Abstract
Cryptosporidium spp. was first described in mice in 1907. The first human case was reported in an acquired immune deficiency syndrome patient after which it gained importance. It is one of the emerging protozoan parasites according to the Centre of Disease Control and Prevention. The special structure which is present in them such as rhoptries and micronemes are responsible for their virulence and pathogenicity. They can be transmitted from animals, human to human, water, food, and tends to cause waterborne outbreaks. The clinical manifestation in immunocompetent patient is self-limiting when compared to immunocompromised individual where it causes chronic diarrhea not responding to treatment. Hence, it is necessary to diagnose them early to prevent any complication in these patients. There are many investigations currently available such as stool microscopy after Sheather's concentration technique, rapid test targeting specific antigen, molecular methods, and imaging techniques.
Keywords: Cryptosporidium, emerging parasite, immunocompromised patients, pathogenicity
HISTORICAL PERSPECTIVE
In 1907, Tyzzer was the first to describe the intestinal protozoan parasite Cryptosporidium in the gastric mucosa of mice.[1] The importance of Cryptosporidium infection was appreciated only in 1955 when there was an outbreak in a turkey flock. The minimum dose of causing Cryptosporidium infection is as low as ten oocysts, and hence, it is considered as highly infectious.[2] Until 1980, it had not gained importance in causing human infection. In 1982, the first case of cryptosporidiosis in acquired immune deficiency syndrome (AIDS) patient was reported.[3] Since then, many cases of cryptosporidiosis have been reported in immunocompromised patients.
CHARACTERISTICS AND LIFECYCLE OF THE PATHOGEN
Cryptosporidium spp. belongs to the phylum: apicomplexa, class: sporozoasida, subclass: coccidia, order: Eucoccidiorida, and family: cryptosporidiidae. The most common species that causes human infection are the Cryptosporidium parvum and Cryptosporidium hominis. They are divided into many species/genotype and subtypes using various molecular methods. In the recent trend, these molecular tools are necessary for epidemiological purposes and our understanding of the transmission of infection to humans and animals. There are nearly 22 species currently known. The zoonotic species C. parvum infects both human and animals while anthroponotic species C. hominis affects only human. These genotyping is based mainly on SSU rRNA. C. parvum and C. hominis are further divided into subtypes based on DNA sequence analysis of the 60 kDa glycoprotein (gp60 also called gp40/15). The subtypes of C. hominis are Ia, Ib, Id, Ie, If, and Ig and C. parvum are IIa, IIb, IIc, IId, IIe, IIf, IIg, IIh, IIi, IIk, and IIl. By knowing the different subtypes, we may understand the biological character of the parasite and the clinical presentation that may differ for each subtype. The other species of little importance that causes human infection are Cryptosporidium meleagridis, Cryptosporidium felis, Cryptosporidium canis, Cryptosporidium muris, Cryptosporidium suis, and Cryptosporidium andersoni. Recently, one waterborne outbreak of C. cuniculus was reported, in which a rabbit genotype was found to be associated.[4]
There has been diversity in the distribution of Cryptosporidium worldwide. In European countries both C. parvum and C. hominis are found to be common while in Middle-East the most common species is C. parvum. In India and other developing countries, the most common species was the C. hominis.[5]
LIFE CYCLE
Cryptosporidium spp. completes its lifecycle in a single host. After ingestion of the oocyst, excystation occurs in the gut followed by the release of sporozoites. It causes infection of the epithelial cells, but it is confined to intracellular and extracytoplasmic location termed as “parasitophorous vacuole.” Here, it undergoes two generations of merogony forming eight and four merozoites, respectively. The second stage merogony is followed by sexual developmental stage, microgamont, and macrogamonts. The microgamete fuses with macrogamete to form zygote and develops into oocyst with four naked sporozoites. There are two types of oocyst, thin-walled and thick-walled oocysts. The former is responsible for autoinfection and the latter persists in the environment for longer periods.[6]
MODES OF TRANSMISSION
Cryptosporidium is ubiquitous in nature. The two-layered thick-walled oocyst is the primary source of transmission. A low dose of 10–100 oocysts can transmit the infection. It is transmitted by zoonotic and nonzoonotic means. They can tolerate various environmental conditions and can survive in water and soil for many months because of suitable moisture and cold temperature. They can be transported long distances in the air and also flushed quickly in the water sources because of its small size.
Zoonotic transmission
The initial outbreaks of Cryptosporidium were zoonotic in nature. The first case was reported in a child living in a cattle rearing farms.[7] Calves, lambs, animal feces, and improper hand washing are considered as factors responsible for zoonotic transmission.
Nonzoonotic transmission
Waterborne transmission
The waterborne transmission is one of the most common sources of infection. There was an outbreak in 1993, commonly involving the immunocompromised patients resulting in 4,03,000 affected cases, 5000 confirmed cases, and 100 fatalities.[8] The Cryptosporidium was listed as a category B pathogen by the Center for Disease Control and Prevention and National Institute of Health because of its threat to cause water contamination.[9] They can be found in surface water, groundwater, treated and untreated drinking water, and also in recreational water such as swimming pools and lakes.[10] These water system gets contaminated by human and animal excreta used as manure for crops, contaminated sewage that enters the water distribution system.[11] The main reason for water being the common source of infection is that the oocysts are resistant to most of the chemical methods of purification such as chlorination. However, they can be reduced by coagulation, filtration, and sedimentation methods. Hence, the only way to control the infection is by following multiple treatment systems.[12]
Foodborne transmission
Food and food products also serve as a source of infection. Foodborne outbreaks have also been reported.[13,14,15] Apple cider, chicken salad, milk, food, seafood-like commercially packed oysters, and raw vegetables are the sources.
Other sources
Nosocomial transmission through hospital personal, daycare centers,[16] mechanical transport through soil, and insects such as cockroaches and houseflies.[17] Oocyst also detected in sputum and bronchial aspirates.[18,19] It can also cause traveler's diarrhea.
PATHOGENICITY AND VIRULENCE FACTORS
The infectious stage of Cryptosporidium is the small (4–6 μm in diameter) sporulated thick-walled oocyst. As low as ten oocysts can cause infection. The entire life cycle takes place in single host on the luminal surface of the intestinal epithelium and it remains the intracellular extracytoplasmic region of the host cell. After ingestion of the oocyst, the attachment to the epithelium occurs with the help of glycoproteins gp900, gp60, and circumsporozoite ligand. It is then followed by the release of motile sporozoites in the intestine, and using rhoptries and micronemes, they penetrate inside the host cell and form parasitophorous vacuoles. Asexual reproduction (merogony) and sexual reproduction (gametogony) occur with the release of thick-walled oocyst in the feces and thin-walled oocyst that causes autoinfection.[20]
These structural alterations in the intestinal epithelium lead to shortening of the villous and lengthening of the crypts. The parasite also causes the release of inflammatory mediators such as interferon-gamma (INF-β), interleukin (IL)-8, and tumor necrosis factor (TNF) which in turn liberates the soluble factors that increase the secretion of chloride and water and decrease the sodium absorption resulting in osmotic diarrhea.
Virulence factors
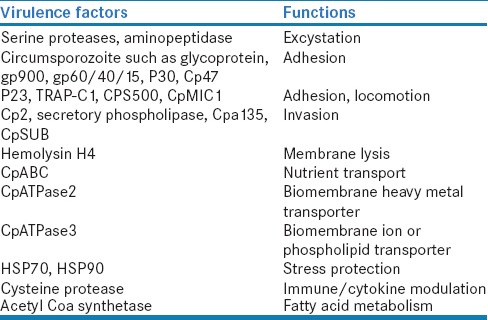
The virulence factors are those that cause the host cell damage. The effect is due to host-parasite interaction. There are various virulence factors that help in the excystation, attachment to the epithelium, gliding motility, invasion, intracellular survival, and host cell damage [Table 1].[21]
Table 1.
Virulence factors of Cryptosporidium spp.
IMMUNITY TO CRYPTOSPORIDIUM INFECTION
Immunity against Cryptosporidium infections is mainly by both innate and adaptive immune responses. Innate immunity is by natural killer cells, dendritic cells, macrophages, chemokines, toll-like receptors, and nitric oxide synthase. Adaptive immunity is mainly by T- and B-cell responses. INF-β plays an important role in innate immunity by activating the macrophages to produce inducible nitric oxide synthase generated nitric oxide which in turn activate the stress signaling pathway. In adaptive immunity, T- and B-cells play an important role. Th1 CD4 helper cells act against intracellular parasites by producing INF-β, IL-2, IL-12, and TNF, and Th2 helper cells help in clearance of parasites. People with very low CD4 count was found to have more severity of infection than with CD4 count >200 cells/mm3. The role of humoral immunity in AIDS patient with Cryptosporidium infection is doubtful. However, they are found to have high IgG/IgA and mucosal IgA.[22]
CLINICAL PRESENTATION
The severity of illness usually depends on the immune status of the individual. It may vary from asymptomatic to fulminant disease.
In immunocompetent individuals
An asymptomatic presentation is seen in both immunocompetent and immunocompromised individuals. It is self-limiting in patients with healthy immune status. It can present as 5–10 episodes of watery diarrhea per day with mucus flecks. Other uncommon presentations are nausea, abdominal cramps, low-grade fever, and anorexia. It resolves within 2 weeks. Failure to thrive is seen in infants with chronic cryptosporidiosis; stunted growth and respiratory tract involvement is also seen in malnourished children.[23]
In immunocompromised individuals
Human immunodeficiency virus individuals
The presentation is asymptomatic to fulminant illness. The symptoms last for many months and they tend to pass 3–6 L of persistent watery stools per day. This large amount of stools is seen mainly in those with CD4 count <200 cells/mm3 and as much as 17 L of the watery stool has been reported.[24] Four different presentations such as chronic diarrhea (36%), cholera-like illness requiring intravenous rehydration therapy (33%), transient diarrhea (15%), and intermittent diarrheal illness (15%) have been reported.[25] The symptom becomes worse with time, reducing the mean survival of the individual. Furthermore, these patients have extraintestinal manifestations involving several organs such as biliary tract with the right upper quadrant nonradiating pain. The typical presentation is primary acalculous cholecystitis and rarely sclerosing cholangitis, pancreatitis with elevated amylase level, and abnormal radiographic findings. Furthermore, respiratory tract disease with symptoms of cough, shortness of breath, wheezing, croup, hoarseness, respiratory failure, and hypoxemia are also seen. Oocyst can be recovered from the sputum, tracheal aspirates, bronchioalveolar lavage, and brush biopsy specimen.[23] It can also involve the stomach resulting in gastric outlet obstruction and esophagus causing dysphagia.
Nonhuman immunodeficiency virus individuals
Other immunosuppressive patients such as transplant recipients, malignancy, primary immunodeficiency, and those on long-term steroids also present with profuse watery diarrhea, anorexia, and weight loss. Extraintestinal involvements such as respiratory tract can also be seen.[23]
EPIDEMIOLOGY
Cryptosporidium spp. is considered as one of the most common causes of diarrhea in both the immunocompetent and immunocompromised patients.[26] C. hominis and C. parvum are the primary causative agents of human cryptosporidiosis, but their prevalence varies in different regions of the world. The other species such as C. felis, C. muris, and C. meleagridis have also been reported to cause human infection due to Cryptosporidium.[27]
Cryptosporidiosis is endemic in developing countries and the individuals more prone are adults with human immunodeficiency virus (HIV) infection and children. The prevalence of Cryptosporidium infection in underdeveloped countries such as Brazil, Venezuela, Indonesia, Thailand, South Africa, Ghana, India, and Bangladesh among children with diarrhea varies from 3% to 13%. In contrast, in developed parts of the world such as Britain, United States, Canada, Australia, and Denmark, it accounts for only 1–4% of childhood diarrhea.[28]
Cryptosporidiosis in India
In India, the prevalence rate was reported to be 4%–13%. In South India, 40% of children in the semiurban slum have multiple episodes of cryptosporidiosis and prolonged oocyst shedding.[29] In Eastern India, the children in the periurban region were found to have a high prevalence of asymptomatic carrier state. These studies show a correlation between the poor sanitation and the cryptosporidial infection.[30]
Cryptosporidiosis in children
Cryptosporidium causes nutritional deficiencies with a significant morbidity and mortality, especially in children and immunodeficient individuals.[31] The primary mechanism of defense against cryptosporidiosis is due to cell-mediated immune responses. Since the immune system of children is not well developed, they are very much prone to infection. The predisposing cause of the immunocompromised state in children is malnutrition and some of the childhood infections such as measles. In India, there are some studies which reported Cryptosporidium spp. in children with diarrhea (ranging from 1.1% to 18.9%)[32] and in asymptomatic children (0%–3%).[33] There are also studies reported up to 9.8% positivity in asymptomatic children (with 13.1% positivity in symptomatic children).[34]
Cryptosporidiosis in primary immunodeficiency
Primary immunodeficiency disorders are either inherited or de novo. It can be due to combined B- and T-lymphocyte deficiency, complement deficiency, or defects in phagocyte number and function. Of various primary immunodeficient conditions, most dangerous Cryptosporidium infection is commonly associated with severe combined immunodeficiency.[3] Only very few case studies on primary immunodeficiency are found.[35,36,37,38,39,40,41] Hence, the available case reports infer only the hazards of infection and do not clearly delineate the risk and prognosis of the disease.
Cryptosporidiosis in secondary immunodeficiency
In human immunodeficiency virus-infected individuals
Among various opportunistic infections, cryptosporidiosis is one of the common infections among HIV-infected individuals. It is also included as an AIDS-defining illness (clinical category C) by the US CDC and Prevention.[42] It causes a life-threatening infection involving the gastrointestinal tract and the extraintestinal sites such as the hepatobiliary tract, pancreas, and respiratory tract. Africa is the first continent to have a significant number of HIV cases in the world with the prevalence of cryptosporidiosis ranging from 3.8% to 73.6%.[43]
Asia is the second continent for increased number of HIV cases (approximately 4.7 million cases) after Africa. There are some case reports from India, with the prevalence ranging from 4.7% to 56.5%.[8] The CD4 count and Cryptosporidium diarrhea are found to be related to one another. It was found that cryptosporidiosis was most common in symptomatic HIV patients with CD4 T-lymphocyte count <200 cells/mm3 than in asymptomatic individuals.[44,45,46,47] In Southeastern Asia, Thailand has a prevalence rate of 8.8%–34.4% while Nepal has 10.7% and Cambodia 45%. Malaysia has a prevalence rate of 3%–23%, Iran and Indonesia 52.5%. In middle Eastern Asia, Iran has a prevalence of 0.9%–26.7%; in Eastern Asia, Korea has 10.5%.[8]
The prevalence ranges from 8% to 39% in European countries, 3.5% to 11.9% in North America, and 4% to 22.8% in South America.
Cryptosporidiosis in transplant recipients
Organ transplant recipients are usually on lifelong immunosuppressive therapy that puts them at risk of developing opportunistic infections. A study by Kang et al. in Vellore found that Cryptosporidium was the most common infection associated with bone marrow transplantation than any other parasitic infections. The same center also has reported 1.7% of Cryptosporidium infection in pediatric bone marrow transplants.[48,49] A survey found 16.6% of infection in renal transplant recipients in North India.[50] The other organ transplant recipients such as liver transplants are also prone to Cryptosporidium infection leading to diffuse cholangitis.[3]
Cryptosporidiosis in malignancy
In malignancy, the immune function is deranged due to various factors such as malnutrition, cachexia, patients receiving chemotherapy, radiotherapy, and in hematological malignancies disease per se can produce defective immune function. The prevalence of Cryptosporidium infection was found to be 76.66% in malignant and 15% in nonmalignant patients. This increase in Cryptosporidium infection is mainly due to immunosuppressive therapy in malignant patients.[51] Children with lymphohematopoietic malignancy are more prone to infection.
LABORATORY DIAGNOSIS
An initial macroscopic examination should be done to look for the consistency of the stool. Usually, it looks watery with mucus flecks. The number of oocysts is related to the consistency of the stool. More oocysts will be seen in the watery stools. However, not in all cases, it can be a solid stool in asymptomatic patients and the oocysts load will be low.
Sample collection
The oocysts show fluctuation in their fecal shedding. Hence, a minimum of three stool samples has to be collected and at least five to six modified acid-fast smears should be examined before it is considered as negative.[20] Concentration techniques either floatation or sedimentation should be done to increase the yield of the oocyst.
Concentration techniques
Floatation techniques
The different floatation techniques are the Sheather's sucrose, zinc sulfate, and saturated sodium chloride. Sheather's solution gives a better result than the other two methods.[52,53] In Sheather's sucrose floatation technique, bright field microscopy shows pink-tinged oocyst and phase-contrast microscopy shows a bright and birefringent image. The oocysts get collapsed and lose their distinct shape when Sheather's solution is kept for more than 15 min.[52] The stool sample can be centrifuged at >500 ×g for 10 min.
Sedimentation technique
Formalin ether and formalin ethyl acetate are the sedimentation methods that is commonly used in the laboratories. It is used for the preparation of acid-fast stains.
Stool microscopy
Iodine-saline wet mount
The wet mount can be made from the concentrated stool sample to look for 4–6 μm oocyst that is highly refractile, round, and double walled. It can be used for the routine screening procedure. However, the limitation is that it cannot be maintained as a permanent record.
Staining procedures
Oocysts of Cryptosporidium are very difficult to detect without special stains. Other specimens such as duodenal fluid, bile, sputum, bronchoalveolar lavage, and biopsy specimens can be used.
Modified acid-fast staining method
Cryptosporidium oocysts are acid-fast and resist 1% concentrated sulfuric acid. They appear pink-colored, round, 4–6 μm in diameter that may contain four sporozoites within the oocyst. The background appears uniformly blue. Either hot or cold staining method can be used. The advantages are of low cost, help in screening a large number of samples, and they are a permanent stain. The limitations include time-consuming procedure, require intensive training, and experience to interpret the results, for interpretation 50,000–5,00,000 oocysts/g of the stool. They have a low sensitivity of 83.7% and specificity of 98.9% [Figure 1].[54]
Figure 1.
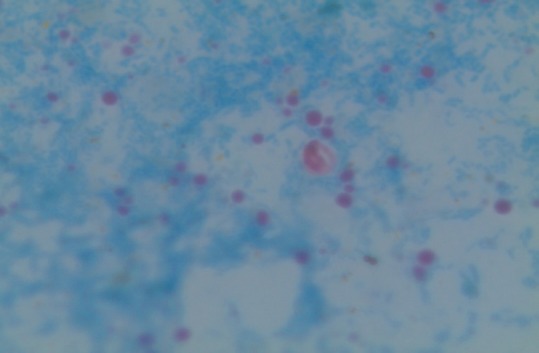
Cryptosporidium in stool sample by modified acid fast staining
Safranin-methylene blue staining method
Here, the smear is initially fixed with 3% HCl in methanol. It is then stained with safranin followed by methylene blue. They appear orange-pink structure, round, and 5 μm in diameter. The sporozoites are stained slightly darker. It is a simple and rapid method, differentiate yeast from oocysts. The problem with this stain is the acid-methanol treatment before and vigorous heating after adding safranin.[55]
Negative staining
In negative staining method of Heine, the fecal material is mixed with carbol fuschsin, spread as thin film, allowed to air dry, immersion oil is added to the smear, and cover slip is placed and observed under bright field microscopy (×400). The oocyst is seen as unstained, strongly refractile, round of 4–6 μm in diameter. Negative staining can also be done with safranin, 2–5% light green, malachite green, and nigrosin.[56] They are rapid and simple. The limitation is the need for phase-contrast microscopy.
Dimethyl sulfoxide modified acid-fast staining
Among various staining method mentioned above, the acid-fast stain is considered as the procedure of choice. With the addition of dimethyl sulfoxide, the method has been modified.[57,58] This shows a bright pink structure against a pale green background. It is a simple way and helps in earlier identification for initiation of therapy. The problem is the variability in stain uptake.
Fluorescent stain
Auramine-rhodamine, auramine-phenol, auramine-carbol fuchsin, and acridine orange can be used. Its main advantage is rapid screening and higher detection efficacy than microscopy and enzyme-linked immunosorbent assay (ELISA). Low sensitivity, low specificity, and high cost are its limitations.[28]
Immunofluorescence stain
The rate of detection of Cryptosporidium is increased using anti-Cryptosporidium specific fluorescent antibody staining over acid-fast staining. It is more sensitive and specific and found to be the gold standard method in many laboratories. In a study done by Anuradha De, DFA-Cryptosporidium had a sensitivity of 99% and specificity of 100% while DFA-Cryptosporidium/Giardia had a sensitivity of 88–98% and specificity of 87–100%. This test is limited by its expense and lack of availability of fluorescence microscope.
Hematoxylin and eosin/Giemsa/Jenner staining
These staining methods are used to describe the developmental stages of the Cryptosporidium. They appear as small, spherical, and basophilic bodies within the microvillous region of the intestinal mucosa.[59,60] However, transmission electron microscopy is required to confirm the diagnosis. It is also an invasive procedure.
Serological techniques
Antibody detection
Specific anti-Cryptosporidium IgG, IgM, or both can be detected by using ELISA. In patients with cryptosporidiosis, 95% are detected at the time of medical presentation and 100% within 2 weeks of presentation.[28] The sensitivity was found to be 66–100% and specificity of 93–100%.[61] It has an advantage of shorter detection time, more economic and detects many samples. The limitation is the high cost of the kits and need for specialist equipment (ELISA reader and plate washer).[62]
Antigen detection
Immunochromatographic method
This method involves the detection of Cryptosporidium/Giardia or triage panel containing Cryptosporidium/Giardia/Entamoeba specific antigens. Agnamey et al. compared four commercial rapid immunochromatographic assays and found that the sensitivity and specificity depend on the kit and species. The mean sensitivities for all Cryptosporidium species were 47.2%, 62.4%, 68.8%, and 70.6% for Crypto-Strip, RIDAQuick, Remel-Xpect, and ImmunoCard STAT, respectively. The specificity was 100% for Remel-Xpect, ImmunoCard STAT, Crypto-Strip, and 98% for RIDAQuick. Although it is a rapid method, the kit cost is more.[63]
Molecular methods
Polymerase chain reaction
Polymerase chain reaction (PCR) is the most sensitive of all the methods for Cryptosporidium detection in both clinical and environmental samples. It helps in genotyping and subtyping of Cryptosporidium.
Nested polymerase chain reaction
It is a two-step PCR using two sets of primers. The common gene targets are gp60, hsp70, 18S rRNA, COWP, and TRAP (C1 and C2). A comparative study was done by Beena Uppal et al. found that nested PCR was able to detect 17.78% more positives than ZN, microscopy, and ELISA. Although it is sensitive and specific, the main drawback is that the species/genotypes can be identified only by sequencing or RFLP analysis and it takes a long time to complete the procedure.[27]
Polymerase chain reaction-Restriction fragment length polymorphism
Restriction fragment length polymorphism (RFLP) helps in the analysis of PCR products after amplification of genomic DNA. Genotyping, subtyping, and species identification can be done. However, it is a time-consuming.
Real-time polymerase chain reaction
This assay is sensitive, specific, reproducible, and improved laboratory workflow and turnaround time. It is a real-time detection of DNA using hybridization probes. The equipment being expensive is the only limitation to the utilization of this method.[64,65]
Multiplex real-time polymerase chain reaction
The main drawback of these entire PCR-based assays is the difficulty in DNA extraction from fecal sample and their possibility of contamination. However, real-time PCR with fluorescent detection probes were designed which reduces the risk of contamination, labor time, and reagent cost. Furthermore, this multiplex assay combines different targets into one assay. There is no difference between the amplification of individual test to that of a multiplex assay. The only problem is that technical expertise is required.[66]
Microsatellite analysis
Simple sequence repeats or microsatellites can serve as polymorphic makers. It helps distinguish between the isolates of same species.[27]
Fluorescent in situ hybridization
In situ hybridization using rRNA-targeted oligonucleotide probe helps in hybridizing the synthetic oligonucleotide probes to particular regions within rRNA of the organism.[67] It identifies the species both in clinical and environmental samples. The time taken for identification is 3 h and a reliable alternative to PCR and RFLP.
Loop-mediated isothermal amplification
This method helps in the amplification of the target sequence of DNA using a constant temperature of 60–65°C. This is in contrast to PCR that needs different temperature for each step. LAMP can use four different primers to identify the six distinct regions of the target gene. Furthermore, loop primers accelerate the reaction. All these increase the specificity of LAMP. The amplification product can be detected visually by the naked eye by turbidity formed due to magnesium pyrophosphate formed as the byproduct during the reaction or using fluorescent dyes. The advantages are the simple procedure, low cost since it does not require expensive equipment such as thermocyclers and shortens the time when compared to PCR-based methods.[68,69]
Diagnosis of extraintestinal cryptosporidiosis
Biliary cryptosporidiosis
Ultrasonography:
It is done to look for the bile duct wall thickening and the gallbladder dilatation.
Computed tomography
-
Endoscopic retrograde cholangiopancreatography (ERCP):
It is the most sensitive of all the methods of diagnosis. It has to be performed when there is high suspicion of biliary disease, but the ultrasound is normal. ERCP may show a papillary stenosis with intrahepatic sclerosing cholangitis.
Serum aminotransferases and alkaline phosphatase levels will be elevated.[28]
Pulmonary cryptosporidiosis
The samples collected for diagnosing are the sputum, tracheal aspirate, bronchoalveolar lavage, and brush biopsy specimens. The presence of acid-fast organism indicates the pulmonary cryptosporidiosis.[28]
Treatment
There are many ways studied for the treatment of cryptosporidiosis. The various therapeutic approaches tried were the macrolide antibiotic, aminoglycoside paromomycin, ionophores such as maduramycin, rifaximin, octreotide, and immunotherapy.[3] Discontinuation of immunosuppressive drugs and restoring the immune function are also found to be effective. In patients with AIDS, antiretroviral therapy has been found to be of value in clearing the Cryptosporidium infection. Nitazoxanide is found to be useful in immunocompetent patients and it is a licensed drug. However, it is not effective in HIV patients.[32]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tyzzer EE. A sporozoan found in the peptic glands of the common mouse. Proc Soc Exp Biol Med. 1907;5:12–3. [Google Scholar]
- 2.Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, et al. Cryptosporidium hominis: Experimental challenge of healthy adults. Am J Trop Med Hyg. 2006;75:851–7. [PubMed] [Google Scholar]
- 3.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin Microbiol Rev. 2002;15:145–54. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp Parasitol. 2010;124:80–9. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Das P, Roy SS, MitraDhar K, Dutta P, Bhattacharya MK, Sen A, et al. Molecular characterization of Cryptosporidium spp. from children in Kolkata, India. J Clin Microbiol. 2006;44:4246–9. doi: 10.1128/JCM.00091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzipori S, Ward H. Cryptosporidiosis: Biology, pathogenesis and disease. Microbes Infect. 2002;4:1047–58. doi: 10.1016/s1286-4579(02)01629-5. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers RM, Giles M. Zoonotic cryptosporidiosis in the UK – Challenges for control. J Appl Microbiol. 2010;109:1487–97. doi: 10.1111/j.1365-2672.2010.04764.x. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal A, Lim YA, Mahdy MA, Dixon BR, Surin J. Epidemiology of cryptosporidiosis in HIV-infected individuals: A global perspective. Open Access Sci Rep. 2012;1:431. [Google Scholar]
- 9.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–30. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose JB. Environmental ecology of Cryptosporidium and public health implications. Annu Rev Public Health. 1997;18:135–61. doi: 10.1146/annurev.publhealth.18.1.135. [DOI] [PubMed] [Google Scholar]
- 11.Meinhardt PL, Casemore DP, Miller KB. Epidemiologic aspects of human cryptosporidiosis and the role of waterborne transmission. Epidemiol Rev. 1996;18:118–36. doi: 10.1093/oxfordjournals.epirev.a017920. [DOI] [PubMed] [Google Scholar]
- 12.Fayer R. Cryptosporidium: A water-borne zoonotic parasite. Vet Parasitol. 2004;126:37–56. doi: 10.1016/j.vetpar.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Ethelberg S, Lisby M, Vestergaard LS, Enemark HL, Olsen KE, Stensvold CR, et al. A foodborne outbreak of Cryptosporidium hominis infection. Epidemiol Infect. 2009;137:348–56. doi: 10.1017/S0950268808001817. [DOI] [PubMed] [Google Scholar]
- 14.Millard PS, Gensheimer KF, Addiss DG, Sosin DM, Beckett GA, Houck-Jankoski A, et al. An outbreak of cryptosporidiosis from fresh-pressed apple cider. JAMA. 1994;272:1592–6. [PubMed] [Google Scholar]
- 15.Quiroz ES, Bern C, MacArthur JR, Xiao L, Fletcher M, Arrowood MJ, et al. An outbreak of cryptosporidiosis linked to a foodhandler. J Infect Dis. 2000;181:695–700. doi: 10.1086/315279. [DOI] [PubMed] [Google Scholar]
- 16.Alpert G, Bell LM, Kirkpatrick CE, Budnick LD, Campos JM, Friedman HM, et al. Outbreak of cryptosporidiosis in a day-care center. Pediatrics. 1986;77:152–7. [PubMed] [Google Scholar]
- 17.Dillingham RA, Lima AA, Guerrant RL. Cryptosporidiosis: Epidemiology and impact. Microbes Infect. 2002;4:1059–66. doi: 10.1016/s1286-4579(02)01630-1. [DOI] [PubMed] [Google Scholar]
- 18.Clavel A, Arnal AC, Sánchez EC, Cuesta J, Letona S, Amiguet JA, et al. Respiratory cryptosporidiosis: Case series and review of the literature. Infection. 1996;24:341–6. doi: 10.1007/BF01716076. [DOI] [PubMed] [Google Scholar]
- 19.Mor SM, Tumwine JK, Ndeezi G, Srinivasan MG, Kaddu-Mulindwa DH, Tzipori S, et al. Respiratory cryptosporidiosis in HIV-seronegative children in Uganda: Potential for respiratory transmission. Clin Infect Dis. 2010;50:1366–72. doi: 10.1086/652140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia LS. Diagnostic Medical Parasitology. 5th ed. Washington, D.C.: American Society for Microbiology; 2007. p. 1236. [Google Scholar]
- 21.Bouzid M, Hunter PR, Chalmers RM, Tyler KM. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 2013;26:115–34. doi: 10.1128/CMR.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitch GJ, He Q. Cryptosporidiosis-an overview. J Biomed Res. 2012;25:1–16. doi: 10.1016/S1674-8301(11)60001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Current WL, Garcia LS. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–58. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control (CDC). Cryptosporidiosis: Assessment of chemotherapy of males with acquired immune deficiency syndrome (AIDS) MMWR Morb Mortal Wkly Rep. 1982;31:589–92. [PubMed] [Google Scholar]
- 25.Manabe YC, Clark DP, Moore RD, Lumadue JA, Dahlman HR, Belitsos PC, et al. Cryptosporidiosis in patients with AIDS: Correlates of disease and survival. Clin Infect Dis. 1998;27:536–42. doi: 10.1086/514701. [DOI] [PubMed] [Google Scholar]
- 26.Harp JA. Parasitic infections of the gastrointestinal tract. Curr Opin Gastroenterol. 2003;19:31–6. doi: 10.1097/00001574-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Snelling WJ, Xiao L, Ortega-Pierres G, Lowery CJ, Moore JE, Rao JR, et al. Cryptosporidiosis in developing countries. J Infect Dev Ctries. 2007;1:242–56. [PubMed] [Google Scholar]
- 28.Vohra P, Sharma M, Chaudhary U. A comprehensive review of diagnostic techniques for detection of Cryptosporidium parvum in stool samples. IOSR J Pharm. 2012;13:15–26. [Google Scholar]
- 29.Ajjampur SS, Sarkar R, Sankaran P, Kannan A, Menon VK, Muliyil J, et al. Symptomatic and asymptomatic Cryptosporidium infections in children in a semi-urban slum community in Southern India. Am J Trop Med Hyg. 2010;83:1110–5. doi: 10.4269/ajtmh.2010.09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palit A, Sur D, MitraDhar K, Saha MR. Asymptomatic cryptosporiosis in a periurban slum setting in Kolkata, India – A pilot study. Jpn J Infect Dis. 2005;58:110–1. [PubMed] [Google Scholar]
- 31.Mak JW. Important zoonotic intestinal protozoan parasites in Asia. Trop Biomed. 2004;21:39–50. [PubMed] [Google Scholar]
- 32.Ajjampur SS, Sankaran P, Kang G. Cryptosporidium species in HIV-infected individuals in India: An overview. Natl Med J India. 2008;21:178–84. [PubMed] [Google Scholar]
- 33.Reinthaler FF, Mascher F, Sixl W, Enayat U, Marth E. Cryptosporidiosis in children in Idukki District in Southern India. J Diarrhoeal Dis Res. 1989;7:89–91. [PubMed] [Google Scholar]
- 34.Kaur N, Diwan N. Cryptosporidiosis in North Indian children. Indian J Med Sci. 1991;45:143–5. [PubMed] [Google Scholar]
- 35.Wolska-Kusnierz B, Bajer A, Caccio S, Heropolitanska-Pliszka E, Bernatowska E, Socha P, et al. Cryptosporidium infection in patients with primary immunodeficiencies. J Pediatr Gastroenterol Nutr. 2007;45:458–64. doi: 10.1097/MPG.0b013e318054b09b. [DOI] [PubMed] [Google Scholar]
- 36.Jo EK, Kim HS, Lee MY, Iseki M, Lee JH, Song CH, et al. X-linked hyper-IgM syndrome associated with Cryptosporidium parvum and Cryptococcus neoformans infections: The first case with molecular diagnosis in Korea. J Korean Med Sci. 2002;17:116–20. doi: 10.3346/jkms.2002.17.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez Morales MA, Ausiello CM, Guarino A, Urbani F, Spagnuolo MI, Pignata C, et al. Severe, protracted intestinal cryptosporidiosis associated with interferon gamma deficiency: Pediatric case report. Clin Infect Dis. 1996;22:848–50. doi: 10.1093/clinids/22.5.848. [DOI] [PubMed] [Google Scholar]
- 38.Hayward AR, Levy J, Facchetti F, Notarangelo L, Ochs HD, Etzioni A, et al. Cholangiopathy and tumors of the pancreas, liver, and biliary tree in boys with X-linked immunodeficiency with hyper-IgM. J Immunol. 1997;158:977–83. [PubMed] [Google Scholar]
- 39.Jacyna MR, Parkin J, Goldin R, Baron JH. Protracted enteric cryptosporidial infection in selective immunoglobulin A and saccharomyces opsonin deficiencies. Gut. 1990;31:714–6. doi: 10.1136/gut.31.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kocoshis SA, Cibull ML, Davis TE, Hinton JT, Seip M, Banwell JG. Intestinal and pulmonary cryptosporidiosis in an infant with severe combined immune deficiency. J Pediatr Gastroenterol Nutr. 1984;3:149–57. doi: 10.1097/00005176-198401000-00028. [DOI] [PubMed] [Google Scholar]
- 41.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, et al. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131(1 Pt 1):47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 42.From the Centers for Disease Control and prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA. 1993;269:460. [PubMed] [Google Scholar]
- 43.Tumwine JK, Kekitiinwa A, Bakeera-Kitaka S, Ndeezi G, Downing R, Feng X, et al. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurrent infection with the human immunodeficiency virus. Am J Trop Med Hyg. 2005;73:921–5. [PubMed] [Google Scholar]
- 44.Attili SV, Gulati AK, Singh VP, Varma DV, Rai M, Sundar S. Diarrhea, CD4 counts and enteric infections in a hospital – based cohort of HIV-infected patients around Varanasi, India. BMC Infect Dis. 2006;6:39. doi: 10.1186/1471-2334-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dwivedi KK, Prasad G, Saini S, Mahajan S, Lal S, Baveja UK. Enteric opportunistic parasites among HIV infected individuals: Associated risk factors and immune status. Jpn J Infect Dis. 2007;60:76–81. [PubMed] [Google Scholar]
- 46.Giri TK, Pande I, Mishra NM, Kailash S, Uppal SS, Kumar A. Spectrum of clinical and laboratory characteristics of HIV infection in Northern India. J Commun Dis. 1995;27:131–41. [PubMed] [Google Scholar]
- 47.Muthusamy D, Rao SS, Ramani S, Monica B, Banerjee I, Abraham OC, et al. Multilocus genotyping of Cryptosporidium sp. isolates from human immunodeficiency virus-infected individuals in South India. J Clin Microbiol. 2006;44:632–4. doi: 10.1128/JCM.44.2.632-634.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang G, Srivastava A, Pulimood AB, Dennison D, Chandy M. Etiology of diarrhea in patients undergoing allogeneic bone marrow transplantation in South India. Transplantation. 2002;73:1247–51. doi: 10.1097/00007890-200204270-00010. [DOI] [PubMed] [Google Scholar]
- 49.George B, Mathews V, Viswabandya A, Srivastava A, Chandy M. Infections in children undergoing allogeneic bone marrow transplantation in India. Pediatr Transplant. 2006;10:48–54. doi: 10.1111/j.1399-3046.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 50.Udgiri N, Minz M, Kashyap R, Heer M, Gupta CS, Mohandas K, et al. Intestinal cryptosporidiasis in living related renal transplant recipients. Transplant Proc. 2004;36:2128–9. doi: 10.1016/j.transproceed.2004.08.107. [DOI] [PubMed] [Google Scholar]
- 51.Al-Warid HS, Mahmood SH, AL-Saqur IM. Cryptosporidiosis among patient with and without lymphohematopoietic malignancy in Baghdad. Adv Biores. 2012;3:38–41. [Google Scholar]
- 52.McNabb SJ, Hensel DM, Welch DF, Heijbel H, McKee GL, Istre GR. Comparison of sedimentation and flotation techniques for identification of Cryptosporidium sp. oocysts in a large outbreak of human diarrhea. J Clin Microbiol. 1985;22:587–9. doi: 10.1128/jcm.22.4.587-589.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parmeshwarappa KD, Chandrakanth C, Sunil B. The prevalence of intestinal parasitic infestations and evaluation of different concentration techniques of the stool examination. J Clin Diagn Res. 2012;6:1188–91. [Google Scholar]
- 54.Morgan UM, Pallant L, Dwyer BW, Forbes DA, Rich G, Thompson RC. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: Clinical trial. J Clin Microbiol. 1998;36:995–8. doi: 10.1128/jcm.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baxby D, Blundell N, Hart CA. The development and performance of a simple, sensitive method for the detection of Cryptosporidium oocysts in faeces. J Hyg (Lond) 1984;93:317–23. doi: 10.1017/s0022172400064858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pohjola S. Negative staining method with nigrosin for the detection of cryptosporidial oocysts: A comparative study. Res Vet Sci. 1984;36:217–9. [PubMed] [Google Scholar]
- 57.Pohjola S, Jokipii L, Jokipii AM. Dimethylsulphoxide-Ziehl-Neelsen staining technique for detection of cryptosporidial oocysts. Vet Rec. 1985;116:442–3. doi: 10.1136/vr.116.16.442. [DOI] [PubMed] [Google Scholar]
- 58.Bronsdon MA. Rapid dimethyl sulfoxide-modified acid-fast stain of Cryptosporidium oocysts in stool specimens. J Clin Microbiol. 1984;19:952–3. doi: 10.1128/jcm.19.6.952-953.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Current WL, Reese NC. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J Protozool. 1986;33:98–108. doi: 10.1111/j.1550-7408.1986.tb05567.x. [DOI] [PubMed] [Google Scholar]
- 60.Goebel E, Brandle U. Ultrastructure of microgametogenesis, microgametes, and gametogony of Cryptosporidium sp. in the small intestine of mice. Protistologica. 1982;18:331–4. [Google Scholar]
- 61.De A. Current laboratory diagnosis of opportunistic enteric parasites in human immunodeficiency virus-infected patients. Trop Parasitol. 2013;3:7–16. doi: 10.4103/2229-5070.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jha AK, Uppal B, Chadha S, Bhalla P, Ghosh R, Aggarwal P, et al. Clinical and microbiological profile of HIV/AIDS cases with Diarrhea in North India. J Pathog. 2012;2012:971958. doi: 10.1155/2012/971958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agnamey P, Sarfati C, Pinel C, Rabodoniriina M, Kapel N, Dutoit E, et al. Evaluation of four commercial rapid immunochromatographic assays for detection of Cryptosporidium antigens in stool samples: A blind multicenter trial. J Clin Microbiol. 2011;49:1605–7. doi: 10.1128/JCM.02074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Limor JR, Lal AA, Xiao L. Detection and differentiation of Cryptosporidium parasites that are pathogenic for humans by real-time PCR. J Clin Microbiol. 2002;40:2335–8. doi: 10.1128/JCM.40.7.2335-2338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higgins JA, Fayer R, Trout JM, Xiao L, Lal AA, Kerby S, et al. Real-time PCR for the detection of Cryptosporidium parvum. J Microbiol Methods. 2001;47:323–37. doi: 10.1016/s0167-7012(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 66.Verweij JJ, Blangé RA, Templeton K, Schinkel J, Brienen EA, van Rooyen MA, et al. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J Clin Microbiol. 2004;42:1220–3. doi: 10.1128/JCM.42.3.1220-1223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alagappan A, Bergquist PL, Ferrari BC. Development of a two-color fluorescence in situ hybridization technique for species-level identification of human-infectious Cryptosporidium spp. Appl Environ Microbiol. 2009;75:5996–8. doi: 10.1128/AEM.00643-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bakheit MA, Torra D, Palomino LA, Thekisoe OM, Mbati PA, Ongerth J, et al. Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet Parasitol. 2008;158:11–22. doi: 10.1016/j.vetpar.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Parida M, Sannarangaiah S, Dash PK, Rao PV, Morita K. Loop mediated isothermal amplification (LAMP): A new generation of innovative gene amplification technique; perspectives in clinical diagnosis of infectious diseases. Rev Med Virol. 2008;18:407–21. doi: 10.1002/rmv.593. [DOI] [PMC free article] [PubMed] [Google Scholar]


