Abstract
Purpose: Careful decontamination and sterilization of reusable flexible ureteroscopes used in ureterorenoscopy cases prevent the spread of infectious pathogens to patients and technicians. However, inefficient reprocessing and unavailability of ureteroscopes sent out for repair can contribute to expensive operating room (OR) delays. Time-driven activity-based costing (TDABC) was applied to describe the time and costs involved in reprocessing.
Materials and Methods: Direct observation and timing were performed for all steps in reprocessing of reusable flexible ureteroscopes following operative procedures. Estimated times needed for each step by which damaged ureteroscopes identified during reprocessing are sent for repair were characterized through interviews with purchasing analyst staff. Process maps were created for reprocessing and repair detailing individual step times and their variances. Cost data for labor and disposables used were applied to calculate per minute and average step costs.
Results: Ten ureteroscopes were followed through reprocessing. Process mapping for ureteroscope reprocessing averaged 229.0 ± 74.4 minutes, whereas sending a ureteroscope for repair required an estimated 143 minutes per repair. Most steps demonstrated low variance between timed observations. Ureteroscope drying was the longest and highest variance step at 126.5 ± 55.7 minutes and was highly dependent on manual air flushing through the ureteroscope working channel and ureteroscope positioning in the drying cabinet. Total costs for reprocessing totaled $96.13 per episode, including the cost of labor and disposable items.
Conclusions: Utilizing TDABC delineates the full spectrum of costs associated with ureteroscope reprocessing and identifies areas for process improvement to drive value-based care. At our institution, ureteroscope drying was one clearly identified target area. Implementing training in ureteroscope drying technique could save up to 2 hours per reprocessing event, potentially preventing expensive OR delays.
Keywords: : ureteroscope, sterile processing, cost analysis, time-driven activity-based costing
Introduction
Ureterorenoscopy with reusable fiber-optic or digital flexible ureteroscopes requires an infrastructure and dedicated technical staff to properly and promptly reprocess ureteroscopes in between cases. Reprocessing of reusable flexible ureteroscopes and other endoscopes encompasses the procedures necessary to prepare them for subsequent cases while preventing the spread of infectious agents from patient to patient or patient to technician.1,2 Optimal reprocessing balances proper decontamination and avoiding damage to fragile components against minimizing personnel time invested, reducing use of disposable supplies, avoiding further damage to ureteroscopes needing repair, and ensuring that ureteroscopes are ready for subsequent cases without causing case delays. This balance must be struck across multiple locations with handoffs between relevant personnel at each transition point. Given the growing emphasis on value in healthcare delivery, the length and complexity of this process present an opportunity to save time and healthcare resources.
Time-driven activity-based costing (TDABC) is a method pioneered by Kaplan and Anderson3 that combines two widely utilized management tools—process mapping and activity-based costing—to increase the efficiency of resource utilization in complex management operations. When applied in the healthcare setting, clinician-managers define higher order phases of care for individuals undergoing treatment for a given condition. Each phase of care is then broken down into process maps defining individual steps each involving one or more clinical resources (personnel and/or equipment).4 Activity-based costing is applied to calculate dollar-per-minute values for resources invested in requisite personnel, equipment, and space. The result is an output that can be used to identify resource-intensive steps for targeted intervention, redundant steps that can be streamlined or eliminated, and idle capacity of staff and equipment that can accommodate a higher volume of use. TDABC has been widely applied across diverse sectors of healthcare delivery.5–8 Within the urological field, studies have used TDABC to define the value of care delivery in localized, low-risk prostate cancer therapy,9 brachytherapy for prostate cancer,10,11 treatment of small renal masses,12 and management of benign prostatic hyperplasia.13
Through applying TDABC to reprocessing of reusable flexible ureteroscopes, this work aimed to characterize the full spectrum of resources utilized in typical reprocessing scenarios and to identify high-variance steps in reprocessing. These calculations will enable us to propose cost reductive methods and strategies for improving ureteroscope reprocessing without sacrificing quality.
Materials and Methods
Process mapping
As part of a prospective time–motion study conducted at University of California, San Francisco, between July 2016 and April 2017, we followed reusable flexible ureteroscopes (URF-P6; Olympus, Tokyo, Japan) after the conclusion of upper urinary tract ureteroscopy operative procedures. We observed and timed the individual steps in reprocessing from the time each ureteroscope left the operating room (OR) to the conclusion of the sterilization process and transfer of the ureteroscope to storage. Steps requiring technician labor as opposed to passive and automated steps were noted. The average time and variance for each step were calculated across all observations of that step. These data were combined to create the overall process map for ureteroscope reprocessing. At multiple checkpoints in reprocessing, ureteroscopes are tested or inspected and those requiring repair are removed to prevent further damage during reprocessing. Ureteroscopes marked for repair are picked up by purchasing department staff and shipped back to the manufacturer. A second process map with time requirements for the steps required to remove damaged ureteroscopes and send them for repair was created based on interviews with purchasing department personnel.
Personnel and disposable items cost calculation
Sterile processing department (SPD), endoscopy department, shipping department, and purchasing department personnel costs were estimated based on the salaries of these positions plus benefits. Benefits for these staff members include bonuses, social security, 401K/403B, disability, healthcare, pension, and paid time off. Staff hours were estimated at 260 paid workdays per year for 8 hours per day. We directly observed and recorded each disposable item used in reprocessing. We applied the average cost of the individual disposables used in one reprocessing episode to demonstrate the overall cost for disposables.
Results
Reprocessing process map
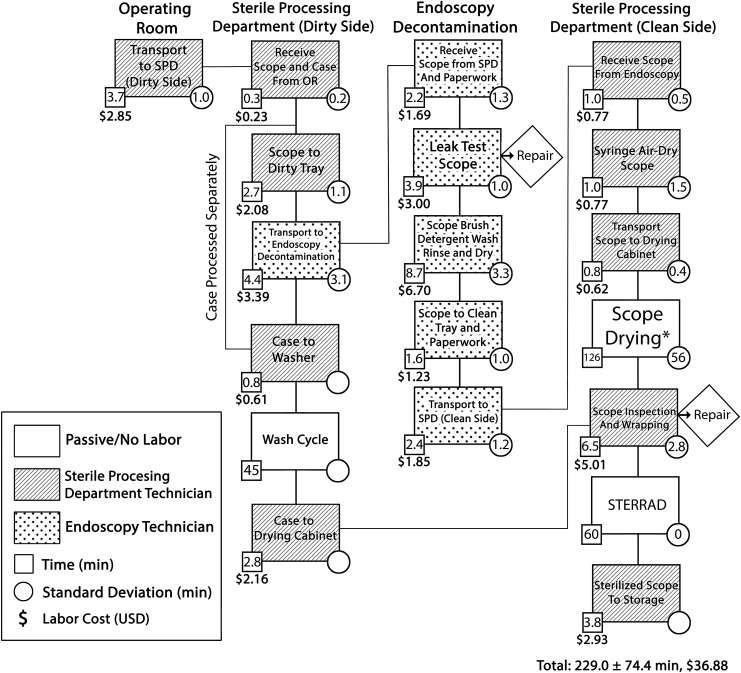
The reusable ureteroscope reprocessing process map was defined based on direct observation of 10 flexible ureteroscopes and is detailed in Figure 1. The ureteroscope storage case is decontaminated separately from the ureteroscope itself, but the ureteroscope and case are eventually wrapped and sterilized together. In all observations, the storage case was ready for sterilization well before the ureteroscope and thus did not change the total start to finish time of reprocessing. Total time for reprocessing averaged 229.0 ± 74.4 minutes from start to finish, of which only 47.7 minutes was dependent on labor by SPD/endoscopy personnel. Labor-dependent steps and machine-automated steps (wash cycle, STERRAD®) demonstrated low variance. Ureteroscope drying, a passive step, was the longest step and had the highest variance at 126.5 ± 55.7 minutes. Observations of ureteroscope drying times ranged from 68.8 to 208.0 minutes. This step is indicated by an asterisk in Figure 1. Ureteroscope drying time was primarily dependent on whether the SPD technician manually flushed the ureteroscope working channel with air and positioned the tip of the ureteroscope in the drying cabinet to allow air to circulate through the ureteroscope.
FIG. 1.
Reusable ureteroscope reprocessing process map. Boxes depict individual steps with patterns corresponding to personnel involved. Average step times, standard deviations and labor costs per step are noted. The sequence of steps is indicated by lines connecting boxes. Checkpoints at which damaged ureteroscopes are removed for repair (diamonds) are noted. Ureteroscope drying (*), a passive step, was the longest and highest-variance step. Average total reprocessing time was 229.0 ± 74.4 minutes. Labor costs for reprocessing averaged $36.88 per ureteroscope. OR = operating room; SPD = sterile processing department.
Repair process map
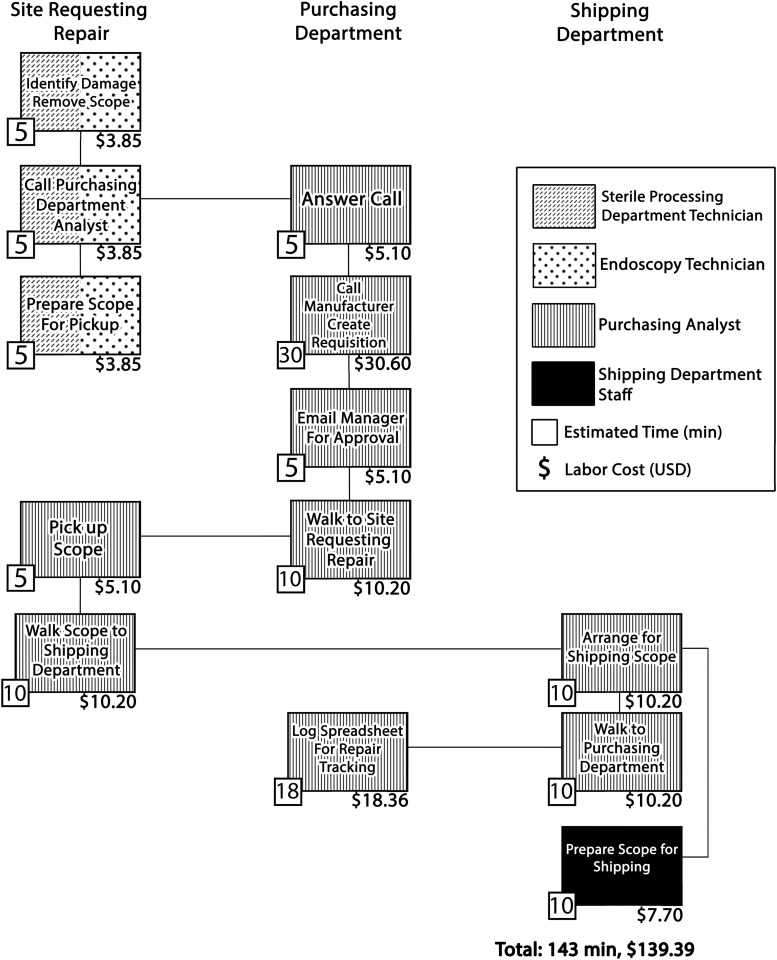
The process map for identifying and sending a ureteroscope for repair was based on interviews with purchasing analyst staff and is detailed in Figure 2. The full process occurs across four physical departments and was estimated to require 143 minutes, all of which involved labor by SPD/endoscopy, purchasing, and shipping personnel.
FIG. 2.
Reusable ureteroscope repair process map after identification and removal of a damaged ureteroscope during reprocessing. Boxes depict individual steps with patterns corresponding to personnel involved. Estimated step times and estimated labor costs per step are noted. The sequence of steps is indicated by lines connecting boxes. Total estimated time for the process was 143 minutes. Labor costs for repair were estimated at $139 per repair and $13.90 per ureteroscopy case.
Personnel costs
SPD, endoscopy, and shipping department personnel cost rates were $0.77/minute for salary plus benefits. The purchasing department analyst cost rate was $1.03/minute. Each ureteroscope reprocessing event required an average of $36.88 of labor by SPD/endoscopy personnel. The average labor cost per step is displayed below each average step time in Figures 1 and 2. Each repair event required an estimated $139.39 of labor by SPD/endoscopy, purchasing, and shipping personnel to remove the ureteroscope from circulation and arrange for its repair. This averaged out to $13.93 in repair labor per ureteroscopy case.
Disposable item costs
Disposables item costs averaged $45.35 per ureteroscope reprocessed. These included personal protective equipment worn by endoscopy decontamination and SPD personnel, brushes, and solutions used in high-level decontamination of ureteroscopes, chemical cassettes, pouches, and gas indicators used in STERRAD sterilization. The single largest contributor to this total was the low-temperature hydrogen peroxide STERRAD cassette at $20.09 per ureteroscope reprocessing event.
Total costs
The total mean cost of one reprocessing event, including SPD personnel labor, endoscopy decontamination personnel labor plus the costs of disposables used, was $82.23. Labor by purchasing and shipping department personnel preparing ureteroscopes for return to their manufacturer pushed our per-event cost to $96.13.
Discussion
In the current era of cost-conscious healthcare, analyses to reduce expenditures and strains on human resources without compromising crucial procedures are increasingly important. Endoscope reprocessing is receiving greater attention. Recent outbreaks of multidrug-resistant infections associated with duodenoscopes have been associated with patient deaths and investigations by the U.S. Food and Drug Administration14 and Centers for Disease Control and Prevention.15 Ureteroscopes have been demonstrated to retain contaminants and viable bacteria despite sterilization.16 Urinary tract infection (UTI) outbreaks associated with contaminated ureteroscopes have been reported in the past17 and may plausibly be lost in the noise of other causes of UTI after ureteroscopy.18–20 Conversely, OR delays waiting for instruments to be properly sterilized are a contributor to wasted healthcare dollars and poorer patient outcomes.21,22 In the context of tensions between reprocessing speed and thoroughness, we have applied TDABC to demonstrate areas for improvement in ureteroscope reprocessing for what is, to our knowledge, the first time in the literature.
At our institution, we found that ureteroscope reprocessing requires 229.0 ± 74.4 minutes. Prior work by our group found that on average a ureteroscopy operation with a flexible reusable ureteroscope took 64.5 ± 37.0 minutes,23 with patient preparation, room cleaning, and anesthesia preparation requiring an additional 67 minutes on average (data not shown). At this pace, a reusable ureteroscope used in the first case of the day would not be ready until the fourth case, necessitating access to a minimum of four ureteroscopes in good condition to allow for multiple back-to-back ureteroscopy cases without delays. Decreasing these times could result in fewer ureteroscopes needing to be purchased to accomplish the same number of cases in a day.
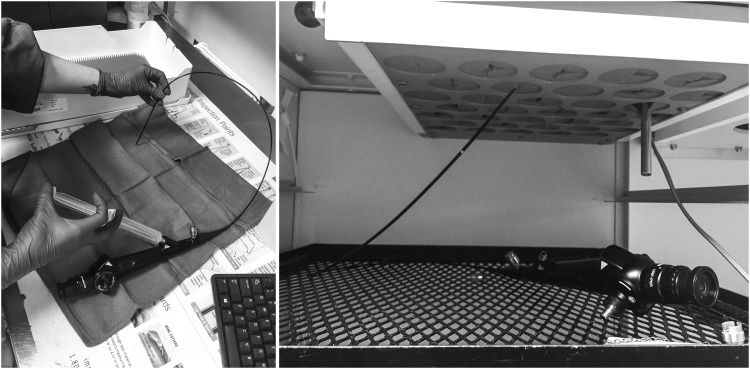
Despite the complexity of reprocessing, we noted that steps in the process generally run expediently with low variability between observations. We did not observe any untoward events inflicted on ureteroscopes by personnel attempting to shortcut the process steps involved. Nearly half of disposable expenses were accounted for by the cost of one STERRAD sterilization cassette. Thus, the potential cost savings of speeding up most steps in the pathway or using fewer disposables are unlikely to make a major financial impact. The notable exception to this was ureteroscope drying. This step alone accounted for 55% of the time ureteroscopes spent in reprocessing and its length was highly variable. As described in the results, proper drying protocol includes the SPD technician manually flushing the ureteroscope working channel with air followed by positioning the ureteroscope in the drying cabinet for maximum air circulation (Fig. 3). When these steps were not conducted, the ureteroscope remained wet and reprocessing was delayed (the STERRAD low-temperature gas–plasma sterilizer will not run if it detects moisture24). Although we did not observe any OR delays attributable to sterile ureteroscope accessibility during our study period, a small investment in training or a redesign of the drying cabinet would be clear interventions that could reduce reprocessing time and avoid this potential source of wasted resources.
FIG. 3.
Ureteroscope drying technique. Left panel: SPD technician syringe-flushing the ureteroscope working channel with air to remove residual moisture. Right panel: ureteroscope positioned in drying cabinet with air running through working channel in the proper position to optimize airflow and drying time.
Fiber-optic and digital flexible ureteroscopes have fragile components that are easily broken. Studies in the literature have characterized sources of damage to ureteroscopes25–30 with one describing mishandling during reprocessing as the single largest source of breakages.26 At our institution, repairs occur once for every 10 cases and have an associated average cost of $9,420 per repair charged by the repair vendor. In this study, we found that repair requires an additional $139 of labor per repair. This rate of repair and our per-case repair value of $956 including labor are consistent with published results from two other large academic medical centers that sent their fiber-optic ureteroscopes for repair once every 9.5 cases at a cost of $605/case28 and once every 6 to 15 cases.30 These values from academic centers are twice the rate of repairs recently reported by a private practice, single-specialty urology group.25 These data support the idea that cost containment can be achieved with process control. Large institutions whose SPDs prepare a diverse array of instruments could potentially reduce costs with different strategies compared with smaller, more specialized centers.
We present here a characterization of the steps and resources involved in reprocessing reusable flexible ureteroscopes at a single institution taking into account multiple sources of costs across all areas of expenditure. Although the cost of performing TDABC is primarily dependent on the cost of personnel resources used to track process steps, the majority of observations and data analyses in our project were performed by medical students and urology residents, demonstrating that this form of analysis can be performed relatively inexpensively. The high level of detail provided by TDABC facilitates novel insights into ureteroscope reprocessing; however, several limitations in our methods should be recognized. Our initial data collection included estimates for department operating costs and the costs (purchasing and maintenance) of capital equipment used in reprocessing. However, given the high volume of instruments processed across multiple services and the relatively long life span of the space and equipment used, these sources contributed only cents to each ureteroscope reprocessing episode and we excluded them from the analysis for simplicity. In addition, the generalizability of our results would benefit from the inclusion of data from more centers using different reprocessing protocols and additional ureteroscopes. We were reliant on interview-based estimates for repair time and workflow. Although this is the practice originally outlined by Kaplan and Anderson3 and adopted in many TDABC studies,5 direct observation would have strengthened our estimates. Finally, this approach to characterization of one aspect of the care pathway of treatment for upper urinary tract pathology would benefit from further studies outlining the full care pathway and allowing for more extensive analysis of the influences of resource expenditure in reprocessing on other settings of care delivery, such as the clinic, OR, and perioperative areas.
Conclusions
In this work, we applied TDABC to the reprocessing of reusable flexible ureteroscopes for what we believe is the first time in the literature. Detailed process maps for decontamination/sterile processing and sending ureteroscopes for repair highlighted areas where standardization could increase value in care delivery when using reusable ureteroscopes. Although the costs of repair remain the single largest target for cost reduction, this work newly underscores the full spectrum of costs associated with the use of reusable ureteroscopes and identifies areas for intervention. At our institution, targeting ureteroscope drying technique may reduce the length of reprocessing by up to 2 hours and reduce OR costs. Further research should expand this analysis to the full care pathway for upper urinary tract pathology.
Abbreviations Used
- SPD
sterile processing department
- TDABC
time-driven activity-based costing
- UTI
urinary tract infection
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Putnam K. Guideline for processing flexible endoscopes. AORN J 2016;103:10–12 [DOI] [PubMed] [Google Scholar]
- 2.Reprocessing Guideline Task Force, Petersen BT, Cohen J, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc 2017;85:282..e1–294.e1. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev 2004;82:131–138, 150. [PubMed] [Google Scholar]
- 4.Kaplan RS, Witkowski M, Abbott M, et al. Using time-driven activity-based costing to identify value improvement opportunities in healthcare. J Healthc Manag 2014;59:399–412 [PubMed] [Google Scholar]
- 5.Keel G, Savage C, Rafiq M, Mazzocato P. Time-driven activity-based costing in health care: A systematic review of the literature. Health Policy 2017;121:755–763 [DOI] [PubMed] [Google Scholar]
- 6.Yu YR, Abbas PI, Smith CM, Carberry KE, Ren H, Patel B, Nuchtern JG, Lopez ME. Time-driven activity-based costing: A dynamic value assessment model in pediatric appendicitis. J Pediatr Surg 2017;52:1045–1049 [DOI] [PubMed] [Google Scholar]
- 7.Najjar PA, Strickland M, Kaplan RS. Time-driven activity-based costing for surgical episodes. JAMA Surg 2017;152:96–97 [DOI] [PubMed] [Google Scholar]
- 8.Yun BJ, Prabhakar AM, Warsh J, Kaplan R, Brennan J, Dempsey KE, Raja AS. Time-driven activity-based costing in emergency medicine. Ann Emerg Med 2016;67:765–772 [DOI] [PubMed] [Google Scholar]
- 9.Laviana AA, Ilg AM, Veruttipong D, et al. Utilizing time-driven activity-based costing to understand the short- and long-term costs of treating localized, low-risk prostate cancer. Cancer 2016;122:447–455 [DOI] [PubMed] [Google Scholar]
- 10.Thaker NG, Orio PF, Potters L. Defining the value of magnetic resonance imaging in prostate brachytherapy using time-driven activity-based costing. Brachytherapy 2017;16:665–671 [DOI] [PubMed] [Google Scholar]
- 11.Thaker NG, Pugh TJ, Mahmood U, et al. Defining the value framework for prostate brachytherapy using patient-centered outcome metrics and time-driven activity-based costing. Brachytherapy 2016;15:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laviana AA, Kundavaram CR, Tan H, Burke MA, Niedzwiecki A, Lee RK, Hu JC. Determining the true costs of treating small renal masses using time driven, activity based costing. Urol Practice 2016;3:180–186 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan AL, Agarwal N, Setlur NP, et al. Measuring the cost of care in benign prostatic hyperplasia using time-driven activity-based costing (TDABC). Healthcare (Amst) 2015;3:43–48 [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. Effective reprocessing of endoscopes used in endoscopic retrograde cholangiopancreatography (ERCP) procedures. FDA executive summary. Prepared for the May 14–15, 2015. Meeting of ther Gastroenterology-Urology Devices Panel of the Medical Devices Advisory Committee [Google Scholar]
- 15.Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-beta-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014;312:1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ofstead CL, Heymann OL, Quick MR, Johnson EA, Eiland JE, Wetzler HP. The effectiveness of sterilization for flexible ureteroscopes: A real-world study. Am J Infect Control 2017;45:888–895 [DOI] [PubMed] [Google Scholar]
- 17.Chang CL, Su LH, Lu CM, Tai FT, Huang YC, Chang KK. Outbreak of ertapenem-resistant Enterobacter cloacae urinary tract infections due to a contaminated ureteroscope. J Hosp Infect 2013;85:118–124 [DOI] [PubMed] [Google Scholar]
- 18.Mitsuzuka K, Nakano O, Takahashi N, Satoh M. Identification of factors associated with postoperative febrile urinary tract infection after ureteroscopy for urinary stones. Urolithiasis 2016;44:257–262 [DOI] [PubMed] [Google Scholar]
- 19.Sohn DW, Kim SW, Hong CG, Yoon BI, Ha US, Cho YH. Risk factors of infectious complication after ureteroscopic procedures of the upper urinary tract. J Infect Chemother 2013;19:1102–1108 [DOI] [PubMed] [Google Scholar]
- 20.Long C, Pulido JE, Weiss DA, et al. Urinary tract infection after ureteroscopy: Can we identify those patients at risk for infection? Abstract presented at: 2013 Pediatric Urology Fall Congress, September2013, Las Vegas, NV [Google Scholar]
- 21.Blackmore CC, Bishop R, Luker S, Williams BL. Applying lean methods to improve quality and safety in surgical sterile instrument processing. Jt Comm J Qual Patient Saf 2013;39:99–105 [DOI] [PubMed] [Google Scholar]
- 22.Wong J, Khu KJ, Kaderali Z, Bernstein M. Delays in the operating room: Signs of an imperfect system. Can J Surg 2010;53:189–195 [PMC free article] [PubMed] [Google Scholar]
- 23.Usawachintachit M, Isaacson DS, Taguchi K, Tzou DT, Hsi RS, Sherer BA, Stoller ML, Chi T. A prospective case-Control Study Comparing LithoVue, a single-use, flexible disposable ureteroscope, with flexible, reusable fiber-optic ureteroscopes. J Endourol 2017;31:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs P, Kowatsch R. STERRAD Sterilization System: A new technology for instrument sterilization. Endosc Surg Allied Technol 1993;1:57–58 [PubMed] [Google Scholar]
- 25.Kramolowsky E, McDowell Z, Moore B, Booth B, Wood N. Cost analysis of flexible ureteroscope repairs: Evaluation of 655 procedures in a community-based practice. J Endourol 2016;30:254–256 [DOI] [PubMed] [Google Scholar]
- 26.Sooriakumaran P, Kaba R, Andrews HO, Buchholz NP. Evaluation of the mechanisms of damage to flexible ureteroscopes and suggestions for ureteroscope preservation. Asian J Androl 2005;7:433–438 [DOI] [PubMed] [Google Scholar]
- 27.McDougall EM, Alberts G, Deal KJ, Nagy JM. Does the cleaning technique influence the durability of the <9F flexible ureteroscope? J Endourol 2004;15:615–618 [DOI] [PubMed] [Google Scholar]
- 28.Tosoian JJ, Ludwig W, Sopko N, Mullins JK, Matlaga BR. The effect of repair costs on the profitability of a ureteroscopy program. J Endourol 2015;29:406–409 [DOI] [PubMed] [Google Scholar]
- 29.Canales BK, Gleason JM, Hicks N, Monga M. Independent analysis of Olympus flexible ureteroscope repairs. Urology 2007;70:11–15 [DOI] [PubMed] [Google Scholar]
- 30.Afane JS, Olweny EO, Bercowsky E, Sundaram CP, Dunn MD, Shalhav AL, McDougall EM, Clayman RV. Flexible ureteroscopes: A single center evaluation of the durability and function of the new endoscopes smaller than 9Fr. J Urol 2000;164:1164–1168 [PubMed] [Google Scholar]