Abstract
Background:
Information on the bioavailability of the essential mineral Mg2+ is sparse.
Objective/Method:
Evaluation of the present knowledge on factors influencing the bioavailability and intestinal absorption of Mg2+.
Results:
Mg2+ is absorbed via a paracellular passive and a transcellular active pathway that involves TRPM6/7 channel proteins. The bioavailability of Mg2+ varies within a broad range, depending on the dose, the food matrix, and enhancing and inhibiting factors. Dietary factors impairing Mg2+ up-take include high doses of other minerals, partly fermentable fibres (e.g., hemicellulose), non-fermentable fibres (e.g., cellulose, lignin), phytate and oxalate, whereas proteins, medium-chain-triglycerides, and low- or indigestible carbohydrates (e.g., resistant starch, oligosaccharides, inulin, mannitol and lactulose) enhance Mg2+ uptake. The Mg2+ dose is a major factor controlling the amount of Mg2+ absorbed. In principle, the relative Mg2+ uptake is higher when the mineral is in-gested in multiple low doses throughout the day compared to a single, large intake of Mg2+. The type of Mg2+ salt appears less relevant than is often thought. Some studies demonstrated a slightly higher bioavailability of organic Mg2+ salts compared to inorganic compounds under standardized conditions, whereas other studies did not.
Conclusion:
Due to the lack of standardized tests to assess Mg2+ status and intestinal absorption, it remains unclear which Mg2+ binding form produces the highest bioavailability. The Mg2+ intake dose combined with the endogenous Mg2+ status is more important. Because Mg2+ cannot be stored but only retained for current needs, a higher absorption is usually followed by a higher excretion of the mineral.
Keywords: Mg-absorption, bioavailability, intestinal uptake, meal composition, dietary fibre, oligosaccharides
1. INTRODUCTION
Magnesium (Mg2+) is the second most abundant intracellular cation, after potassium, and is the fourth most abundant cation in the human body [1]. This essential mineral is needed for a broad variety of physiological and biochemical functions. As a co-factor in more than 300 enzymatic reactions, which often depend on ATP, Mg2+ is involved in many biochemical pathways of key importance, including the degradation of macronutrients, oxidative phosphorylation, DNA and protein synthesis, neuro-muscular excitability, and regulation of parathyroid hormone (PTH) secretion (for a review, see [2]). As a physiological calcium channel antagonist, Mg2+ affects processes that are regulated by intracellular calcium concentration fluxes and is therefore essential for normal neurological and muscular function [3, 4]. Furthermore, Mg2+ regulates membrane permeability via interactions with phospholipids and affects vessel tone and blood pressure.
Data vary on total body content of Mg2+ and its distribution in adults. The total Mg2+ amount varies between 22 and 26 g [3]. More than 99% of the total body Mg2+ is located in the intracellular space, mainly stored in bone (60-65%), muscle and soft tissues (34-39%), whereas less than 1% is located in the extracellular space [5, 6]. Up to 70% of all plasma Mg2+ exists in the ionized (free) active form, which is important for physiological processes, including neuromuscular transmission and cardiovascular tone [7].
The reference range for serum ionized Mg2+ is 0.54-0.67 mmol/l [3]. Deviations from this physiological Mg2+ range cause neural excitability, arrhythmia, bone formation and several other pathological consequences [8]. Thus, Mg2+ stores are tightly regulated via a balanced interplay between intestinal absorption and renal excretion under normal conditions. Renal elimination removes approximately 100 mg of Mg2+ per day, whereas the losses via sweat are generally low. However, during intense exercise, these losses can rise substantially.
Balance studies suggest a daily Mg2+ requirement of 3.0-4.5 mg per kg body weight. The recommended intake derived from these data varies in different countries. Whereas the Institute of Medicine [9] recommends 310-320 mg per day for women and 400-420 mg per day for men as adequate, the European Food Safety Authority [10] recently defined an adequate intake of 300 and 350 mg per day for women and men, respectively.
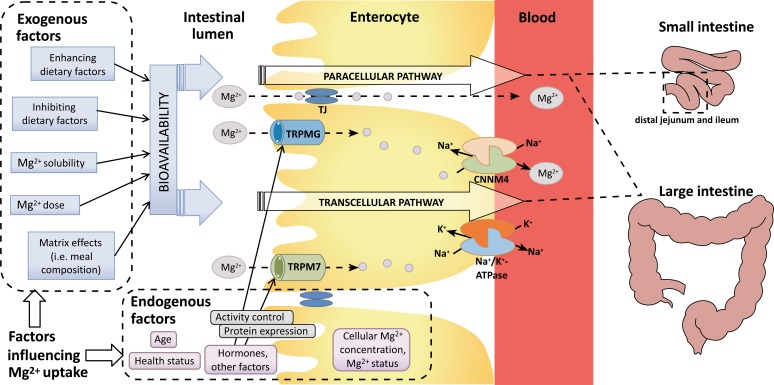
The mechanism of Mg2+ absorption through the enterocytes into the bloodstream shows a dual kinetic process that involves two mechanisms: a saturable (transcellular) active pathway and a non-saturable (paracellular) passive pathway. The intestinal absorption occurs predominantly in the small intestine-mainly in the distal jejunum and ileum [11] via the paracellular pathway, which is regulated by the paracellular Tight Junctions (TJ). The fine-tuning of Mg2+ uptake occurs in the caecum and colon of the large intestine via the transcellular pathway mediated by membrane TRPM6/7 channel proteins and the paracellular pathway. It has been suggested that the basolateral Mg2+ extrusion mechanism of the enterocyte is performed via CNNM4, a Na+/ Mg2+-Antiporter [12]. The driving force is a Na+-gradient that is established via the Na+/K+-ATPase. The bioavailability and intestinal absorption efficacy of orally ingested Mg2+ are influenced by various endogenous and exogenous factors. For more details, see the text.
CNNM4, cyclin M4; TRPM6, transient receptor potential melastatin type 6; TRPM7, transient receptor potential melastatin type 7.
Various factors influence the intestinal uptake of Mg2+ and are of substantial importance for the supply of the mineral. Dietary Mg2+ uptake in the intestine varies within a broad range and depends on dose, the food matrix, and enhancing and inhibiting factors. Furthermore, several studies have shown that the absorption of Mg2+ from food supplements and pharmaceutical preparations under standard conditions is slightly influenced by the type of Mg2+ salt. Nevertheless, an approach that focuses on one or a few aspects is insufficient from a nutritional and medical point of view. To understand the true absorption of Mg2+, numerous endogenous and exogenous factors must be considered. Overall, the understanding of Mg2+ absorption and its influencing factors is still limited, which has been due to methodological limitations. This article gives an overview of this issue.
2. MECHANISMS OF MG2+ ABSORPTION IN THE INTESTINE
Intestinal Mg2+ absorption (Fig. 1) [11, 12] occurs predominantly in the small intestine via a paracellular pathway, and smaller amounts are absorbed in the colon, mainly via a transcellular pathway [13]. In humans, Mg2+ absorption starts approximately 1 h after oral intake, reaches a plateau after 2-2.5 h up to 4-5 h and then declines. At 6 h, the Mg2+ absorption is approximately 80% complete [14].
Fig. (1).
Intestinal Mg2+ absorption and influencing factors.
With a daily intake of 370 mg, the absorption rate of Mg2+ in the intestine ranges from 30-50% [13]. However, the efficiency of Mg2+ uptake is dependent on the ingested dose [15, 16]. For example, early studies with a low dietary Mg2+ intake showed that the relative absorption rate can reach 80% [17], whereas it is reduced to 20% with Mg2+ surfeits [18].
In general, Mg2+ is absorbed as an ion. It is not known if the mineral is absorbed together with other nutrients or if Mg2+ is absorbed in the form of complexes [19].
2.1. Transcellular Pathway
With the identification and characterization of the Mg2+ transporters TRPM6 and TRPM7, which are members of the Transient Receptor Potential (TRP) melastatin family of cation channels, our understanding of Mg2+ absorption mechanisms has greatly improved (for a review, see [18]). TRP channels contribute to the saturable active transcellular movement of divalent cations from the intestinal lumen into the cells [8]. The tight regulation of TRPM6, induced by intracellular Mg2+, provides a feedback mechanism in Mg2+ influx and implies that intracellular Mg2+ buffering and Mg2+ extrusion mechanisms strongly impact channel functioning [20].
2.2. Paracellular Pathway
It has been hypothesized that the paracellular pathway exclusively contributes to Mg2+ absorption in the small intestine because a) Mg2+ absorption in this region linearly correlates with luminal Mg2+ concentrations [13, 18, 21]; and b) the TRPM6 channel is not expressed in the small intestine [22].
Paracellular Mg2+ absorption occurs via simple diffusion and involves the transport of Mg2+ through small spaces between the epithelial cells. The driving force for the passive Mg2+ transport in the distal jejunum and ileum is established by the high luminal Mg2+ concentration and the lumen-positive transepithelial voltage of ~15 mV [23]. The process relies on tight junction permeability, which is still poorly understood [10]. The small transmembrane proteins, claudins, are the key components of the paracellular channel because they control ion permeability. The relatively low expression of ‘tightening’ claudins 1, 3, 4, 5 and 8 in the small intestine enables Mg2+ permeability [24].
3. INTESTINAL ABSORPTION OF Mg2+-METHO-DOLOGICAL ASPECTS
Studies on the absorption and bioavailability of Mg2+ have produced different results and are often not comparable because of the different methods used. Different parameters, such as retention and urinary excretion must be used to evaluate Mg2+ bioavailability.
3.1. Direct Bioavailability Studies
The investigation of Mg2+ absorption and its kinetics is complex. Conventional bioavailability studies, which monitor the plasma Mg2+ levels after oral administration (direct method), are insufficient to investigate the rate and amount of Mg2+ absorption because the plasma Mg2+ levels are subject to rapid homeostasis, which is mainly driven by renal excretion and storage in compartments such as bone [25]. The active reabsorption of Mg2+ from primary urine in the kidney produces approximately 20 times more Mg2+ transported into the plasma compared to Mg2+, which is absorbed in the intestinal tract. The remaining Mg2+ is excreted in urine. In the net balance, the complete amount of Mg2+ absorbed in the intestinal tract is excreted via the kidney. Therefore, the basic plasma Mg2+ levels are quickly regulated, thereby impeding evaluation of precise concentration time curves.
3.2. Indirect Chemical Balance Studies
The absorption of Mg2+ should be studied in human studies by using indirect methods of dietary balance that are based on measuring faecal or urinary Mg2+ excretion after oral Mg2+ administration. However, such chemical balance studies also have a number of limitations. Usually, these studies are carried out over a period of several days or weeks, where a strict diet has to be followed. Long-term balance studies are susceptible to low compliance, and it is questionable whether the results of such long-term balance studies are suitable for extrapolation on bioavailability. These studies instead provide data on the required intake amounts. However, a short balance period may yield inaccurate absorption results because the meals given during the balance period might mix with preceding meals in the intestine, an effect that might vary between subjects due to varying gastrointestinal passage time. At a minimum, probands must be given food low in Mg2+ throughout the studies, especially through beverages (e.g., water). Nevertheless, mineral excretion in faeces cannot be strictly related to intake. In addition, endogenous faecal Mg2+ is lost through bile, the pancreas, and other ways; thus, ‘true absorption’ cannot be determined because there is no ability to distinguish between endogenous and dietary Mg2+.
3.3. Isotopic Methods
In contrast, absorption studies using labelled Mg2+ (isotopic methods) allow the amount of Mg2+ that is absorbed from a certain food or drink to be calculated. Because the addition of radioisotopes (28Mg2+) in meals is not useful in terms of either ethical considerations or its half-life (21 h), stable isotope techniques are preferable [26]. Combined with inductively coupled plasma mass spectrometry (ICP-MS), 25Mg2+ and 26Mg2+ can be used to follow exogenous Mg2+ in plasma, urine, or faeces after the oral administration of labelled test meals and to calculate the absolute bioavailability of Mg2+ [26]. However, the two isotopes of Mg2+, 25Mg2+ and 26Mg2+, are highly abundant in nature (10 and 11%, respectively), which reduces the sensitivity [26]. Furthermore, it remains unclear whether the addition of isotopes to a food leads to similar properties in terms of solubility and binding to the matrix compared to the unlabelled Mg2+ in the respective source.
3.4. Other Issues
The long-term collection of urine and faeces is very cumbersome. Therefore, Sabatier et al. (2003) compared several multiple blood sample protocols with complete urine and faecal samples [27]. All protocols were combined with stable-isotope-tracer methods. The authors found that double-labelling methods are an alternative to faecal monitoring methods, which are simpler and less invasive [27].
Hansen et al. (2014) performed a bioavailability study with stable Mg2+ isotopes to identify a more convenient method of measuring Mg2+absorption that did not require 72-h urine or ≥ 6-d stool collection [28]. Mg2+ absorption values using means of the 0-24 h urine collection and 3-h serum samples were found to most accurately reflect 72-h Mg2+ absorption.
Mg2+ retention depends on absorption and other mechanisms that contribute to homeostasis, such as excretion via the renal pathway, which is the most important organ for regulating Mg2+ homeostasis. Therefore, to prevent any sub-Mg2+ deficiency and minimise the differences in Mg2+ status, subjects of Mg2+ bioavailability studies need to be supplemented for ≥ 4 weeks before evaluation [29]. Indeed, under this condition, Mg2+ bioavailability is comparable. However, the observation is meaningless because the additional absorbed Mg2+ is immediately eliminated renally in case of sufficient Mg2+ status. Such data are only limitedly transferable to a situation where the Mg2+ supply status in insufficient. It is unclear whether the type of Mg2+ salt or other exogenous factors influencing Mg2+ bioavailability are important under conditions of insufficient Mg2+.
4. DATA ON INTESTINAL MG2+ ABSORPTION
The absorption rate of orally ingested Mg2+ for healthy individuals is influenced by various endogenous and exogenous factors (Table 1). In particular, the amount of ingested Mg2+ and, to a variable extent, the presence of inhibiting and enhancing dietary components (Fig. 1) are important. More-over, the meal composition (i.e., matrix effects), the type of Mg2+ salt and galenic formulation (e.g., gastric acid resistant capsules, pH-dependent release systems, or retard formulation) may influence the absorption efficacy.
Table 1.
Overview of endogenous and exogenous factors affecting absorption of Mg2+.
| Improve Absorption | Impair Absorption | |
|---|---|---|
| Endogenous Factors | • Low Mg2+ status | • Increasing age |
| • Balanced Mg2+ status • Intestinal dysfunction (e.g., in CD, IBD, or SBS) |
||
| Exogenous Factors | • MCT (SFA) (?) • Proteins (?) • Casein phosphopeptides (?) • Low- or indigestible carbohydrates (i.e. oligosaccharides, inulin, mannitol and lactulose) • High solubility of Mg2+ • Solubilized Mg2+ (e.g., effervescent tablets) |
• High single Mg2+ intake dose • Partly fermentable fibers (hemicellulose) • Non-fermentable fibers (cellulose and lignin) • LCT (?) • Phytate • Oxalate • Pharmacological doses of calcium, phosphorus, iron, copper, manganese and zinc • Slow-release formulations (?) |
CD, celiac disease; IBD, inflammatory bowel disease; LCT, long chain triglycerides; MCT, medium chain triglycerides; SBS, short bowel syndrome; SFA, saturated fatty acids.
In previous balance studies, various protocols have been applied, including true bioavailability studies with stable Mg2+-isotopes [30-39]. Furthermore, the Mg2+ load administered varied widely among studies (from <100 to >1,000 mg/d), notwithstanding the age of subjects (infants to adults), their physical condition or the proximity of meals to administration. As a result, the data often appear confusing and conflicting.
The absorption of Mg2+ and other minerals is impaired in patients with gastrointestinal disorders such as Celiac Disease (CD) [40], Inflammatory Bowel Disease (IBD) [41] and Short Bowel Syndrome (SBS) [42] due to a malabsorption syndrome. Hence, a Mg2+-enriched diet and a thorough Mg2+ supplementation is therefore advised to prevent or treat Mg2+ deficiency. Little is known on the bioavailability of dietary Mg2+ and other minerals in CD, IBD and SBS patients. The following data outline the Mg2+ absorption in healthy subjects.
4.1. Endogenous Factors Influencing Absorption
4.1.1. Homeostasis and Mg Status
The kidney is the primary organ that regulates Mg2+ homeostasis [39]. Approximately 2,400 mg of the mineral is filtered through the glomeruli, and 15-20% of the filtered Mg2+ is reabsorbed in the proximal convoluted tubule. Approximately 65% is reabsorbed in the Henle loop via active transport [39], and approximately 10% is reabsorbed in the distal convoluted tubule [11]. Thus, only approximately 5% of the filtered Mg2+ is excreted under normal conditions. Excessive Mg2+ is almost entirely excreted through the kidneys, which is also the case in hypermagnesaemia. Consequently, supplementation with Mg2+ usually increases renal Mg2+ excretion to varying degrees, depending on the quantity absorbed. Renal handling of Mg2+ is comprehensively discussed elsewhere [43]. Systematic studies comparing the intestinal uptake efficiency of Mg2+ between Mg2+ depleted and saturated subjects cannot be executed for ethical reasons.
4.1.2. Age
The efficiency of the gastrointestinal tract in absorbing micronutrients is negatively affected by increasing age [44]. This trend also applies to Mg2+. Coudray et al. (2006) investigated the effect of ageing on mineral absorption in the intestine using a stable isotope approach in rats [45]. The authors showed that aged rats exhibited less efficient intestinal absorption of 25Mg2+. Young and adult rats absorbed 56%, whereas Mg2+ absorption decreased to 45% in old and very old rats. Additionally, a human study found a significant, inverse relation between 28Mg2+ absorption from mineral water and age [46]. However, the study by Verhas et al. [46] had a restricted sample size, and the subjects had only a two-decade age range, which are limitations of their study.
4.2. Exogenous Factors Influencing Absorption
4.2.1. Absolute Mg Intake Per Dose
In studies with humans, a wide range (10-75%) of Mg2+ absorption rates have been reported. Such variability is most likely due to the Mg2+ load than to the analytical method, the formulation or the food matrix [29]. It is generally thought that the relative absorption of Mg2+ is inversely related to the ingested dose; in other words, the quantity of Mg2+ in the digestive tract is the major factor controlling the amount of Mg2+ absorbed. For example, in 1991, Fine et al. showed that in humans, the relative Mg2+ absorption rate from a daily dose of 36 mg was 65%, whereas, only 11% was absorbed from a daily dose of 973 mg, apparently due to the greater restriction of intestinal permeability to Mg2+ [47]. However, it should be noted that absolute absorption increased with each increment in intake [47].
Nakamura et al. (2012) conducted two experiments where the effects of the Artificial Mineral Water (AMW) serving volume and consumption pattern and the Mg2+ concentration on Mg2+ absorption in rats were examined [48]. In experiment 1, rats received 1 ml of AMW containing 200 mg Mg2+/l at 4 times, 400 mg Mg2+/l twice, or 800 mg Mg2+/l at 1 time. In experiment 2, the rats received 1 ml of AMW containing 200 mg Mg2+/l or 0.25 ml of AMW containing 800 mg Mg2+/l at 4 times or 1 ml of AMW containing 800 mg Mg2+/l at 1 time. The absorption of Mg2+ decreased with increasing Mg2+ concentrations in the same serving volume of AMW with different serving frequencies. When the AMW containing 800 mg Mg2+/l was portioned into 4 servings, Mg2+ absorption increased to the level of absorption in the group exposed to AMW containing 200 mg Mg2+/l served at the same frequency. These results suggest that the Mg2+ concentration and the volume of AMW do not affect Mg2+ absorption per se but that Mg2+ absorption from AMW decreases when the amount of Mg2+ in each serving is increased. Thus, frequent consumption is preferable for mineral water that is rich in Mg2+ when the total consumption of mineral water is the same.
Additionally, several human studies observed higher bioavailabilities when a given amount of Mg2+ was distributed over the span of a day rather than being consumed in a single bolus [29, 47, 49, 50]. Ekmekcioglu et al. (2000) showed that the upper range of Mg2+ absorption was obtained for the lowest ingested amount of Mg2+ in a study with adults [31]. Likewise, in a study with infants, the fractional absorption of Mg2+ of the same Mg2+ load (20 mg) was increased after distributed (64.0±3.9%) vs. bolus administration (54.3±5.9%) [49].
In a 2-d, cross-over, single-dose study with 12 healthy men, Sabatier et al. (2011) determined that the mode of administration (bolus vs. consumption throughout the day) could influence Mg2+ bioavailability from Mg2+-rich natural mineral water comparing the same nutritional Mg2+ amount (126 mg from 2x750 ml or 7x212 ml) [29]. Two stable isotopes (25Mg2+ and 26Mg2+) were used to label the water and distinguish both regimens. Fractional apparent Mg2+ absorption was determined by faecal monitoring, and Mg2+ retention was determined by measuring the urinary excretion of Mg2+ isotopes. The authors confirmed the results of the rat study by Nakamura et al. (2012) [48] and observed higher Mg2+ absorption and retention from Mg2+-rich mineral water when it was consumed in seven servings compared with two larger servings, suggesting that regular water consumption throughout the day is an effective way to increase Mg2+ bioavailability from Mg2+-rich mineral water. This increase in Mg2+ absorption after distributed vs. a bolus administration can most likely be explained by the absorption of low Mg2+ amounts via the TRPM6 channels [51, 52].
4.2.2. Meal Composition/Matrix Effects
Mg2+ is typically consumed as a part of complex meal, even in the case of supplementation. Hence, it is important to design studies with real food systems.
In a cross-over study with 25Mg2+ and 26Mg2+ isotopes, Sabatier et al. (2002) investigated the bioavailability of mineral water consumed with or without a simultaneous meal [53]. Apparent Mg2+ absorption was determined by faecal monitoring, and Mg2+ retention was determined from urinary excretion of Mg2+ isotopes. The mean Mg2+ absorption from mineral water consumed alone was 45.7±4.6% but was sig-nificantly greater (p = 0.0001) when consumed with a meal (52.3±3.9%), which is a relative difference of 14.4%. Therefore, the Mg2+ bioavailability from mineral water is enhanced when the water is consumed with a meal, perhaps because of a slower gastrointestinal transit time or the presence of other food constituents (or both). A slower transit time may lead to an increased exposure of the mucosal cells of the intestine to Mg2+ and thus a higher total absorption. Surprisingly, Verhas et al. (2002) [46] observed a mean Mg2+ bioavailability rate of 59±13.6% from carbonated water consumed without a meal, which lies in the upper reported range for solid foods. However, in this study, the bioavailability of Mg2+ from water was not compared to solid food.
Bergillos et al. (2015) determined the bioavailability of Mg2+ from ultrafiltered goats’ milk fermented with the pr-obiotic L. plantarum C4 in an in vitro model with Caco-2 cells combining simulated gastrointestinal digestion and mineral retention [54]. The highest Mg2+ bioavailability was found in the probiotic-fermented goats’ milk compared with ultrafiltered fermented goats’ milk without the probiotic and commercial fermented goats’ milks. The authors indicated that the casein concentration from the ultrafiltration process could increase the Mg2+ bioavailability.
In many western countries, bread is an important source of Mg2+. Lopez et al. (2004) compared the effects of different kinds of bread fermentation on Mg2+ bioavailability in rats [55]. The authors found that although yeast fermentation minimizes the unfavourable effects of phytic acid on Mg2+ bioavailability, sourdough bread is the better source of available Mg2+. Consumption of Maillard reaction products present in food (e.g., bread crust) has been related to deterioration of protein digestibility and changes in mineral bioavailability [56-58]. However, in a balance study with rats, no influence of Maillard reaction products from bread crust on Mg2+ balance was observed [59].
4.2.3. Enhancing Factors
Various dietary factors that promote Mg2+ bioavailability have been investigated in animal and human studies. Several early human studies showed that higher protein intake increased Mg2+ absorption compared to lower intake [60-63], possibly by preventing the precipitation of calcium-Mg2+-phosphate complexes in the ileum resulting in an increased solubility of Mg2+ [64]. Likewise, lipids impact the absorbability of Mg2+, whereby the lipid composition is suggested to be the influencing factor. Rat studies showed that a replacement of Medium Chain Triglycerides (MCT) for Long Chain Triglycerides (LCT) increased Mg2+ absorption [65, 66], possibly due to more soluble Mg2+ soaps of saturated fatty acids compared to insoluble Mg2+ salts formed with unsaturated fatty acids [67]. Conversely, studies on the influence of absolute fat mass on Mg2+ absorption have not produced consistent results ([68-70], reviewed in [64]).
Many studies examined the effect of low or indigestible carbohydrates (Table 2) and of lactose. A stimulatory effect of these carbohydrates on Mg2+ absorption has been predominantly shown in animal studies [37, 71-79] and some human studies [31, 80, 81]. The tested carbohydrates include resistant starch (especially raw resistant starch) [67-70], short-chain fructo-oligosaccharides [30, 80], resistant maltodextrin [82], a mixture of chicory oligofructose and long-chain inulin [31], galactooligosaccharides (GOS) [75, 76], inulin [37, 77, 78], polydextrose [78], maltitol and the hydrogenated polysaccharide fraction of Lycasin®HBC [81], mannitol [79] or lactulose [36]. Only one human study with short-chain fructo-oligosaccharides found no effect on Mg2+ uptake [30].
Table 2.
Low- or indigestible carbohydrates supposed to enhance bioavailability of Mg2+.
Studies are sorted by dietary factors. Mg2+ intake is consistently indicated in mg. Specifications in mmol were converted to mg.
| Species | Design | Duration |
Dietary Factor
Investigated |
Diet/Doses | Target Parameter for Mg2+ Bioavailability | Core Result | Refs. |
|---|---|---|---|---|---|---|---|
| 11 Healthy Postmenopausal Women | Randomized, placebo-controlled, double-blind, cross-over (3 weeks wash-out), stable isotope 25Mg2+ |
5 weeks | Short-chain fructo-oligosaccharides (sc-FOS) |
Diet with sc-FOS (10 g/d) or sucrose (placebo) 250 mg Mg2+ + 87.5 mg 25Mg2+ |
Mg2+ excretion in faeces and urine, Mg2+ in blood | sc-FOS increase Mg2+ absorption | [80] |
| 14 Healthy Girls | Randomized, placebo-controlled, double-blind, cross-over (12 days wash-out), stable isotopes 24Mg2+, 25Mg2+ and 26Mg2+ | 36 days (8-d of c-FOS intake) | sc-FOS | Diets with maltodextrin (placebo) or 10 g sc-FOS 41.0 mg Mg2+ + 52.5 mg 25Mg2+ + 21.1 mg 26Mg2+ intravenously |
Mg2+ excretion in urine | No significant differences | [30] |
| 15 Postmenopausal Women | Randomized, placebo-controlled, double-blind, cross-over (6 weeks wash-out), stable isotopes 25Mg2+ 26Mg2+ | 6 weeks | Mixture of chicory oligofructose (c-OF) and long-chain inulin (lc-In) | Diet with digestible maltodextrin (placebo) or 5 g c-OF and lc-In 58.0 mg total Mg2+ incl. 23.0 mg of 26Mg2+ + 11.5 mg 25Mg2+ intravenously |
Mg2+ excretion in urine | c-OF and lc-Is increase Mg2+ absorption | [31] |
| 20 Male Fischer Rats | Parallel group, control-diet | 7 days | Galactooligosaccharides (GOS) | Control diet or diet with 5 g GOS/ 100 g Control: 20.0±2.0 mg Mg2+/3 d GOS: 18.7±2.7 mg Mg2+/3 d |
Mg2+ excretion in faeces | GOS increase Mg2+ absorption, action of intestinal bacteria is necessary for the stimulatory effect of GOS | [75] |
| 75 Male Sprague Dawley Rats | Randomized, parallel group, control-diet | 8 weeks | GOS, dose-response effect | Control diet or diet containing 2, 4, 6 or 8% GOS Control: 30.4±2.7 mg Mg2+/3 d 2% GOS: 27.9±1.6 mg Mg2+/3 d 4% GOS: 30.2±2.6 mg Mg2+/3 d 6% GOS: 30.9±2.1 mg Mg2+/3 d 8% GOS: 31.2±3.6 mg Mg2+/3 d |
Mg2+ excretion in faeces and urine | GOS increase Mg2+ absorption | [76] |
| 80 Male Wistar Rats | Randomized, control-diet, stable isotope 25Mg2+ | 25 days | Inulin (In) |
Control diet or diet with 3.75% In for 4 days and then 7.5% In for 21 days Control: 495 mg Mg2+/kg (+ once ~ 2.5 mg 25Mg2+) In diets: 514 mg Mg2+/kg (+once 2.5 mg 25Mg2+) |
Mg2+ excretion in faeces and urine | In increase Mg2+ absorption |
[37] |
| Species | Design | Duration |
Dietary Factor Investigated |
Diet/Doses | Target Parameter for Mg2+ Bioavailability | Core Result | Refs. |
| 60 Male Wistar Rats | Randomized, parallel group, control-diet | 40 days | In + different calcium levels |
Control diet or diet with 5% In for 4 days and then 10% In, each group divided in 3 subgroups receiving 0.25%, 0.50% and 0.75% calcium short-term balance study: 0.25%: 9.9±1.1 mg Mg2+ 0.50%: 9.9±0.6 mg Mg2+ 0.75%: 9.5±0.6 mg Mg2+ 0.25%+In: 8.2±1.0 mg Mg2+ 0.50%+In: 8.1±0.8 mg Mg2+ 0.75%+In: 7.4±0.7 mg Mg2+ long-term balance study: 0.25%: 10.1±1.4 mg Mg2+ 0.50%: 9.2±0.7 mg Mg2+ 0.75%: 9.3±0.8 mg Mg2+ 0.25%+In: 8.3±1.3 mg Mg2+ 0.50%+In: 8.4±1.0 mg Mg2+ 0.75%+In: 8.0±1.0 mg Mg2+ |
Mg2+ excretion in urine | In increase Mg2+ absorption, efficiency of intestinal Mg2+ absorption (%) was negatively affected by calcium intake levels |
[77] |
| Ovariectomized (OVX) Sprague-Dawley Rats | Parallel group, control-diet | 4 weeks | In polydextrose | 6 treatment groups: Control, OVX-Control, OVX rats receiving daily estradiol (E2) injections, and OVX rats receiving a diet supplement with either In-based fiber (SYN or Fruitafit HD) or polydextrose fiber at 5% wt. of diet no information on Mg2+ intake |
Mg2+ excretion in faeces and urine | In and polydextrose increase Mg2+ absorption |
[78] |
| 50 Male Wistar Rats | Parallel group, control-diet | 3 weeks | Lactulose, pectin, guar gum, amylomaize starch | 6 treatment groups: control, 10% lactulose, 10% pectin, 10% guar gum, 25% amylomaize starch, 50% amylomaize starch no information on Mg2+ intake |
Mg2+ excretion in faeces | Fermentable carbohydrates increase Mg2+ absorption | [71] |
| 36 Female Wistar Rats | Parallel-group, control-diet | 3 weeks | Lactose, lactulose | 3 treatment groups: control: 4.8±0.2 mg Mg2+/d, lactose: 4.6±0.1mg Mg2+/d, lactulose: 4.6±0.1 mg Mg2+/d |
Mg2+ excretion in faeces and urine | Lactose and lactulose increase Mg2+ absorption | [86] |
| 36 Female Wistar Rats | Parallel-group, control-diet | 13 days | Maize starch Resistant Starch (RS) |
3 treatment groups: Low RS1: 4.1 mg Mg2+/d High RS22: 4.1 mg Mg2+/d High RS33: 4.4 mg Mg2+/d |
Mg2+ excretion in faeces and urine | RS2 increases Mg2+ absorption, no differences in RS3 | [74] |
| 64 Male Wistar Rats | Parallel-group, control-diet | 3 weeks | Raw potato starch (RPS), high amylose starch (HAS) | 3 treatment groups: Control: 14.5±0.6 mg Mg2+/d RPS: 15.8±0.7 mg Mg2+/d HAS: 16.1±0.8 mg Mg2+/d |
Mg2+ excretion in faeces | RPS and HAS increase Mg2+
absorption |
[73] |
| Species | Design | Duration |
Dietary Factor Investigated |
Diet/Doses | Target Parameter for Mg2+ Bioavailability | Core Result | Refs. |
| 32 Male Wistar Rats | Parallel-group, control-diet | 3 weeks | In, RS | 4 treatment groups: Control: 20.8±1.1 mg Mg2+/d In: 22.4±1.2 mg Mg2+/d RS: 23.0±1.0 mg Mg2+/d In+RS: 21.9±1.1 mg Mg2+/d |
Mg2+ excretion in faeces and urine | In and RS increase Mg2+ absorption |
[72] |
|
Exp. 1: 40 Male Sprague-Dawley Rats Exp. 2: 32 Male Sprague-Dawley Rats |
Parallel-group, control-diet |
Exp. 1: 2 weeks Exp. 2: 1 week |
Resistant maltodextrin (Fibersol 2, FS2), hydrogenated resistant maltodextrin (Fibersol 2H, FS2H) |
Exp. 1: 5 treatment groups: control: 17.3±0.6 mg Mg2+/d 1.5% FS2: 15.0±0.6 mg Mg2+/d 3%FS2: 15.7±0.6 mg Mg2+/d 1.5%FS2H: 16.0±0.4 mg Mg2+/d 3% FS2H: 15.4±0.5 mg Mg2+/d Exp. 2: 4 treatment groups CX-Ct: 13.3±0.1 mg Mg2+/d CX-FS2H: 13.2±0.1 mg Mg2+/d Sham-Ct: 13.5±0.1 mg Mg2+/d Sham-FS2H: 13.6±0.1 mg Mg2+/d |
Mg2+ excretion in faeces | Resistant maltodextrin and hydrogenated resistant maltodextrin increase Mg2+ absorption |
[82] |
|
Exp. 1: 35 Male Wistar Rats Exp. 2: 21 Male Wistar Rats |
Parallel-group, control-diet |
Exp. 1: 4 weeks Exp. 2: 7 days |
Mannitol |
Exp. 1: 5 treatment groups: Control, 2M (2% Mannitol), 4M, 6M, 8M Exp. 2: 3 treatment groups: Control, 4M, 8M no information on Mg2+ intake |
Mg2+ excretion in faeces | Mannitol increases Mg2+ absorption | [79] |
| 9 Healthy Young Men | Placebo-controlled, Latin-square (3x3) with three repetitions | 32 days (each) | Hydrogenated polysaccharide fraction of Lycasin®HBC (polyol) | Diet with dextrose (control) or hydrogenated polysaccharide fraction of Lycasin®HBC 320-330 mg Mg2+/d |
Mg2+ excretion in faeces and urine | Hydrogenated polysaccharides increase Mg2+ absorption | [81] |
| 10 Healthy Young Men | Randomized, cross-over (4 weeks wash-out) | 31 days | Glucose-polymer (NUTRIOSE FB) | 2 diets 1) control (+ 212±6.0 mg Mg2+/d) 2) 100 g NUTRIOSE FB/d (+ 232±7.0 mg Mg2+/d) |
Mg2+ excretion in urine and faeces | NUTRIOSE FB enhanced Mg2+ absorption | [97] |
| 24 Healthy Adult Males | Randomized, placebo-controlled, double-blind, cross-over (2 weeks wash-out), stable isotopes 24Mg2+ and 25Mg2+ | Single test meals | Lactulose | Test foods containing lactulose at a dose of 0 g (placebo), 2.0 g (low-dose), or 4.0 g (high-dose) 150 mg Mg2+ + 28.0 mg 25Mg2+ |
Mg2+ excretion in urine | Lactulose increase Mg2+ absorption | [36] |
1 Cooked normal starch, 2 Uncooked high amylose starch, 3 Cooked and cooled high amylose starch.
The stimulatory effect of GOS-and possibly other low- or indigestible carbohydrates-on mineral uptake might be attributed to the effects of short-chain fatty acids (lactate, acetate, propionate, butyrate) and reduced pH in the large intestine produced through fermentation of the carbohydrates by intestinal bacteria (mainly bifidobacteria) [75, 83]. The resulting lower caecal pH may increase solubility of minerals, thereby enhancing their absorption from the colon and caecum [84]. A rat study observed that the promoting effect of GOS on Mg2+ absorption was diminished by neomycin treatment (bacteria-suppressing), suggesting that the GOS-effect is dependent on the action of intestinal bacteria [75]. Weaver et al. (2011) observed that supplementing rats with GOS stimulates Mg2+ absorption and results in a decreased caecal pH, increased caecal wall and content weight and an increased proportion of bifidobacteria [76]. The authors proposed that these effects were either directly or indirectly attributed to changes in caecal pH, caecal content and wall weight (increased surface area available for Mg2+ absorption) and to the number of bifidobacteria. The proposed explanations cannot be verified, especially because the bulk of Mg2+ is absorbed in the small intestine and not in the large intestine. However, the increased Mg2+ absorption following prebiotic exposure associated with a shift in gut microbiome would occur in the large intestine. Moreover, there may be further explanations. For example, Rondón et al. (2008) showed that inulin ingestion also modulated TRPM6 and TRPM7 expression in the large intestine of mice, which suggests ameliorated active Mg2+ absorption in the large intestine [85].
An enhancing effect of lactose on Mg2+ absorption has been demonstrated in two studies with lactase-deficient rats [86, 87], but human studies have shown mixed results. An early study by Ziegler and Fomon (1983) observed an enhanced Mg2+ absorption of lactose in healthy infants compared to sucrose and polyose [88], whereas other studies with preterm infants [89] or term infants [90] did not find significant differences. There have been no studies with human adults investigating the effect of lactose on Mg2+ absorption. Xiao et al. (2013) observed that resistant sugar mannitol improves apparent Mg2+ absorption in growing Wistar rats, possibly by the fermentation of mannitol in the caecum resulting in a reduced pH [79]. Moreover, lactulose-an indigestible synthetic disaccharide of D-galactose and fructose-increased Mg2+ absorption in rat studies [81, 86] and a human study [36]. Seki et al. (2007) performed a clinical trial with a double-blind, randomized cross-over design and stable isotopes 24Mg2+ and 25Mg2+ to evaluate the effect of lactulose on Mg2+ absorption in healthy men. The test foods contained lactulose at a dose of 0 g (placebo), 2 g (low-dose), or 4 g (high-dose) [36]. The authors demonstrated that lactulose enhanced the absorption of Mg2+. The stimulatory effect on Mg2+ absorption is possibly also due to acidification in the ileal lumen [86].
4.2.4. Inhibiting Factors
The number of studies investigating dietary factors with a negative influence on the availability and uptake of Mg2+ is limited (Table 3). Early studies reported that increasing calcium in the diet significantly depressed Mg2+ absorption [91, 92]. The same depressive effect on Mg2+ absorption was shown with excess phosphorus, iron, copper, manganese [93] and zinc [94]. However, in these studies, unphysiological doses of the minerals were used. When these substances are consumed within a physiological range, such as present in a regular diet, the inhibiting effects have not been observed [64]. For example, long-term Mg2+ balance studies with calcium doses >1.000 mg/d did not produce a negative effect on Mg2+ uptake [35, 94, 95]. Andon et al. (1996) demonstrated in a human study with 26 adolescent girls that high calcium intake (1.667 mg/d) had no relevant impact on measures of Mg2+ utilization, including the absorption rate or urinary or faecal excretion [95]. Likewise, a balance study with adolescent girls showed that high calcium intake (1.800 mg/d) did not alter Mg2+ kinetics or balance compared to a calcium intake of 800 mg/d [35].
Table 3.
Dietary factors supposed to inhibit bioavailability of Mg2+.
Studies are sorted by dietary factors. Mg2+ intake is consistently indicated in mg. Specifications in mmol were converted to mg.
| Species | Design | Duration |
Dietary Factor
Investigated |
Diet/ Doses | Target Parameter For Mg2+ Bioavailability | Core Result | Refs. |
|---|---|---|---|---|---|---|---|
| 9 Healthy Adults | Cross-over (1 day wash-out), stable isotopes 25Mg2+ and 26Mg2+ |
Single test meals | Oxalic acid (OA) | 2 diets: 1) 300 g spinach (6.6 mmol OA; 122 mg Mg2+ incl. 17.0 mg 25Mg2+) 2) 300 g kale (0.1 mmol OA; 117 mg Mg2+ incl. 29.2 mg 26Mg2+) |
Mg2+ excretion in faeces | OA reduce Mg2+
absorption |
[32] |
| Male Wister Rats | Parallel group, control-diet | 8 days | OA | 6 diets: 1) Mg2+-deficient diet (control, 0.3 mg Mg2+) 2) raw powdered spinach (R-sp + 34.5 mg Mg2+) 3) boiled powdered spinach (B-sp + 34.8 mg Mg2+) 4) fried powdered spinach (F-sp + 35.9 mg Mg2+) 5) control diet with OA (Ox-C + 33.6 mg Mg2+) 6) control diet + 31.1 mg Mg2+ |
Mg2+ excretion in faeces and urine | OA reduce Mg2+
absorption Rate of absorbed Mg2+: control 88.9%, R-sp 80.2%, B-sp 88.4%, F-sp 90.4%, Ox-C 88.1%, + Mg2+ 87.7% |
[96] |
| 20 Healthy Adults | Cross-over (1 day wash-out), placebo-controlled, stable isotopes 25Mg2+ and 26Mg2+ | Single test meals | Phytic acid (PA) |
2 diets with 200 g wheat bread: 1) 0.75 mmol PA (+ 88.5 mg Mg2+ incl. 17.0 mg 25Mg2+) 2) 1.49 mmol PA (+ 88.5 mg Mg2+ incl. 26.7 mg 26Mg2+) |
Mg2+ excretion in faeces | PA reduce Mg2+
absorption, PA inhibiting effect was dose dependent |
[33] |
| 78 Male Sprague-Dawley Rats | Randomized, control-diet | 1, 3, or 5 weeks | Potato starch (PS) with esterified phosphorus (EP) |
4 diets: 1) EP-free control diet (+ 11.0 mg Mg2+) 2) 600 g Cornstarch (+ 8.0 mg Mg2+) 3) 600 g Benimaru PS (+ 8.3 mg Mg2+) 4) 600 g Konafubuki PS (+ 9.3 mg Mg2+) |
Mg2+ excretion in faeces | PS-EP reduce Mg2+ absorption | [98] |
| 40 Premenopausal and Post Menopausal Women |
Randomized, placebo-controlled, single-blind, cross-over (2 weeks wash-out), stable isotope 26Mg2+ | 2 weeks + single test meals for Mg2+ absorption |
Wheat dextrin (WD) | Cookies with 15.0 g WD/d or without (placebo) 120 mg Mg2+ incl. 29.2 mg 26Mg2+ |
Mg2+ excretion in urine | No significant differences |
[34] |
| 26 Adolescent Girls | Randomized, placebo-controlled, double-blind, parallel-group | 2 weeks | Calcium | 3 diets with basal Mg2+ intake of 176 mg Mg2+: 1) Placebo diet 2) low calcium (667 mg/d) 3) high calcium (1,667 mg/d) |
Mg2+ excretion in urine and faeces | No significant differences |
[95] |
| 5 Adolescent Girls | Randomized, cross-over (5 weeks wash-out), stable isotopes 25Mg2+ and 26Mg2+ | 2 weeks | Calcium | 2 diets (each + 40.0 mg 26Mg2+ oral + 20.0 mg 25Mg2+ intravenously): 1) low calcium (800 mg/d) + 305±30.0 mg Mg2+/d 2) high calcium (1,800 mg/d) + 286±9.0 mg Mg2+/d |
Mg2+ excretion in urine and faeces | No significant differences |
[35] |
Oxalic Acid (OA) is present in high amounts in members of the spinach family and in brassicas (cabbage, broccoli, brussels sprouts). The conjugate base of OA, oxalate, is a chelating agent for metal cations and thus affects the gastrointestinal bioavailability of Mg2+. The effect of OA on Mg2+ absorption has been studied in rats [96] and humans [32]. Kikunaga et al. (1995) investigated Mg2+ availability from OA-rich spinach in Mg2+-deficient rats [96]. The authors demonstrated that OA in spinach impairs Mg2+ absorption. In a cross-over study with healthy humans and stable isotopes 25Mg2+ and 26Mg2+, Bohn et al. (2004) evaluated Mg2+ absorption from a test meal served with an OA-rich vegetable, spinach (6.6 mmol OA), compared to a test meal with kale, a vegetable with low OA content (0.1 mmol) [32]. The authors demonstrated that Mg2+ absorption from the OA-rich spinach meal was significantly lower compared to the kale meal. The same group investigated the effect of Phytic Acid (PA) on Mg2+ bioavailability in another human study. PA is typically found in the outer layers of cereal grains (aleurone layer). Therefore, high amounts of PA are present in cereal products such as bran and whole-meal bread. PA, a myo-inositol hex-akisphosphate, has a strong binding affinity to important minerals and forms insoluble precipitates, which are not absorbable in the intestine. In a bioavailability study, Bohn et al. (2004) demonstrated that PA dose-dependently lowers Mg2+ absorption [33]. The amounts of PA tested in the study were similar to those naturally present in whole-meal (1.49 mmol) and in brown bread (0.75 mmol) [97, 98].
Human studies also found an inhibiting effect of partly and non-fermentable fibres such as wheat bran, cellulose and lignin on Mg2+ absorption [99, 100]. Two other human studies also observed a significant increase in faecal Mg2+ when cellulose was added to the diet [101, 102]. However, neither study matched the Mg2+ concentrations between the diet groups. Fibres such as hemicellulose and pectin are partly fermentable by intestinal bacteria. Two human studies with healthy males showed an inhibitory effect of hemicellulose on Mg2+ absorption [100, 103]. The effect of pectin on Mg2+ absorption remains controversial. A rat study observed a positive effect of pectin on Mg2+ flux from the caecum to the blood [81]. In contrast, two human studies found no significant difference in Mg2+ absorption when feeding healthy subjects citrus pectin [100, 104].
Unlike starch derived from cereals or other plants, potato starch contains considerable amounts of phosphorus [105], which is esterified on the carbon-6-hydroxyl group of the glucose molecule [106]. Other esterified phosphorus-bonded compounds in food sources, e.g., casein phosphopeptide, are known to enhance the absorption of calcium and other minerals [107]. Therefore, Mineo et al. (2009) examined the effect of potato starch feeding for 1, 3, and 5 weeks on apparent Mg2+ absorption in bone using a balance study in rats [98]. Two kinds of potato starch (Benimaru potato starch and Konafubuki potato starch) containing different phosphorus contents were used as carbohydrate sources. However, instead of increasing the absorption rate, the ingestion of potato decreased the absorption of Mg2+. The inhibiting effect is likely due to the binding effect of esterified phosphorus on Mg2+ and, thus, to enhanced faecal excretion. The study results, which were obtained in growing male rats, are difficult to extrapolate directly to humans. To evaluate the effect of potato starch and esterified phosphorus on Mg2+ bioavailability in humans, further experiments are needed.
Two human intervention studies investigated the effect of low-digestible carbohydrates on Mg2+ absorption [34, 97]. Armas (2011) determined the effect of chronic ingestion of Wheat Dextrin (WD) on Mg2+ absorption in premenopausal and postmenopausal women [34]. WD is a non-viscous soluble fibre that is used as a supplement to increase fibre intake. In a randomized, two-way cross-over, placebo-controlled, single-blind trial over two weeks, the authors showed that 15 g WD per day had no effect on Mg2+ absorption. Supplementation with a low-digestible glucose-polymer (NUTRIOSE FB, 100 g/d) versus dextrose in experimental meals was tested by Vermorel et al. (2004) in 10 healthy young men [97]. This study, likewise, reported no inhibiting effects of the low-digestible carbohydrate. Instead, the apparent Mg2+ absorption increased from 30.4% in the control group to 50.9% in the NUTRIOSE FB group. A possible explanation for the improvement in Mg2+ absorption is a high-regulation of the active intestinal absorption in the upper part of the intestine after ingesting fermentable carbohydrates for several weeks [81].
4.2.5. Type of Mg2+ Salt/Chemical and Physical Properties
In the past, attention has been given to the type of Mg2+ salt that should be administered, especially with respect to supplements. This aspect should be critically discussed in view of other factors influencing bioavailability and retention of the mineral. Surprisingly, there are only a few animal and human studies investigating the bioavailability of different Mg2+ salts (Table 4). In a rat study, Coudray et al. (2005) determined the intestinal Mg2+ absorption and urinary excretion of various organic and inorganic Mg2+ salts using stable isotopes (26Mg2+) [38]. Eighty male Mg2+-depleted Wistar rats were fed the same diet replete with Mg2+ (550 mg Mg2+/kg) as oxide, chloride, sulphate, carbonate, acetate, pidolate, citrate, gluconate, lactate or aspartate. The Mg2+ absorption values obtained varied from 50% to 67%. Organic Mg2+ salts were slightly more available than inorganic Mg2+ salts, whereas Mg2+ gluconate exhibited the highest Mg2+ bioavailability. However, the study demonstrated that all Mg2+ salts were equally efficient in restoring rats’ blood Mg2+ levels in plasma and red blood cells. Although humans and rats have some differences in intestinal physiology, these results may be extrapolated to human Mg2+ nutrition with necessary precautions.
Table 4.
Comparative studies on Mg2+ bioavailability from different types of Mg2+ salts.
Mg2+ intake is consistently indicated in mg. Specifications in mmol were converted to mg.
| Species | Design | Duration |
Type of Mg2+
Salt/Formulation Doses |
Mg2+-Intake on Empty Stomach or with Diet | Assessment/Adjust-ment of Mg2+-Status Before Intervention |
Control of Mg2+ in
Diet During Treatment/Intervention Period |
Target
Parameter for Mg2+ Bioavailability |
Core Result | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 18 Healthy Female Adults | Randomized, placebo-controlled, cross-over (4 d wash out) | Single intake | 3 Mg2+ salts: 1) 330 mg Mg2+ as Mg-lactate + 30.0 mg Mg2+ as Mg-citrate tablets 2) 270 mg Mg2+ as Mg-lactate + 90.0 mg Mg2+ as Mg-hydroxide tablets 3) 500 mg Mg2+ as Mg-chloride solution 4) 500 mg Mg2+ as Mg-hydroxide tablets |
Before meals | No information (“normal Mg-status”) | Subjects were asked to avoid Mg rich foods (no control) | Mg2+ excretion in 24-h urine | No significant differences | [108] |
| 17 Healthy Adults | Randomized, parallel-group | Single intake | 2 Mg2+ salts: 1) 607.6 mg Mg2+ as Mg-oxide 2) 607.6 mg Mg2+ as Mg-citrate |
Sober | Restricted diet 3 d before (200 mg Mg2+) | Subjects were asked to avoid Mg preparations | Mg2+ excretion in urine (4-h and 2nd 2-h post-load) | Mg-citrate showed significantly greater absorption than Mg-oxide | [112] |
| 8 Healthy Male Adults |
Randomized, placebo-controlled, parallel-group | Single intake | 2 Mg2+ salts: 1) Placebo: 36.5±0.6 mg Mg2+ 2) Mg acetate (MgAc) 1130 mg: 162±1.0 mg Mg2+ 3) MgAc 2,145 mg: 273±2.4 mg Mg2+ 4) MgAc 4,289 mg: 510±2.4 mg Mg2+ 5) MgAc 8,578 mg: 974±7.3 mg Mg2+ 6) Slow-Mag® (MgCl): 164±0.7 mg Mg2+ 7) Almonds: 177±2.4 mg Mg2+ 8) Fast: 0 mg Mg2+ |
Unclear condition |
No information (“normal Mg-status”) | Standardized diet | Mg2+ excretion in urine (4-h, 10-h urine collection) | Mg-acetate showed greater absorption than Mg-chloride, Mg from almonds was as bioavailable as from MgAc supplement | [47] |
| 14 Healthy Adults | Randomized, cross-over (3 d wash out) | Single intake | 2 Mg2+ salts: 1) 304 mg Mg2+ as Mg-citrate 2) 304 mg Mg2+ as K-Mg-citrate |
With diet | Constant metabolic diet 3 d before (no information on Mg2+) | No information on Mg2+ | Mg2+ excretion in 24-h urine | No significant differences | [110] |
| Species | Design | Duration |
Type of Mg2+ Salt/Formulation Doses |
Mg2+-Intake on Empty Stomach or with Diet | Assessment/Adjust-ment of Mg2+-Status Before Intervention |
Control of Mg2+ in Diet During Treatment/Intervention Period |
Target Parameter for Mg2+ Bioavailability |
Core Result | Refs. |
| 24 Healthy Adults | Randomized, placebo-controlled, parallel-group | 7 days | 3 Mg2+ salts/ 2 concentrations: 1) 729 mg/d Mg2+ as Mg-L-aspartate-HCl tablets 2) 1,093 mg/d Mg2+ as Mg-L-aspartate-HCl tablets 3) 729 mg/d Mg2+ as Mg-L-aspartate-HCl granules 4) 1,093 mg/d Mg2+ as Mg-L-aspartate-HCl granules 5) 729 mg/d Mg2+ as Mg-oxide capsules 6) 1,093 mg/d Mg2+ as Mg-oxide capsules |
With usual diet | One control and one placebo week before | No special diet | Mg2+ excretion in urine (7-d cumulative) | Mg-L-aspartate-HCl showed significantly greater absorption than Mg-oxide | [114] |
| 18 Healthy Male Adults 40 Healthy Age-Matched Controls |
Randomized, cross-over (2 d wash out) | Single intake | 3 Mg2+ formulations: 300 mg Mg2+ as 1) Mg-phosphate + Mg-oxide 2) Mg-oxide in smooth gelatin capsules 3) Mg-oxide in hard gelatin capsules |
After standardized breakfast | 6 d Mg2+-saturation period | Standardized diet rich in Mg2+ | Mg2+ excretion in urine (2-h intervals first in first 12-h, 4-h intervals in next 12-h intervals, 8- and 12-h intervals until 48-h post-load) | No significant differences | [109] |
| 12 Healthy Adults | Randomized, cross-over (1 week wash out) | Single intake | 3 Mg2+ formulations: 389 mg Mg2+ as 1) Mg-chloride solution 2) slow release Mg-chloride tablets 3) Mg-gluconate tablets |
Standard low Mg diet intake after fasting state | 1 day Mg2+ low diet | Low Mg diet | Mg2+ excretion in urine at baseline, 0 to 4, 4 to 8, 8 to 12, 12 to 24 h Mg2+ in blood at baseline, 1, 2, 3, 4, 8, 12, and 24 h |
No significant differences | [111] |
| 16 Healthy Adults | Randomized, cross-over (3 d wash out) | Single intake | 3 Mg2+ salts: 1) 243 mg Mg2+ as Mg-oxide 2) 267 mg Mg2+ as Mg- l-lactate 3) 267 mgl Mg2+ as Mg-aspartate |
With usual diet | No supplement intake (“normal Mg-status”) | Subjects were asked to avoid Mg rich foods (no control) | Mg2+ excretion in 24-h urine | Mg-chloride, Mg-l-lactate, Mg-aspartate showed a significantly higher bioavailability than Mg- oxide, Mg2+ bioavailability from Mg-chloride, Mg- l-lactate and Mg-aspartate was equivalent |
[115] |
| Species | Design | Duration |
Type of Mg2+ Salt/Formulation Doses |
Mg2+-Intake on Empty Stomach or with Diet | Assessment/Adjust-ment of Mg2+-Status Before Intervention |
Control of Mg2+ in Diet During Treatment/Intervention Period |
Target Parameter for Mg2+ Bioavailability |
Core Result | Refs. |
| 46 Healthy Adults | Randomized, parallel-group, placebo-controlled, double-blind | 60 days | 3 Mg2+ salts: 1) 269±34.4 mg/d Mg2+ as Mg-amino acid chelate 2) 255±18.3 mg/d Mg2+ as Mg-citrate 3) 280±29.8 mg/d Mg2+ as Mg-oxide |
With usual diet | Subjects were asked to avoid Mg rich foods | Subjects were asked to avoid Mg rich foods (no control) | Mg2+ excretion in 24-h urine Mg2+ in saliva and plasma |
Mg-citrate and Mg-amino-acid chelate showed significantly greater absorption than Mg-oxide | [113] |
| 16 Healthy Adults | Cross-over (5 days wash-out) | Single intake | 2 Mg2+ salts: 1) 600 mg Mg-oxide 2) 600 mg Mg-hydroxide carbonate |
Sober | 10 days strict diet with 350 mg Mg2+, first 3 days + 300 mg Mg-citrate | Standardized diet (350 mg Mg2+) | Mg2+ excretion in 24h urine Mg2+ in plasma |
No significant differences | [117] |
| 20 Healthy Male Adults | Randomized, cross-over | Single intake | 2 Mg2+ salts: 1) Mg citrate 2) Mg oxide |
With diet | Supplementation with Mg2+ to saturate Mg-pools (5 days) | Balanced, mixed diet (300-400 mg Mg/d) | Mg2+ excretion in 24-h urine, Mg2+ in serum, red blood cells, leukocytes |
Mg-citrate showed greater absorption than Mg-oxide | [116] |
| 120 Male Sprague-Dawley Rats | Parallel-group | 2 weeks | 6 Mg2+ salts: 1) Mg-carbonate: 2.8 mg Mg2+/d 2) Mg-chloride: 2.7 mg Mg2+/d 3) Mg-oxide: 2.8 mg Mg2+/d 4) Mg-phosphate: 2.5 mg Mg2+/d 5) Mg-sulfate: 2.6 mg Mg2+/d 6) Mg-silicate: 2.6 mg Mg2+/d |
With diet | 5-day pretest period with 9 g diet per day (400 mg Mg2+/kg) 8 g diet per day during the experimental period (200 or 400 mg Mg2+/kg) |
Yes, same diet | Mg2+ excretion in faeces and urine, Mg2+ in plasma |
No significant differences | [118] |
| 80 Sprague-Dawley Rats | Parallel-group, control-diet | 4 weeks | 8 Mg2+ salts: 1) Control: 4.0 mg Mg2+/100 g 19.0 mg Mg2+/100 g (2-10) 2) wheat flour 3) Mg-sulfate 4) Mg-oxide 5) Mg-chloride 6) Mg-phosphate 7) Mg-carbonate 8) Mg-lactate 9) Mg-citrate 10) Mg-acetate |
With diet | No information (“normal Mg2+-status”) | Yes, same mild Mg2+-deficient diet | Mg2+ excretion in faeces and urine, Mg2+ in plasma |
No significant differences | [119] |
| Species | Design | Duration |
Type of Mg2+ Salt/Formulation Doses |
Mg2+-Intake on Empty Stomach or with Diet | Assessment/Adjust-ment of Mg2+-Status Before Intervention |
Control of Mg2+ in Diet During Treatment/Intervention Period |
Target Parameter for Mg2+ Bioavailability |
Core Result | Refs. |
| 40 Male Wistar Rats | Parallel-group, control-diet | 4 weeks | 1 Mg2+ salt: 1) Control: 10.7±0.7 mg Mg2+/d 2) Mg-chloride: 12.5±1.2 mg Mg2+/d 3) sulphate rich water: 13.7±1.2 mg Mg2+/d 4) carbonate rich water: 13.6±2.0 mg Mg2+/d Day 24: 3 mg of 26Mg2+ orally + 0.5 mg 25Mg intravenously |
With diet | No information | Yes, same diet | Mg2+ excretion in faeces and urine, Mg2+ in plasma, red blood cells |
No significant differences | [120] |
| 80 Male Wistar Rats | Randomized, parallel-group, stable isotope 26Mg2+ | 2 weeks | 10 Mg2+ salts: 600 mg Mg2+/kg diet for two weeks + 1.8 mg 26Mg2+ 1) Mg-oxide 2) Mg-chloride 3) Mg-sulphate 4) Mg-carbonate 5) Mg-acetate 6) Mg-pidolate 7) Mg-citrate 8) Mg-gluconate 9) Mg-lactate 10) Mg-aspartate |
With diet | Run in phase: 3 w 150 mg Mg2+/kg diet | Yes, same diet | Mg2+ excretion in faeces and urine, Mg2+ in plasma |
Organic Mg-salts were slightly more available than inorganic Mg-salts, Mg-gluconate exhibited the highest Mg2+ bioavailability | [38] |
The few human studies, which were predominantly conducted in the early 1990’s, that compared the bioavailability of different Mg2+ salts led to mixed results (Table 4). Several chemical balance studies investigating urinary Mg2+ excretion in humans found no significant differences between various Mg2+ salts, including the comparison of organic with inorganic Mg2+ salts [108-120], which indicated that all types of Mg2+ are suitable to maintain or restore the Mg2+ status. Some other studies observed a slightly better bioavailability of organic Mg2+ salts under standardized conditions [112-116].
Lindberg et al. (1990) compared Mg2+ citrate and Mg2+ oxide with respect to in vitro solubility and in vivo gastrointestinal absorbability in healthy volunteers [112]. The authors observed that the bioavailability of Mg2+ citrate was higher than that of Mg2+ oxide, possibly due to its better solubility. Likewise, a product study by Kappeler et al. (2017) observed a slightly higher bioavailability of Mg2+ citrate as compared to Mg2+ oxide using 24-h urinary Mg2+ excretion as biomarker [116]. However, the difference in urinary Mg2+ levels between Mg2+ citrate (7.2±1.48 mmol) and Mg2+ oxide (6.7±1.43 mmol)-although statistically significant-is marginal. The difference of 0.565 mmol Mg2+ is equivalent to 13.7 mg (!) and, thus, physiologically irrelevant, especially with regard to a total Mg2+ intake of about 800 mg or more in the study during the test day. Walker et al. (2003) compared the bioavailability of Mg2+ oxide, Mg2+ citrate and Mg2+ amino acid chelate after single ingestion (24 h) and chronic administration (2 months) [113]. The authors also reported that the bioavailabilities of Mg2+ citrate and Mg2+ amino-acid chelate were higher that of Mg2+ oxide. Mühlbauer et al. (1991) observed that Mg2+ L-asparate was more bioavailable than Mg2+ oxide in healthy volunteers [114]. Firoz & Graber (2001) determined the Mg2+ bioavailability in four commercial Mg2+ preparations (Mg2+ oxide,
Mg2+ chloride, Mg2+ lactate and Mg2+ aspartate) in human subjects by using urinary Mg2+ excretion [115]. They observed a relatively poor bioavailability of Mg2+ oxide but a greater or equivalent bioavailability of the other three Mg2+ salts. Dolinska & Ryszka (2004) studied the influence of three different salts at different concentrations on Mg2+ absorption in the small intestine of rats using the area under the curve as the endpoint for Mg2+ bioavailability [121]. Mg2+ absorption was shown to be most efficient from Mg2+ gluconate compared to Mg2+ fumarate or Mg2+ chloride forms.
Together, most of the studies have shown that the availability of organic Mg2+ salts is slightly higher than that of inorganic compounds. However, the results of the different studies are hardly comparable because the designs of the studies were different (Table 4). For example, Mg2+ supplements were ingested together with a meal in some studies [38, 108-111, 113-116] or on an empty stomach or unclear conditions in others [47, 112, 117]. A study by Sabatier et al. (2002) demonstrated higher Mg2+ bioavailability when Mg2+-rich mineral water was consumed with a simultaneous meal [53]. It is questionable whether such food matrix effects simi-larly affect the bioavailability of Mg2+ salts and formulations. The target parameters used to evaluate Mg2+ bioavailability vary between studies. Most studies used Mg2+ excretion in urine but at different time points ranging from 2 h to 24 h. Another study used the 7-d cumulative Mg2+ excretion in urine [114].
Moreover, the validity of numerous studies is limited due to methodological weaknesses. Several studies did not adjust (or did not even assess) Mg2+ status by using a Mg2+-defined diet before the intervention period [108, 113, 115]. A similar Mg2+ status between the probands is a prerequisite to compare the bioavailability of Mg2+. In other words, several studies did not adequately control Mg2+ intake in the background diet or water intake during the treatment or intervention period [110, 112, 114, 116]. Other studies simply encouraged subjects to avoid Mg2+-rich foods or avoid Mg2+ supplements [108, 113, 115]. In a recent study [116], the concomitant diet during the test day contained more Mg2+ (300-400 mg) than the actual Mg2+ content in comparable supplements (300 mg Mg2+ citrate or Mg2+ oxide). Likewise, the drinking volume was not standardized over the 24 h test day. For example, subjects were allowed to drink Mg2+-containing water ad libitum until 1 h prior to administration. Moreover, the consumption of Mg2+-containing water was not adequately controlled during the test day. As a result, variations in the Mg2+ intake during the test day could have taken place, which question the standardization of the study conditions. In several cross-over studies with a single intake of Mg2+, the wash-out periods were very short (1-3 days) between the treatments [109, 110, 115]. Finally, only one study (with Wistar rats) used stable isotopes (26Mg2+), in contrast to all human studies.
Against this background, it is quite difficult to judge the importance of the type of salt for Mg2+ absorption. It has to be assumed that it is only one factor in the complex process and not of importance to maintain or restore Mg2+ status. Consequently, for legal reasons, several inorganic and organic Mg2+ salts are allowed for use in Mg2+-containing drugs and food supplements because they are all suitable for restoring Mg2+ status under physiological conditions.
4.2.6. Galenic Properties
In a randomized, controlled, cross-over trial with 22 healthy male volunteers, Karagülle et al. (2006) showed that the Mg2+ absorption from a single dose of mineral water with comparable pH value (test water I with 120 mg Mg2+/l, or test water II with 281 mg Mg2+/l) was similar to that from a pharmaceutical Mg2+ oxide (150.8 mg Mg2+) preparation [122]. The complete ionization of Mg2+ in the mineral water and the Mg2+ intake in diluted form might account for the good absorbability of Mg2+ from mineral waters [123, 124]. In addition, it has been suggested that Mg2+ in water, which appears as hydrated ions, can be more readily absorbed than Mg2+ from food [125].
This result is consistent with data from a randomized cross-over study with 13 healthy male volunteers that investigated the bioavailability of two different pharmaceutical Mg2+ oxide formulations (each 450 mg Mg2+) using urinary Mg2+ excretion (24-h urine) as an endpoint [126]. Better bioavailability of Mg2+ from Mg2+ oxide-effervescent tablets than from Mg2+ oxide-capsules was observed. The results showed that although the same Mg2+ amount was given with each preparation, the increase in Mg2+ excretion with effervescent tablets was twice that obtained with capsules. The authors assumed that the dissolution of Mg2+ tablets in water before ingestion results in an ionization of Mg2+, which is an important precondition for absorption. During solution CO2 production, acidic pH and excess citric acid achieve complete solubility of the Mg2+ salt such that Mg2+ becomes readily ionized. As a result, the bioavailability of Mg2+ from Mg2+ oxide effervescent tablets is comparable to that of the organic Mg2+salts, e.g., Mg2+ lactate, aspartate, amino acid chelate, and citrate [113, 115].
The few studies examining the effect of slow-release formulations on Mg2+ absorption produced different results. In a randomized, cross-over study with 12 healthy volunteers, White et al. (1992) compared the bioavailability of a Mg2+ chloride solution and slow-release Mg2+ chloride tablets by using urinary Mg2+ excretion (24-h urine) as the endpoint [111]. The authors observed no significant differences between the galenic forms, which suggests that the delayed-release tablet formulations had no influence on intestinal Mg2+ uptake. In contrast, Fine et al. (1991) showed that “slow release” Mg2+ formulations such as gastric acid resistant capsules also impacted the bioavailability of Mg2+ [47]. In their study, it was demonstrated that the Mg2+ absorption from enteric-coated tablets (cellulose acetate phthalate) of Mg2+ chloride was 67% less than that from Mg2+ acetate in gelatin capsules, suggesting that an enteric coating can impair Mg2+ bioavailability. Cellulose acetate phthalate requi-res 3-5-h before it is completely dissolved and the Mg2+ chloride is expelled. This delay would presumably reduce the absorptive area in the small intestine, where Mg2+ is predominantly absorbed.
SUMMARY AND CONCLUSION
The intestinal absorption of Mg2+ is a complex process that involves a saturable (transcellular) active pathway and a non-saturable (paracellular) passive pathway. At physiological luminal concentrations of the mineral, an active, saturable, and transcellular process dominates, whereas at higher doses, the passive, paracellular pathway gains importance. In principle, the relative bioavailability of Mg2+ is higher when the mineral is taken up in multiple low doses throughout the day compared to a single intake of a high amount of Mg2+. However, absolute absorption increases with the dose. The uptake of Mg2+ can be influenced by physiological factors, such as age and the other food components in a meal. Inhibitory effects can be exerted by high levels of partly fermentable fibres (i.e., hemicellulose), non-fermentable fibres (i.e., cellulose and lignin) and phytate and oxalate. In contrast, the inhibitory effect of other minerals, such as calcium, was not supported because it only occurs when unphysiological amounts are given within a meal. In addition to inhibiting factors, several dietary factors are known to enhance Mg2+ uptake, including proteins, MCT, and low- or indigestible carbohydrates such as resistant starch, oligosaccharides, inulin, mannitol and lactulose. Some studies have demonstrated a slightly higher bioavailability of organic Mg2+ salts compared to inorganic compounds under standardized conditions, which is probably due to variations in solubility. Other studies did not find significant differences between various Mg2+ salts. The design of the few studies investigating the differences in Mg2+ salts was heterogeneous. In addition, many of these studies had methodological weaknesses that limited the significance of the results. Due to the lack of standardized tests to assess Mg2+ status and intestinal absorption, it remains unclear which Mg2+ binding form shows the highest bioavailability. Animal studies showed that organic and inorganic Mg2+ salts were equally efficient at restoring depleted Mg2+ levels in plasma and red blood cells, despite a slightly higher bioavailability of organic Mg2+ compounds. Because Mg2+ cannot be stored but only retained for current needs, this aspect is less relevant than it is often thought to be. Higher absorption is followed by higher excretion of the mineral in most cases. In practice, especially in the case of additional administration of Mg2+ with a meal, absorption is superimposed by individual physiological conditions and the other food compounds. Due to the importance of passive paracellular Mg2+ absorption, the quantity of Mg2+ in the intestinal tract is the major factor controlling the amount of Mg2+ absorbed from the diet. In addition to standardized markers for Mg2+ uptake and status, there is a need for further studies investigating the intestinal absorption and bioavailability of Mg2+ that also include endogenous and exogenous factors.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
The manuscript was written through contributions of both authors.
CONFLICT OF INTEREST
The authors declare that this article content has no conflict of interest.
REFERENCES
- 1.Ayuk J., Gittoes N.J. Contemporary view of the clinical relevance of magnesium homeostasis. Ann. Clin. Biochem. 2014;51:179–188. doi: 10.1177/0004563213517628. [DOI] [PubMed] [Google Scholar]
- 2.Whang R., Hampton E.M., Whang D.D. Magnesium homeostasis and clinical disorders of magnesium deficiency. Ann. Pharmacother. 1994;28:220–226. doi: 10.1177/106002809402800213. [DOI] [PubMed] [Google Scholar]
- 3.Saris N.E., Mervaala E., Karppanen H., Khawaja J.A., Lewenstam A. Magnesium. An update onphysiological, clinical and analytical aspects. Clin Chim Acta Int J Clin Chem. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 4.Topf J.M., Murray P.T. Hypomagnesemia and hypermagnesemia. Rev. Endocr. Metab. Disord. 2003;4:195–206. doi: 10.1023/a:1022950321817. [DOI] [PubMed] [Google Scholar]
- 5.Konrad M., Schlingmann K.P., Gudermann T. Insights into the molecular nature of magnesiumhomeostasis. Am. J. Physiol. Renal Physiol. 2004;286:F599–F605. doi: 10.1152/ajprenal.00312.2003. [DOI] [PubMed] [Google Scholar]
- 6.O’Dell B.L., Sunde R.A. Handbook of Nutritionally Essential Mineral Elements. Boca Raton: CRC Press; 1997. [Google Scholar]
- 7.Assadi F. Hypomagnesemia: An evidence-based approach to clinical cases. Iran. J. Kidney Dis. 2010;4:13–19. [PubMed] [Google Scholar]
- 8.Dimke H., Hoenderop J.G., Bindels R.J. Molecular basis of epithelial Ca2+ and Mg2+ transport: Insights from the TRP channel family. J. Physiol. 2011;589:1535–1542. doi: 10.1113/jphysiol.2010.199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine (U.S.) Dietary reference intakes: for calcium, phosphorus,magnesium, vitamin D, and fluoride. Washington, D.C: National Academy Press; 1997. [PubMed] [Google Scholar]
- 10.European Food Safety Authority (EFSA) Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015;13:4186. [Google Scholar]
- 11.Rude R.K. Magnesium deficiency: A cause of heterogeneous disease in humans. J Bone Miner Res Off J Am Soc Bone Miner Res. 1998;13:749–758. doi: 10.1359/jbmr.1998.13.4.749. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki D., Funato Y., Miura J., et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 2013;9:e1003983. doi: 10.1371/journal.pgen.1003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Baaij J.H., Hoenderop J.G., Bindels R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 14.Hardwick L.L., Jones M.R., Brautbar N., Lee D.B. Site and mechanism of intestinal magnesium absorption. Miner. Electrolyte Metab. 1990;16:174–180. [PubMed] [Google Scholar]
- 15.Hardwick L.L., Jones M.R., Buddington R.K., Clemens R.A., Lee D.B. Comparison of calcium and magnesium absorption: In vivo and in vitro studies. Am. J. Physiol. 1990;259:G720–G726. doi: 10.1152/ajpgi.1990.259.5.G720. [DOI] [PubMed] [Google Scholar]
- 16.Schweigel M., Martens H. Magnesium transport in the gastrointestinal tract. Front Biosci J Virtual Libr. 2000;5:D666–D677. doi: 10.2741/schweigel. [DOI] [PubMed] [Google Scholar]
- 17.Graham L.A., Caesar J.J., Burgen A.S. Gastrointestinal absorption and excretion of Mg 28 in man. Metabolism. 1960;9:646–659. [PubMed] [Google Scholar]
- 18.Quamme G.A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008;24:230–235. doi: 10.1097/MOG.0b013e3282f37b59. [DOI] [PubMed] [Google Scholar]
- 19.Vormann J. Magnesium: Nutrition and metabolism. Mol. Aspects Med. 2003;24:27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 20.Voets T., Nilius B., Hoefs S., et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 21.Karbach U., Rummel W. Cellular and paracellular magnesium transport across the terminal ileumof the rat and its interaction with the calcium transport. Gastroenterology. 1990;98:985–992. doi: 10.1016/0016-5085(90)90023-t. [DOI] [PubMed] [Google Scholar]
- 22.Groenestege W.M., Hoenderop J.G., Van den Heuvel L., Knoers N., Bindels R.J. The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content andestrogens. J. Am. Soc. Nephrol. 2006;17:1035–1043. doi: 10.1681/ASN.2005070700. [DOI] [PubMed] [Google Scholar]
- 23.Fordtran J.S., Rector F.C., Carter N.W. The mechanisms of sodium absorption in the human small intestine. J. Clin. Invest. 1968;47:884–900. doi: 10.1172/JCI105781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amasheh S., Fromm M., Günzel D. Claudins of intestine and nephron-a correlation of molecular tight junction structure and barrier function. Acta Physiol. (Oxf.) 2011;201:133–140. doi: 10.1111/j.1748-1716.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 25.Benech H., Pruvost A., Batel A., Bourguignon M., Thomas J.L., Grognet J.M. Use of the stable isotopes technique to evaluate the bioavailability of a pharmaceutical form of magnesium in man. Pharm. Res. 1998;15:347–351. doi: 10.1023/a:1011947525377. [DOI] [PubMed] [Google Scholar]
- 26.Benech H., Grognet J.M. Recent data on the evaluation of magnesium bioavailability in humans. Magnes. Res. 1995;8:277–284. [PubMed] [Google Scholar]
- 27.Sabatier M., Arnaud M.J., Turnlund J.R. Magnesium absorption from mineral water. Eur. J. Clin. Nutr. 2003;57:801–802. doi: 10.1038/sj.ejcn.1601750. [DOI] [PubMed] [Google Scholar]
- 28.Hansen K.E., Nabak A.C., Johnson R.E., et al. Isotope concentrations from 24-h urine and 3-h serum samples can be used to measure intestinal magnesium absorption in postmenopausal women. J. Nutr. 2014;144:533–537. doi: 10.3945/jn.113.186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabatier M., Grandvuillemin A., Kastenmayer P., et al. Influence of the consumption pattern of magnesium from magnesium-rich mineral water on magnesium bioavailability. Br. J. Nutr. 2011;106:331–334. doi: 10.1017/S0007114511001139. [DOI] [PubMed] [Google Scholar]
- 30.Van den Heuvel E.G., Muijs T., Brouns F., Hendriks H.F. Short-chain fructo-oligosaccharides improve magnesium absorption in adolescent girls with a low calcium intake. Nutr. Res. 2009;29:229–237. doi: 10.1016/j.nutres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Holloway L., Moynihan S., Abrams S.A., Kent K., Hsu A.R., Friedlander A.L. Effects of oligofructose-enriched inulin on intestinal absorption of calcium and magnesium and bone turnover markers in postmenopausal women. Br. J. Nutr. 2007;97:365. doi: 10.1017/S000711450733674X. [DOI] [PubMed] [Google Scholar]
- 32.Bohn T., Davidsson L., Walczyk T., Hurrell R.F. Fractional magnesium absorption is significantly lower in human subjects from a meal served with an oxalate-rich vegetable, spinach, as compared with a meal served with kale, a vegetable with a low oxalate content. Br. J. Nutr. 2004;91:601. doi: 10.1079/BJN20031081. [DOI] [PubMed] [Google Scholar]
- 33.Bohn T., Davidsson L., Walczyk T., Hurrell R.F. Phytic acid added to white-wheat bread inhibits fractional apparent magnesium absorption in humans. Am. J. Clin. Nutr. 2004;79:418–423. doi: 10.1093/ajcn/79.3.418. [DOI] [PubMed] [Google Scholar]
- 34.Armas L.G., Rafferty K., Hospattankar A., Abrams S.A., Heaney R.P. Chronic dietary fiber supplementation with wheat dextrin does not inhibit calcium and magnesium absorption in premenopausal and postmenopausal women. J. Int. Med. Res. 2011;39:1824–1833. doi: 10.1177/147323001103900525. [DOI] [PubMed] [Google Scholar]
- 35.Sojka J., Wastney M., Abrams S., et al. Magnesium kinetics in adolescent girls determined using stable isotopes: effects of high and low calcium intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;273:R710–R715. doi: 10.1152/ajpregu.1997.273.2.R710. [DOI] [PubMed] [Google Scholar]
- 36.Seki N., Hamano H., Iiyama Y., et al. Effect of lactulose oncalcium and magnesium absorption: a study using stable isotopes in adult men. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53:5–12. doi: 10.3177/jnsv.53.5. [DOI] [PubMed] [Google Scholar]
- 37.Coudray C., Rambeau M., Feillet-Coudray C., et al. Dietaryinulin intake and age can significantly affect intestinal absorption of calcium and magnesium in rats: A stable isotope approach. Nutr. J. 2005:4. doi: 10.1186/1475-2891-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coudray C., Rambeau M., Feillet-Coudray C., et al. Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnes. Res. 2005;18:215–223. [PubMed] [Google Scholar]
- 39.Quamme G.A., Dirks J.H. The physiology of renal magnesium handling. Ren. Physiol. 1986;9:257–269. doi: 10.1159/000173090. [DOI] [PubMed] [Google Scholar]
- 40.Caruso R., Pallone F., Stasi E., Romeo S., Monteleone G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013;45:522–531. doi: 10.3109/07853890.2013.849383. [DOI] [PubMed] [Google Scholar]
- 41.Kruis W., Phuong Nguyen G. Iron deficiency, zinc, magnesium, vitamin deficiencies in Crohn’s disease: Substitute or not? Dig. Dis. 2016;34:105–111. doi: 10.1159/000443012. [DOI] [PubMed] [Google Scholar]
- 42.Braga C.B., Ferreira I.M., Marchini J.S., Cunha S.F. Copper and magnesium deficiencies in patients with short bowel syndrome receiving parenteral nutrition or oral feeding. Arq. Gastroenterol. 2015;52:94–99. doi: 10.1590/S0004-28032015000200004. [DOI] [PubMed] [Google Scholar]
- 43.Houillier P. Mechanisms and regulation of renal magnesium transport. Annu. Rev. Physiol. 2014;76:411–430. doi: 10.1146/annurev-physiol-021113-170336. [DOI] [PubMed] [Google Scholar]
- 44.Saltzman J.R., Russell R.M. The aging gut. Nutritional issues. Gastroenterol. Clin. North Am. 1998;27:309–324. doi: 10.1016/s0889-8553(05)70005-4. [DOI] [PubMed] [Google Scholar]
- 45.Coudray C., Feillet-Coudray C., Rambeau M., et al. The effect ofaging on intestinal absorption and status of calcium, magnesium, zinc, and copper in rats: A stable isotope study. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. 2006;20:73–81. doi: 10.1016/j.jtemb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Verhas M., De La Gueronniere V., Grognet J.M., Paternot J., et al. Magnesium bioavailability from mineral water. A study in adult men. Eur. J. Clin. Nutr. 2002;56:442. doi: 10.1038/sj.ejcn.1601333. [DOI] [PubMed] [Google Scholar]
- 47.Fine K.D., Santa Ana C.A., Porter J.L., Fordtran J.S. Intestinal absorption of magnesium from food and supplements. J. Clin. Invest. 1991;88:396–402. doi: 10.1172/JCI115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura E., Tai H., Uozumi Y., Nakagawa K., Matsui T. Magnesium absorption from mineral water decreases with increasing quantities of magnesium per serving in rats. Nutr. Res. 2012;32:59–65. doi: 10.1016/j.nutres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Schuette S.A., Ziegler E.E., Nelson S.E., Janghorbani M. Feasibility of using the stable isotope 25Mg to study Mg metabolism in infants. Pediatr. Res. 1990;27:36–40. doi: 10.1203/00006450-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Lönnerdal B. Magnesium nutrition of infants. Magnes. Res. 1995;8:99–105. [PubMed] [Google Scholar]
- 51.Ekmekcioglu C., Ekmekcioglu A., Marktl W. Magnesium transport from aqueous solutions acrossCaco-2 cells--an experimental model for intestinal bioavailability studies. Physiological considerations and recommendations. Magnes. Res. 2000;13:93–102. [PubMed] [Google Scholar]
- 52.Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochim Biophys Acta . 1772 doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Sabatier M., Arnaud M.J., Kastenmayer P., Rytz A., Barclay D.V. Meal effect on magnesium bioavailability from mineral water in healthy women. Am. J. Clin. Nutr. 2002;75:65–71. doi: 10.1093/ajcn/75.1.65. [DOI] [PubMed] [Google Scholar]
- 54.Bergillos-Meca T., Cabrera-Vique C., Artacho R., et al. Does Lactobacillus plantarum or ultrafiltration process improve Ca, Mg, Zn and P bioavailability from fermented goats’ milk? Food Chem. 2015;187:314–321. doi: 10.1016/j.foodchem.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 55.Lopez H.W., Leenhardt F., Remesy C. New data on the bioavailability of bread magnesium. Magnes. Res. 2004;17:335–340. [PubMed] [Google Scholar]
- 56.Rendleman J.A. Complexation of calcium by melanoidin and its role in determining bioavailability. J. Food Sci. 1987;52:1699–1705. [Google Scholar]
- 57.Andrieux C., Sacquet E. Effects of Maillard’s reaction products on apparent mineral absorption in different parts of the digestive tract. The role of microflora. Reprod. Nutr. Dev. 1984;24:379–386. doi: 10.1051/rnd:19840404. [DOI] [PubMed] [Google Scholar]
- 58.Delgado-Andrade C., Seiquer I., Pilar N.M. Maillard reaction products consumption: Magnesium bioavailability and bone mineralization in rats. Food Chem. 2008;107:631–639. [Google Scholar]
- 59.Roncero-Ramos I., Delgado-Andrade C., Morales F.J., Navarro M.P. Influence of Maillard products from bread crust on magnesium bioavailability in rats. J. Sci. Food Agric. 2013;93:2002–2007. doi: 10.1002/jsfa.6006. [DOI] [PubMed] [Google Scholar]
- 60.McCance R.A., Widdowson E.M., Lehmann H. The effect of protein intake on the absorption of calcium and magnesium. Biochem. J. 1942;36:686–691. doi: 10.1042/bj0360686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz R., Woodcock N.A., Blakely J.D., MacKellar I. Metabolic responses of adolescent boys to two levels of dietary magnesium and protein. II. Effect of magnesium and and protein level on calcium balance. Am. J. Clin. Nutr. 1973;26:519–523. doi: 10.1093/ajcn/26.5.519. [DOI] [PubMed] [Google Scholar]
- 62.Hunt S.M., Schofield F.A. Magnesium balance and protein intake level in adult human female. Am. J. Clin. Nutr. 1969;22:367–373. doi: 10.1093/ajcn/22.3.367. [DOI] [PubMed] [Google Scholar]
- 63.Lakshmanan F.L., Rao R.B., Kim W.W., Kelsay J.L. Magnesium intakes, balances, and blood levels of adults consuming self-selected diets. Am. J. Clin. Nutr. 1984;40:1380–1389. doi: 10.1093/ajcn/40.6.1380. [DOI] [PubMed] [Google Scholar]
- 64.Bohn T. Dietary factors influencing magnesium absorption in humans. Curr. Nutr. Food Sci. 2008;4:53–72. [Google Scholar]
- 65.López Aliaga I., Barrionuevo M., Campos M.S., Coves F., Lisbona F. Influence of intestinal resection and type of diet on digestive utilization and metabolism of magnesium in rats. J Int Vitaminol Nutr. 1991;61:61–66. [PubMed] [Google Scholar]
- 66.Vaquero M.P., Sarria B. Long-chain fatty acid supplemented infant formula does not influence calcium and magnesium bioavailability in weanling rats. J. Sci. Food Agric. 2005;85(8):1292–1298. [Google Scholar]
- 67.Renaud S.C., Ruf J.C., Petithory D. The positional distribution of fatty acids in palm oil and lard influences their biologic effects in rats. J. Nutr. 1995;125:229–237. doi: 10.1093/jn/125.2.229. [DOI] [PubMed] [Google Scholar]
- 68.Van Dokkum W., Cloughley F.A., Hulshof K.F., Oosterveen L.A. Effect of variations in fat and linoleic acid intake on the calcium, magnesium and iron balance of young men. Ann. Nutr. Metab. 1983;27:361–369. doi: 10.1159/000176707. [DOI] [PubMed] [Google Scholar]
- 69.Ricketts C., Kies C., Garcia P., Fox H. Manganese and magnesium utilization of humans as affected by level and kind of dietary-fat. Fed. Proc. 1985;44:1850. [Google Scholar]
- 70.Kies C., Samudio V., Ricketts C. Calcium, magnesium, and manganese utilization by healthy females as affected by dietary fat alterations.Ames Ed Diet Modif Des Affect Lipid Metab. Iowa State University: Iowa Agriculture and Home Economics Experiment Station; 1992. [Google Scholar]
- 71.Demigné C., Levrat M-A., Rémésy C. Effects of feeding fermentable carbohydrates on the cecal concentrations of minerals and their fluxes between the cecum and blood plasma in the rat. J. Nutr. 1989;119:1625–1630. doi: 10.1093/jn/119.11.1625. [DOI] [PubMed] [Google Scholar]
- 72.Younes H., Coudray C., Bellanger J., Demigné C., Rayssiguier Y., Rémésy C. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesiumbalance in rats. Br. J. Nutr. 2001;86:479–485. doi: 10.1079/bjn2001430. [DOI] [PubMed] [Google Scholar]
- 73.Lopez H.W., Levrat-Verny M-A., Coudray C., et al. Class 2 resistant starches lower plasma and liver lipids and improve mineral retention in rats. J. Nutr. 2001;131:1283–1289. doi: 10.1093/jn/131.4.1283. [DOI] [PubMed] [Google Scholar]
- 74.Schulz A.G., Van Amelsvoort J.M., Beynen A.C. Dietary native resistant starch but not retrograded resistant starch raises magnesium and calcium absorption in rats. J. Nutr. 1993;123:1724–1731. doi: 10.1093/jn/123.10.1724. [DOI] [PubMed] [Google Scholar]
- 75.Chonan O., Takahashi R., Watanuki M. Role of activity of gastrointestinal microflora inabsorption of calcium and magnesium in rats fed beta1-4 linked galactooligosaccharides. Biosci. Biotechnol. Biochem. 2001;65:1872–1875. doi: 10.1271/bbb.65.1872. [DOI] [PubMed] [Google Scholar]
- 76.Weaver C.M., Martin B.R., Nakatsu C.H., et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011;59:6501–6510. doi: 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- 77.Coudray C., Feillet-Coudray C., Tressol J.C., et al. Stimulatory effectof inulin on intestinal absorption of calcium and magnesium in rats is modulated by dietary calciumintakes: Short- and long-term balance studies. Eur. J. Nutr. 2005;44:293–302. doi: 10.1007/s00394-004-0526-7. [DOI] [PubMed] [Google Scholar]
- 78.Legette L.L., Lee W., Martin B.R., Story J.A., Campbell J.K., Weaver C.M. Prebiotics enhance magnesium absorption and inulin-based fibers exert chronic effects on calcium utilization in a postmenopausal rodent model. J. Food Sci. 2012;77:H88–H94. doi: 10.1111/j.1750-3841.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- 79.Xiao J., Li X., Min X., Sakaguchi E. Mannitol improves absorption and retention of calcium andmagnesium in growing rats. Nutrition. 2013;29:325–331. doi: 10.1016/j.nut.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 80.Tahiri M., Tressol J.C., Arnaud J., et al. Five-week intake of short-chain fructo-oligosaccharides increases intestinal absorption and status ofmagnesium in postmenopausal women. J. Bone Miner. Res. 2001;16:2152–2160. doi: 10.1359/jbmr.2001.16.11.2152. [DOI] [PubMed] [Google Scholar]
- 81.Coudray C., Bellanger J., Vermorel M., et al. Two polyol, low digestible carbohydrates improve the apparent absorption of magnesium but not of calcium in healthy young men. J. Nutr. 2003;133:90–93. doi: 10.1093/jn/133.1.90. [DOI] [PubMed] [Google Scholar]
- 82.Miyazato S., Nakagawa C., Kishimoto Y., Tagami H., Hara H. Promotive effects of resistant maltodextrin on apparent absorption of calcium, magnesium, iron and zinc in rats. Eur. J. Nutr. 2010;49:165–171. doi: 10.1007/s00394-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bongers A., Van den Heuvel E.G. Prebiotics and the bioavailability of minerals and trace elements. Food Rev. Int. 2003;19:397–422. [Google Scholar]
- 84.Younes H., Demigné C., Rémésy C. Acidic fermentation in the caecum increases absorption ofcalcium and magnesium in the large intestine of the rat. Br. J. Nutr. 1996;75:301–314. doi: 10.1079/bjn19960132. [DOI] [PubMed] [Google Scholar]
- 85.Rondón L.J., Rayssiguier Y., Mazur A. Dietary inulin in mice stimulates Mg2+ absorption and modulates TRPM6 and TRPM7 expression in large intestine and kidney. Magnes. Res. 2008;21:224–231. [PubMed] [Google Scholar]
- 86.Heijnen A.M., Brink E.J., Lemmens A.G., Beynen A.C. Ileal pH and apparent absorption of magnesium in rats fed on diets containing either lactose or lactulose. Br. J. Nutr. 1993;70:747. doi: 10.1079/bjn19930170. [DOI] [PubMed] [Google Scholar]
- 87.Yanahira S., Morita M., Aoe S., et al. Effects of lactitololigosaccharideson calcium and magnesium absorption in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 1997;43:123–132. doi: 10.3177/jnsv.43.123. [DOI] [PubMed] [Google Scholar]
- 88.Ziegler E., Fomon S. Lactose enhances mineral absorption in infancy. J. Pediatr. Gastroenterol. Nutr. 1983;2:288–294. [PubMed] [Google Scholar]
- 89.Wirth F.H., Numerof B., Pleban P., Neylan M.J. Effect of lactose on mineral absorption in preterm infants. J. Pediatr. 1990;117:283–287. doi: 10.1016/s0022-3476(05)80548-7. [DOI] [PubMed] [Google Scholar]
- 90.Moya M., Lifschitz C., Ameen V., Euler A.R. A metabolic balance study in term infants fed lactose containing or lactose-free formula. Acta Paediatr. 1999;88:1211–1215. doi: 10.1080/080352599750030301. [DOI] [PubMed] [Google Scholar]
- 91.Clarkson E.M., Warren R.L., McDonald S.J., De Wardener H.E. The effect of a high intake of calcium on magnesium metabolism in normal subjects and patients with chronic renal failure. Clin. Sci. 1967;32:11–18. [PubMed] [Google Scholar]
- 92.Norman D.A., Fordtran J.S., Brinkley L.J., et al. Jejunal andileal adaptation to alterations in dietary calcium: Changes in calcium and magnesium absorption and pathogenetic role of parathyroid hormone and 1, 25-dihydroxy vitamin D. J. Clin. Invest. 1981;67:1599. doi: 10.1172/JCI110194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De H., Basu K. Mutual influence of minerals in metabolism. Indian J. Med. Res. 1949;37:213–231. [Google Scholar]
- 94.Spencer H., Norris C., Williams D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J. Am. Coll. Nutr. 1994;13:479–484. doi: 10.1080/07315724.1994.10718438. [DOI] [PubMed] [Google Scholar]
- 95.Andon M.B., Ilich J.Z., Tzagournis M.A., Matkovic V. Magnesium balance in adolescent females consuming a low- or high-calcium diet. Am. J. Clin. Nutr. 1996;63:950–953. doi: 10.1093/ajcn/63.6.950. [DOI] [PubMed] [Google Scholar]
- 96.Kikunaga S., Ishii H., Takahashi M. The bioavailability of magnesium in spinach and the effectof oxalic acid on magnesium utilization examined in diets of magnesium-deficient rats. J. Nutr. Sci. Vitaminol. (Tokyo) 1995;41:671–685. doi: 10.3177/jnsv.41.671. [DOI] [PubMed] [Google Scholar]
- 97.Vermorel M., Coudray C., Wils D., et al. Energy value of alow-digestible carbohydrate, Nutriose Fb, and its impact on magnesium, calcium and zinc apparentabsorption and retention in healthy young men. Eur. J. Nutr. 2004;43:344–352. doi: 10.1007/s00394-004-0477-z. [DOI] [PubMed] [Google Scholar]
- 98.Mineo H., Ohmi S., Ishida K., et al. Ingestion of potato starch containing high levels of esterified phosphorus reduces calcium and magnesium absorption andtheir femoral retention in rats. Nutr. Res. 2009;29:648–655. doi: 10.1016/j.nutres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 99.McHale M., Kies C., Fox H. Calcium and magnesium nutritional status of adolescent humans fed cellulose or hemicellulose supplements. J. Food Sci. 1979;44:1412–1417. [Google Scholar]
- 100.Drews L.M., Kies C., Fox H.M. Effect of dietary fiber on copper, zinc, and magnesium utilization by adolescent boys. Am. J. Clin. Nutr. 1979;32:1893–1897. doi: 10.1093/ajcn/32.9.1893. [DOI] [PubMed] [Google Scholar]
- 101.Slavin J.L., Marlett J.A. Influence of refined cellulose on human bowel function and calcium and magnesium balance. Am. J. Clin. Nutr. 1980;33:1932–1939. doi: 10.1093/ajcn/33.9.1932. [DOI] [PubMed] [Google Scholar]
- 102.Behall K.M., Scholfield D.J., Lee K., Powell A.S., Moser P.B. Mineral balance in adult men: Effect of four refined fibers. Am. J. Clin. Nutr. 1987;46:307–314. doi: 10.1093/ajcn/46.2.307. [DOI] [PubMed] [Google Scholar]
- 103.Taper L.J., Milam R.S., McCallister M.S., Bowen P.E., Thye F.W. Mineral retention in young men consuming soy-fiber-augmented liquid-formula diets. Am. J. Clin. Nutr. 1988;48:305–311. doi: 10.1093/ajcn/48.2.305. [DOI] [PubMed] [Google Scholar]
- 104.Stasse-Wolthuis M., Albers H.F., Van Jeveren J.G., et al. Influence of dietary fiber from vegetables and fruits, bran or citrus pectin on serum lipids, fecal lipids, and colonic function. Am. J. Clin. Nutr. 1980;33:1745–1756. doi: 10.1093/ajcn/33.8.1745. [DOI] [PubMed] [Google Scholar]
- 105.Posternak T. On the phosphorus of potato starch. J. Biol. Chem. 1951;188:317–325. [PubMed] [Google Scholar]
- 106.Hizukuri S., Tabata S. Kagoshima, Nikuni Z. Studies on starch phosphate Part 1. Estimation ofglucose-6-phosphate residues in starch and the presence of other bound phosphate(s). Starke. 1970;22:338–343. [Google Scholar]
- 107.Tsuchita H., Suzuki T., Kuwata T. The effect of casein phosphopeptides on calcium absorption from calcium-fortified milk in growing rats. Br. J. Nutr. 2001;85:5–10. doi: 10.1079/bjn2000206. [DOI] [PubMed] [Google Scholar]
- 108.Bøhmer T., Røseth A., Holm H., Weberg-Teigen S., Wahl L. Bioavailability of oral magnesium supplementation in female students evaluated from elimination of magnesium in 24-hour urine. Magnes. Trace Elem. 1990;9:272–278. [PubMed] [Google Scholar]
- 109.Altura B.T., Wilimzig C., Trnovec T., Nyulassy S., Altura B.M. Comparative effects of a Mg enriched diet and different orally administered magnesium oxide preparations on ionized Mg, Mg metabolism and electrolytes in serum of human volunteers. J. Am. Coll. Nutr. 1994;13:447–454. doi: 10.1080/07315724.1994.10718433. [DOI] [PubMed] [Google Scholar]
- 110.Koenig K., Padalino P., Alexandrides G., Pak C.Y. Bioavailability of potassium and magnesium, and citraturic response from potassium-magnesium citrate. J. Urol. 1991;145:330–334. doi: 10.1016/s0022-5347(17)38330-1. [DOI] [PubMed] [Google Scholar]
- 111.White J., Massey L., Gales S.K., Dittus K., Campbell K. Blood and urinary magnesium kinetics after oral magnesium supplements. Clin. Ther. 1992;14:678–687. [PubMed] [Google Scholar]
- 112.Lindberg J.S., Zobitz M.M., Poindexter J.R., Pak C.Y. Magnesium bioavailability from magnesium citrate and magnesium oxide. J. Am. Coll. Nutr. 1990;9:48–55. doi: 10.1080/07315724.1990.10720349. [DOI] [PubMed] [Google Scholar]
- 113.Walker A.F., Marakis G., Christie S., Byng M. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes. Res. 2003;16:183–191. [PubMed] [Google Scholar]
- 114.Mühlbauer B., Schwenk M., Coram W.M., et al. Magnesium-L-aspartate-HCl and magnesium-oxide: Bioavailability in healthy volunteers. Eur. J. Clin. Pharmacol. 1991;40:437–438. doi: 10.1007/BF00265863. [DOI] [PubMed] [Google Scholar]
- 115.Firoz M., Graber M. Bioavailability of US commercial magnesium preparations. Magnes. Res. 2001;14:257–262. [PubMed] [Google Scholar]
- 116.Kappeler D., Heimbeck I., Herpich C., et al. Higher bioavailability of magnesium citrate as compared to magnesium oxide shown by evaluation of urinary excretion and serum levels after single-dose administration in a randomized cross-over study. BMC Nutr. 2017;3:7. [Google Scholar]
- 117.Tobolski O., Pietrzik K., Schlebusch H., Friedbarg R. Excretion of magnesium and calcium after a single dose of two magnesium preparations in a cross-over trial. Magnes-Bull. 1997;19:92–97. [Google Scholar]
- 118.Cook D.A. Availability of magnesium: Balance studies in rats with various inorganic magnesium salts. J. Nutr. 1973;103:1365–1370. doi: 10.1093/jn/103.9.1365. [DOI] [PubMed] [Google Scholar]
- 119.Ranhotra G., Loewe R., Puyat L. Bioavailability of magnesium from wheat flour and various organic and inorganic salts. Cereal Chem. 1976;53:770–776. [Google Scholar]
- 120.Feillet-Coudray C., Lafay S., Tressol J., Gueux E., Mazur A., Coudray C. Effects of sulphate- and bicarbonate-rich mineral waters on net and fractional intestinal absorption and urinary excretion of magnesium in rats. Eur. J. Nutr. 2003;42:279–286. doi: 10.1007/s00394-003-0423-5. [DOI] [PubMed] [Google Scholar]
- 121.Dolinska B., Ryszka F. Influence of salt form and concentration on the absorption of magnesiumin rat small intestine. Boll. Chim. Farm. 2004;143:163–165. [PubMed] [Google Scholar]
- 122.Karagülle O, Kleczka T, Vidal C, et al. Magnesium absorption from mineral waters of different magnesium content in healthy subjects. Forsch Komplementarmedizin. 2006. [DOI] [PubMed]
- 123.Gutenbrunner C., Hildebrandt G. Handbuch der Heilwasser-Trinkkuren: Theorie und Praxis; 20 Tabellen. Sonntag; 1994. [Google Scholar]
- 124.Gutenbrunner C., Hildebrandt G. Handbuch der Balneologie und medizinischen Klimatologie. Springer-Verlag; 1998. [Google Scholar]
- 125.Rubenowitz E., Axelsson G., Rylander R. Magnesium in drinking water and body magnesium status measured using an oral loading test. Scand. J. Clin. Lab. Invest. 1998;58:423–428. doi: 10.1080/00365519850186409. [DOI] [PubMed] [Google Scholar]
- 126.Siener R., Jahnen A., Hesse A. Bioavailability of magnesium from different pharmaceutical formulations. Urol. Res. 2011;39:123–127. doi: 10.1007/s00240-010-0309-y. [DOI] [PubMed] [Google Scholar]