Abstract
Background:
Human hookworm infection is caused mainly by Necator americanus and Ancylostoma duodenale. Among the zoonotic hookworm species, only Ancylostoma ceylanicum causes potent human infections where dogs and cats act as reservoir of infection. Hence, species differentiation is imperative because the eradication of both anthroponotic and zoonotic hookworm depends on the concurrent human and animal health programs, hygienic practices, and mass drug administration for humans and dogs.
Objective:
This study was performed to evaluate the utility of polymerase chain reaction (PCR) for detection of hookworm infections.
Materials and Methods:
A total of 209 stool samples were collected and subjected to stool microscopy, Kato-Katz method to identify the intensity of the infection, coproculture for L3 larval identification and species differentiation and semi-nested PCR with sequencing.
Results:
The prevalence of hookworm was estimated as 7.6%. Highest hookworm prevalence was seen in 20–30 years of age group. Majority of the infections were mild intensity infections. Sensitivity of stool microscopy was found to be 81.2% and the specificity was 100%. Sensitivity of Kato-Katz method was 87.5% and specificity was 100%. True positivity by agar plate culture was 83.3% and false positivity rate was 16.6%.
Conclusion:
Stool microscopy is the major mode of detection, but it has a higher false negative rate. Coproculture is time-consuming and needs the expertise to differentiate the species. On the other hand, PCR is known to be a sensitive, specific, and a reliable investigative tool which can help in diagnosis as well as in species differentiation.
Keywords: Hookworm, polymerase chain reaction, species differentiation
INTRODUCTION
Soil-transmitted helminths are cosmopolitan in distribution; however, tropical and subtropical regions are majorly affected areas. Hookworm affects nearly 560–740 million people worldwide.[1] Human hookworm infections are mainly caused by Necator americanus and Ancylostoma duodenale. In these, the former is present widely whereas the latter is geographically restricted. Hookworm infection is the most important cause of iron deficiency anemia and protein energy malnutrition in women and children.[2,3] The clinical manifestations depend on the intensity of the infection and also there is a positive correlation exists between the infecting hookworm species and the rate of anemia.[4,5]
Among the zoonotic hookworm species, only Ancylostoma ceylanicum causes potent human infections whereas the other zoonotic species like A. braziliensis and A. caninum cause cutaneous larva migrans.[6] Humans acquire zoonotic infections from dogs and cats. Nearly, 19–73 million people are infected with hookworm in endemic regions.[7] Each species differ in their morphology, pathogenesis, life cycle, and clinical features, therefore, species differentiation is imperative for the eradication of both anthroponotic and zoonotic hookworm. Hookworm eradication requires concurrent human and animal health programs, hygienic practices, and mass drug administration for humans and dogs.
The most common modality used for diagnosing hookworm infection is stool smear microscopy. However, this is not useful in species differentiation, as the eggs of all three species are morphologically analogs.[8] On the other hand, coproculture aids in species differentiation based on the morphological feature of the L3 larva, but this is time-consuming, highly prone to contamination and highly skilled microscopist is required to differentiate the species.[9] Contrary to that molecular methods such as semi-nested polymerase chain reaction (PCR) have high specificity and helps in exact identification and differentiation of hookworm species.
This study was undertaken to evaluate the effectiveness of semi-nested PCR for the diagnosis of hookworm infection including A. ceylanicum. Parallel to molecular diagnosis conventional methods such as stool microscopy, coproculture, and Kato-Katz methods were also performed for diagnosis and intensity detection. Further, all the conventional, nonmolecular methods were compared with semi-nested PCR for specificity and sensitivity.
MATERIALS AND METHODS
This study was a prospective clinical study, conducted at the Department of Microbiology, JIPMER from October 2014 to April 2016 on Institute Ethics Committee approval. Consent waiver was also obtained and anonymity of samples was maintained. All stool samples received in clinical pathology laboratory for routine stool examination during the study were included in the study.
A total of 209 stool samples collected from the clinical pathology laboratory were divided into two parts; one part was subjected to microscopy, Kato-Katz, and culture while the other part was exclusively used for PCR. The presence of ova, cyst, trophozoites, larva, pus cells, and red blood cells were identified by saline and iodine wet mount examination. Intensity of infection was measured for all the microscopy positive samples by Kato-Katz method as described by Kato-Katz.[10] Further, according to the protocol of Arakaki et al. microscopically hookworm positive stool samples were subjected to agar plate culture method and based on the morphological features of L3 larva, hookworm species were identified.[11]
DNA extraction
The DNA was extracted using the QIAGEN stool DNA extraction kit (QIAGEN Cat No./ID: 51304, Germany) according to the manufacturer instructions with minor modifications. Approximately, 0.2 g of stool was weighed and aliquot into a 1.5 ml microcentrifuge tube. Then, 1 ml ASL buffer was added to the sample. Then, a cap full of silica glass beads were added to the sample mixture. Three cycles of continuous bead beating with bead beater were done. Each cycle lasted for 3 min. Pretreatment with heat shock and liquid nitrogen was done (water bath at 95°C for 5 min and liquid nitrogen for 2 min for five cycles). The DNA samples were stored at −20°C until further processing.
Further, semi-nested PCR was performed to amplify the internal transcribed spacer 1, 2 and 5.8 s region to detect N. americanus, A. duodenale, and A. ceylanicum. Primers were used as per George et al.,[12] which includes common forward primer – UGHWF (5'-GTT GGGAGTATCRCCMMCCK-3') first round reverse primer – NC1R (5'-AACA ACCCTGAACCAGACGT-3') second round reverse primer – UGHWR (5'-ATGCCTTCAAAATTTCACCA-3') to amplify 552 bp (N. americanus) and 404 bp (Ancylostoma spp.), respectively. 50 μl PCR mix consisted of 2.5 μl DNA, 1 μl of each primer, 1 μl dNTP, 1.5 μl MgCl2 (25 mM), 5 μl buffer, 35.2 μl water, and 0.3 μl DNA polymerase. The following PCR conditions were used: initial denaturation at 94°C for 5 min followed by 39 cycles of denaturation 94°C for 40 s, annealing at 59°C for 50 s, and extension at 72°C, followed by final extension at 72°C for 10 min.
RESULTS
The age of the study participants ranged from 1 to 58 years. The median age was 26 years in which 65% were males and 35% were females. Intestinal parasitic prevalence was 25.3% and hookworm prevalence was 6.2% by stool microscopy. In the present study, total intestinal parasitic prevalence was 25.3%. Ascaris lumbricoides (10.5%), hookworm (6.2%), and cysts of Entamoeba spp. (5.2%) were observed commonly during microscopic observation of stool wet mount. Apart from this, Strongyloides stercoralis (2.3%) and cysts of Giardia (0.9%) were also recorded in low frequency.
A total of 13 samples (6.2%) were positive by stool microscopy whereas, 14 samples (6.7%) were positive by Kato-Katz method. Hookworm intensity profile was assessed by Kato-Katz method and analyzed with the WHO threshold[13] according to which 71.4% were mild infections, 21.4% were moderate, and 7.2% were heavily infected. The highest prevalence of hookworm was recorded in the age group of 20–30 years followed by 1–10 years age group. The highest prevalence of hookworm was recorded in the age group of 20–30 years (43%) followed by 36% in 1–10 years age group, 14% in 11–20 years age group, and 7% in 31–40 years of age group. Stool samples which were positive for hookworm eggs (n = 14) by both microscopy and Kato-Katz method were subjected to agar plate culture. After incubation, two samples showed contamination with maggots, hence were discarded. Further, of the remaining 12 positive samples, two were identified as A. duodenale and 10 samples were identified as N. americanus. Maximum number of agar plate culture positives were identified during 4th and 5th day of incubation. A. ceylanicum, A. caninum, and N. americanus DNA were used as the positive controls. A total of 16 samples were positive for hookworm by PCR. All the PCR positive samples were identified as N. americanus. No Ancylostoma spp. (duodenale and ceylanicum) or any mixed infection were seen in our study population. Hence, the prevalence of hookworm was calculated as 7.6% by PCR.
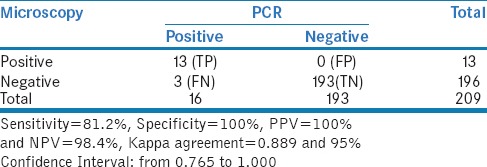
In the present study, total of 13 stool samples were identified as positive for hookworm by stool microscopy [Figure 1]. In addition, hookworm DNA was detected in three samples by PCR which were negative by stool microscopy. Hence, the sensitivity of stool microscopy was 81.2% and specificity was 100% when compared to PCR [Table 1].
Figure 1.
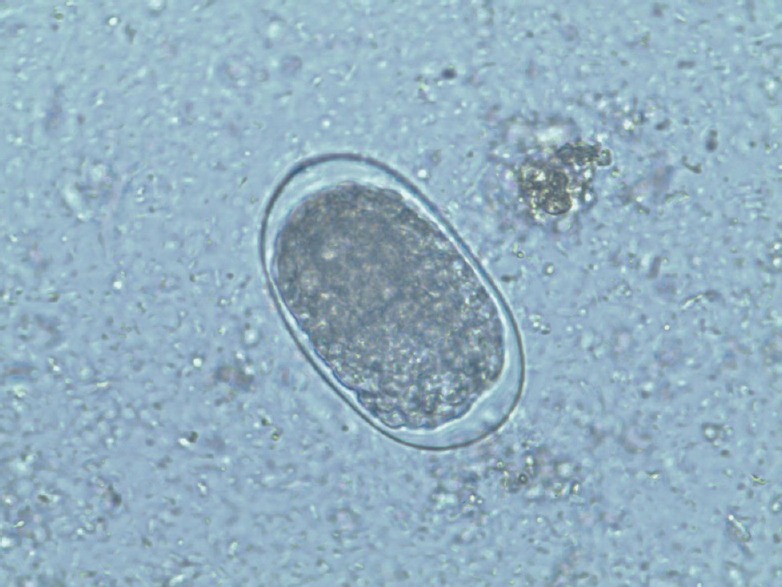
Hookworm egg, stool sample, saline wet mount (×40)
Table 1.
Comparing Microscopy with PCR
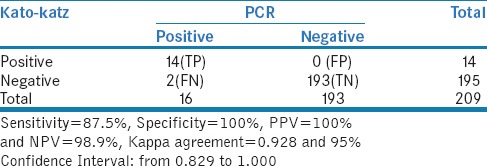
In our study, 14 samples were identified as positive by Kato-Katz method and two samples which were negative by Kato-Katz method were identified positive by PCR. The sensitivity of Kato-Katz method was estimated as 87.5% and specificity was 100% [Figure 2 and Table 2].
Figure 2.
Kato-Katz kit
Table 2.
Comparing Kato-katz method with PCR
DISCUSSION
The prevalence of intestinal parasites has changed over a time period. An earlier study from Puducherry showed an overall parasitic prevalence to be 37.9%, of which 44% were positive for hookworm.[14] On the other hand, a recent study conducted at Vellore detected 7.8% of positivity for intestinal parasites in stool samples with 8.4% being positive for hookworm infections.[15] In our study, the overall parasitic prevalence was estimated to be 25.3% whereas, the prevalence of hookworm was found to be 7.6% by PCR. The low prevalence of hookworm in this current study could be due to the annual mass drug administration with 400 mg of albendazole along with 100 mg of diethylcarbamazine to school children since 2004.
The prevalence of hookworm is found to be associated with the age of the patient. In our study, the maximum prevalence of hookworm was common in the age group of 20–30 years (43%) followed by 1–10 years (36%), whereas, a study from Nigeria showed that the hookworm prevalence was higher in 9–13 years of age group.
In our study, hookworm infection seems to be prevalent in male (57%) when compared to female (47%). In contrary to this, a study done in Malaysia revealed that the majority of the hookworm infections were seen in females (62.7%) than males (37.3%).[16] Male predominance seen in our study may be due to the habit of walking barefoot and open air defecation.
In the present study, the prevalence of hookworm infection by both stool microscopy and Kato-Katz method was estimated to be 6.2% and 6.6%, respectively. In another study by Hailu and Abera,[17] the prevalence of hookworm was found to be 63.9% with Kato-Katz method whereas; it was only 57.2% with direct stool smear microscopy. Hence, the sensitivity of Kato-Katz was higher (69.5%) when compared with direct stool microscopy (48.5%). The similar prevalence in our study population by both methods could be due to the predominant occurrence of mild intensity infections (4.8%) in the study population, and only small amount of stool sample was being used in Kato-Katz method. The high sensitivity of the Kato-Katz method observed in our study may be due to multiple reasons, such as the smears being prepared immediately after the sample collection and screened within 20–30 min of smear preparation.
Culture was performed for all 14 microscopically positive hookworm samples of which two samples were contaminated. The contamination rate was 14.2%. The agar plates were incubated at 25°C for 7 days. The long incubation period for hookworm could be explained by the fact that the hookworm requires further development in the feces before hatching. The other reason may be due to the low levels of egg load in stool samples collected for the study. All the 12 samples showed furrows on the agar plate due to the crawling of the L3 larvae. The furrows were thicker and the larva had moved in the forward direction as described by Arakaki et al.,[18] the furrows showed some characteristic features such as sudden change in the track and irregular arrangement [Figures 3 and 4]. This observation was in concordance with the remarks made by dos Santos Neto, and Jongwutiwes et al., in previous studies.[19,20] Out of total 12 samples 2 were identified as A. duodenale and remaining ten samples were identified as N. americanus based on the morphological changes seen in the L3 larva. About 75% of the hookworm larvae were detected during 4th–5th day and only 25% during 6th and 7th day of incubation.
Figure 3.
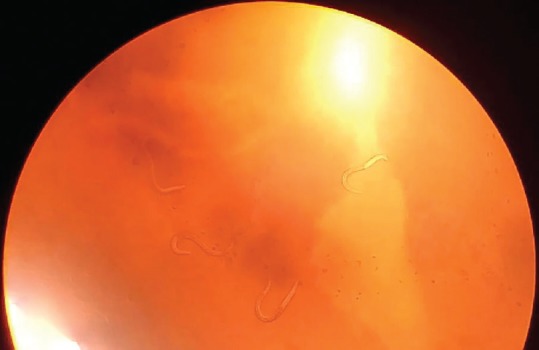
Hookworm larvae in Agar plate culture (×10)
Figure 4.
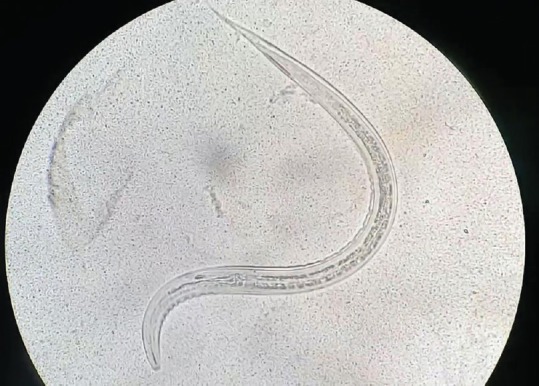
Filariform larvae (third stage), agar culture method (×40)
In this study, the true positivity of agar plate culture was 83.3% and the false positivity rate was 16.6% when compared to PCR. The false positivity was because of misidentification of 2 samples as A. duodenale, which was later identified as N. americanus by PCR [Figure 5].
Figure 5.
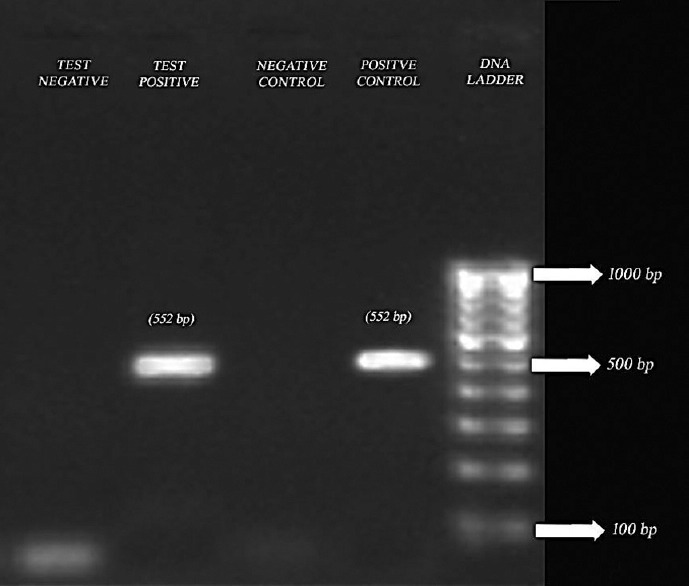
Detection of hookworm by polymerase chain reaction. Lane 1: 100–1000 bp; Lane 2: Positive control of Necator americanus; Lane 3: Negative control; Lane 4: Test sample showing band for positive for Necator americanus
In stool microscopy, many hookworm look alike eggs of Oesophagostomum bifurcum and Trichostrongylus species may be misidentified as hookworm.[21] However, PCR helps in identifying different hookworm species affecting humans and also aids in the differentiation of hookworm like eggs.
In this study, out of the total 209 samples, we found 16 samples were positive by conventional PCR, and all of them were identified as N. americanus. There was no detectable A. duodenale, A. ceylanicum, or any mixed infection found in the study population.
In an earlier study carried out in JIPMER by Parija et al., a total of 184 microscopically hookworm positive stool samples were subjected to Harada-Mori culture, and it detected 60 A. duodenale, 49 N. americanus, and three mixed infections.[22] In a recent study conducted in Vellore, 50 microscopically hookworm positive samples were subjected to PCR, out of which 39 samples were positive for N. americanus, six samples were positive for A. duodenale, and only two samples were identified as A. ceylanicum.[23]
A recent community-based study from Cambodia reported 57.45% of hookworm prevalence by PCR. Among them, the prevalence of A. ceylanicum was found to be higher (51.4%) followed by N. americanus (51%). Approximately, 89% of the infections were single infection. The authors have suggested that the close association of pet animals and study population could be the possible reasons for the higher prevalence of A. ceylanicum.[24]
Although stool microscopy is the major mode of detection of hookworm in many parasitology laboratories. It shows higher false negative rate in mild intensity infection. The morbidity and the intensity of the hookworm infection are reduced by the mass drug administration such as albendazole. However, the rate of reinfection and resistance to antihelminthic treatment depends mainly on the infecting species.[25] Hence, the application of Kato-Katz method helps to identify the intensity of the infection. However, it does not differentiate between N. americanus and A. duodenale. Hence, for species identification, coproculture or molecular techniques are required.
In coproculture, there is a risk of auto exposure to the infection, it is time-consuming, and expertise is required to differentiate the species based on the morphological features. All the features outweighed the advantage of coproculture over PCR for species differentiation. On the other hand, PCR is known to be a sensitive, specific, reliable investigative tool which can help in differentiating the species.
The total number of positives were too small to make any significant conclusion. This is the main limitation of the study. Only fresh stool samples can be used for agar plate culture method not the refrigerated samples. Refrigerated samples do not favor the survival of the rhaditiform or filariform larvae of hookworm, which in turn results in an increase in false negativity rate.
CONCLUSION
This study emphasis is in need for more advanced diagnostic modalities for diagnosis of hookworm infection and the importance of human and animal health programs and hygienic practices along with the mass drug administration of albendazole for the complete elimination of both human and zoonotic hookworm species.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L, et al. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Brooker S, Hotez PJ, Bundy DA. Hookworm-related anaemia among pregnant women: A systematic review. PLoS Negl Trop Dis. 2008;2:e291. doi: 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gezahegn A, Menkir S. Hookworm Infection and Hemoglobin Concentration Among Students in Three Selected Primary Schools, AboboWoreda, Gambella Region, Western Ethiopia. 2012 [Google Scholar]
- 4.Bundy DA, Cooper ES, Thompson DE, Didier JM, Simmons I. Effect of age and initial infection intensity on the rate of reinfection with Trichuris trichiura after treatment. Parasitology. 1988;97(Pt 3):469–76. doi: 10.1017/s003118200005887x. [DOI] [PubMed] [Google Scholar]
- 5.Bethony J, Chen J, Lin S, Xiao S, Zhan B, Li S, et al. Emerging patterns of hookworm infection: Influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin Infect Dis. 2002;35:1336–44. doi: 10.1086/344268. [DOI] [PubMed] [Google Scholar]
- 6.Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43:1009–15. doi: 10.1016/j.ijpara.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RC. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in bangkok. Vet Parasitol. 2008;155:67–73. doi: 10.1016/j.vetpar.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Parija SC. Textbook of Medical Parasitology: Protozoology and Helminthology. 4th ed. New Delhi: All India Publishers and Distributors; 2013. [Google Scholar]
- 9.Shahid S, Chowdhury A, Shamsuzzaman S, Mamun K. Identification of hookworm species in stool by Harada Mori culture. Bangladesh J Med Microbiol. 2012;4:3–4. [Google Scholar]
- 10.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 11.Arakaki T, Hasegawa H, Asato R, Ikeshiro T, Kinjo F, Saito A, et al. A new method to detect Strongyloides stercoralis from human stool. Jpn J Trop Med Hyg. 1988;16:11–7. [Google Scholar]
- 12.George S, Levecke B, Kattula D, Velusamy V, Roy S, Geldhof P, et al. Molecular identification of hookworm isolates in humans, dogs and soil in a tribal area in Tamil Nadu, India. PLoS Negl Trop Dis. 2016;10:e0004891. doi: 10.1371/journal.pntd.0004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Soil-Transmitted Helminthiases: Eliminating as Public Health Problem Soil-Transmitted Helminthiases in Children: Progress Report 2001-2010 and Strategic Plan 2011-2020. Geneva: World Health Organization; 2012. [Google Scholar]
- 14.Parija SC, Rao R. Prevalence of parasitic infections in Pondicherry. Indian J Parasitol. 1987;11:63–5. [Google Scholar]
- 15.Kattula D, Sarkar R, Rao Ajjampur SS, Minz S, Levecke B, Muliyil J, et al. Prevalence & risk factors for soil transmitted helminth infection among school children in South India. Indian J Med Res. 2014;139:76–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Walana W, Aidoo EN, Tay SC. Prevalence of hookworm infection: A retrospective study in Kumasi. Asian Pac J Trop Biomed. 2014;4:S158–61. doi: 10.12980/APJTB.4.2014APJTB-2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hailu T, Abera B. Performance evaluation of direct saline stool microscopy, formol ether concentration and Kato Katz diagnostic methods for intestinal parasitosis in the absence of gold standard methods. Trop Doct. 2015;45:178–82. doi: 10.1177/0049475515581127. [DOI] [PubMed] [Google Scholar]
- 18.Arakaki T, Iwanaga M, Kinjo F, Saito A, Asato R, Ikeshiro T, et al. Efficacy of agar-plate culture in detection of Strongyloides stercoralis infection. J Parasitol. 1990;76:425–8. [PubMed] [Google Scholar]
- 19.dos Santos Neto JG. Movement of the rhabditiform larva of Strongyloides stercoralis. Lancet. 1993;342:1310. [PubMed] [Google Scholar]
- 20.Jongwutiwes S, Charoenkorn M, Sitthichareonchai P, Akaraborvorn P, Putaporntip C. Increased sensitivity of routine laboratory detection of Strongyloides stercoralis and hookworm by agar-plate culture. Trans R Soc Trop Med Hyg. 1999;93:398–400. doi: 10.1016/s0035-9203(99)90132-3. [DOI] [PubMed] [Google Scholar]
- 21.Verweij JJ, Brienen EA, Ziem J, Yelifari L, Polderman AM, Van Lieshout L, et al. Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg. 2007;77:685–90. [PubMed] [Google Scholar]
- 22.Parija SC, Malini G, Rao RS. Prevalence of hookworm species in Pondicherry, India. Trop Geogr Med. 1992;44:378–80. [PubMed] [Google Scholar]
- 23.George S, Kaliappan SP, Kattula D, Roy S, Geldhof P, Kang G, et al. Identification of Ancylostoma ceylanicum in children from a tribal community in Tamil Nadu, India using a semi-nested PCR-RFLP tool. Trans R Soc Trop Med Hyg. 2015;109:283–5. doi: 10.1093/trstmh/trv001. [DOI] [PubMed] [Google Scholar]
- 24.Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis. 2014;20:976–82. doi: 10.3201/eid2006.131770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall A, Horton S, de Silva N. The costs and cost-effectiveness of mass treatment for intestinal nematode worm infections using different treatment thresholds. PLoS Negl Trop Dis. 2009;3:e402. doi: 10.1371/journal.pntd.0000402. [DOI] [PMC free article] [PubMed] [Google Scholar]


