Abstract
Soil-transmitted helminths (STH) consist of Ascaris lumbricoides, Trichuris trichiura, and hookworm (Necator americanus and Ancylostoma duodenale). It affects nearly 1.7 billion people globally in which Ascaris contributes nearly 1.2 billion cases. The main mode for transmission of Ascaris and Trichuris is through contaminated food and water, whereas hookworm transmitted by skin penetration. STH were mainly seen in areas with poverty, overcrowding, and poor sanitation. The prevalence is more in rural areas compared to urban areas. It affects mainly children and causes lack of school attendance, anemia, and cognitive deficits. This review emphasizes on the epidemiology and clinical features of all STH and emphasizes on the role on preventive measures in containing STH.
Keywords: Epidemiology, hookworm, iron deficiency anemia, prevalence, soil-transmitted helminths
INTRODUCTION
Soil-transmitted helminths (STH) are distributed worldwide mostly in tropical and subtropical regions. It consists of mainly roundworm (Ascaris lumbricoides), whipworm (Trichuris trichiura), and hookworm (Necator americanus and Ancylostoma duodenale). Ascaris and Trichuris affect mainly children whereas hookworm affects both children and young adults. It leads to iron deficiency anemia, protein energy malnutrition, and stunted growth. Severe infections lead to intestinal obstructions and gangrene. Recently, many newer diagnostic modalities have emerged to identify STH. It is important to know the epidemiology and their clinical features to take necessary preventive measures to eradicate them completely.
GLOBAL EPIDEMIOLOGY
Globally, 1.7 billion people are infected with one or other STH. According to 2003 survey, the global prevalence of A. lumbricoides is more than 1.2 billion, of which >50% of cases are seen in China. T. trichiura prevalence was 795 million, whereas hookworm prevalence was estimated to be 740 million. Sub-Saharan Africa and China contribute nearly 50% of hookworm prevalence.[1] 2010 survey by Pullan et al. showed the prevalence rate of A. lumbricoides was 819 million and T. trichiura was 464 million and hookworm was 439 million in which >50% of cases were seen in South Asia and Sub-Saharan Africa.[2] The difference in prevalence rate can be due to many reasons such as the 2010 review used only the global atlas of helminthic infections data and it assessed only the population at risk of acquiring the infection and not the entire general population.[2]
Asia contributes 67% of the global prevalence of STH and in Asia; the highest prevalence is seen in India (21%) followed by China (18%).[3] Overall, the STH prevalence decreased to 30% in 2010 from 38.6% in 1990. The Republic of China and Indonesia showed a major decline and others parts of Asia; Sub-Saharan Africa showed only a small change in the prevalence.[2]
STH prevalence is more common in rural areas when compared to urban areas. A community-based study from Lucknow, India, showed that infection rate was 20% in rural areas compared to 5% in urban areas, in which A. lumbricoides contributes 11.4% and hookworm contributes 2.4%.[4] A recent study done at Vellore showed the prevalence of STH 9% and 4.8% in rural and urban areas, respectively. However, the prevalence rate of T. trichiura (2.2%) and A. lumbricoides (3.3%) was higher in urban areas and hookworm prevalence was more in rural areas (8.4%), and it may be due to the socioeconomic status, sanitary measures, and improper water supply.[5] Meta-analysis done in India also showed that the STH prevalence was higher in rural areas compared to urban areas due to poor sanitary measures, inadequate water supply, and overcrowding.[6]
Despite the high prevalence, STH was considered as a neglected tropical disease because of three main features.
More prevalent in the underdeveloped countries
Chronic illness not an acute illness
Effect of this infection on economic and education burden is not quantified.
INDIAN EPIDEMIOLOGY
The southeast Asian region consists of eight nations – India, Sri Lanka, Nepal, Pakistan, Afghanistan, Bangladesh, Bhutan, and Maldives. The total population covered in this region is 1.5 billion (one-fourth of the global population), of which 75% of them reside in India.
According to 2010 global estimates, South Asia contributes nearly 70% of global prevalence of STH, in which 298, 140, and 101 million people are infected with A. lumbricoides,T. trichiura, and hookworm, respectively. India contributes 21% of overall global prevalence.[3]
In India, the hookworm prevalence is estimated to be 71 million cases and A. lumbricoides contributes 140 million and T. trichiura 73 million cases. In India, the maximum prevalence is seen in Karnataka (47%) followed by Andhra Pradesh (40%). The prevalence rate in Tamil Nadu is 3.2% whereas in Puducherry, it is 4.8%.[7] A study done in Vizianagaram, Andhra Pradesh, showed 55.6% STH prevalence and the most common parasite was Entamoeba (37.7%) followed by hookworm (8.7%) and the most common age group affected is 8–10 years.[8] Another study in Andhra Pradesh also showed similar prevalence (49%).[9] A recent survey done in Vellore also showed a similar prevalence rates for hookworm (8.4%).[5] In Karaikal region of Puducherry, the overall prevalence of parasitic infection is found to be 30%, of which 35% was due to hookworm.[10] The overall prevalence of parasitic diseases in Puducherry in 1998 was 67% in slum children aged 1–10 years.[11] In 1987, a hospital-based study done in Puducherry showed the overall parasitic prevalence was 38%. Of these, hookworm constitutes 44% followed by T. trichiura, 26% and A. lumbricoides, 22%.[12] In a study from Puducherry which was done in school children to find out the prevalence of parasitic infections, the estimated prevalence was 34.56% and the most common infection was A. lumbricoides (43.21%) followed by hookworm (28.8%), T. trichura (10.87%), and Hymenolepis nana (7.68%). It was found that polyparasitism was common among the infected children and out of 28.8% of microscopically positive stool samples for hookworm, 55.5% was positive by stool culture.[13] Another hospital-based study done in Puducherry showed the overall parasitic prevalence of 16%, in which 68% had helminth infection. The most common helminthic infection was due to hookworm (86%) followed by Strongyloides stercoralis (6.3%) and A. lumbricoides (2.8%).[14]
N. americanus is most common in South India, whereas A. duodenale is the most common in North India and mixed infections are also seen.[15] In 1992, a study carried out in Puducherry to know the prevalence of hookworm species by coproculture, demonstrated that both species were prevalent in Puducherry with the lesser prevalence of N. americanus.[16]
RISK OF DISABILITY
STH infections tend to cause silent chronic infection. Hence, it is important to estimate the fraction of the infected population with heavy wormload who are at a risk of disability. Disability risk is determined as a range between the lower threshold and higher threshold of worm load to cause the disability. The lower worm threshold is associated with cognitive deficits and higher threshold is associated with significant clinical consequences such as anemia.[17]
The disability risk for to A. lumbricoides and T. trichiura is more common during the 5–15 years of age group, whereas hookworm disability risk is seen more in the adults 20–25 years of age. School going children with hookworm infection have a negligible risk of disability. In STH, it has been found that 70% had Class II, 24% had Class III, and 6% had Class IV disability. In 2010, the years lost due to disability (YLD) was calculated as 4.98 million globally of which 22% due to A. lumbricoides, 13% due to T. trichiura, and 65% due to hookworm. South Asia shares approximately 35%, 45%, and 47% of YLD of Hookworm, A. lumbricoides, and T. trichiura.[3]
Disability-adjusted life years (DALY) indicate the burden of the disease in the community. It is calculated by adding the YLD and years of life lost due to the disease. In 2010, DALY estimated was 5.18 million globally. DALY for hookworm increased from 1.5 in 1990 to 1.8 million in 2001.[18]
INTENSITY OF THE INFECTION
The intensity of the infection determines the rate of prevalence and morbidity. Worm burden is not uniformly distributed among the population. Few people harbor very high worm burden whereas most individual harbor less intense infections.[19] Chan et al. showed 15% of the infected people harbored 70% of the total worm burden. These heavily infected people were the major source of environmental contamination and transmission of infection.[20] Based on the egg count, the WHO proposed three classes of the intensity of STH – light, moderate, and heavy intensity infections [Table 1].[21]
Table 1.
WHO classification of intensity of STH
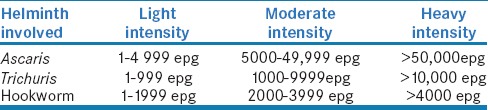
Literature has proved that a direct correlation exists between the initial worm burden before treatment and worm burden during reinfection.[22,23]
If high-intensity infections were present before treatment, then during reinfection, the individual is predisposed to have high-intensity infections. This pattern is seen in children affected with A. lumbricoides, T. trichura, and adults with hookworm.[19]
Researchers have established the correlation between the infection intensity and age of the host. In A. lumbricoides and T. trichiura, heavy intensity infection is seen in children who are 5–10 years old, and then, the intensity plateaus in adulthood whereas in hookworm, infection increases with age and heavy infection peaks during 20–25 years of age.[24]
CLINICAL FEATURES
Hookworm
The transmission depends on many factors such as warm and moist climate, improper water supply, and poor sanitation. The most common route of transmission is skin penetration. Oral ingestion also has been postulated as the mode of transmission for A. duodenale. Transplacental transmission with A. duodenale has been reported in some studies. In China, Banwell and Schad in 1972 proved the transplacental transmission of A. duodenale larva in utero.[25]
At the site of skin penetration, some allergic reactions occur such as maculopapular rashes and itching (ground itch). Pulmonary symptoms such as a cough, sneezing, bronchitis, and pneumonia may appear when the larva migrates through the lungs. When the worm load is less, gastrointestinal discomfort and diarrhea occur.[26]
After skin penetration, it reaches the upper part of small intestine, and it attaches to the mucosa by its buccal capsules, leading to mucosal erosion and blood loss. One adult worm can cause up to six mucosal lesions per day.
Heavy intensity infections cause high blood loss if not treated, this will lead to depletion of iron stores and iron deficiency anemia. Anticoagulant substances secreted from the hookworm enhance the blood loss and the rate of blood loss depends on the species. A. duodenale causes 0.14–0.4 ml of blood loss, whereas N. americanus causes 0.01–0.03 ml of blood loss and A. ceylanicum causes 0.04–0.1 ml of blood loss.[26] It shows that A. ceylanicum causes less blood loss than A. duodenale but more than N. americanus. The rate of anemia depends on the host iron stores, number of pregnancies in females, infecting species, intensity and duration of the infection, and any other concurrent medical illness.[27]
Studies on anemia associated with hookworm showed that there is a disproportionate reduction in the hemoglobin level after a threshold increase in the worm burden, and there exists an inverse relationship between the hookworm intensity and hemoglobin level. This nonlinear relationship is determined by the host iron stores and chronic infection status.[17] A study by Pal et al. in West Bengal estimated the hookworm prevalence and its association with anemia, in which hookworm prevalence was 24.8% and it was associated with mild to moderate degree of anemia in 50% of the affected population.[28] Hill et al. showed a positive correlation between the hookworm intensity and fall in hemoglobin level. The hemoglobin decline was markedly increased when the hookworm intensity was 4000–8000 egg per gram of feces.[29]
Pregnant women and children are at an increased risk for hookworm anemia because of the increased physiological need for iron during pregnancy. Nearly, 44 million pregnant women are infected with hookworm and it has an impact on both maternal and child health. Hookworm-infected pregnant women have severe iron deficiency anemia, preterm labor, and low birth weight babies. The maternal mortality rate increases three times in case of coinfection with hookworm.[30] Randomized control trial done on pregnant women with hookworm anemia showed that maternal hemoglobin levels fall with an increase in the worm load. Treatment with antihelminths in the first trimester leads to increased maternal iron stores, hemoglobin concentration, and weight gain, thus reducing maternal, fetal mortality, and rate of preterm delivery.[31]
Hookworm infection impacts on children
Hookworm-infected children suffer from anemia, protein energy malnutrition, and stunted growth.[32] A study carried out in Ethiopia revealed the impact of hookworm infection in children and they found that 69% of children with hookworm had iron deficiency anemia.[33]
Ascaris
It causes infection by eating contaminated food and water. The Ascaris egg is the infective form. It causes mostly asymptomatic infection. Symptoms occur due to larval migration in lungs or due to the presence of adult worm in the intestine. Morbidity depends on intensity of the infection. Most commonly, it causes pulmonary infections such as pneumonitis referred to as Loeffler syndrome. Severe infections can cause intestinal obstructions also. Infection is more common in children aged between 1 and 5 years. It can also lead to complications such as intussusception and intestinal gangrene. A review compared the worm burden and its association with intestinal obstruction and it illustrated that the rate of obstruction is ten times more in the high worm burden individuals.[34] A meta-analysis by de Silva et al. showed that the most common complication was intestinal obstruction and it contributes 38%–87% of all complications.[35]
Ascaris is proved as a cause of childhood malnutrition in many studies. A study done by Gupta et al. showed that children gained significant weight after receiving antihelminthic drugs when compared to children who received placebo.[36] Similar results were recorded by Thein-Hlaing et al. and Stephenson et al.[37,38]
Trichuris
Infection occurs by ingestion of contaminated food and water with embryonated eggs of T. trichiura. It then develops into adult worm in the intestine, mainly in the cecum, in heavy infections, it also seen in colon and rectum. It leads to abdominal colitis resembles inflammatory bowel syndrome and presents with diarrhea and abdominal pain. In case of severe infections, it can also cause Trichuris dysentery syndrome associated with rectal prolapse. It can also leads to malnutrition, growth stunting, clubbing of fingers, and anemia rarely in children.[39]
CONCLUSION
In 2001, the WHO made a goal to reduce the prevalence of STH in children by reducing the intensity of STH to <50% and administration of preventive chemotherapy to at least 75% and up to 100% all preschool going (1–4 years) and school going (5–14 years) children by 2020.[21] Preventive chemotherapy is the main goal of treatment, but it is associated with recurrent infections with high intensity. To prevent this, along with chemotherapeutic measures, water and sanitation programs and health education are necessary to completely eliminate the STH.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pullan RL, Brooker SJ. The global limits and population at risk of soil-transmitted helminth infections in 2010. Parasit Vectors. 2012;5:81. doi: 10.1186/1756-3305-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitin S, Venkatesh V, Husain N, Masood J, Agarwal GG. Overview of intestinal parasitic prevalence in rural and urban population in Lucknow, North India. J Commun Dis. 2007;39:217–23. [PubMed] [Google Scholar]
- 5.Kattula D, Sarkar R, Rao Ajjampur SS, Minz S, Levecke B, Muliyil J, et al. Prevalence & risk factors for soil transmitted helminth infection among school children in South India. Indian J Med Res. 2014;139:76–82. [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S, Dwivedi A, Shrivastava A, Vijayananth P, Vidyavardhini R, Venkatesh S. Prevalence of Soil-Transmitted Helminthic Infection in India in Current Scenario: A Systematic Review. J Commun Dis. 2016;48:24–35. [Google Scholar]
- 7.Ananthakrishnan S, Nalini P, Pani SP. Intestinal geohelminthiasis in the developing world. Natl Med J India. 1997;10:67–71. [PubMed] [Google Scholar]
- 8.Panda D. Prevalence of intestinal parasitic infections among school children in rural area of Vizianagaram. IOSJPBS. 2012;3:42–4. [Google Scholar]
- 9.Padmaja N, Sai Swaroop P, Nageswararao P. Prevalence of intestinal parasitic infections among school children in and around Amalapuram. J Public Health Med Res. 2014;2:36–8. [Google Scholar]
- 10.Karthikeyan MS. Prevalence of intestinal parasitic infection among school going children in Karaikal. Asia Pac J Res. 2016;2:181–6. [Google Scholar]
- 11.Rau PV, Rao RS, Sharma S, Shah M, Ramachandiran, Bansal RD. Concomitant helminthiasis and recurrent upper respiratory tract infection in children of an urban community in Pondicherry. Indian J Public Health. 1988;32:39–40. [PubMed] [Google Scholar]
- 12.Parija SC, Rao R. Prevalence of parasitic infections in Pondicherry. Indian J Parasitol. 1987;11:63–5. [Google Scholar]
- 13.Ragunathan L, Kalivaradhan SK, Ramadass S, Nagaraj M, Ramesh K. Helminthic infections in school children in Puducherry, South India. J Microbiol Immunol Infect. 2010;43:228–32. doi: 10.1016/S1684-1182(10)60036-9. [DOI] [PubMed] [Google Scholar]
- 14.Sunil S, Pavan C, Gopal R. 16.Soil transmitted helminths in a rural population of Puducherry – A hospital based study. Int J Pharma Biosci. 2011;2:29–37. [Google Scholar]
- 15.Singh P, Gupta ML, Thakur TS, Vaidya NK. Intestinal parasitism in Himachal Pradesh. Indian J Med Sci. 1991;45:201–4, 200. [PubMed] [Google Scholar]
- 16.Parija SC, Malini G, Rao RS. Prevalence of hookworm species in Pondicherry, India. Trop Geogr Med. 1992;44:378–80. [PubMed] [Google Scholar]
- 17.Bundy DA, Chan MS, Medley GF, Jamison D, Savioli L. Intestinal nematode infections. In: Murray CJ, Lopez AD, Mathers CD, editors. Global Epidemiology of Infectious Disease. Geneva: World Health Organization; 2004. pp. 243–300. [Google Scholar]
- 18.World Health Organization. The World Health Report 2002. Geneva: World Health Organization; 2002. [Google Scholar]
- 19.Schad GA, Anderson RM. Predisposition to hookworm infection in humans. Science. 1985;228:1537–40. doi: 10.1126/science.4012307. [DOI] [PubMed] [Google Scholar]
- 20.Bundy DA, Chan MS, Savioli L. Hookworm infection in pregnancy. Trans R Soc Trop Med Hyg. 1995;89:521–2. doi: 10.1016/0035-9203(95)90093-4. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Soil-Transmitted Helminthiases: Eliminating as Public Health Problem Soil-Transmitted Helminthiases in Children: Progress Report 2001-2010 and Strategic Plan 2011-2020. Geneva: World Health Organization; 2012. [Google Scholar]
- 22.Anderson RM. The population dynamics and epidemiology of intestinal nematode infections. Trans R Soc Trop Med Hyg. 1986;80:686–96. doi: 10.1016/0035-9203(86)90367-6. [DOI] [PubMed] [Google Scholar]
- 23.Bundy DA, Cooper ES, Thompson DE, Didier JM, Simmons I. Effect of age and initial infection intensity on the rate of reinfection with Trichuris trichiura after treatment. Parasitology. 1988;97(Pt 3):469–76. doi: 10.1017/s003118200005887x. [DOI] [PubMed] [Google Scholar]
- 24.Bethony J, Chen J, Lin S, Xiao S, Zhan B, Li S, et al. Emerging patterns of hookworm infection: Influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin Infect Dis. 2002;35:1336–44. doi: 10.1086/344268. [DOI] [PubMed] [Google Scholar]
- 25.Banwell JG, Schad GA. Hookworm. Clin Gastroenterol. 1978;7:129–56. [PubMed] [Google Scholar]
- 26.Roche M, Layrisse M. The nature and causes of “hookworm anemia”. Am J Trop Med Hyg. 1966;15:1029–102. [PubMed] [Google Scholar]
- 27.Conlan JV, Sripa B, Attwood S, Newton PN. A review of parasitic zoonoses in a changing Southeast Asia. Vet Parasitol. 2011;182:22–40. doi: 10.1016/j.vetpar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Pal D, Chattopadhyay UK, Sengupta G. A study on the prevalence of hookworm infection in four districts of West Bengal and its linkage with anaemia. Indian J Pathol Microbiol. 2007;50:449–52. [PubMed] [Google Scholar]
- 29.Hill AW, Andrews J. Relation of hookworm burden to physical status in Georgia. Am J Trop Med Hyg. 1942;22:499–506. [Google Scholar]
- 30.Brooker S, Hotez PJ, Bundy DA. Hookworm-related anaemia among pregnant women: A systematic review. PLoS Negl Trop Dis. 2008;2:e291. doi: 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torlesse H, Hodges M. Albendazole therapy and reduced decline in haemoglobin concentration during pregnancy (Sierra Leone) Transactions of the Royal Society of Tropical Medicine and Hygienes. 2001;95:195–201. doi: 10.1016/s0035-9203(01)90164-6. [DOI] [PubMed] [Google Scholar]
- 32.Rao V, Aggrawal M, Yadav R, Das S, Sahare L, Bondley M, et al. Intestinal parasitic infections, anaemia and undernutrition among tribal adolescents of Madhya Pradesh. Indian J Community Med. 2003;28:26–9. [Google Scholar]
- 33.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 34.de Silva NR, Guyatt HL, Bundy DA. Worm burden in intestinal obstruction caused by Ascaris lumbricoides. Trop Med Int Health. 1997;2:189–90. doi: 10.1046/j.1365-3156.1997.d01-241.x. [DOI] [PubMed] [Google Scholar]
- 35.de Silva NR, Guyatt HL, Bundy DA. Morbidity and mortality due to Ascaris-induced intestinal obstruction. Trans R Soc Trop Med Hyg. 1997;91:31–6. doi: 10.1016/s0035-9203(97)90384-9. [DOI] [PubMed] [Google Scholar]
- 36.Gupta M, Arora KL, Mithal S, Tandon BN. Effect of periodic deworming on nutritional status of ascaris-infested preschool children receiving supplementary food. Lancet. 1977;2:108–10. doi: 10.1016/s0140-6736(77)90119-2. [DOI] [PubMed] [Google Scholar]
- 37.Thein H, Thane T, Than S, Myat LK, Myint L. A controlled chemotherapeutic intervention trial on the relationship between Ascaris lumbricoides infection and malnutrition in children. Trans R Soc Trop Med Hyg. 1991;85:523–8. doi: 10.1016/0035-9203(91)90242-q. [DOI] [PubMed] [Google Scholar]
- 38.Stephenson LS, Crompton DW, Latham MC, Schulpen TW, Nesheim MC, Jansen AA. Relationships between Ascaris infection and growth of malnourished preschool children in Kenya. Am J Clin Nutr. 1980;33:1165–72. doi: 10.1093/ajcn/33.5.1165. [DOI] [PubMed] [Google Scholar]
- 39.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]


