Abstract
Colorectal cancer (CRC), more of lifestyle-related disorder, is one of the deadliest types of cancer across the globe. Nevertheless, infectious agents could be responsible for 20% of cancer. Recent findings have indicated the association of Blastocystis in CRC and recommend routine screening for Blastocystis. Herein, we describe a case of CRC with severe Blastocystis infection.
Keywords: Blastocystis, colorectal cancer, genetic diversity, inflammatory bowel disorder
INTRODUCTION
Blastocystis is an atypical stramenopile, cosmopolitan in distribution with wide host range, and it is one of the most frequently encountered parasites in human fecal samples in the developing countries.[1] Blastocystis is highly polymorphic, complicating morphological diagnosis. Currently, based on ribosomal lineages, different species of Blastocystis are designated as various subtypes (STs). The ST system is primarily based on the consensus developed by Stensvold et al. in 2007.[2] So far, there are nine STs have been identified in humans. However, ST1, ST2, ST3, and ST4 together make up 90% of all human Blastocystis.[3] Accumulating epidemiological data suggests that Blastocystis is associated with various gastrointestinal complications including colorectal cancer (CRC). Furthermore, recent findings indicate that the association of particular ST of Blastocystis augments disease progression in CRC.[4,5] Therefore, we describe a case of CRC with severe Blastocystis ST3 infection.
CASE REPORT
A 35-year-old male taxi driver presented to the medical outpatient department with nonbilious vomiting after feeds, early satiety, and significant weight loss (15–20 kg) for the past 3 months. He also had complaints of altered bowel habits for the past 6 months with diarrhea. On upper gastrointestinal endoscopy, Grade I esophagitis Lax O-G junction was detected.
On preliminary examination, there was no history of hematemesis, melena, per rectal bleeding, jaundice, fever, or dysuria. The patient was conscious, oriented with mild pallor, icterus, and pedal edema. The patient was admitted to the medical gastroenterology, Jawaharlal Institute of Postgraduate Medical Education and Research, for further evaluation. His blood and stool samples were sent for biochemical and microbiological examinations. On colonoscopy, a circumferential growth in the distal transverse colon was noted. Contrast-enhanced computed tomography scan of the abdomen indicated a transverse colon growth measuring 8 cm × 8 cm with dilatation of the proximal colon, with no other complications such as free fluid or liver, peritoneal metastasis. Abdominal ultrasonography revealed asymmetrical wall thickening of the descending colon. Biopsies were taken, and the patient was referred to surgical gastroenterology for further management. There was no similar illness seen in the family. Furthermore, the patient was a nonsmoker and an occasional drinker without any comorbid illness such as diabetes and hypertension.
The patient had chronic diarrhea, and macroscopic examination of the stool showed that it was liquid in consistency with the copious amount of mucus, but no evidence of any helminthic infections. On microscopic examination, the presence of many fecal leukocytes, red blood cells, and vacuolar forms of Blastocystis was appreciated. Furthermore, trichrome staining (Wheatley modification) was done to observe the presence of Blastocystis [Figure 1a], and the intensity of infection was calculated as previously explained.[6] More than 10 Blastocystis were found per 10 oil immersion fields which indicated severe infection. Modified Ziehl–Neelsen stain for coccidian parasites was performed which was negative. The stool sample was inoculated into the diphasic NIH modification of Boeck and Drbohlav medium and Lowenstein–Jensen medium[7] for Blastocystis isolation. After 48 and 72 h of incubation at 37°C, the culture was observed for the presence of Blastocystis. Vacuolar, granular, and amoeboid forms of Blastocystis were seen on microscopic observation. However, the vacuolar form of Blastocystis was predominant with varying diameters ranging between 10μm to 50 μm [Figure 1b and c]. Stool DNA was extracted by QIAamp DNA Stool Mini Kit according to the manufacturer's instructions (Qiagen, Germany). Further, it was subje calculated as previously cted to polymerase chain reaction (PCR) with Blastocystis-specific primers which target barcoding region (600 bp) of 18S SSU rRNA gene of Blastocystis.[8] PCR result confirmed the presence of Blastocystis DNA in the sample [Figure 2]. The purified amplicon was subjected to sequencing (Applied Biosystems. Model: 3730 × l/AB/3730XL-15104-028, Foster City, CA, USA), and sequence results obtained from both the strands were assembled and ST analysis was done using the sequence database available http://www.pubmlst.org/blastocystis/. The sequence represents Blastocystis ST3 and allele 34. Further, the assembled sequence was uploaded into NCBI GenBank (accession number KR000003). On the other hand, a nested multiplex PCR was carried out for detecting Entamoeba spp., which was found to be negative.[9] Bacteriological culture of stool sample showed the absence of stool pathogens such as Salmonella, Shigella, and Vibrio group of organisms. Serum samples sent for other investigations such as anti-HIV antibody detection, hepatitis B virus surface antigen detection, anti-hepatitis C virus antibody (IgM and IgG) detection, and Widal tests were negative. Thyroid profile and other routine biochemical parameters were found to be normal. Based on the microbiological findings, the patient was prescribed therapy with oral metronidazole 400 mg 8th hourly for 7–10 days.
Figure 1.
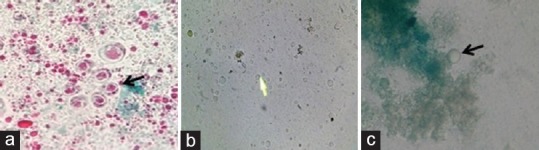
(a) Trichrome staining (×1000) of unconcentrated stool, (b) wet mount (×400), and (c) lactophenol cotton blue mount (×400) of culture showing vacuolar forms of Blastocystis
Figure 2.
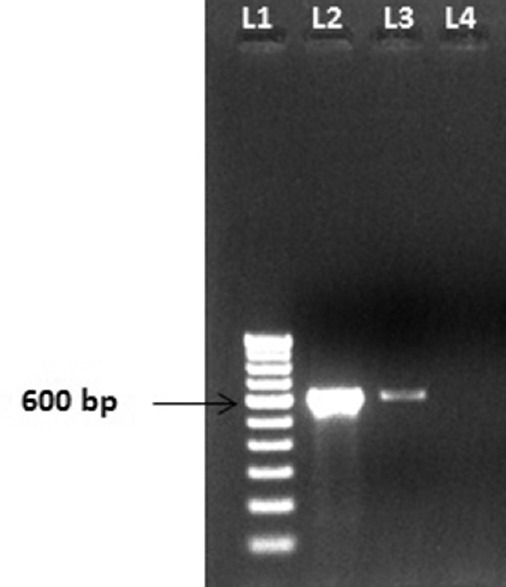
Blastocystis DNA (600 bp) identified by polymerase chain reaction. Lane 1-100 bp ladder, Lane 2 – stool DNA extracted from patient sample, Lane 3 – Blastocystis DNA-positive control, Lane 4 – negative control
Histopathological and radiological findings confirmed carcinoma of the distal transverse colon. Following which, preoperatively, antibiotics and antiparasitic drugs were administered and extended left hemicolectomy with double stapled colorectal anastomosis and gastric sleeve resection procedure was performed. After the operation, the patient was shifted to ICU and put on the liquid diet. The patient was discharged after 15 days of stay in the hospital with cancer chemotherapy drugs as treatment and was advised to turn up for regular follow-ups. As the patient was on a liquid diet, we were not able to receive repeat stool sample. On histopathological examination, we could not appreciate any invasion or adhesion of Blastocystis in hematoxylin and eosin-stained slides of biopsy specimen taken from the patient.
DISCUSSION AND CONCLUSION
CRC is one of the widespread and deadliest types of cancer across the globe, and increasing incidence of CRC has been observed in Asia.[10,11] Development of CRC has multiple explanations; recent in vitro studies have shown the possible role of Blastocystis in the exacerbation of pre-existing cancer; in particular, Blastocystis ST3 can elicit higher proliferation of colon cancer cell line.[4,5] On the other hand, Blastocystis is cosmopolitan in distribution with wide host range. Thus, humans can easily acquire Blastocystis through consumption of contaminated water and due to human-to-human transmissions.[12] Furthermore, inflammatory bowel disorder (IBD) is one of the major risk factors which would facilitate the development and progression of CRC.[13] Considering all these facts, we believe that there is a need to understand the association of Blastocystis with CRC and its involvement in disease progression. This can be achieved by large-scale molecular epidemiological studies alongside with more focused pathogenesis studies which in turn discern the actual role of Blastocystis in CRC. Meanwhile, it is prudent to screen and treat Blastocystis on routine basis among IBD and CRC patients.
Financial support and sponsorship
This study is supported by JIPMER, Intramural Research Grant.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We sincerely thank Dr. Christen Rune Stensvold, Statens Serum Institut, Copenhagen S, Denmark, and Dr. Prashant Kumar Pandey, Universidad Nacional Agraria La Molina, Lima, Perú, for providing us with Blastocystis DNA. We also thank Department of Surgical Gastroenterology, JIPMER, Puducherry, for sharing clinical details related to this case report.
REFERENCES
- 1.Parija SC, Jeremiah S. Blastocystis: Taxonomy, biology and virulence. Trop Parasitol. 2013;3:17–25. doi: 10.4103/2229-5070.113894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stensvold CR, Suresh GK, Tan KS, Thompson RC, Traub RJ, Viscogliosi E, et al. Terminology for Blastocystis subtypes – A consensus. Trends Parasitol. 2007;23:93–6. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Stensvold CR. Thoughtson Blastocystis. Copenhagen: Amazon Kindle; 2013. [Google Scholar]
- 4.Chandramathi S, Suresh K, Kuppusamy UR. Solubilized antigen of Blastocystis hominis facilitates the growth of human colorectal cancer cells, HCT116. Parasitol Res. 2010;106:941–5. doi: 10.1007/s00436-010-1764-7. [DOI] [PubMed] [Google Scholar]
- 5.Kumarasamy V, Kuppusamy UR, Samudi C, Kumar S. Blastocystis sp. subtype 3 triggers higher proliferation of human colorectal cancer cells, HCT116. Parasitol Res. 2013;112:3551–5. doi: 10.1007/s00436-013-3538-5. [DOI] [PubMed] [Google Scholar]
- 6.Garcia LS. Diagnostic Medical Parasitology. 5th ed. Washington, DC: ASM Press; 2007. [Google Scholar]
- 7.Basak S, Rajurkar MN, Mallick SK. Detection of Blastocystis hominis: A controversial human pathogen. Parasitol Res. 2014;113:261–5. doi: 10.1007/s00436-013-3652-4. [DOI] [PubMed] [Google Scholar]
- 8.Scicluna SM, Tawari B, Clark CG. DNA barcoding of Blastocystis. Protist. 2006;157:77–85. doi: 10.1016/j.protis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Khairnar K, Parija SC. A novel nested multiplex polymerase chain reaction (PCR) assay for differential detection of Entamoeba histolytica, E. moshkovskii and E. dispar DNA in stool samples. BMC Microbiol. 2007;7:47. doi: 10.1186/1471-2180-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart BW, Kleihues P. World cancer report. Lyon: IARC press; 2003. [Google Scholar]
- 11.Sung JJ, Lau JY, Goh KL, Leung WK. Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: Implications for screening. Lancet Oncol. 2005;6:871–6. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 12.Anuar TS, Ghani MK, Azreen SN, Salleh FM, Moktar N. Blastocystis infection in Malaysia: Evidence of waterborne and human-to-human transmissions among the Proto-Malay, Negrito and Senoi tribes of Orang Asli. Parasit Vectors. 2013;6:40. doi: 10.1186/1756-3305-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20:9872–81. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]


