and AD assessment scale was shown in several studies [93, 94]. Moreover, naproxen sodium application could also result in AD patients outcome, leading to reduced ratio of CSF tau to Aβ1-42 [95]. Unfortunately, no apparent effects of new generation NSAIDs, such as selective cyclooxygenase-2 inhibitors or nimesulide on AD were observed in clinical trials [40].
Moreover, the effect of various systemic comorbidities, including diabetes and hypertension as well as systemic inflammation could be considered as possible targets of AD therapy in future studies. Despite known anti-inflammatory action of statins, the clinical trials with these drugs (simvastatin and atorvastatin) gave no positive effects on CSF levels of Aβ isoforms [96] or neuropsychological tests results [97, 98], respectively.
In addition, aerobic exercise has been shown to decrease the proliferation of microglia in the brain and hippocampal expression of immune-related genes, as well as to reduce the expression of inflammatory cytokines such as TNF-α. Therefore physical exercise could be considered as the preventing factor and supplementary treatment for various neuroinflammatory diseases, including AD [99]. An interesting study on this field, concerning strategies for cognitive wellness promotion, including nutritional guidance, physical exercise, cognitive training, and social intervention was The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). The researchers concluded that these developing interventions are needed as soon as possible (even before the onset of the disease clinical manifestation), since it might improve patients’ cognitive functions and prevent or delay dementia symptoms [100].
Although the biophysiological activity of YKL-40 is poorly understood, this protein is believed to be involved not only in AD development and progression, but also to be associated with proliferation of connective tissue cells [101, 102] and activation of vascular endothelial cells [103]. Furthermore, the elevation of YKL-40 serum levels in a variety of chronic inflammatory diseases [104, 105] suggests the relationship of this protein with the process of extra- cellular matrix remodeling [58, 106]. Within last few years, the pathologic role of YKL-40 in development of a broad type of human cancers has been highlighted. Moreover, increasing evidence has indicated the particular role of YKL-40 as an angiogenic factor in cancer development. The study of Faibish et al. demonstrated blockade of angiogenesis and progression using YKL-40 neutralizing monoclonal antibodies (mAY) [107]. Therefore, the potential utility of mAY could implicate its potential therapeutic value in cancers. Taking these findings into account, it seems reasonable to focus on inhibiting the YKL-40 function by using mAY as potential therapeutic target in AD and other neurodegenerative conditions.
Conclusion
This review summarizes recent data regarding a suggested role of YKL-40 as a candidate inflammatory biomarker of AD. It is already known that neuroinflammation plays an important role in AD pathology. The latest studies have pointed out the elevated CSF levels of YKL-40 in neuro- degenerative disorders, especially in AD. The correlations between CSF concentrations of YKL-40 and classical AD biomarkers, such as Tau protein and its phosphorylated form (pTau181) have been found. Moreover, it has been demonstrated that YKL-40 might be useful in the diagnosis and prognosis of the AD progression. Additionally, YKL-40 might be associated in the pathophysiology of other neuro- degenerative diseases ongoing with inflammatory background, such as Huntington’s disease as well as Parkinson’s disease. Furthermore, the interactions between neuroinflammation and neurodegenerative diseases, especially AD, might represent a possible hypothetic target for novel AD drugs, modulating YKL-40 activity, although this issue requires further investigation.
ACKNOWLEDGEMENTS
This study was supported by funds from the Leading National Research Centre (KNOW) and grants for neuro- degenerative diseases, Medical University of Białystok, Poland. BM, PM and AK-P are supported by funds from KNOW. BM has received consultation and/or lecture honoraria from Roche, Cormay and Biameditek.
List of Abbreviations
- AChE
Inhibitors of Acetylcholinesterase
- AD
Alzheimer’s Disease
- ALS
Amyotrophic Lateral Sclerosis
- aMCI
Amnestic Mild Cognitive Impairment
- APOE
Apoliporotein E
- APP
Amyloid Precursor Protein
- AUC
Area Under the ROC Curve
- Aβ
Amyloid Beta
- BBB
Blood-Brain Barrier
- BD
Bipolar Disorder
- CAG
Cytosine-Adenine-Guanine
- CBD
Corticobasal Degeneration
- CD14
Cluster of Differentiation 14
- CD33
Cluster of Differentiation 33
- cDNA
Complementary DNA
- CDR
Clinical Dementia Rating
- CHI3L1
Chitinase 3-Like Protein 1
- CHI3L2
Chitinase 3-Like Protein 2
- CHI3L3
Chitinase 3-Like Protein 3
- CRP
C-Reactive Protein
- CSF
Cerebrospinal Fluid
- CTh
Cortical Thickness
- DLB
Dementia with Lewy Bodies
- ECM
Extracellular Matrix
- GM
Gray Matter
- HC-gp39
Human Cartilage Glycoprotein 39
- IL
Interleukin
- mAY
YKL-40 Neutralizing Monoclonal Antibodies
- mCAP-1
Membrane-Bound Channel-Activating Serine Protease 1
- MCI
Mild Cognitive Impairment
- MCP-1
Monocyte Chemoattractant Protein 1
- mHtt
Mutated Huntingtin Protein
- MIP-1
Macrophage Inflammatory Protein 1
- MMPs
Matrix Metalloproteinases
- MMSE
Mini-Mental State Examination
- mRNA
Messenger RNA
- MS
Multiple Sclerosis
- MSA
Multiple System Atrophy
- NDs
Neurodegenerative Diseases
- NFTs
Neurofibrillary Tangles
- NIA-AA
National Institute on Aging and the Alzheimer’s Association
- NMDA
Agonists of N-Methyl-D-Aspartate
- NSAIDs
Non-Steroidal Anti-Inflammatory Drugs
- PD
Parkinson’s Disease
- PGs
Prostaglandins
- PiB
Pittsburgh Compound B
- PSP
Progressive Supranuclear Palsy
- pTau181
Phosphorylated Tau Protein
- SNAP
Suspected Non-Alzheimer Pathology
- Tau
Tau Protein
- TIM
Triose-Phosphate Isomerase
- TIMPs
Tissue Inhibitors of Matrix Metalloproteinases
- TNF-α
Tumor Necrosis Factor α
- TREM2
Triggering Receptor Expressed on Myeloid Cells 2
- TX
Thromboxane
- VaD
Vascular Dementia
- YKL-40
Chitinase 3-Like Protein 1
AUTHORS CONTRIBUTION
BM designed the study and contributed to acquisition of data. MG and PM performed research and wrote the manuscript. AK and AK-P have been involved in revising critically the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Scheltens N.M., Galindo-Garre F., Pijnenburg Y.A., van der Vlies A.E., Smits L.L., Koene T., Teunissen C.E., Barkhof F., Wattjes M.P., Scheltens P., van der Flier W.M. The identification of cognitive subtypes in Alzheimer’s disease dementia using latent class analysis. J. Neurol. Neurosurg. Psychiatry. 2015;87:235–243. doi: 10.1136/jnnp-2014-309582. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K., Dubois B., Fagan A.M., Lewczuk P., de Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtzman D.M. CSF biomarkers for Alzheimer’s disease: current utility and potential future use. Neurobiol. Aging. 2011;32:4–9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Report W.A. The Global Impact of Dementia. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 6.Fagan A.M. CSF biomarkers of Alzheimer’s disease: impact on disease concept, diagnosis, and clinical trial design. Advances in Geriatrics; 2014. [Google Scholar]
- 7.Beason-Held L.L., Goh J.O., An Y., Kraut M.A., O’Brien R.J., Ferrucci L., Resnick S.M. Changes in brain function occur years before the onset of cognitive impairment. J. Neurosci. 2013;33:18008–18014. doi: 10.1523/JNEUROSCI.1402-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [http://dx.doi.org/ 10.1016/S0140-6736(15)01124-1]. [DOI] [PubMed] [Google Scholar]
- 9.Dubois B., Feldman H.H., Jacova C., Dekosky S.T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P.J., Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 10.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Jr, Kaye J., Montine T.J., Park D.C., Reiman E.M., Rowe C.C., Siemers E., Stern Y., Yaffe K., Carrillo M.C., Thies B., Morrison-Bogorad M., Wagster M.V., Phelps C.H. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr, Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., Mohs R.C., Morris J.C., Rossor M.N., Scheltens P., Carrillo M.C., Thies B., Weintraub S., Phelps C.H. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J., Jack C.R., Jr, Jagust W.J., Shaw L.M., Toga A.W., Trojanowski J.Q., Weiner M.W. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay J., Laurin D., Verreault R., Hébert R., Helliwell B., Hill G.B., McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 15.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [https://doi.org/10. 1038/nrneurol.2011.2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtzman D.M., Herz J., Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(3):a006312. doi: 10.1101/cshperspect.a006312. [http://dx.doi.org/10.1101/cshperspect.a006312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinney L. Alzheimer’s disease: The forgetting gene. Nature. 2014;510:26–28. doi: 10.1038/510026a. [DOI] [PubMed] [Google Scholar]
- 18.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2010;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson L.V., Leitner W.P., Rivest A.J., Staples M.K., Radeke M.J., Anderson D.H. The Alzheimer’s A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama H., Arai T., Kondo H., Tanno E., Haga C., Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis. Assoc. Disord. 2010;14:47–53. doi: 10.1097/00002093-200000001-00008. [https://doi.org/ 10.1097/00002093-200000001-00008]. [DOI] [PubMed] [Google Scholar]
- 22.Veerhuis R., Van Breemen M.J., Hoozemans J.M., Morbin M., Ouladhadj J., Tagliavini F., Eikelenboom P. Amyloid beta plaque-associated proteins C1q and SAP enhance the Abeta1-42 peptide-induced cytokine secretion by adult human microglia in vitro. Acta Neuropathol. 2003;105:135–144. doi: 10.1007/s00401-002-0624-7. [DOI] [PubMed] [Google Scholar]
- 23.Rogers J., Lue L.F. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem. Int. 2001;39:333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 24.Tuppo E.E., Arias H.R. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [https://doi.org/10.1016/j.biocel.2004.07.009]. [DOI] [PubMed] [Google Scholar]
- 25.Selkoe D.J. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2011;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 26.Moore A.H., O’Banion M.K. Neuroinflammation and anti-inflammatory therapy for Alzheimer’s disaese. Adv. Drug Deliv. Rev. 2002;54:1627–1656. doi: 10.1016/s0169-409x(02)00162-x. [DOI] [PubMed] [Google Scholar]
- 27.Heneka M.T., Nadrigny F., Regen T., Martinez-Hernandez A., Dumitrescu-Ozimek L., Terwel D., Jardanhazi-Kurutz D., Walter J., Kirchhoff F., Hanisch U.K., Kummer M.P. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA. 2010;107:6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eikelenboom P., Veerhuis R., Scheper W., Rozemuller A.J., van Gool W.A., Hoozemans J.J. The significance of neuroinflammation in understanding Alzheimer’s disease. J. Neural Transm. (Vienna) 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzl S., Albers D.S., LeWitt P.A., Chirichigno J.W., Hilgenberg S.L., Cudkowicz M.E., Beal M.F. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J. Neurol. Sci. 2003;207:71–76. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- 30.Jensen C.J., Massie A., De Keyser J. Immune players in the CNS: the astrocyte. J. Neuroimmune Pharmacol. 2013;8:824–839. doi: 10.1007/s11481-013-9480-6. [DOI] [PubMed] [Google Scholar]
- 31.Sekar S., McDonald J., Cuyugan L., Aldrich J., Kurdoglu A., Adkins J., Serrano G., Beach T.G., Craig D.W., Valla J., Reiman E.M., Liang W.S. Alzheimer’s disease is associated with altered expression of genes involved in immune response and mitochondrial processes in astrocytes. Neurobiol. Aging. 2013;36:583–591. doi: 10.1016/j.neurobiolaging.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., Sher A., Litke A.M., Lambris J.D., Smith S.J., John S.W., Barres B.A. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 33.Dickson D.W. The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi H., Sugihara S., Ogawa A., Saido T.C., Ihara Y. Diffuse plaques associated with astroglial amyloid ß protein, possibly showing a disappearing stage of senile plaques. Acta Neuropathol. 1998;95:217–222. doi: 10.1007/s004010050790. [DOI] [PubMed] [Google Scholar]
- 35.Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F., Silverstein S.C., Husemann J. Adult mouse astrocytes degrade amyloid ß in vitro and in situ. Nat. Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 36.Chao C.C., Hu S., Sheng W.S., Bu D., Bukrinsky M.I., Peterson P.K. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16:276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AID-GLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S., Younkin S., Hazrati L., Collinge J., Pocock J., Lashley T., Williams J., Lambert J.C., Amouyel P., Goate A., Rademakers R., Morgan K., Powell J., St George-Hyslop P., Singleton A., Hardy J. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradshaw E.M., Chibnik L.B., Keenan B.T., Ottoboni L., Raj T., Tang A., Rosenkrantz L.L., Imboywa S., Lee M., Von Korff A. Morris, M.C.; Evans, D.A.; Johnson, K.; Sperling, R.A.; Schneider, J.A.; Bennett, D.A.; De Jager, P.L. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat. Neurosci. 2013;16:848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham C., Wilcockson D.C., Campion S., Lunnon K., Perry V.H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes C., Cunningham C., Zotova E., Woolford J., Dean C., Kerr S., Culliford D., Perry V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes C., Cunningham C., Zotova E., Culliford D., Perry V.H. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology. 2011;77:212–218. doi: 10.1212/WNL.0b013e318225ae07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry V.H., Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013;35:601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alzheimer A. A. Uber eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie und psychisch-gerichtliche Medizin, 1907;64:146–148. [Google Scholar]
- 46.Braak H., Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 47.Braak H., Del Tredici K., Schultz C., Braak E. Vulnerability of select neuronal types to Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000;924:53–61. doi: 10.1111/j.1749-6632.2000.tb05560.x. [https://doi.org/10.1111/j.1749-6632.2000. tb05560.x]. [DOI] [PubMed] [Google Scholar]
- 48.Lewczuk P., Popp J., Lelental N., Kölsch H., Maier W., Kornhuber J., Jessen F. Cerebrospinal fluid soluble amyloid-β protein precursor as a potential novel biomarkers of alzheimer’s disease. J. Alzheimers Dis. 2012;28:119–125. doi: 10.3233/JAD-2011-110857. [DOI] [PubMed] [Google Scholar]
- 49.Lewczuk P., Zimmermann R., Wiltfang J., Kornhuber J. Neurochemical dementia diagnostics: a simple algorithm for interpretation of the CSF biomarkers. J. Neural Transm. (Vienna) 2009;116:1163–1167. doi: 10.1007/s00702-009-0277-y. [https://doi.org/10.1007/s00702-009-0277-y]. [DOI] [PubMed] [Google Scholar]
- 50.Marksteiner J., Pirchl M., Ullrich C., Oberbauer H., Blasko I., Lederer W., Hinterhuber H., Humpel C. Analysis of cerebrospinal fluid of Alzheimer patients. Biomarkers and toxic properties. Pharmacology. 2008;82:214–220. doi: 10.1159/000156487. [https://doi.org/10. 1159/000156487]. [DOI] [PubMed] [Google Scholar]
- 51.Sonnen J.A., Montine K.S., Quinn J.F., Kaye J.A., Breitner J.C., Montine T.J. Biomarkers for cognitive impairment and dementia in elderly people. Lancet Neurol. 2008;7:704–714. doi: 10.1016/S1474-4422(08)70162-5. [https://doi. org/10.1016/S1474-4422(08)70162-5]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Craig-Schapiro R., Perrin R.J., Roe C.M. Xiong. C.; Carter, D.; Cairns, N.J.; Mintun, M.A.; Peskind, E.R.; Li, G.; Galasko, D.R.; Clark, C.M.; Quinn, J.F.; D’Angelo, G.; Malone, J.P.; Townsend, R.R.; Morris, J.C.; Fagan, A.M.; Holtzman, D.M. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol. Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brosseron F., Krauthausen M., Kummer M., Heneka M.T. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol. Neurobiol. 2014;50:534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarkowski E., Andreasen N., Tarkowski A., Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galimberti D., Fenoglio C., Scarpini E. Inflammation in neurodegenerative disorders: friend or foe? Curr. Aging Sci. 2008;1:30–41. doi: 10.2174/1874609810801010030. [DOI] [PubMed] [Google Scholar]
- 56.Rehli M., Niller H.H., Ammon C., Langmann S., Schwarzfischer L., Andreesen R., Krause S.W. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J. Biol. Chem. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 57.Bonneh-Barkay D., Bissel S.J., Kofler J., Starkey A., Wang G., Wiley C.A. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012;22:530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansen J.S. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan. Med. Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 59.Hakala B.E., White C., Recklies A.D. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 60.Renkema G.H., Boot R.G., Au F.L., Donker-Koopman W.E., Strijland A., Muijsers A.O., Hrebicek M., Aerts J.M. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur. J. Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 61.Rehli M., Krause S.W., Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–225. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 62.Kumar H.A., Kumar J.S., Mahavir Y., Archana T. Expression of YKL-40, an Inflammatory Glycoprotein and its Prognostic Implications in Cancer. J. Mol. Biomark. Diagn. 2012;3:130. [Google Scholar]
- 63.Kirkpatrick R.B., Emery J.G., Connor J.R., Dodds R., Lysko P.G., Rosenberg M. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inflammatory and peripheral blood monocyte-derived macrophages. Exp. Cell Res. 1997;237:46–54. doi: 10.1006/excr.1997.3764. [DOI] [PubMed] [Google Scholar]
- 64.Recklies A.D., Ling H., White C., Bernier S.M. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J. Biol. Chem. 2005;280:41213–41221. doi: 10.1074/jbc.M510146200. [DOI] [PubMed] [Google Scholar]
- 65.Létuvé S., Kozhich A., Arouche N., Grandsaigne M., Reed J., Dombret M.C., Kiener P.A., Aubier M., Coyle A.J., Pretolani M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J. Immunol. 2008;181:5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 66.Colton C.A., Mott R.T., Sharpe H., Xu Q., Van Nostrand W.E., Vitek M.P. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J. Neuroinflammation. 2006;3:27–39. doi: 10.1186/1742-2094-3-27. [https://doi.org/10.1186/1742-2094-3-27]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roslind A., Johansen J.S. YKL-40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol. Biol. 2009;511:159–184. doi: 10.1007/978-1-59745-447-6_7. [https://doi.org/10.1007/978-1-59745-447-6_7]. [DOI] [PubMed] [Google Scholar]
- 68.Rosén C., Andersson C.H., Andreasson U., Molinuevo J.L., Bjerke M., Rami L., Lladó A., Blennow K., Zetterberg H. Increased Levels of Chitotriosidase and YKL-40 in Cerebrospinal Fluid from Patients with Alzheimer’s Disease. Dement. Geriatr. Cogn. Dis. Extra. 2014;4:297–304. doi: 10.1159/000362164. [https://doi.org/10.1159/ 000362164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antonell A., Mansilla A., Rami L., Lladó A., Iranzo A., Olives J., Balasa M., Sánchez-Valle R., Molinuevo J.L. Cerebrospinal fluid level of YKL-40 protein in preclinical and prodromal Alzheimer’s disease. J. Alzheimers Dis. 2014;42:901–908. doi: 10.3233/JAD-140624. [DOI] [PubMed] [Google Scholar]
- 70.Kester M.I., Teunissen C.E., Sutphen C., Herries E.M., Ladenson J.H., Xiong C., Scheltens P., van der Flier W.M., Morris J.C., Holtzman D.M., Fagan A.M. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimers Res. Ther. 2015;7:59. doi: 10.1186/s13195-015-0142-1. [https://doi.org/10.1186/s13195-015-0142-1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sutphen C.L., Jasielec M.S., Shah A.R., Macy E.M., Xiong C., Vlassenko A.G., Benzinger T.L., Stoops E.E., Vanderstichele H.M., Brix B., Darby H.D., Vandijck M.L., Ladenson J.H., Morris J.C., Holtzman D.M., Fagan A.M. Longitudinal cerebrospinal fluid biomarker changes in preclinical alzheimer disease during middle age. JAMA Neurol. 2015;72:1029–1042. doi: 10.1001/jamaneurol.2015.1285. [https://doi.org/10.1001/jamaneurol.2015.1285]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alcolea D., Martínez-Lage P., Sánchez-Juan P., Olazarán J., Antúnez C., Izagirre A., Ecay-Torres M., Estanga A., Clerigué M., Guisasola M.C., Sánchez Ruiz D., Marín Muñoz J., Calero M., Blesa R., Clarimón J., Carmona-Iragui M., Morenas-Rodríguez E., Rodríguez-Rodríguez E., Vázquez Higuera J.L., Fortea J., Lleó A. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology. 2015;85:626–633. doi: 10.1212/WNL.0000000000001859. [https://doi.org/10.1212/WNL. 0000000000001859]. [DOI] [PubMed] [Google Scholar]
- 73.Racine A.M., Koscik R.L., Nicholas C.R., Clark L.R., Okonkwo O.C., Oh J.M., Hillmer A.T., Murali D., Barnhart T.E., Betthauser T.J., Gallagher C.L., Rowley H.A., Dowling N.M., Asthana S., Bendlin B.B., Blennow K., Zetterberg H., Carlsson C.M., Christian B.T., Johnson S.C. Cerebrospinal fluid ratios with Aβ42 predict preclinical brain β-amyloid accumulation. Alzheimers Dement. 2016;2:27–38. doi: 10.1016/j.dadm.2015.11.006. [https://doi.org/10.1016/j. dadm.2015.11.006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alcolea D., Vilaplana E., Pegueroles J., Montal V., Sánchez-Juan P., González-Suárez A., Pozueta A., Rodríguez-Rodríguez E., Bartrés-Faz D., Vidal-Piñeiro D., González-Ortiz S., Medrano S., Carmona-Iragui M., Sánchez-Saudinós M., Sala I., Anton-Aguirre S., Sampedro F., Morenas-Rodríguez E., Clarimón J., Blesa R., Lleó A., Fortea J. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer’s disease. Neurobiol. Aging. 2015;36:2018–2023. doi: 10.1016/j.neurobiolaging.2015.03.001. [https://doi.org/10.1016/j.neurobiolaging.2015.03.001]. [DOI] [PubMed] [Google Scholar]
- 75.Gispert J.D., Monté G.C., Falcon C., Tucholka A., Rojas S., Sánchez-Valle R., Antonell A., Lladó A., Rami L., Molinuevo J.L. CSF YKL-40 and pTau181 are related to different cerebral morphometric patterns in early AD. Neurobiol. Aging. 2016;38:47–55. doi: 10.1016/j.neurobiolaging.2015.10.022. [https://doi.org/10.1016/j.neurobiolaging.2015.10.022]. [DOI] [PubMed] [Google Scholar]
- 76.Olsson B., Hertze J., Lautner R., Zetterberg H., Nägga K., Höglund K., Basun H., Annas P., Lannfelt L., Andreasen N., Minthon L., Blennow K., Hansson O. Microglial markers are elevated in the prodromal phase of Alzheimer’s disease and vascular dementia. J. Alzheimers Dis. 2013;33:45–53. doi: 10.3233/JAD-2012-120787. [DOI] [PubMed] [Google Scholar]
- 77.Rubinsztein D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 78.Bredesen D.E., Rao R.V., Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Möller T. Neuroinflammation in Huntington’s disease. J. Neural Transm. (Vienna) 2010;117:1001–1008. doi: 10.1007/s00702-010-0430-7. [DOI] [PubMed] [Google Scholar]
- 80.Vinther-Jensen T., Budtz-Jørgensen E., Simonsen A.H., Nielsen J.E., Hjermind L.E. YKL-40 in cerebrospinal fluid in Huntington’s disease: a role in pathology or a nonspecific response to inflammation? Parkinsonism Relat. Disord. 2014;20:1301–1303. doi: 10.1016/j.parkreldis.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Arima K., Hirai S., Sunohara N., Aoto K., Izumiyama Y., Uéda K., Ikeda K., Kawai M. Cellular co-localization of phosphorylated tau- and NACP/alpha-synuclein-epitopes in Lewy bodies in sporadic Parkinson’s disease and in dementia with Lewy bodies. Brain Res. 1999;843:53–61. doi: 10.1016/s0006-8993(99)01848-x. [DOI] [PubMed] [Google Scholar]
- 82.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 83.Mezey E., Dehejia A., Harta G., Papp M.I., Polymeropoulos M.H., Brownstein M.J. Alpha synuclein in neurodegenerative disorders: murderer or accomplice? Nat. Med. 1998;4:755–757. doi: 10.1038/nm0798-755. [DOI] [PubMed] [Google Scholar]
- 84.Olsson B., Constantinescu R., Holmberg B., Andreasen N., Blennow K., Zetterberg H. The glial marker YKL-40 is decreased in synucleinopathies. Mov. Disord. 2013;28:1882–1885. doi: 10.1002/mds.25589. [DOI] [PubMed] [Google Scholar]
- 85.Berk M., Kapczinski F., Andreazza A.C., Dean O.M., Giorlando F., Maes M., Yucel M., Gama C.S., Dodd S., Dean B., Magalhaes P.V., Amminger P., McGorry P., Malhi G.S. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Rao J.S., Harry G.J., Rapoport S.I., Kim H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jakobsson J., Bjerke M., Sahebi S., Isgren A., Ekman C.J., Sellgren C., Olsson B., Zetterberg H., Blennow K., Pålsson E., Landén M. Monocyte and microglial activation in patients with mood-stabilized bipolar disorder. J. Psychiatry Neurosci. 2015;40:250–258. doi: 10.1503/jpn.140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rolstad S., Jakobsson J., Sellgren C., Isgren A., Ekman C.J., Bjerke M., Blennow K., Zetterberg H., Pålsson E., Landén M. CSF neuroinflammatory biomarkers in bipolar disorder are associated with cognitive impairment. Eur. Neuropsychopharmacol. 2015;25:1091–1098. doi: 10.1016/j.euroneuro.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 89.Bonneh-Barkay D., Wang G., Starkey A., Hamilton R.L., Wiley C.A. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J. Neuroinflammation. 2010;11:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malmeström C., Axelsson M., Lycke J., Zetterberg H., Blennow K., Olsson B. CSF levels of YKL-40 are increased in MS and replaces with immunosuppressive treatment. J. Neuroimmunol. 2014;269:87–89. doi: 10.1016/j.jneuroim.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hung C.H., Hoa Y.S., Changa R.C. Modulation of mitochondrial calcium as a pharmacological target for Alzheimer’s disease. Ageing Res. Rev. 2010;9:447–456. doi: 10.1016/j.arr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 93.Rogers J., Kirby L.C., Hempelman S.R., Berry D.L., McGeer P.L., Kaszniak A.W., Zalinski J., Cofield M., Mansukhani L., Willson P. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;43:1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 94.de Jong D., Jansen R., Hoefnagels W., Jellesma-Eggenkamp M., Verbeek M., Borm G., Kremer B. No effect of one-year treatment with indomethacin on Alzheimer;s disease progression: a randomized controlled trial. PLoS One. 2008;3:e1475. doi: 10.1371/journal.pone.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Breitner J.C., Baker L.D., Montine T.J., Meinert C.L., Lyketsos C.G., Ashe K.H., Brandt J., Craft S., Evans D.E., Green R.C., Ismail M.S., Martin B.K., Mullan M.J., Sabbagh M., Tariot P.N. Extended results of the Alzheimer’s disease antiinflammatory prevention trial. Alzheimers Dement. 2011;7:402–411. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simons M., Schwärzler F., Lütjohann D., von Bergmann K., Beyreuther K., Dichgans J., Wormstall H., Hartmann T., Schulz J.B. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann. Neurol. 2002;52:346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- 97.Sparks D.L., Sabbagh M.N., Connor D.J., Lopez J., Launer L.J., Browne P., Wasser D., Johnson-Traver S., Lochhead J., Ziolwolski C. Atorvastatin for the treatment of mild to moderate Alzheimer disease: preliminary results. Arch. Neurol. 2005;62:753–757. doi: 10.1001/archneur.62.5.753. [DOI] [PubMed] [Google Scholar]
- 98.Feldman H.H., Doody R.S., Kivipelto M., Sparks D.L., Waters D.D., Jones R.W., Schwam E., Schindler R., Hey-Hadavi J., DeMicco D.A., Breazna A., LEADe investigators randomized controlled trial of atorvastatin in mild to moderate alzheimer disease: LEADe. Neurology. 2010;74:956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 99.Kohman R.A., Bhattacharya T.K., Wojcik E., Rhodes J.S. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J. Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kivipelto M., Solomon A., Ahtiluoto S., Ngandu T., Lehtisalo J., Antikainen R., Bäckman L., Hänninen T., Jula A., Laatikainen T., Lindström J., Mangialasche F., Nissinen A., Paajanen T., Pajala S., Peltonen M., Rauramaa R., Stigsdotter-Neely A., Strandberg T., Tuomilehto J., Soininen H. The finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimers Dement. 2013;9:657–965. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Recklies A.D., White C., Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HCgp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signaling pathways. Biochem. J. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Ceuninck F., Gaufillier S., Bonnaud A., Sabatini M., Lesur C., Pastoureau P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem. Bioph. Res. 2001;285:926–931. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 103.Malinda K.M., Ponce L., Kleinman H.K., Shackelton L.M., Millis A.J. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp. Cell Res. 1999;250:168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 104.Sharif M., Granell R., Johansen J., Clarke S., Elson C., Kirwan J.R. Serum cartilage oligomeric matrix protein and other biomarker profiles in tibiofemoral and patellofemoral osteoarthritis of the knee. Rheumatology. 2006;45:522–526. doi: 10.1093/rheumatology/kei216. [DOI] [PubMed] [Google Scholar]
- 105.Johansen J.S., Christoffersen P., Moller S., Price P.A., Henriksen J.H., Garbarsch C., Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J. Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 106.Nordenbaek C., Johansen J.S., Junker P., Borregaard N., Sorensen O., Price P.A. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community acquired pneumonia requiring hospitalization. J. Infect. Dis. 1999;180:1722–1726. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- 107.Faibish M., Francescone R., Bentley B. Yan. W.; Shao, R. YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol. Cancer Ther. 2011;10:742–751. doi: 10.1158/1535-7163.MCT-10-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]