Abstract
Since its discovery in 1984, the beta amyloid peptide has treaded the boards of neurosciences as the star molecule in Alzheimer’s disease pathogenesis. In the last decade, however, this vision has been challenged by evidence-based medicine showing the almost complete failure of clinical trials that experimented anti-amyloid therapies with great hopes. Moreover, data have accumulated which clearly indicate that this small peptide plays a key role in the physiological processes of memory formation. In the present review, we will discuss the different aspects of the amyloid cascade hypothesis, highlighting its pros and cons, and we will analyse the results of the therapeutic approaches attempted to date that should change the direction of Alzheimer’s disease research in the future.
Keywords: Alzheimer's disease, beta amyloid, clinical trials, LTP, memory, anti-amyloid therapy
1. The amyloid cascade hypothesis: pros
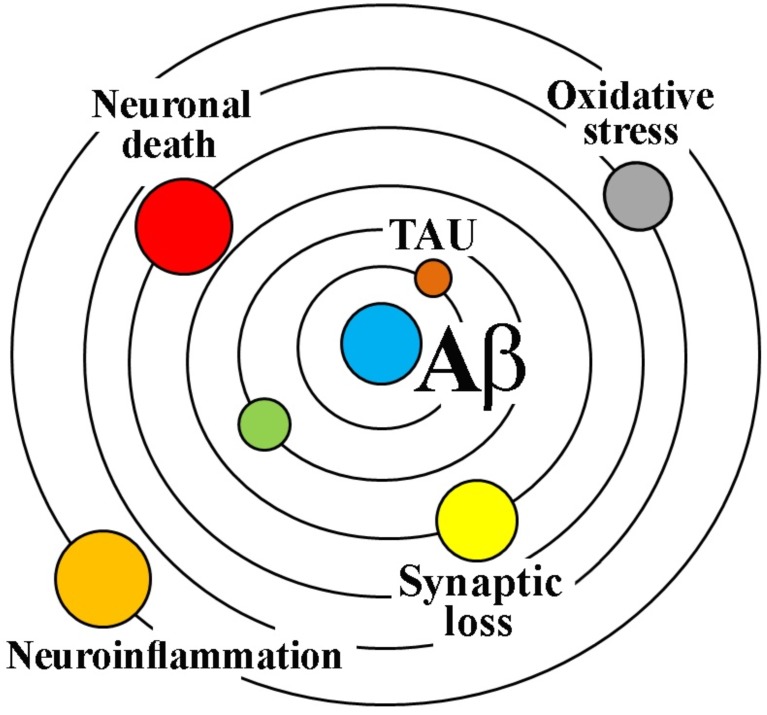
“Our hypothesis is that deposition of amyloid β protein (AβP), the main component of the plaques, is the causative agent of Alzheimer’s pathology and that the neurofibrillary tangles, cell loss, vascular damage, and dementia follow as a direct result of this deposition”. These words represented the dawn of the amyloid cascade hypothesis that dates back in 1992 when Hardy and Higgins [1] for the first time posed the accumulation of Aβ peptides in the brain parenchyma as the central event in the pathogenesis of Alzheimer’s disease (AD) (Fig. 1).
Fig. (1).
The amyloidocentric theory of AD. The amyloid cascade hypothesis represented as the geocentric Ptolemy's theory of the solar system, which placed the Earth at the center and was accepted for many centuries.
Certainly, the most compelling pieces of evidence supporting this view are: a) the occurrence of the pathology in individuals carrying autosomal-dominant mutations in the genes encoding the amyloid precursor protein (APP) or the γ-secretase complex proteins presenilin 1/2 (PSEN1/2), and b) the considerable portion of patients with Down syndrome showing clinical manifestation of AD early in life, an event that has been attributed to triplication and overexpression of the gene coding for APP, which is located on chromosome 21.
In the brain, the main form of APP is a 695 amino acid membrane protein that, in the so-called amyloidogenic pathway, is sequentially cleaved by two enzymes: the β-site APP cleavage enzyme (BACE) and γ-secretase. BACE processes APP at the N-terminal domain of the Aβ sequence, whereas γ-secretase, a multiprotease complex that includes PSEN1/2, acts on the transmembrane domain of APP through endopeptidase/carboxypeptidase cleavages. Due to the cleavage at variable sites, γ-secretase yields Aβ peptides of different length, with Aβ40 and Aβ42 being the most common forms in the human brain [2, 3].
Indeed, APP and PSEN1/2 mutations, which are responsible for aggressive forms of familial AD (FAD), do alter the proteolytic cleavage of APP and lead to an increased ratio between longer (and more self-aggregating) and shorter forms of Aβ peptides.
A huge body of evidence has accumulated over these last 25 years showing that different forms of Aβ, from insoluble aggregates to soluble dimers/oligomers, either synthetic or derived from AD brains, can cause synaptotoxic effects and neuronal death in a variety of in vitro and in vivo models. However, with respect to the original premise, soluble Aβ peptides, rather than their insoluble fibrillar aggregates, are now thought to be the main responsible of the neuro- degenerative disorder, as they seem to better correlate with AD symptoms and severity [4, 5]. Aβ oligomers cause different types of synaptic defects, such as alteration of neurotransmitter uptake/release, cytoskeletal abnormalities, changes in receptor cellular localization and disruption of synaptic plasticity (i.e., inhibition of long-term potentiation, LTP, and enhancement of long-term depression, LTD), effects that would be causative of memory deficits, although they have never been proven in AD patients [5-7]. Ultimately, Aβ causes cell death and it has been proposed that its neurotoxic properties depend on its assembly state, with AD diffusible ligands (ADDLs) receiving more and more interest [8]. These Aβ ligands have been shown to interact with a variety of targets, from α7-nicotinic receptors to cellular prion protein [9], thus triggering multiple interacting mechanisms (Ca2+ homeostasis dysregulation, mitochondrial damage, oxidative stress, alteration of axonal transport, glial activation, etc.) responsible for the synapto- and neurotoxic effects [5, 10-12].
The amyloid cascade hypothesis has received additional support by the generation of a variety of APP or APP/PS1 transgenic mouse models that have been shown to recapitulate some of the main anatomopathological and behavioural features of FAD, such as formation of amyloid plaques, synaptic loss, synaptic plasticity alterations and memory impairment [13]. Moreover, different pharmacological, genetic and immunological approaches aimed at reducing the cerebral Aβ load in AD mice, resulted in the decrease of synaptic loss and in the rescue of memory deficits [14, 15]. These results further convinced the scientific community on the validity of the amyloid hypothesis, giving the green light to a series of clinical trials (see below).
2. The amyloid cascade hypothesis: cons
A huge amount of evidence, however, opposes the amyloid cascade hypothesis as the central event in AD pathogenesis.
As a matter of fact, the vast majority of AD cases are sporadic (SAD) and, although they also show Aβ plaques and tau neurofibrillary tangles, it is not so straightforward to conclude that FAD and SAD share the same pathogenic trigger.
It has been consistently shown that Aβ accumulation and deposition do not correlate with neuronal loss and cognitive decline, and that many individuals have significant amyloid plaque burden, assessed with PET scan, without showing symptoms of memory impairment [16-21]. Furthermore, in one PET study with 189 cognitively normal patients (age range 43-89 years), a positive correlation between CSF tau and pospho-tau (ptau181) with the amount of cortical amyloid was found. However, no correlations between brain/CSF Aβ42 or tau/ptau with psychometric test performance were observed [22].
Even for Aβ oligomers, there are a couple of points that need to be clarified. First of all, extraction and purification of soluble oligomers present in AD patients or mice are not an easy task and it is not clear whether the different isolated oligomeric species are really endogenously produced or are artifactually created during the analytical procedures. A second point is that, while Aβ oligomers are known to kill neurons in vitro [23-27], neuronal cell death is virtually absent in APP or APP/PS1 transgenic mice modelling human AD, an observation that can only lead to the conclusion that high levels of endogenous Aβ peptides, no matter what assembly state, do not trigger neurodegeneration in vivo.
This inference has changed, at least in part, the amyloidocentric scenario of AD, with tau protein coming into action to justify such a conundrum. Thus, it was shown that tau deficient neurons are resistant to Aβ neurotoxicity in vitro, and that reduction of endogenous tau in AD mouse models protects from Aβ-induced synaptotoxicity and memory deficits [28-31]. On the other hand, crossing mice carrying human tau mutations with AD mice have been shown to induce accelerated tangle formation and neuronal cell death [32-34]. In addition, several lines of evidence indicate that the amyloid peptide drives tau hyper- phosphorylation and that the two proteins can act in a synergistic fashion to cause cell death [35]. On the basis of these results, it has been postulated that, in the AD pathocascade, Aβ is “the trigger and tau is the bullet” [36].
Yet, it does not add up because both temporal and regional distribution of neurofibrillary tangles and Aβ plaques do not correlate in AD patients. Actually, tangles seem to precede plaques formation [37-39] and their distribution correlates much better than plaques with the clinical picture. Furthermore, APP and APP/PS1 mice, which indeed produce elevated levels of Aβ, do not show evidence of tangle formation, although it has been argued that mouse and human brains express different tau isoform profile [40].
Indeed, the most critical issue, in our opinion, is that much of our knowledge on Aβ pathophysiology derives from transgenic AD mice, which are increasingly put into question as to whether or not they can represent adequate models of the human pathology. As these mice carry the mutations found in FAD, it is obvious that they are not representative of late onset SAD, which affects more than 95% of AD patients. Although the two forms of AD have similar anatomo- pathological features, it is well known that they manifest at different ages, and with distinctive cognitive symptoms and disease progression [41-43]. Even in respect to FAD, the APP and APP/PS1 transgenic models have some critical drawbacks since they overexpress APP (no evidence in AD patients) and, as highlighted above, do not show tangles and a frank neuronal death. In addition, memory deficits in these animals can be almost completely rescued by pharmacological/genetic reduction of Aβ, indicating some sort of reversible cognitive damage, which does not certainly occur in AD patients. Moreover, even if the more recent triple transgenic mouse (APP/PS1/tau) reproduces the anatomo-pathological features of human AD, its relevance may be doubted, since mutations of tau are not associated with AD but with fronto-temporal dementia. In addition, using a novel approach with adeno-associated viral vectors, it has been reported that in vivo expression of human wild type Tau4R causes dramatic cell death in cortex and in CA1/2 hippocampal pyramidal neurons in the absence of accumulation of Aβ peptides [44, 45], suggesting that, contrary to the amyloid cascade hypothesis, tau-induced neurodegeneration can occur independently of Aβ.
Therefore, while these models have been certainly useful to spotlight the complexity of APP processing and Aβ formation, their importance in understanding the etiopathogenesis of AD is at best questionable, especially if we take into account their poor translational value. Indeed, we need to develop alternative models that must take into account the various genetic and environmental elements identified in human studies as main risk factors for sporadic AD (e.g. age, gender, APOE genotype) [46].
3. The physiological roles of APP and AΒ
Since the discovery of Aβ in 1984, research on AD has been almost exclusively focused on the pathological role of this small peptide. Already in 1990, however, Aβ physiological functions came into the limelight for the first time, when Yankner and collaborators [47] showed that this peptide was neurotrophic to immature hippocampal neurons. In line with this finding, it was later reported that inhibition of β/γ-secretases or Aβ immunodepletion in primary neuronal cultures resulted in a significant reduction of cell viability, which was prevented by the addition of physiological concentrations of Aβ40 [48].
Apart from neurotrophic effects, Aβ40 was also reported to enhance hippocampal LTP in the dentate gyrus [49], a finding whose real physiological implication was overlooked since, at that time, memory was only known to be impaired by this peptide.
Later on, with the application of gene knockout and RNA interference strategies, evidence started to accumulate showing physiological roles of APP and its soluble fragments in neurogenesis, neurite outgrowth, cell adhesion, modulation of ion channels, neuroprotection and vesicle exocytosis [50-52]. In particular, hippocampal LTP impairment and cognitive deficits have been reported in APP as well as BACE knockout mice, a finding that supports the critical role of APP and APP-derived peptides in the physiological processes of learning and memory [53-56], although it has to be bore in mind that BACE is involved in the processing of many substrates other than APP (see below).
With regards to Aβ42, it was found that, contrary to high (nanomolar) concentrations, picomolar levels of the peptide enhanced, rather than inhibited, hippocampal LTP in normal mice, an effect that was paralleled by the improvement of hippocampal-dependent memory assessed using the Morris water maze and the fear conditioning tasks [57]. However, the very conclusive demonstration of the physiological role of Aβ in the process of memory formation was provided by behavioural and electrophysiological experiments in which the endogenous peptide was blocked by selective antibodies. As a matter of fact, under these conditions, mice showed significant cognitive impairment [58, 59] and hippocampal LTP was abrogated [59], both effects being rescued by the addition of exogenous Aβ42. These results were confirmed using APP siRNA and supported by the evidence that endogenous levels of the peptide are increased during learning and memory formation [59].
Interestingly, the physiological effects of Aβ on LTP and memory seem to be mediated by α7 nicotinic receptors (α7-nAChRs), as they are not observed in α7-nAChRs knockout mice [57, 59]. Accordingly, physiological concentrations of Aβ were shown to potentiate presynaptic α7-nAChRs, stimulating the release of glutamate and aspartate, the two major excitatory neurotransmitters involved in hippocampal LTP [60].
Our laboratory has recently demonstrated that APP expression and Aβ production are under the control of the adenylyl cyclase/cAMP/PKA pathway and that Aβ is necessary for cAMP to maintain LTP and memory consolidation [61-63]. In particular, we showed that increasing intracellular cAMP by blocking its degradation with the selective PDE4 inhibitor rolipram, activates the translation of APP mRNA with the consequent increase of Aβ production. Most importantly, we demonstrated that the well-known rolipram-induced enhancement of hippocampal LTP is prevented by anti-Aβ antibodies in normal mice and is lost in APP knockout mice [62].
Finally, several in vitro and in vivo evidence suggest that soluble Aβ oligomers may play a protective role against microbial infections, thus taking part in the innate immune response [64, 65].
In conclusion, Aβ peptides, whatever their main role, are physiologically produced in the central nervous system, as clearly indicated by their presence in the extracellular milieu of normal mouse brain [66], as well as in the cerebrospinal fluid of healthy individuals [67, 68].
4. The failure of anti-AΒ therapies
On the basis of the amyloid hypothesis and the promising results obtained in AD mouse models, different therapeutic strategies, aimed at clearing Aβ from the brain, have been the object of several clinical trials. Here, we summarize the results of the three main approaches: Aβ immunization, γ- and β-secretase inhibitors.
4.1. Active and Passive Immunization Against Aβ
In 2000, AN-1792, the first vaccine against Aβ42 was trialled in phase II on AD patients and, although the trial had to be stopped due to severe side effects, the outcomes in antibody responders were not different from placebo-treated controls [69]. These negative effects occurred despite the decrease of amyloid plaques observed in autoptic brains of vaccinated patients [69-73]. Yet, analysis of the z-score composite in the small antibody-responder population showed some reduced cognitive decline [69], which seemed to be maintained over time as reported in a follow-up study that, however, enrolled only part of these patients [74]. Nevertheless, progression to severe stages of AD was not halted [73].
Other two anti-Aβ vaccines have been tested, namely CAD-106 and ACC-001. In a phase I trial, CAD-106 did not cause serious adverse effects and almost 75% of the treated AD patients showed an adequate antibody production [75]. Safety and tolerability of this vaccine in long-term treatments (52 weeks) has been recently confirmed in phase II [76], yet no data on its clinical efficacy are available. As for ACC-001, the results of two phase IIa trials have been recently published, showing no differences between treatment and control groups in exploratory cognitive evaluations, volumetric brain MRI and CSF biomarkers [77].
With respect to other approaches, passive immunization has been certainly the more investigated so far. Bapineuzumab has been the first N-terminus (Aβ1-5) directed anti-Aβ antibody (able to bind fibrillar, oligomeric and monomeric forms) to be tested in humans. In a first phase II trial, primary efficacy outcomes with this humanized monoclonal antibody were not significant, thus indicating that there was no cognitive amelioration in treated AD patients; however, exploratory analysis suggested potential efficacy in APOE ε4 noncarriers [78]. Unfortunately, two large, double-blind, randomized, placebo-controlled phase III clinical trials undoubtedly concluded that bapineuzumab is ineffective in ameliorating cognitive deficits, irrespective of APOE genotype, and revealed significant adverse effects [79]. Despite this failure, another humanized monoclonal antibody named solanezumab was developed that, at variance with bapineuzumab, recognizes soluble monomeric, not fibrillar, Aβ. In a first phase II trial, solanezumab was shown to increase Aβ plasma and CSF levels in a dose-dependent manner, a result compatible with the enhanced clearance of plaques in brain, but it had no significant effects on cognition as assessed by ADAS-Cog [80]. Nevertheless, solanezumab entered two double-blind, randomized, placebo controlled phase III trials (EXPEDITION 1 and EXPEDITION 2) with an identical design for a total of 2052 patients. Also in this case, the studies did not meet the primary outcomes (changes in ADAS-Cog11 and ADCS-ADL) and only EXPEDITION 2 showed some reduction in cognitive decline using a different analysis (ADAS-Cog14) [81]. In a subsequent secondary analysis of efficacy, data from the two trials were pooled and less cognitive and functional decline was observed in treated patients (503 individuals) compared to those receiving placebo (521), with a percentage of slowing ranging from 18 (ADCS-ADL) to 34% (ADAS-Cog14, MMSE), whilst no differences were found in other tests [82]. It is worth noting, however, that the difference in the ADAS-Cog analysis was of 2 points on an 80-point scale, which is rather disappointing. On the basis of the reasoning that the sooner the treatment starts, the better the functional outcome and the clinical results can be expected, a third phase III trial (EXPEDITION 3; NCT01900665) investigated for the first time the effects of solanezumab only in patients with a clinical diagnosis of early mild AD. The study’s negative results, although not yet published, were announced on 23 November 2016 by the company sponsoring the trial [83]. Solanezumab did not meet the primary endpoint as patients did not show a significant slowing in cognitive decline measured by the ADAS-Cog14 and also the effects on secondary endpoints were small.
At present, two phase III trials are recruiting participants to further investigate the effects of solanezumab: the A4 study in asymptomatic patients who shows biomarker evidence of amyloid deposition (NCT02008357) and the ExpeditionPRO in patients with a clinical diagnosis of MCI or prodromal AD (NCT02760602) that are estimated to complete in 2020 and 2021, respectively.
Gantenerumab is a human antibody directed against the aggregated forms of Aβ, which recognizes both the N-terminus and the mid-domain of Aβ [84, 85]. In a first double-blind, randomized, placebo controlled PET trial, gantenerumab was shown to reduce cerebral amyloid levels in a dose-dependent fashion in mild to moderate AD patients (36% difference from placebo at the highest dose tested) [86]. The study, however, was not powered to detect efficacy on cognitive parameters. At the moment, there are two active phase III trials evaluating gantenerumab in prodromal (NCT01224106) and mild AD patients (NCT02051608), and one phaseII/III trial in which gantenerumab and solanezumab will be tested in individuals at risk for FAD who are cognitively normal or with mild AD or dementia (NCT01760005).
The first results of a phase Ib trial with aducanumab (antibody selective for aggregated Aβ) on prodromal or mild AD patients have been recently reported [87]. A small, though significant, reduction (approx.19%) of PET-monitored brain Aβ levels was observed after one year of monthly i.v. infusions of the highest dose of aducanumab and this decrease was similar in patients with prodromal or mild AD. Also a significant slowing of cognitive decline has been measured by MMSE and CDR-SB but no effects were seen in NTB and FCSRT. In any case, the number of patients was rather low (30 placebo-treated patients and 4 groups of antibody-treated patients with approx. 24 subjects/group) and, unfortunately, there are loads of examples of promising results observed in phase I trials that have not been confirmed in large phase II/III trials.
In this context, it is also worth mentioning the ADAD (Autosomal Dominant Alzheimer’s Disease) study by the Alzheimer’s Prevention Initiative, that is a phase II clinical trial designed to test crenezumab, another anti-Aβ antibody, in 300 cognitively healthy individuals of an extended family in Colombia, who are destined to develop AD since they carry a rare autosomal dominant mutation (PSEN1 E280A; NCT01998841). This study is estimated to complete by September 2020.
As one of the reasons called into question to explain the therapeutic failure of the anti-Aβ immunization is that treatments have been tested in patients with ongoing irreparable neurodegenerative processes, the results of the above-mentioned AD prevention trials will be of fundamental importance to determine the final fate of the amyloid hypothesis.
4.2. β-and γ-secretase Inhibitors
In the attempt to lower cerebral Aβ levels, BACE1 and γ-secretase, the two enzymes sequentially involved in its production, have been investigated as possible therapeutic targets.
BACE1 is a transmembrane aspartyl protease that acts on APP to yield sAPPβ and CTFβ, and catalyses the rate-limiting step in the synthesis of Aβ. However, it is now clear that this β-secretase has many others substrates. One of the first identified and characterized is Neuregulin 1 whose processing by BACE1 is necessary to regulate axon myelination in the CNS and PNS, but proteomic analysis has identified at least 40 novel BACE1 substrates over the last few years [88].
By analysing the phenotype of homozygote Bace1 knockout mice, it has now clearly emerged that the absence of BACE1 activity induces multiple anatomical and functional alterations in the CNS including, but not limited to, impairment of synaptic plasticity (e.g., LTP) and cognitive deficits [55, 89-92]. In addition, BACE1 knockout has been shown to provoke muscle spindle reduction and retinal degeneration [93, 94]. Finally, a recent study has reported that BACE1, which is also present in cardiac myocytes, modulates gating of the voltage-dependent K+ channels KCNQ1 and KCNE1 [95].
Pioglitazone and rosiglitazone, two thiazolidinediones used in the type 2 diabetes therapy for their action on insulin and carbohydrate metabolism, have been recently trialled in AD as they are able to stimulate the clearance of Aβ [96, 97] and suppress BACE1 transcription [98] by activating the nuclear factor PPAR-γ. Initial clinical trials with these drugs produced diverse results, with some of them showing cognitive improvement in AD or MCI patients, but a large randomized experimentation failed to demonstrate a significant benefit, even when considering the APOE-ε4 genotype [99].
Following a series of phase I studies demonstrating the safety, tolerability and great efficacy in reducing plasma/ CSF Aβ levels (up to 80% decrease), novel β-secretase inhibitors have advanced to phase II/III trial on prodromal as well as mild to moderate AD patients (MK-8931 NCT01739348, NCT01953601; E2609, NCT02322021; LY3314814 NCT02783573, NCT02245737; https://www.clinicaltrials.gov/ct2/home). However, no results are available at the moment. Other phase II clinical trials on BACE1 inhibitors, such as LY2886721 or RG7129, were terminated because of liver toxicity that seemed to be due to off-target effects [88].
Semagacestat (LY-450139) is a γ-secretase inhibitor that has been tested in two large randomized, double-blind, placebo-controlled phase III trials on mild to moderate AD patients (NCT00594568, NCT00762411). Both studies were terminated before completation since an interim futility analysis showed that the treatment with Semagacestat was ineffective or even worsened cognitive decline, and there were more adverse events (e.g., skin cancer, infections) in comparison with the placebo arm [100, 101]. These adverse effects have been especially linked to the inhibition of γ-secretase-mediated cleavage of Notch, a protein that plays a fundamental role in development and cell differentiation [2].
Begacestat (GSI-953) and Avagacestat (BMS-708163) are other two γ-secretase inhibitors that have been claimed to possess Notch-sparing properties [102, 103] although their selectivity has also been questioned [104, 105]. The first has been shown to reduce Aβ in plasma of healthy subjects [106] but no other results have been reported. The results of a randomized, double-blind, placebo-controlled phase II study with Avagacestat have been recently published [107]. This trial was carried out on a small but significant population of patients with prodromal AD (263 individuals), identified on the basis of CSF biomarker criteria and MCI symptoms. Avagacestat did not demonstrate any disease modifying effect at 2 years, when the progression to dementia was similar to that of the placebo-treated population, as it was also the brain atrophy rate assessed by volumetric MRI. On the contrary, a trend toward more progression was observed in the treatment group at one year, although it was not statistically significant. In addition, Avagacestat increased the frequency of adverse events, including non-melanoma skin cancer, thus casting doubts on its Notch sparing properties.
Beside full inhibitors, γ-secretase modulators have also been developed and tested on AD. Although the exact mechanism of action still remains unclear, these modulators are able to decrease the production of Aβ42 without reducing overall Aβ levels, do not increase APP CTFs and do not alter Notch processing [108]. On the other hand, they enhance Aβ38 production and it has been hypothesised that this is due to the alteration of processing events subsequent to the initial γ-secretase cleavage [109].
Tarenflurbil has been the first γ-secretase modulator to be tested in AD patients. In a first phase II study [110], it showed slowing of decline in mild but not in moderate AD, with significant changes in some scores (ADCS-ADL and CDR-SB) but not in others (ADAS-cog). Again, when this drug was trialled in a large randomized, double-blind, placebo-controlled phase III study, it showed no beneficial effects [111].
Conclusion
After 25 years of experimentations driven by the amyloid cascade hypothesis, we certainly know much more about the processing of APP and the biochemistry of Aβ peptides. Yet, we have still a lot to learn on the physiological functions of these proteins, as well as of the enzymes involved in the amyloidogenic and non-amyloidogenic pathways. In fact,
evidence has been accumulating showing that APP and Aβ play key functions in a variety of central processes, including memory formation and consolidation [112]. Moreover, Aβ40 and Aβ42 are not the only peptides originating from canonical APP processing, since many other fragments are produced during α-, β- and γ-secretase cleavage (e.g. sAPPα, sAPPβ, Aβ38,46,49) and we know very little about their functional roles. In addition, it is now clear that β- and γ-secretases process a vast number of substrates that are involved in a variety of physiological events [2, 88], thus suggesting that these enzymes may not be the optimal target for therapeutic interventions. Finally, other APP cleavage mechanisms are emerging (e.g. δ- and η-secretases), which lead to many different fragments that have shown biological activity [113] and that need to be further investigated to comprehensively understand their physiological role, if any.
Keeping this in mind, all the negative results of the anti-Aβ strategies trialled so far in AD patients undoubtedly demonstrate that this peptide is not the pathogenetic factor we were seeking, although it might participate to the evolution of the disease. Indeed, in the best-case scenario, lowering cerebral Aβ levels has resulted in some delay of the cognitive decline but it has not arrested the progression to dementia, thus demonstrating an efficacy similar to that of existing therapies (e.g., cholinesterase inhibitors).
Although a possible pathogenetic role of Aβ cannot be completely ruled out at present, the large and solid body of evidence accumulated to date points to the waning of the amyloid cascade hypothesis and indicates that the scientific community should still devote its utmost efforts to identify the real culprit of Alzheimer’s disease.
ACKNOWLEDGEMENTS
Roberta Ricciarelli and Ernesto Fedele equally contributed to the collection and review of the relevant literature, and to the draft and revision of the manuscript.
List of abbreviations
- ADAS-cog
Alzheimer’s disease assessment scale-cognition
- ADCS-ADL
Alzheimer’s disease cooperative study-activities of daily living
- APOE
APOlipoprotein E
- cAMP
Cyclic adenosine mono phosphate
- CDR-SB
Clinical dementia rating-sum of boxes
- CNS
Central nervous system
- CSF
Cerebro-spinal fluid
- CTFβ
Carboxy-terminal fragment beta
- FCSRT
Free and cued selective reminding test
- LTP
Long-term potentiation
- LTD
Long-term depression
- MCI
Mild cognitive impairment
- MMSE
Mini mental state examination
- MRI
Magnetic resonance imaging
- NTB
Neuropsychological test battery
- PET
Positron emission tomography
- PKA
Protein kinase A
- PPAR-γ
Perixosome proliferator-activated receptor-γ
- sAPPα/β
Soluble APPα/β
- siRNA
Small interference RNA
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hardy J.A., Higgings G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [http://dx.doi.org/ 10.1126/science.1566067]. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X., Li Y., Xu H., Zhang Y. The γ-secreatse complex: from structure to function. Front. Cell. Neurosci. 2014;8:427. doi: 10.3389/fncel.2014.00427. [http:// dx.doi.org/10.3389/fncel.2014.00427]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiwari M.K., Kepp K.P. β-Amyloid pathogenesis: Chemical properties versus cellular levels. Alzheimers Dement. 2016;12:184–194. doi: 10.1016/j.jalz.2015.06.1895. [http://dx.doi.org/10.1016/j.jalz.2015.06.1895]. [DOI] [PubMed] [Google Scholar]
- 4.Mucke L., Selkoe D.J. Neurotoxicity of amylod β-protein: synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [http://dx.doi.org/10.1101/cshperspect. a006338]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira S.T., Lourenco M.V., Oliveira M.M., De Felice F.G. Soluble amyloid-β oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell. Neurosci. 2015;26:9–191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira S.T., Klein W.L. The Aβ oligomer hypothesis for synapse failure and mmemory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011;96:529–543. doi: 10.1016/j.nlm.2011.08.003. [http://dx.doi.org/10.1016/j.nlm. 2011.08.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koffie R.M., Hyman B.T., Soires-Jones T.L. Alzheimer’s disease: synapses gone cold. Mol. Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [http://dx.doi.org/10.1186/1750-1326-6-63]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krafft G.A., Klein W.L. ADDLs and the signalling web that leads to Alzheimer’s disease. Neuropharmacology. 2010;59:230–242. doi: 10.1016/j.neuropharm.2010.07.012. [http://dx.doi.org/10.1016/j.neuropharm.2010.07.012]. [DOI] [PubMed] [Google Scholar]
- 9.Xia M., Cheng X., Yi R., Gao D., Xiong J. The binding receptors of Aβ: an alternative therapeutic target for Alzheimer’s disease. Mol. Neurobiol. 2016;53:455–471. doi: 10.1007/s12035-014-8994-0. [http://dx.doi.org/10. 1007/s12035-014-8994-0]. [DOI] [PubMed] [Google Scholar]
- 10.Garwood C.J., Ratcliffe L.E., Simpson J.E., Health P.R., Ince P.G., Wharton S.B. Review: Astrocytes in Alzheimer’s disease and other age-associated dementias; a supporting player with a central role. Neuropathol. Appl. Neurobiol. 2016;43:281–298. doi: 10.1111/nan.12338. [http://dx. doi.org/10.1111/nan.12338]. [DOI] [PubMed] [Google Scholar]
- 11.Kamat P.K., Kalani A., Rai S., Swarnkar S., Tota S., Nath C., Tyagi N. Mechanisms of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutic strategies. Mol. Neurobiol. 2016;53:648–661. doi: 10.1007/s12035-014-9053-6. [http:// dx.doi.org/10.1007/s12035-014-9053-6]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrillo-Mora P., Luna R., Colìn-Barenque L. Amyloid beta: multiple mechanisms of toxicity and only some protective effects? Oxid. Med. Cell. Longev. 2014. [DOI] [PMC free article] [PubMed]
- 13.Puzzo D., Lee L., Palmeri A., Calabrese G., Arancio O. Behavioral assays with mouse models of Alzheimer’s disease: practical considerations and guidelines. Biochem. Pharmacol. 2014;88:450–467. doi: 10.1016/j.bcp.2014.01.011. [http://dx.doi.org/10.1016/j.bcp.2014.01.011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Götz J., Streffer J.R., David D., Schild A., Hoerndli F., Pennanen L., Kurosinski P., Chen F. Transgenic animal models of Alzheimer’s disease and related disorders: hystopathology, behavior and therapy. Mol. Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 15.Li C., Ebrahimi A., Schluesener H. Drug pipeline in neuro- degeneration based on transgenic mice models of Alzheimer’s disease. Ageing Res. Rev. 2013;12:116–140. doi: 10.1016/j.arr.2012.09.002. [http://dx.doi.org/10. 1016/j.arr.2012.09.002]. [DOI] [PubMed] [Google Scholar]
- 16.Katzman R., Terry R., DeTeresa R., Brown T., Davies P., Fuld P., Rebing X., Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [http://dx.doi.org/10.1002/ana.410230206]. [DOI] [PubMed] [Google Scholar]
- 17.Delaère P., Duyckaerts C., Masters C., Beyreuther K., Piette F., Hauw J.J. Large amounts of neocortical beta A4 deposits without neuritic plaques nor tangles in a psychometrically assessed, non-demented person. Neurosci. Lett. 1990;116:87–93. doi: 10.1016/0304-3940(90)90391-l. [http://dx.doi.org/10.1016/0304-3940(90)90391-L]. [DOI] [PubMed] [Google Scholar]
- 18.Dickson D.W., Crystal H.A., Mattiace L.A., Masur D.M., Blau A.D., Davies P., Yen S.H., Aronson M.K. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol. Aging. 1992;13:178–189. doi: 10.1016/0197-4580(92)90027-u. [http://dx. doi.org/10.1016/0197-4580(92)90027-U]. [DOI] [PubMed] [Google Scholar]
- 19.Aizenstein H.J., Nebes R.D., Saxton J.A., Price J.C., Mathis C.A., Tsopelas N.D., Ziolko S.K., James J.A., Snitz B.E., Houck P.R., Bi W., Cohen A.D., Lopresti B.J., DeKosky S.T., Halligan E.M., Klunk W.E. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [http://dx.doi.org/10.1001/archneur.65.11.1509]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klunk W.E., Mathis C.A., Price J.C., DeKosky S.T., Lopresti B.J., Tsopelas N.D., Saxton J.A., Nebes R.D. Amyloid imaging with PET in Alzheimer’s disease, mild cognitive impairment, and clinically unimpaired subjects. 2009. pp. 119–147.
- 21.Villemagne V.L., Pike K.E., Chételat G., Ellis K.A., Mulligan R.S., Bourgeat P., Ackermann U., Jones G., Szoeke C., Salvado O., Martins R., O’Keefe G., Mathis C.A., Klunk W.E., Ames D., Masters C.L., Rowe C.C. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann. Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [http://dx.doi.org/10.1002/ana.22248]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagan A.M., Mintun M.A., Shah A.R., Aldea P., Roe C.M., Mach R.H., Marcus D., Morris J.C., Holtzman D.M. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol. Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [http://dx.doi.org/10.1002/emmm.200900048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., Wals P., Zhang C., Finch C.E., Krafft G.A., Klein W.L. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [http://dx.doi.org/10.1073/pnas.95.11.6448]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H.J., Chae S.C., Lee D.K., Chromy B., Lee S.C., Park Y.C., Klein W.L., Krafft G.A., Hong S.T. Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 2003;17:118–120. doi: 10.1096/fj.01-0987fje. [DOI] [PubMed] [Google Scholar]
- 25.De Felice F.G., Velasco P.T., Lambert M.P., Viola K., Fernandez S.J., Ferreira S.T., Klein W.L. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [http:// dx.doi.org/10.1074/jbc.M607483200]. [DOI] [PubMed] [Google Scholar]
- 26.Ono K., Condron M.M., Teplow D.B. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc. Natl. Acad. Sci. USA. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [http://dx.doi.org/10.1073/pnas. 0905127106]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed M., Davis J., Aucoin D., Sato T., Ahuja S., Aimoto S., Elliott J.I., Van Nostrand W.E., Smith S.O. Structural conversion of neurotoxic amyloid-β(1–42) oligomers to fibrils. Nat. Struct. Mol. Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [http://dx.doi.org/10.1038/nsmb.1799]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapoport M., Dawson H.N., Binder L.I., Vitek M.P., Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [http://dx.doi.org/10.1073/ pnas.092136199]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [http://dx. doi.org/10.1126/science.1141736]. [DOI] [PubMed] [Google Scholar]
- 30.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A., Eckert A., Staufenbiel M., Hardeman E., Götz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [http://dx.doi.org/ 10.1016/j.cell.2010.06.036]. [DOI] [PubMed] [Google Scholar]
- 31.Leroy K., Ando K., Laporte V., Dedecker R., Suain V., Authelet M., Héraud C., Pierrot N., Yilmaz Z., Octave J.N., Brion J.P. Lack of tau proteins rescues neuronal cell death and decreases amyloidogenic processing of APP in APP/PS1 mice. Am. J. Pathol. 2012;181:1928–1940. doi: 10.1016/j.ajpath.2012.08.012. [http://dx.doi.org/10.1016/j. ajpath.2012.08.012]. [DOI] [PubMed] [Google Scholar]
- 32.Lewis J., Dickson D.W., Lin W.L., Chisholm L., Corral A., Jones G., Yen S.H., Sahara N., Skipper L., Yager D., Eckman C., Hardy J., Hutton M., McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [http://dx.doi.org/10.1126/science. 1058189]. [DOI] [PubMed] [Google Scholar]
- 33.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [http://dx.doi.org/10.1016/S0896-6273(03)00434-3]. [DOI] [PubMed] [Google Scholar]
- 34.Ribé E.M., Pérez M., Puig B., Gich I., Lim F., Cuadrado M., Sesma T., Catena S., Sánchez B., Nieto M., Gómez-Ramos P., Morán M.A., Cabodevilla F., Samaranch L., Ortiz L., Pérez A., Ferrer I., Avila J., Gómez-Isla T. Accelerated amyloid deposition, neurofibrillary degeneration and neuronal loss in double mutant APP/tau transgenic mice. Neurobiol. Dis. 2005;20:814–822. doi: 10.1016/j.nbd.2005.05.027. [http://dx.doi.org/10.1016/j.nbd.2005.05.027]. [DOI] [PubMed] [Google Scholar]
- 35.Ittner L.M., Götz J. Amyloid-β and tau-a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [http:// dx.doi.org/10.1038/nrn2967]. [DOI] [PubMed] [Google Scholar]
- 36.Bloom G.S. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [http://dx.doi.org/10.1001/jamaneurol.2013.5847]. [DOI] [PubMed] [Google Scholar]
- 37.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [http://dx. doi.org/10.1007/BF00308809]. [DOI] [PubMed] [Google Scholar]
- 38.Price J.L., Davis P.B., Morris J.C., White D.L. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol. Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [http://dx.doi.org/10.1016/0197-4580(91)90006-6]. [DOI] [PubMed] [Google Scholar]
- 39.Schönheit B., Zarski R., Ohm T.G. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol. Aging. 2004;25:697–711. doi: 10.1016/j.neurobiolaging.2003.09.009. [http://dx.doi.org/10.1016/ j.neurobiolaging.2003.09.009]. [DOI] [PubMed] [Google Scholar]
- 40.Drummond E., Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2016;133:155–175. doi: 10.1007/s00401-016-1662-x. [http://dx.doi.org/10.1007/s00401-016-1662-x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farrer L.A., Myers R.H., Cupples L.A., St. George-Hyslop P.H., Bird T.D., Rossor M.N., Mullan M.J., Polinsky R., Nee L., Heston L., van Broeckoven C., Martin J.J., Crapper MacLachlan D., Growdon J.H. Transmission and age-at-onset patterns in familial Alzheimer’s disease: evidence for heterogeneity. Neurology. 1990;40:394–403. doi: 10.1212/wnl.40.3_part_1.395. [http://dx.doi.org/10.1212/WNL.40.3_Part_1.395]. [DOI] [PubMed] [Google Scholar]
- 42.Stopford C.L., Snowden J.S., Thompson J.C., Neary D. Variability in cognitive presentation of Alzheimer’s disease. Cortex. 2008;44:185–195. doi: 10.1016/j.cortex.2005.11.002. [http://dx.doi.org/10.1016/j.cortex. 2005.11.002]. [DOI] [PubMed] [Google Scholar]
- 43.Komarova N.L., Thalhauser C.J. High degree of heterogeneity in Alzheimer’s disease progression patterns. PLOS Comput. Biol. 2011;7:e1002251. doi: 10.1371/journal.pcbi.1002251. [http://dx.doi.org/10.1371/journal.pcbi.1002251]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaworski T., Dewachter I., Lechat B., Croes S., Termont A., Demedts D., Borghgraef B., Devijver H., Filipkowski R., Kaczmarek L., Kügler S., Van Leuven F. AAV-Tau mediates pyramidal neuro-degeneration by cell-cycle re-entry without neurofibrillary tangle formation in wild-type mice. PLoS One. 2009;4:e7280. doi: 10.1371/journal.pone.0007280. [http://dx.doi.org/10.1371/journal.pone.0007280]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaworski T., Dewachter I., Seymore C.M., Borghgraef B., Devijver H., Kügler S., Van Leuven F. Alzheimer’s disease: old problem, new views from transgenic and viral models. Biochim. Biophys. Acta, 2010:808–818. doi: 10.1016/j.bbadis.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Onos K.D., Sukoff R.S., Howell G.R., Sasner M. Toward more predictive genetic mouse models of Alzheimer’s disease. Brain Res. Bull. 2016;122:1–11. doi: 10.1016/j.brainresbull.2015.12.003. [http://dx.doi.org/10.1016/j.brainresbull. 2015.12.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yankner B.A., Duffy L.K., Kirschner D.A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [http://dx.doi.org/ 10.1126/science.2218531]. [DOI] [PubMed] [Google Scholar]
- 48.Plant L.D., Boyle J.P., Smith I.F., Peers C., Pearson H.A. The production of amyloid beta peptide is a critical requirement for the viability of central neurons. J. Neurosci. 2003;23:5531–5535. doi: 10.1523/JNEUROSCI.23-13-05531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J., Anwyl R., Rowan M.J. beta-Amyloid-(1-40) increases long-term potentiation in rat hippocampus in vitro. Eur. J. Pharmacol. 1995;284:R1–R3. doi: 10.1016/0014-2999(95)00539-w. [http://dx.doi.org/10.1016/0014-2999(95)00539-W]. [DOI] [PubMed] [Google Scholar]
- 50.Aydin D., Weyer S.W., Müller U.C. Functions of the APP gene family in the nervous system: insights from mouse models. Exp. Brain Res. 2012;217:423–434. doi: 10.1007/s00221-011-2861-2. [http://dx.doi.org/10.1007/s00221-011-2861-2]. [DOI] [PubMed] [Google Scholar]
- 51.Müller U.C., Zheng H. Physiological functions of APP family proteins. Cold Spring Harb. Perspect. Med. 2012;2:a006288. doi: 10.1101/cshperspect.a006288. [http://dx.doi.org/10.1101/cshperspect.a006288]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nalivaeva N.N., Turner A.J. The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett. 2013;587:2046–2054. doi: 10.1016/j.febslet.2013.05.010. [http://dx.doi.org/10.1016/j. febslet.2013.05.010]. [DOI] [PubMed] [Google Scholar]
- 53.Dawson G.R., Seabrook G.R., Zheng H., Smith D.W., Graham S., O’Dowd G., Bowery B.J., Boyce S., Trumbauer M.E., Chen H.Y., Van der Ploeg L.H., Sirinathsinghji D.J. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [http://dx.doi.org/10.1016/ S0306-4522(98)00410-2]. [DOI] [PubMed] [Google Scholar]
- 54.Seabrook G.R., Smith D.W., Bowery B.J., Easter A., Reynolds T., Fitzjohn S.M., Morton R.A., Zheng H., Dawson G.R., Sirinathsinghji D.J., Davies C.H., Collingridge G.L., Hill R.G. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [http://dx.doi.org/10.1016/ S0028-3908(98)00204-4]. [DOI] [PubMed] [Google Scholar]
- 55.Laird F.M., Cai H., Savonenko A.V., Farah M.H., He K., Melnikova T., Wen H., Chiang H.C., Xu G., Koliatsos V.E., Borchelt D.R., Price D.L., Lee H.K., Wong P.C. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [http://dx.doi.org/10.1523/JNEUROSCI.2766-05.2005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., Song L., Laird F., Wong P.C., Lee H.K. BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J. Neurosci. 2008;28:8677–8681. doi: 10.1523/JNEUROSCI.2440-08.2008. [http://dx.doi.org/ 10.1523/JNEUROSCI.2440-08.2008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puzzo D., Privitera L., Leznik E., Fa M., Staniszewski A., Palmeri A., Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008;28:14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.2692-08.2008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Osta A., Alberini C.M. Amyloid beta mediates memory formation. Learn. Mem. 2009;16:267–272. doi: 10.1101/lm.1310209. [http://dx.doi.org/10. 1101/lm.1310209]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puzzo D., Privitera L., Fa’ M., Staniszewski A., Hashimoto G., Aziz F., Sakurai M., Ribe E.M., Troy C.M., Mercken M., Jung S.S., Palmeri A., Arancio O. Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann. Neurol. 2011;69:819–830. doi: 10.1002/ana.22313. [http://dx.doi.org/10.1002/ana.22313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mura E., Zappettini S., Preda S., Biundo F., Lanni C., Grilli M., Cavallero A., Olivero G., Salamone A., Govoni S., Marchi M. Dual effect of beta-amyloid on α7 and α4β2 nicotinic receptors controlling the release of glutamate, aspartate and GABA in rat hippocampus. PLoS One. 2012;7:e29661. doi: 10.1371/journal.pone.0029661. [http://dx.doi.org/ 10.1371/journal.pone.0029661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canepa E., Domenicotti C., Marengo B., Passalacqua M., Marinari U.M., Pronzato M.A., Fedele E., Ricciarelli R. Cyclic adenosine monophosphate as an endogenous modulator of the amyloid-β precursor protein metabolism. IUBMB Life. 2013;65:127–133. doi: 10.1002/iub.1109. [http://dx.doi.org/10.1002/iub.1109]. [DOI] [PubMed] [Google Scholar]
- 62.Ricciarelli R., Puzzo D., Bruno O., Canepa E., Gardella E., Rivera D., Privitera L., Domenicotti C., Marengo B., Marinari U.M., Palmeri A., Pronzato M.A., Arancio O., Fedele E. A novel mechanism for cyclic adenosine monophosphate-mediated memory formation: Role of amyloid beta. Ann. Neurol. 2014;75:602–607. doi: 10.1002/ana.24130. [http://dx.doi.org/10.1002/ana.24130]. [DOI] [PubMed] [Google Scholar]
- 63.Rivera D., Fedele E., Marinari U.M., Pronzato M.A., Ricciarelli R. Evaluating the role of hnRNP-C and FMRP in the cAMP-induced APP metabolism. Biofactors. 2015;41:121–126. doi: 10.1002/biof.1207. [http:// dx.doi.org/10.1002/biof.1207]. [DOI] [PubMed] [Google Scholar]
- 64.Bourgade K., Dupuis G., Frost E.H., Fülöp T. Anti-viral properties of amyloid- β peptides. J. Alzheimers Dis. 2016;54:859–878. doi: 10.3233/JAD-160517. [http://dx.doi.org/10.3233/JAD-160517]. [DOI] [PubMed] [Google Scholar]
- 65.Kumar D.K., Choi S.H., Washicosky K.J., Eimer W.A., Tucker S., Ghofrani J., Lefkowitz A., McColl G., Goldstein L.E., Tanzi R.E., Moir R.D. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [http://dx.doi.org/10.1126/ scitranslmed.aaf1059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cirrito J.R., May P.C., O’Dell M.A., Taylor J.W., Parsadanian M., Cramer J.W., Audia J.E., Nissen J.S., Bales K.R., Paul S.M., DeMattos R.B., Holtzman D.M. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J. Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehta P.D., Pirttila T. Increased cerebrospinal fluid A beta38/A beta42 ratio in Alzheimer disease. Neurodegener. Dis. 2005;2:242–245. doi: 10.1159/000090363. [http://dx.doi.org/10.1159/000090363]. [DOI] [PubMed] [Google Scholar]
- 68.Giedraitis V., Sundelöf J., Irizarry M.C., Gårevik N., Hyman B.T., Wahlund L.O., Ingelsson M., Lannfelt L. The normal equilibrium between CSF and plasma amyloid beta levels is disrupted in Alzheimer’s disease. Neurosci. Lett. 2007;427:127–131. doi: 10.1016/j.neulet.2007.09.023. [http://dx.doi.org/10.1016/j.neulet.2007.09.023]. [DOI] [PubMed] [Google Scholar]
- 69.Gilman S., Koller M., Black R.S., Jenkins L., Griffith S.G., Fox N.C., Eisner L., Kirby L., Rovira M.B., Forette F., Orgogozo J.M. AN1792(QS-21)-201 Study Team. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [http://dx. doi.org/10.1212/01.WNL.0000159740.16984.3C]. [DOI] [PubMed] [Google Scholar]
- 70.Nicoll J.A., Wilkinson D., Holmes C., Steart P., Markham H., Weller R.O. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [http://dx.doi.org/10.1038/nm840]. [DOI] [PubMed] [Google Scholar]
- 71.Ferrer I., Boada R.M., Sánchez G.M., Rey M.J., Costa-Jussá F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [http://dx.doi.org/10.1111/j.1750-3639.2004.tb00493.x]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masliah E., Hansen L., Adame A., Crews L., Bard F., Lee C., Seubert P., Games D., Kirby L., Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [http://dx.doi. org/10.1212/01.WNL.0000148590.39911.DF]. [DOI] [PubMed] [Google Scholar]
- 73.Holmes C., Boche D., Wilkinson D., Yadegarfar G., Hopkins V., Bayer A., Jones R.W., Bullock R., Love S., Neal J.W., Zotova E., Nicoll J.A. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [http://dx.doi.org/10. 1016/S0140-6736(08)61075-2]. [DOI] [PubMed] [Google Scholar]
- 74.Vellas B., Black R., Thal L.J., Fox N.C., Daniels M., McLennan G., Tompkins C., Leibman C., Pomfret M., Grundman M. AN1792 (QS-21)-251 Study Team. Long-term follow-up of patients immunized with AN1792: reduced functional decline in antibody responders. Curr. Alzheimer Res. 2009;6:144–151. doi: 10.2174/156720509787602852. [http://dx.doi.org/10.2174/156720509787602852]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winblad B., Andreasen N., Minthon L., Floesser A., Imbert G., Dumortier T., Maguire R.P., Blennow K., Lundmark J., Staufenbiel M., Orgogozo J.M., Graf A. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [http://dx.doi.org/10.1016/S1474-4422(12)70140-0]. [DOI] [PubMed] [Google Scholar]
- 76.Farlow M.R., Andreasen N., Riviere M.E., Vostiar I., Vitaliti A., Sovago J., Caputo A., Winblad B., Graf A. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res. Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [http://dx.doi.org/10.1186/s13195-015-0108-3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pasquier F., Sadowsky C., Holstein A., Leterme Gle P., Peng Y., Jackson N., Fox N.C., Ketter N., Liu E., Ryan J.M. Two Phase 2 Multiple Ascending-Dose Studies of Vanutide Cridificar (ACC-001) and QS-21 Adjuvant in Mild-to-Moderate Alzheimer’s Disease. J. Alzheimers Dis. 2016;51:1131–1143. doi: 10.3233/JAD-150376. [http://dx.doi. org/10.3233/JAD-150376]. [DOI] [PubMed] [Google Scholar]
- 78.Salloway S., Sperling R., Gilman S., Fox N.C., Blennow K., Raskind M., Sabbagh M., Honig L.S., Doody R., van Dyck C.H., Mulnard R., Barakos J., Gregg K.M., Liu E., Lieberburg I., Schenk D., Black R., Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [http://dx.doi.org/10. 1212/WNL.0b013e3181c67808]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P., Ferris S., Reichert M., Ketter N., Nejadnik B., Guenzler V., Miloslavsky M., Wang D., Lu Y., Lull J., Tudor I.C., Liu E., Grundman M., Yuen E., Black R., Brashear H.R. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [http://dx.doi.org/10.1056/ NEJMoa1304839]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farlow M., Arnold S.E., van Dyck C.H., Aisen P.S., Snider B.J., Porsteinsson A.P., Friedrich S., Dean R.A., Gonzales C., Sethuraman G., DeMattos R.B., Mohs R., Paul S.M., Siemers E.R. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [http://dx.doi.org/10.1016/j.jalz.2011.09.224]. [DOI] [PubMed] [Google Scholar]
- 81.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., Raman R., Sun X., Aisen P.S., Siemers E., Liu-Seifert H., Mohs R., Solanezumab Study Group Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [http://dx.doi.org/10.1056/ NEJMoa1312889]. [DOI] [PubMed] [Google Scholar]
- 82.Siemers E.R., Sundell K.L., Carlson C., Case M., Sethuraman G., Liu-Seifert H., Dowsett S.A., Pontecorvo M.J., Dean R.A., Demattos R. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [http://dx.doi.org/10.1016/j.jalz.2015.06.1893]. [DOI] [PubMed] [Google Scholar]
- 83.2016 https://investor.lilly.com/releasedetail.cfm?ReleaseID=100087
- 84.Bohrmann B., Baumann K., Benz J., Gerber F., Huber W., Knoflach F., Messer J., Oroszlan K., Rauchenberger R., Richter W.F., Rothe C., Urban M., Bardroff M., Winter M., Nordstedt C., Loetscher H. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J. Alzheimers Dis. 2012;28:46–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 85.Novakovic D., Feligioni M., Scaccianoce S., Caruso A., Piccinin S., Schepisi C., Errico F., Mercuri N.B., Nicoletti F., Nisticò R. Profile of gantenerumab and its potential in the treatment of Alzheimer’s disease. Drug Des. Devel. Ther. 2013:1359–1364. doi: 10.2147/DDDT.S53401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ostrowitzki S., Deptula D., Thurfjell L., Barkhof F., Bohrmann B., Brooks D.J., Klunk W.E., Ashford E., Yoo K., Xu Z.X., Loetscher H., Santarelli L. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch. Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [http://dx.doi.org/10.1001/archneurol. 2011.1538]. [DOI] [PubMed] [Google Scholar]
- 87.Sevigny J., Chiao P., Bussière T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., O’Gorman J., Qian F., Arastu M., Li M., Chollate S., Brennan M.S., Quintero-Monzon O., Scannevin R.H., Arnold H.M., Engber T., Rhodes K., Ferrero J., Hang Y., Mikulskis A., Grimm J., Hock C., Nitsch R.M., Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [http://dx.doi.org/10.1038/nature19323]. [DOI] [PubMed] [Google Scholar]
- 88.Barão S., Moechars D., Lichtenthaler S.F., De Strooper B. BACE1 physiological functions may limit its use as therapeutic target for Alzheimer’s disease. Trends Neurosci. 2016;39:158–169. doi: 10.1016/j.tins.2016.01.003. [http://dx.doi.org/10.1016/j.tins.2016.01.003]. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi D., Zeller M., Cole T., Buttini M., McConlogue L., Sinha S., Freedman S., Morris R.G., Chen K.S. BACE1 gene deletion: impact on behavioral function in a model of Alzheimer’s disease. Neurobiol. Aging. 2008;29:861–873. doi: 10.1016/j.neurobiolaging.2007.01.002. [http://dx.doi. org/10.1016/j.neurobiolaging.2007.01.002]. [DOI] [PubMed] [Google Scholar]
- 90.Wang H., Song L., Laird F., Wong P.C., Lee H.K. BACE1 knockouts display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J. Neurosci. 2008;28:8677–8681. doi: 10.1523/JNEUROSCI.2440-08.2008. [http://dx.doi.org/ 10.1523/JNEUROSCI.2440-08.2008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filser S., Ovsepian S.V., Masana M., Blazquez-Llorca L., Brandt E.A., Volbracht C., Müller M.B., Jung C.K., Herms J. Pharmacological inhibition of BACE1 impairs synaptic plasticity and cognitive functions. Biol. Psychiatry. 2015;77:729–739. doi: 10.1016/j.biopsych.2014.10.013. [http://dx.doi.org/10.1016/j.biopsych.2014.10.013]. [DOI] [PubMed] [Google Scholar]
- 92.Yan R., Vassar R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014;13:319–329. doi: 10.1016/S1474-4422(13)70276-X. [http://dx.doi.org/10.1016/S1474-4422(13)70276-X]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai J., Qi X., Kociok N., Skosyrski S., Emilio A., Ruan Q., Han S., Liu L., Chen Z., Bowes Rickman C., Golde T., Grant M.B., Saftig P., Serneels L., de Strooper B., Joussen A.M., Boulton M.E. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol. Med. 2012;4:980–991. doi: 10.1002/emmm.201101084. [http://dx.doi.org/10. 1002/emmm.201101084]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cheret C., Willem M., Fricker F.R., Wende H., Wulf-Goldenberg A., Tahirovic S., Nave K.A., Saftig P., Haass C., Garratt A.N., Bennett D.L., Birchmeier C. Bace1 and Neuregulin-1 cooperate to control formation and maintenance of muscle spindles. EMBO J. 2013;32:2015–2028. doi: 10.1038/emboj.2013.146. [http://dx.doi.org/ 10.1038/emboj.2013.146]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Agsten M., Hessler S., Lehnert S., Volk T., Rittger A., Hartmann S., Raab C., Kim D.Y., Groemer T.W., Schwake M., Alzheimer C., Huth T. BACE1 modulates gating of KCNQ1 (Kv7.1) and cardiac delayed rectifier KCNQ1/KCNE1 (IKs). J. Mol. Cell. Cardiol. 2015;89:35–348. doi: 10.1016/j.yjmcc.2015.10.006. [http://dx.doi.org/10.1016/ j.yjmcc.2015.10.006]. [DOI] [PubMed] [Google Scholar]
- 96.Camacho I.E., Serneels L., Spittaels K., Merchiers P., Dominguez D., De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J. Neurosci. 2004;24:10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [http://dx.doi. org/10.1523/JNEUROSCI.3987-04.2004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.d’Abramo C., Massone S., Zingg J.M., Pizzuti A., Marambaud P., Dalla P.B., Azzi A., Marinari U.M., Pronzato M.A., Ricciarelli R. Role of peroxisome proliferator-activated receptor gamma in amyloid precursor protein processing and amyloid beta-mediated cell death. Biochem. J. 2005;391:693–698. doi: 10.1042/BJ20050560. [http://dx. doi.org/10.1042/BJ20050560]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Landreth G., Jiang Q., Mandrekar S., Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics. 2008;5:481–489. doi: 10.1016/j.nurt.2008.05.003. [http://dx.doi.org/10.1016/j. nurt.2008.05.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miller B.W., Willett K.C., Desilets A.R. Rosiglitazone and pioglitazone for the treatment of Alzheimer’s disease. Ann. Pharmacother. 2011;45:1416–1424. doi: 10.1345/aph.1Q238. [http://dx.doi.org/10.1345/ aph.1Q238]. [DOI] [PubMed] [Google Scholar]
- 100.Doody R.S., Raman R., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., He F., Sun X., Thomas R.G., Aisen P.S., Siemers E., Sethuraman G., Mohs R. Semagacestat Study Group. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [http://dx.doi.org/ 10.1056/NEJMoa1210951]. [DOI] [PubMed] [Google Scholar]
- 101.Doody R.S., Raman R., Sperling R.A., Seimers E., Sethuraman G., Mohs R., Farlow M., Iwatsubo T., Vellas B., Sun X., Ernstrom K., Thomas R.G., Aisen P.S. Peripheral and central effects of γ-secretase inhibition by semagacestat in Alzheimer’s disease. Alzheimers Res. Ther. 2015;7:36. doi: 10.1186/s13195-015-0121-6. [http://dx.doi.org/ 10.1186/s13195-015-0121-6]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mayer S.C., Kreft A.F., Harrison B., Abou-Gharbia M., Antane M., Aschmies S., Atchison K., Chlenov M., Cole D.C., Comery T., Diamantidis G., Ellingboe J., Fan K., Galante R., Gonzales C., Ho D.M., Hoke M.E., Hu Y., Huryn D., Jain U., Jin M., Kremer K., Kubrak D., Lin M., Lu P., Magolda R., Martone R., Moore W., Oganesian A., Pangalos M.N., Porte A., Reinhart P., Resnick L., Riddell D.R., Sonnenberg-Reines J., Stock J.R., Sun S.C., Wagner E., Wang T., Woller K., Xu Z., Zaleska M.M., Zeldis J., Zhang M., Zhou H., Jacobsen J.S. Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer’s disease. J. Med. Chem. 2008;51:7348–7351. doi: 10.1021/jm801252w. [http://dx.doi.org/10.1021/jm801252w]. [DOI] [PubMed] [Google Scholar]
- 103.Gillman K.W., Starrett J.E., Jr, Parker M.F., Xie K., Bronson J.J., Marcin L.R., McElhone K.E., Bergstrom C.P., Mate R.A., Williams R., Meredith J.E., Jr, Burton C.R., Barten D.M., Toyn J.H., Roberts S.B., Lentz K.A., Houston J.G., Zaczek R., Albright C.F., Decicco C.P., Macor J.E., Olson R.E. Discovery and Evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med. Chem. Lett. 2010;1:120–124. doi: 10.1021/ml1000239. [http://dx.doi.org/10.1021/ml1000239]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crump C.J., Castro S.V., Wang F., Pozdnyakov N., Ballard T.E., Sisodia S.S., Bales K.R., Johnson D.S., Li Y.M. BMS-708,163 targets presenilin and lacks notch-sparing activity. Biochemistry. 2012;51:7209–7211. doi: 10.1021/bi301137h. [http://dx.doi.org/10.1021/ bi301137h]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chávez-Gutiérrez L., Bammens L., Benilova I., Vandersteen A., Benurwar M., Borgers M., Lismont S., Zhou L., Van Cleynenbreugel S., Esselmann H., Wiltfang J., Serneels L., Karran E., Gijsen H., Schymkowitz J., Rousseau F., Broersen K., De Strooper B. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31:2261–2274. doi: 10.1038/emboj.2012.79. [http://dx.doi.org/10.1038/emboj.2012.79]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martone R.L., Zhou H., Atchison K., Comery T., Xu J.Z., Huang X., Gong X., Jin M., Kreft A., Harrison B., Mayer S.C., Aschmies S., Gonzales C., Zaleska M.M., Riddell D.R., Wagner E., Lu P., Sun S.C., Sonnenberg-Reines J., Oganesian A., Adkins K., Leach M.W., Clarke D.W., Huryn D., Abou-Gharbia M., Magolda R., Bard J., Frick G., Raje S., Forlow S.B., Balliet C., Burczynski M.E., Reinhart P.H., Wan H.I., Pangalos M.N., Jacobsen J.S. Begacestat (GSI-953): A novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein γ-secretase for the treatment of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2009;331:598–608. doi: 10.1124/jpet.109.152975. [http://dx.doi.org/10.1124/jpet.109.152975]. [DOI] [PubMed] [Google Scholar]
- 107.Coric V., Salloway S., van Dyck C.H., Dubois B., Andreasen N., Brody M., Curtis C., Soininen H., Thein S., Shiovitz T., Pilcher G., Ferris S., Colby S., Kerselaers W., Dockens R., Soares H., Kaplita S., Luo F., Pachai C., Bracoud L., Mintun M., Grill J.D., Marek K., Seibyl J., Cedarbaum J.M., Albright C., Feldman H.H., Berman R.M. Targeting prodromal alzheimer disease with avagacestat: A randomized clinical trial. JAMA Neurol. 2015;72:1324–1333. doi: 10.1001/jamaneurol.2015.0607. [http://dx.doi.org/10.1001/jamaneurol. 2015.0607]. [DOI] [PubMed] [Google Scholar]
- 108.Weggen S., Eriksen J.L., Das P., Sagi S.A., Wang R., Pietrzik C.U., Findlay K.A., Smith T.E., Murphy M.P., Bulter T., Kang D.E., Marquez-Sterling N., Golde T.E., Koo E.H. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [http://dx. doi.org/10.1038/35102591]. [DOI] [PubMed] [Google Scholar]
- 109.Golde T.E., Koo E.H., Felsenstein K.M., Osborne B.A., Miele L. γ-Secretase inhibitors and modulators. Biochim. Biophys. Acta. 2013:2898–2907. doi: 10.1016/j.bbamem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilcock G.K., Black S.E., Hendrix S.B., Zavitz K.H., Swabb E.A., Laughlin M.A. Tarenflurbil Phase II Study investigators. Efficacy and safety of tarenflurbil in mild to moderate Alzheimer’s disease: a randomised phase II trial. Lancet Neurol. 2008;7:483–493. doi: 10.1016/S1474-4422(08)70090-5. [http://dx.doi.org/10.1016/S1474-4422(08)70090-5]. [DOI] [PubMed] [Google Scholar]
- 111.Green R.C., Schneider L.S., Amato D.A., Beelen A.P., Wilcock G., Swabb E.A., Zavitz K.H. Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [http://dx.doi. org/10.1001/jama.2009.1866]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fedele E., Rivera D., Marengo B., Pronzato M.A., Ricciarelli R. Amyloid β: Walking on the dark side of the moon. Mech. Ageing Dev. 2015;152:1–4. doi: 10.1016/j.mad.2015.09.001. [http://dx.doi.org/10.1016/j.mad.2015.09.001]. [DOI] [PubMed] [Google Scholar]
- 113.Andrew R.J., Kellett K.A., Thinakaran G., Hooper N.M. A Greek Tragedy: The growing complexity of alzheimer amyloid precursor protein proteolysis. J. Biol. Chem. 2016;291:19235–19244. doi: 10.1074/jbc.R116.746032. [http://dx.doi.org/10.1074/jbc.R116.746032]. [DOI] [PMC free article] [PubMed] [Google Scholar]