Abstract
Background:
Cyclin-dependent kinase (CDK) 4/6 inhibitor-based therapies have shown great promise in improving clinical outcomes for patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer.
Objectives:
1. Discuss the mode of action of the three CDK4/6 inhibitors in late clinical development: palbociclib (PD-0332991; Pfizer), ribociclib (LEE011; Novartis), and abemaciclib (LY2835219; Lilly). 2. Describe the efficacy and safety data relating to their use in HR+, HER2– advanced breast cancer. 3. Discuss the key side effects associated with CDK4/6 inhibitors along with considerations for adverse event management and patient monitoring.
Method:
Relevant information and data were assimilated from manuscripts, congress publications, and online sources.
Results:
CDK4/6 inhibitors have demonstrated improved progression-free survival in combination with endocrine therapy compared with endocrine therapy alone. The side-effect profile of each agent is described, along with implications for patient monitoring, and considerations for patient care providers and pharmacists.
Conclusion:
Addition of a CDK4/6 inhibitor to endocrine therapy increases efficacy and delays disease progression. Insight into the unique side-effect profiles of this class of agents and effective patient monitoring will facilitate the successful use of CDK4/6 inhibitor-based therapies in the clinic.
Keywords: Abemaciclib, advanced breast cancer, CDK4/6 inhibitor, hormone receptor-positive, palbociclib, ribociclib
1. INTRODUCTION
Breast cancer presents a significant health burden worldwide [1]. In 2016, breast cancer was expected to result in more than 40,000 deaths in the US alone, accounting for approximately 7% of all US cancer-related deaths [2]. Around 75% of all breast cancer cases are diagnosed as hormone receptor-positive (HR+) [3]. HR+ breast cancers express the estrogen receptor (ER) and/or the progesterone receptor (PgR) and are typically dependent on the ER signaling pathway for growth and survival. Driven by the female hormone estrogen, the ER signaling pathway regulates a variety of cellular functions including cell proliferation, apoptosis, and angiogenesis [4]. HR+ breast cancers harness the biological functions of the ER pathway to promote breast cancer growth, development, and progression [5]. The reliance of HR+ breast cancer on ER signaling led to the development of agents that target the estrogen signaling pathway, such as aromatase inhibitors (AIs; including letrozole, anastrozole, and exemestane), selective ER modulators (tamoxifen), and selective ER down-regulators (fulvestrant), collectively termed endocrine therapy [6].
Endocrine therapy makes up the treatment backbone for HR+ breast cancer [7-9]. However, the efficacy of endocrine therapy is limited by high rates of both pre-existing de novo resistance, leading to a proportion of patients that fail to respond to endocrine therapy, and resistance that is acquired during treatment with endocrine therapy [4]. A key factor in the shift from estrogen dependency lies in alternative survival pathways, often referred to as ‘escape’ pathways, that are co-opted by the tumor to replace the reliance on ER signaling [10]. The ER pathway and many of the known escape pathways act through the cyclin D–cyclin-dependent kinase (CDK) 4/6–inhibitor of CDK4 (INK4)–retinoblastoma (Rb) pathway to promote tumor growth [11]. As such, it can be hypothesized that targeting the ER and cyclin D–CDK4/6–INK4–Rb pathways in combination will lead to a more extensive inhibition of tumor growth and prevent the activation of escape pathways, precluding the development of endocrine therapy resistance. Recently, the addition of a CDK4/6 inhibitor to endocrine therapy has demonstrated improved clinical outcomes, with delayed onset of tumor progression [12-14]. The combination of endocrine therapy and a CDK4/6 inhibitor is now included in the treatment guidelines for advanced HR+ breast cancer and is being widely prescribed [7, 8].
The advent of CDK4/6 inhibitor-based combination therapies presents a new challenge for health care providers to understand the toxicity profiles of the inhibitors in this class of agents and to deliver effective monitoring and management of the associated side effects. In this review, we described the mode of action of the following three CDK4/6 inhibitors, palbociclib (PD-0332991; Pfizer), ribociclib (LEE011; Novartis), and abemaciclib (LY2835219; Lilly), the efficacy and safety data relating to their use in HR+, human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer, and the implications for patient monitoring when these agents are combined with endocrine therapy.
2. THE CYCLIN D–CDK4/6–INK4–RB PATHWAY AS A THERAPEUTIC TARGET IN BREAST CANCER
2.1. The CDK4/6 and ER Pathways in Cell Cycle Control
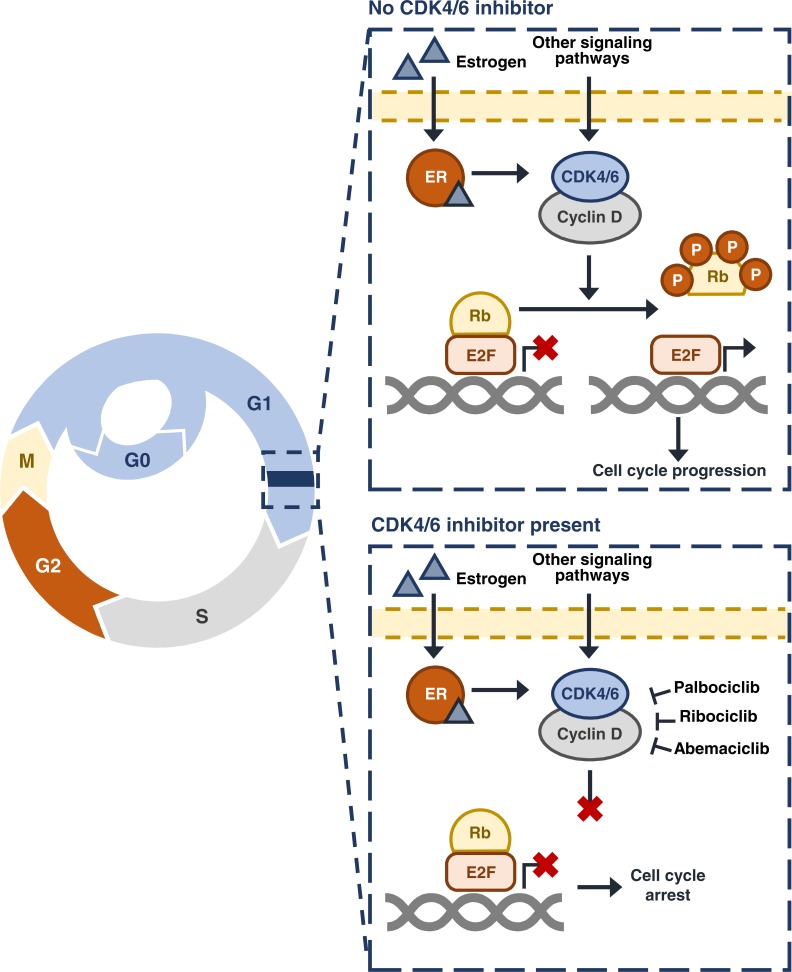
Individual cells are subject to stringent controls from external growth signals and cell cycle machinery before growth and proliferation can occur [15, 16]. Cell cycle progression from the first growth phase (G1), through the DNA synthesis (S) phase and the second growth phase (G2), to cell division in mitosis (M), is tightly controlled by a series of ‘checkpoints’ [15]. Cell cycle checkpoints allow the detection of cellular damage and the repair of any defects prior to mitosis in order to avoid the transfer of DNA damage to subsequent daughter cells [15]. Unrestricted passage through the cell cycle checkpoints as a result of cell cycle dysregulation is a classic hallmark of cancer, leading to uncontrolled proliferation and genomic instability that is characteristic of tumor cells [16].
A crucial point in the cell cycle is the G1–S cell cycle checkpoint, or the ‘restriction point’, after which a cell is irreversibly committed to mitosis irrespective of any external signals [17]. The cyclin D–CDK4/6–INK4–Rb pathway acts to control cellular progression through the G1–S checkpoint (Fig. 1) [17-21]. During G1, the Rb protein can be found in an inactive complex with the E2 transcription factor (E2F). This inactive complex prevents the expression of genes required for entry into S phase. At the G1–S checkpoint, mitogenic signaling pathways including the ER pathway, drive the expression of cyclin D. In turn, cyclin D associates with and activates the protein kinases CDK4 and CDK6. The active cyclin D–CDK4/6 complexes phosphorylate the Rb protein. Phosphorylated Rb is unable to interact with E2F; this renders E2F active and able to drive the expression of genes necessary for entry into S phase.
Fig. (1).
The cyclin D–CDK4/6–INK4–Rb pathway and cell cycle control. CDK, cyclin-dependent kinase; E2F, E2 transcription factor; ER, estrogen receptor; G, growth phase; INK4, inhibitor of CDK4; M, mitosis; P, phosphorylation; Rb, retinoblastoma; S, synthesis phase.
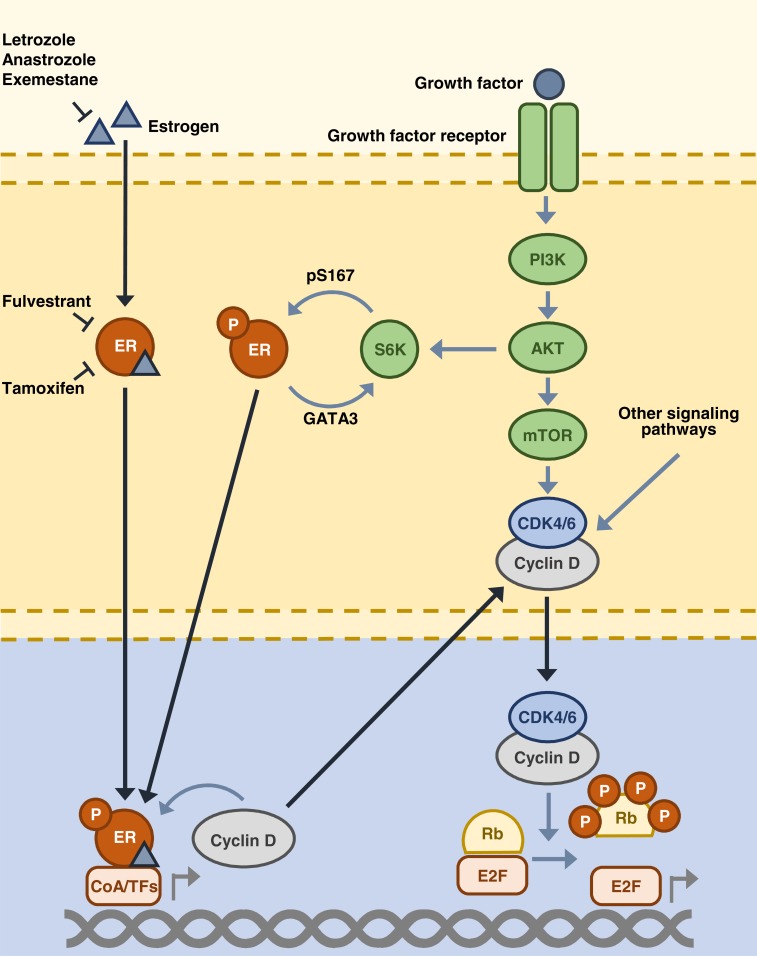
There are multiple layers of cross talk between the cyclin D–CDK4/6–INK4–Rb and ER signaling pathways (Fig. 2). The ER signaling pathway acts to directly upregulate cyclin D mRNA and protein expression, promoting cell cycle progression through activation of the cyclin D–CDK4/6–INK4–Rb pathway [20]. In addition, cyclin D is able to enhance the activity of ER through interactions with ER and its co-regulators [22]. Upon treatment with endocrine therapy, the ER pathway is inhibited, which prevents activation of the cyclin D–CDK4/6–INK4–Rb pathway. The resulting reduced cyclin D–CDK4/6 activity causes cell cycle arrest at the G1–S checkpoint, preventing cell division [3]. However, the cyclin D–CDK4/6–INK4–Rb pathway is frequently disrupted in favor of cell cycle progression in HR+ breast cancer [23, 24]. Increased cyclin D–CDK4/6 activity can result from the amplification of genes that encode cyclin D, CDK4, and CDK6 [25], or loss of the p16 protein [26], in addition to other mechanisms such as the activation of upstream signaling pathways [10]. This activation of the cyclin D–CDK4/6–INK4–Rb pathway has been associated with poor response and resistance to endocrine therapy [27, 28], with some tumor cells able to maintain pathway activity despite endocrine therapy [27].
Fig. (2).
ER and cyclin D–CDK4/6–INK4–Rb pathway cross-talk. AKT, protein kinase B; CDK, cyclin-dependent kinase; CoA, co-activator; E2F, E2 transcription factor; ER, estrogen receptor; GATA3, GATA-binding protein 3; mTOR, mammalian target of rapamycin; P, phosphorylation; PI3K, phosphatidylinositol 3-kinase; pS167, phosphorylated serine-167; TFs, transcription factors; Rb, retinoblastoma; S6K, S6 kinase.
In addition to cyclin D–CDK4/6–INK4–Rb pathway activation, multiple additional mechanisms exist in cells with pre-existing or acquired resistance to endocrine therapy, through which cell cycle progression can occur independently of estrogen signaling. These include both ligand-free ER signaling and the activation of alternative signaling pathways, as previously mentioned [10, 29]. Ligand-free ER signaling enables cells to activate E2F expression independently of estrogen presence; however, in these cases, the cell is still responsive to endocrine therapies that target the ER such as fulvestrant [29]. Following ER downregulation or loss, the upregulation of substitute signaling pathways, including growth factor receptor, cytokine, and cell survival pathways, promotes cancer growth [10]. In both the examples of endocrine resistance mechanisms, cell cycle progression remains reliant on activation of the cyclin D–CDK4/6–INK4–Rb pathway [11]. As such, targeting the cyclin D–CDK4/6–INK4–Rb pathway is an effective strategy both to enhance the efficacy of endocrine therapy and to overcome the problem of endocrine therapy resistance.
2.2. CDK4/6 Inhibitor Mode of Action
Palbociclib, ribociclib, and abemaciclib are orally bioavailable, selective inhibitors of CDK4 and CDK6 [30, 31]1 designed to bind to the ATP-binding pocket contained within the protein kinases, and thereby block CDK4/6-mediated phosphorylation of Rb [32]. Consistent with this observation, treatment with CDK4/6 inhibitors results in a lack of Rb phosphorylation in cancer cell lines [30, 31].1 Un-phosphorylated Rb remains bound to E2F in an inactive complex; hence E2F is unable to activate the expression of genes that favor cell cycle progression [3]. As such, the cell is arrested at the G1–S checkpoint and unable to proceed to cell division (Fig. 1). This is demonstrated by the inhibition of cell proliferation in Rb-positive cell lines and dose-dependent tumor growth inhibition in Rb-positive xenograft tumor models upon treatment with the CDK4/6 inhibitors palbociclib, ribociclib, and abemaciclib [30, 31].1
Palbociclib, ribociclib, and abemaciclib all inhibit cyclin D–CDK4/6 complexes to a similar extent, with half maximal inhibition (IC50) values of <40 nM, yet the ratios of CDK4 versus CDK6 inhibition vary (Table 1) [30, 31]. Ribociclib and abemaciclib are 4- and 5-times more selective toward CDK4 over CDK6, respectively [31].1 In addition, palbociclib, ribociclib, and abemaciclib vary in their selectivity toward other cyclin–CDK complexes [30, 31]. The differences in the ratios of inhibition may be clinically relevant, influencing the main toxicities of each individual drug [33]. All three agents are associated with hematologic toxicities; neutropenia and leukopenia are the most common side effects for each single agent [34, 35].2 Hematologic toxicities have
Table 1.
IC50 inhibition values (nmol/L) against cyclin–CDK complexes.
| Cyclin D1–CDK4 | CyclinD1/2/3–CDK6 | CDK4:CDK6 inhibition ratio | Cyclin B–CDK1 | Cyclin A/E–CDK2 | Cyclin T–CDK9 | |
|---|---|---|---|---|---|---|
| Palbociclib [30] | 11 | 16 | 1:1.5 | >10,000 | >10,000 | NR |
| Ribociclib* | 10 | 39 | 1:4 | 113,000 | 76,000 | NR |
| Abemaciclib [31] | 2 | 10 | 1:5 | 1627 | 504 | 57 |
CDK, cyclin-dependent kinase; IC50, concentration at which 50% inhibitory activity is exerted; NR, not reported.
*Kim, S.; Loo, A.; Chopra, R.; Caponigro, G.; Huang, A.; Vora, S.; Parasuraman, S.; Howard, S.; Keen, N.; Sellers, W.; Brain, C. LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6–Reactivating Rb in cancer. Mol. Cancer Ther., 2013, 12(11), Abstract PR02 (Oral presentation).
been ascribed to the consequence of CDK6 inhibition as the kinase plays crucial roles in the proliferation and development of hematologic precursors [32, 36]. Abemaciclib treatment is associated with a higher incidence of both all-grade and Grade 3/4 gastrointestinal toxicities [37].2 Abemaciclib also demonstrates inhibitory activity against other cyclin–CDK complexes in vitro including cyclin B–CDK1, cyclin A/E–CDK2, and cyclin T–CDK9 [31], although the impact of this activity on the side-effect profile of abemaciclib remains unknown.
In addition to the highlighted differences with respect to the levels of cyclin–CDK inhibition and side-effect profiles, each CDK4/6 inhibitor possesses individual properties to be taken into account for drug administration. Palbociclib dose exposure is subject to a food effect and must be taken with food [38], whereas ribociclib exposure remains comparable when taken both with and without regard to meal times.3 The time to maximum concentration (Tmax) also differs for palbociclib, ribociclib, and abemaciclib. Ribociclib is rapidly absorbed, with a Tmax of 1–5 hours [34], abemaciclib has a Tmax of 4–6 hours [39], whereas palbociclib absorption is slightly slower with a Tmax of 6–12 hours [38]. Out of the three agents, ribociclib has the longest mean half-life (T1/2) of approximately 33 hours [34], palbociclib mean T1/2 is approximately 26 hours [40, 41], while the mean T1/2 of abemaciclib ranges between 17 and 38 hours [39]. Steady-state levels of both palbociclib and ribociclib are generally reached within 8 days of once-daily drug administration [34, 38]. In contrast, the maintenance of abemaciclib steady-state plasma concentration and sustained cell cycle arrest requires more frequent dosing due to the shorter half-life of this agent [39]. Taken together, the side-effect profiles and drug properties have contributed to the recommended dosing schedule for each agent. The recommended Phase II dose of both single-agent palbociclib and single-agent ribociclib was determined to be once daily on a 3-weeks-on/1-week-off intermittent schedule [34, 41], although continuous once-daily dosing schedules in combination with endocrine therapy are under investigation for palbociclib and ribociclib [42, 43]. Abemaciclib is dosed twice daily on a continuous schedule.4
3. CDK4/6 INHIBITORS IN BREAST CANCER TREATMENT
3.1. Single-Agent CDK4/6-Inhibitor Therapy
The interest in the use of selective CDK4/6 inhibitors in breast cancer therapy transpired the following preclinical data, supporting the activity of these agents in HR+ breast cancer models. Across a panel of 47 breast cancer cell lines, growth inhibition upon palbociclib treatment was observed in luminal HR+ breast cancer cell lines, while breast cancers with negative ER status such as non-luminal and basal subtypes demonstrated high levels of resistance to palbociclib [44]. A similar activity was observed for ribociclib and abemaciclib, with high levels of activity in HR+ cell lines.5,6,7 This translated to growth inhibition of HR+ breast cancer tumor xenograft models.6,7,8 The first Phase I single-agent studies of palbociclib, ribociclib, and abemaciclib in adult patients demonstrated promising signs of clinical activity for this class of agents, with manageable safety profiles. Out of 31 evaluable patients with Rb-positive advanced solid tumors or non-Hodgkin’s lymphoma treated with single-agent palbociclib, 9 patients experienced stable disease and one patient had a partial response [40]. Following single-agent ribociclib treatment of patients with Rb-positive advanced solid tumors or lymphomas (N=132), 41 patients experienced stable disease and 3 patients had partial responses (NCT01237R236) [34]. In a Phase I study, abemaciclib treatment of patients with advanced cancers also showed signs of clinical activity; out of 47 evaluable patients with breast cancer and 68 evaluable patients with non-small cell lung cancer, 22 and 31 patients experienced stable disease, and 11 and 2 patients had a partial response, respectively (NCT01394016) [39]. The MONARCH-1 Phase II trial of single-agent abemaciclib in 132 patients with pretreated HR+, HER2– breast cancer demonstrated promising results; 26 patients had a partial response and 63 patients experienced stable disease (NCT02102490).9 Following MONARCH-1, an expanded access program for single-agent abemaciclib in patients with HR+, HER2– advanced breast cancer who experienced disease progression on prior therapies opened in June 2016 (NCT02792725) [45].
3.2. Combined CDK4/6-Inhibitor and Endocrine Therapy
The majority of clinical trials involving palbociclib, ribociclib, and abemaciclib concentrate on the benefit of combining CDK4/6 inhibitors with endocrine therapies as a treatment for HR+ breast cancer. Given the importance of the cyclin D–INK4–CDK4/6–Rb pathway in the development of resistance to endocrine therapy, the combination of both therapies is expected to increase efficacy and prevent endocrine therapy resistance [46]. Moreover, studies on breast cancer cell lines revealed synergistic activity between cell cycle and anti-estrogen therapies. As the repression of target genes in response to anti-estrogens primarily takes place when the cell is in the S phase of the cell cycle, increased apoptosis is observed when cell cycle-arrested cells are treated with endocrine therapy compared with non-arrested cells [47]. Preclinical in vivo studies with palbociclib and ribociclib also confirmed the potential for improved efficacy when combining CDK4/6 inhibition and endocrine therapy. In both ER+ breast cancer cell lines and HR+ breast cancer xenograft models, the combination of CDK4/6 inhibitors and endocrine therapy resulted in greater cell and tumor growth inhibition than with either agent alone [44].10,11,12
Out of the three CDK4/6 inhibitors under development, palbociclib has been approved by US Food and Drug Administration (FDA) in two indications in HR+, HER2– advanced breast cancer; in combination with letrozole as the first-line therapy, and in combination with fulvestrant in the second-line setting [38]. The approval of palbociclib plus letrozole was based on results from the PALOMA-1 Phase II trial (NCT00721409), in which the addition of palbociclib to letrozole therapy in patients who had received no prior treatment for ER+, HER2– advanced breast cancer significantly improved progression-free survival (PFS) compared with single-agent letrozole (20.2 versus 10.2 months, hazard ratio 0.488, 95% confidence interval [CI] 0.319–0.748, one-sided p=0.0004) [48]. The clinical activity of palbociclib plus letrozole was confirmed in the PALOMA-2 Phase III trial (NCT01740427), in which the addition of palbociclib to letrozole significantly improved PFS compared with placebo plus letrozole with a hazard ratio of 0.58 (95% CI 0.46–0.72, one-sided p<0.000001) [12]. Similarly, for the combination of palbociclib plus fulvestrant as a second-line therapy, Phase III results (NCT01942135) demonstrated that median PFS was increased to 9.5 months in patients who received combination therapy, from 4.6 months in patients who received fulvestrant alone (hazard ratio 0.46, 95% CI 0.36–0.59, two-sided p<0.0001) [13]. Neither amplification of cyclin D1 (CCND1), loss of p16, or PIK3CA mutational status were shown to affect response to combined palbociclib and endocrine therapy [13, 48]. Recent results from the MONALEESA-2 trial (NCT01958021) have shown that ribociclib in combination with letrozole significantly improved PFS in patients with HR+, HER2– advanced breast cancer who had received no systemic therapy for their advanced disease; median PFS in the ribociclib plus letrozole arm was not reached compared with 14.7 months in patients who received placebo plus letrozole (hazard ratio 0.56, 95% CI 0.43–0.72, p=0.00000329) [14]. Based on these data, FDA approval has been granted to ribociclib in combination with letrozole for the first-line treatment of HR+, HER2– advanced breast cancer [49]. Abemaciclib currently has FDA Breakthrough Therapy Designation as a single agent, following the previously mentioned data from the breast cancer cohort expansion in the Phase I single-agent trial (NCT01394016) [50]. There are a number of other ongoing key Phase III trials in the first- and second-line setting for pre- and postmenopausal patients with HR+ advanced breast cancer that are currently investigating the combination of CDK4/6 inhibitors with endocrine therapy agents, including fulvestrant, anastrozole/letrozole, tamoxifen, and exemestane (Table 2).
Table 2.
Key phase III CDK4/6 inhibitor-based clinical trials in advanced/metastatic breast cancer.*
| Trial | Setting | Patient Population | Treatment | Enrollment | Trial Status |
|---|---|---|---|---|---|
| PALOMA-4 (NCT02297438) [72] | 1L | Asian postmenopausal women with ER+, HER2– ABC | Letrozole ± palbociclib | ≈330 | Recruiting |
| PALOMA-2 (NCT01740427) [12, 73] |
1L | Postmenopausal women with ER+, HER2– ABC | Letrozole ± palbociclib | 666 | Active, not recruiting |
| PALOMA-3 (NCT01942135) [74] | 2L | Pre/peri- and postmenopausal women with HR+, HER2– MBC | Fulvestrant ± palbociclib | 521 | Active, not recruiting |
| PEARL (NCT02028507) [75] |
2L | Postmenopausal women with HR+, HER2– MBC | Palbociclib + exemestane or fulvestrant vs palbociclib + capecitabine | 600 | Recruiting |
| MONALEESA-2 (NCT01958021) [76] |
1L | Postmenopausal women with HR+, HER2– ABC | Letrozole ± ribociclib | 668 | Active, not recruiting |
| MONALEESA-7 (NCT02278120) [77] | 1L | Premenopausal women with HR+, HER2– ABC | Tamoxifen or NSAI ± ribociclib + goserelin |
671 | Active, not recruiting |
| MONALEESA-3 (NCT02422615) [78] | 1L, 2L | Men and postmenopausal women with HR+, HER2 ABC | Fulvestrant ± ribociclib | 725 | Active, not recruiting |
| MONARCH-3 (NCT02246621) [79] | 1L | Postmenopausal women with HR+, HER2– ABC | Letrozole/anastrozole ± abemaciclib |
≈450 | Active, not recruiting |
| MONARCH-2 (NCT02107703) [80, 81] |
1L, 2L | Pre/peri- and postmenopausal women with HR+, HER2– ABC | Fulvestrant ± abemaciclib | 669 | Active, not recruiting |
1L, first-line; 2L, second-line; ABC, advanced breast cancer; CDK, cyclin-dependent kinase; ER+, estrogen receptor-positive; HER2–, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; MBC, metastatic breast cancer; NSAI, non-steroidal aromatase inhibitor.
*Phase I and II trials for palbociclib, ribociclib, and abemaciclib in combination with endocrine therapy and alternative therapies can be found on ClinicalTrials.gov.uk.
4. SAFETY PROFILES AND SIDE-EFFECT MONITORING OF CDK4/6 INHIBITOR-BASED THERAPIES
The efficacy of CDK4/6 inhibitors in combination with endocrine therapy has brought CDK4/6 inhibitor-based therapy to the forefront of HR+ breast cancer treatment. Successful implementation of CDK4/6 inhibitor-based combination therapies in the clinic requires an understanding of the associated side effects and effective patient monitoring. Table 3 details the most common side effects associated with each CDK4/6 inhibitor based on data from the key single-agent and Phase III combination trials. The main toxicity associated with palbociclib, ribociclib, and abemaciclib treatment is bone marrow suppression resulting in neutropenia and leukopenia; other cytopenias, such as anemia and thrombocytopenia, are less common [12-14, 34, 35].13 Hematologic adverse events are thought to result from the impact of on-target CDK4/6 inhibition on bone marrow progenitor cells [51]. Similar to other therapeutic agents for advanced breast cancer, fatigue and low-severity gastrointestinal toxicities, including nausea, diarrhea, and vomiting, have been observed for each agent [12, 13, 34].14 All three CDK4/6 inhibitors in late development demonstrated similar toxicity profiles with the exception of higher rates of gastrointestinal toxicities upon abemaciclib treatment, including Grade 3 diarrhea [12, 13, 34].14 Overall, the side effects associated with CDK4/6 inhibitor therapy are less severe than those experienced with chemotherapy [37], and in most cases dose delays or reductions result in prompt recovery [52]. In the PALOMA-2 and PALOMA-3 trials, palbociclib dose reductions due to adverse events were required for 36% and 34% of patients, respectively [12, 13]. In the MONALEESA-2 trial of ribociclib in combination with letrozole, 50.6% of patients received a dose reduction due to adverse events [14].
Table 3.
Common side effects associated with CDK4/6 inhibitor-based therapy.
| Treatment Details | Patient Population |
Most Common Side Effects
(>30% any grade) |
Most Common Severe Side Effects
(≥20% Grade 3/4) |
|
|---|---|---|---|---|
| Palbociclib | ||||
| Palbociclib monotherapy*,† (NCT01037790) [35] | ABC N=37 |
Leukopenia (100%), neutropenia (92%), thrombocytopenia (76%), anemia (70%), fatigue (68%), lymphopenia (65%) |
Neutropenia (54%), leukopenia (51%), lymphopenia (30%) | |
| Palbociclib + letrozole† (PALOMA-2; NCT01740427) [12] |
ER+, HER2– ABC N=444 |
All-causality AEs: Neutropenia (80%), leukopenia (39%), fatigue (37%), nausea (35%), arthralgia (33%), alopecia (33%) |
Neutropenia (66%), leukopenia (25%) | |
| Palbociclib + fulvestrant*,†
(PALOMA-3; NCT01942135) [13] |
HR+, HER2– MBC N=345 |
All-causality AEs: Neutropenia (81%), leukopenia (50%), infections (42%), fatigue (39%), nausea (32%) |
Neutropenia (65%) leukopenia (28%) |
|
| Ribociclib | ||||
| Ribociclib monotherapy (NCT01237236) [34] | Advanced solid tumors/lymphomas N=132 |
TEAEs: Neutropenia (46%), fatigue (45%), leukopenia (43%), nausea (42%), thrombocytopenia (30%) |
Neutropenia (27%) | |
| Ribociclib + letrozole (MONALEESA-2; NCT01958021) [14] |
HR+, HER2– ABC N=334 |
All-causality AEs: Neutropenia (74%), nausea (52%), infections (50%), fatigue (37%), diarrhea (35%), alopecia (33%), leukopenia (33%) |
Neutropenia (59%), leukopenia (21%) | |
| Abemaciclib | ||||
| Abemaciclib monotherapy†
(MONARCH-1; NCT02102490)‡ |
HR+, HER2– MBC N=132 |
TEAEs: Leukopenia (91%), diarrhea (90%), neutropenia (88%), anemia (69%), fatigue (65%), nausea (64%), decreased appetite (46%), thrombocytopenia (41%), abdominal pain (39%), vomiting (35%) |
Leukopenia (28%), neutropenia (27%), diarrhea (20%) | |
ABC, advanced breast cancer; AE, adverse event; CDK, cyclin-dependent kinase; ER+, estrogen receptor-positive; HER2–, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; MBC, metastatic breast cancer; TEAEs, treatment-emergent AEs.
*Any-grade incidence values were calculated by the addition of the number of patients listed as experiencing Grade 1–4 adverse events;
†Grade 3/4 incidence values were calculated by the addition of the number of patients listed as experiencing Grade 3 and Grade 4 adverse events.
‡Dickler, M.; Tolaney, S.; Rugo, H.; Cortes, J.; Diéras, V.; Patt, D.A.; Wildiers, H.; Frenzel, M.; Koustenis, A.; Baselga, J. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J. Clin. Oncol., 2016, 34(Suppl), Abstract 510 (Oral presentation).
Side effects are generally well managed, with low rates of treatment discontinuation as a result; adverse events were the cause of patient discontinuation in 9.7%, 2.0%, 7.5%, and 7.6% of patients in the PALOMA-2, PALOMA-3, MONALEESA-2, and MONARCH-1 clinical trials, respectively [12-14].15 The monitoring processes for the following key side effects are listed in Table 4: hematologic side effects, gastrointestinal toxicities, liver enzyme elevation, pulmonary embolism, and cardiac toxicity.
Table 4.
Monitoring of CDK4/6 inhibitor-associated side effects.
|
Hematologic
Side Effects |
Gastrointestinal Toxicity |
Liver Enzyme
Elevation |
Pulmonary Embolism | QT Prolongation | |
|---|---|---|---|---|---|
| Symptoms | Fatigue, shortness of breath, increased tendency to bleed, and/or bruise [82] |
Diarrhea, nausea, vomiting* [12, 13, 14] |
Weight loss, jaundice, dark urine, itching, abdominal swelling [64] | Shortness of breath, chest pain, cough, rapid breathing, rapid heart rate [38, 66] |
Palpitations, fainting episodes [69] |
| Clinical assessment | Complete blood counts [7, 38] | Electrolyte levels [60] | Liver function tests [64] | Monitor patient symptoms [38] |
ECG [83] |
| Crucial time window | 2 weeks after drug administration in treatment Cycles 1 and 2 (palbociclib and ribociclib) [34, 38, 53] |
1 week after drug administration (abemaciclib)* |
Throughout treatment [14] |
Throughout treatment [38] | First 4 weeks of treatment [14] |
| Frequency of monitoring | Start of each treatment cycle. Additional assessments during Cycles 1 and 2 [7, 38] | Throughout treatment* | Throughout treatment [14] |
Throughout treatment [38] | Throughout treatment [14] |
| Additional risk factors | Infections, fever, Asian ethnicity, low baseline neutrophil count [53, 82] |
Fever, dizziness, abdominal pain [60] |
Concomitant medication [84] |
Deep-vein thrombosis [66] | Diarrhea, vomiting, concomitant medication [69] |
CDK, cyclin-dependent kinase; ECG, electrocardiogram.
*Dickler, M.; Tolaney, S.; Rugo, H.; Cortes, J.; Diéras, V.; Patt, D.A.; Wildiers, H.; Frenzel, M.; Koustenis, A.; Baselga, J. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J. Clin. Oncol., 2016, 34(suppl), Abstract 510 (Oral presentation).
4.1. Hematologic Side Effects
Hematologic side effects are among the most common effects experienced by patients who receive CDK4/6
inhibitor-based therapy, with CDK4/6 inhibitor-associated neutropenia the most frequent Grade 3/4 adverse event reported in clinical trials. Neutropenia is regularly experienced by patients treated with chemotherapy, yet neutropenia associated with CDK4/6 inhibitors differs both mechanistically (see below) in that, it is rapidly reversible [32]. Grade 3/4 neutropenia experienced by patients treated with palbociclib and fulvestrant therapy in the PALOMA-3 trial generally resolves within 7 days [53]. In addition, CDK4/6 inhibitor-associated neutropenia is less severe than that experienced by patients who have undergone chemotherapy: there is an absence of related pancytopenia, and low rates of infection [12, 13, 34]. Chemotherapy-induced severe Grade 4 neutropenia is experienced by over 37% of breast cancer patients during the first four cycles of treatment, and up to 23% of these patients develop subsequent febrile neutropenia [54] with mortality rates of around 5% in patients with solid tumors [55]. In contrast, in CDK4/6 inhibitor studies, less than 10% of patients developed Grade 4 neutropenia, and low rates of febrile neutropenia have been reported (in 2.5%, 0.9%, 1.5%, and 0.8% of patients in the PALOMA-2, PALOMA-3, MONALEESA-2, and MONARCH-1 trials, respectively) [12-14].14 The reversible nature of CDK4/6 inhibitor-associated neutropenia is a result of the mode of action of CDK4/6 inhibitors that suppress the bone marrow through cell cycle arrest, as opposed to apoptotic cell death [56]. Preclinical studies have demonstrated that exposure of hematopoietic stem cells to CDK4/6 inhibitors reversibly decreased proliferation but did not decrease total marrow cellularity, alter apoptosis levels, or affect cell viability [57]. As such, the majority of neutropenia cases resulting from palbociclib or ribociclib therapy are resolved rapidly and Grade 3/4 neutropenia can be managed with dose interruptions or reductions [14, 37]. Dose modifications for lymphopenia are not typically necessary unless concurrent opportunistic infection occurs [38].
To prevent any complications such as severe myelosuppression with related infection, fever and/or bleeding, and to avoid prolonged Grade 3/4 neutropenia, all patients who receive CDK4/6 inhibitors require frequent monitoring throughout the course of CDK4/6 inhibitor treatment [46]. The onset of neutropenia is around 15 days following the first dose of either palbociclib or ribociclib [34, 38, 53]; therefore, the timing of patient monitoring is crucial, particularly during the first two treatment cycles. American Society of Clinical Oncology guidelines suggest that complete blood counts should be examined prior to starting CDK4/6 inhibitor-based therapy, at the beginning of each new treatment cycle, and on Day 14 of Cycles 1 and 2 [7]. It is important to note that due to baseline assessments, dose interruptions, and cycle delays, the patient may not always begin study treatment on the initially scheduled day. This can result in clinical assessments taking place at the wrong point in the cycle, leaving an opportunity for early signs of neutropenia to be missed. Occurrences such as this can be minimized by encouraging clear communication between the patient and the monitoring team. A follow-up call with the patient, the day after the proposed start of each treatment cycle to confirm whether the dose was administered is a simple and effective method to ensure that assessments take place within the required time-frame. There is a mild increased risk of infection including influenza and upper respiratory infections with CDK4/6 inhibition [48, 53]; although as previously mentioned, neutropenic fever and sepsis are rare. Due to this increased risk of infection associated with neutropenia and CDK4/6 inhibitors, in addition to monitoring complete blood counts, patients should be strongly encouraged to maintain a high standard of personal hygiene, avoid contact with people who have an infection, and report any instances of fever above 38.3°C or a persistent fever of over 38°C that lasts 1 hour or more [58, 59].
4.2. Gastrointestinal Toxicities
CDK4/6 inhibitor treatment-related gastrointestinal side effects include diarrhea, nausea, and vomiting. These toxicities are generally low-grade occurrences for both palbociclib and ribociclib, with the exception of higher rates of Grade 3 diarrhea observed following abemaciclib treatment.16 During the MONARCH-1 trial of abemaciclib monotherapy for patients with HR+, HER2– metastatic breast cancer, diarrhea was generally experienced within one week of therapy initiation, leading to dose reduction in 21% of patients.16 The majority of cases were resolved quickly with a median duration of 7.5 days (Grade 2) and 4.5 days (Grade 3).16 Diarrhea can be a debilitating side effect of cancer treatment as the loss of fluids and electrolytes can result in serious dehydration, renal insufficiency, and electrolyte imbalances; therefore, patients with persistent diarrhea should be monitored for these symptoms [60]. Regular blood tests during treatment with CDK4/6 inhibitors may help to identify alterations in electrolyte levels such as hypokalemia and hypophosphatemia [60]. Persistent diarrhea can result from alternative causes such as infectious or viral etiologies, which should be considered before ascribing diarrhea as a side effect of CDK4/6 inhibitor treatment. If infectious causes are ruled out, anti-motility agents such as loperamide or diphenoxylate/atropine can be offered for persistent diarrhea. Reciprocally, diarrhea increases the risk of infections, which can have serious consequences in patients concurrently experiencing neutropenia, emphasizing the importance of managing this condition [61]. Proactive management of diarrhea at the first sign of loose stools as well as patient assessments for added risk factors including fever, dizziness, abdominal pain, and weakness may help to prevent any complications associated with the condition [60].
4.3. Liver Enzyme Elevation
Asymptomatic abnormal liver function with corresponding increases in the liver enzymes alanine transaminase (ALT) and aspartate transaminase (AST) have been observed following treatment with CDK4/6 inhibitors in combination with endocrine therapy. In clinical trials of patients with HR+, HER2– breast cancer, Grade 3 increments in ALT and AST levels have been reported in 4% and 2% of patients treated with palbociclib and anastrozole, and 2% and 3% of patients who received palbociclib plus fulvestrant, respectively [13].17 Palbociclib in combination with letrozole has been associated with hepatic failure and liver-related death in two patients with HR+, HER2– advanced breast cancer [62]. In the MONALEESA-2 study, Grade 3/4 ALT and AST elevations were observed in 9% and 6% of patients treated with ribociclib in combination with letrozole [14]. Four patients in the ribociclib plus letrozole arm experienced ALT/AST elevations with a concurrent increase in total bilirubin; however, there were no resulting deaths and all the cases were reversible following ribociclib discontinuation [14]. Out of 20 patients with HR+, HER2– metastatic breast cancer treated with abemaciclib plus letrozole, increase in Grade 3 in ALT or AST levels was observed in two patients.18 Routine liver function tests may help the early identification of any abnormal liver function during CDK4/6 inhibitor-based therapy. Additionally, concomitant medication including patient use of over-the-counter herbal medications should be regularly reviewed to avoid liver enzyme elevations as a result of drug–drug interactions, and patients should be counselled to limit alcohol intake [63]. Mild impairment of liver function is unlikely to result in any noticeable side effects; however, patients should be encouraged to report any of the following symptoms: unexplained weight loss, jaundice, dark urine, itching, or abdominal pain [64]. Based on the aforementioned data, patients with significant hepatic impairment may be poor candidates for CDK4/6 inhibitor-based therapy and should inform their health care provider about their condition.
4.4. Pulmonary Embolism
Thromboembolism is a leading cause of death in patients receiving anticancer therapy; in a study of patients receiving outpatient chemotherapy (n=4466), 9% died as a result of thromboembolism [65]. Some cases of thromboembolism have also been found associated with CDK4/6 inhibitor-based combination therapy. Thromboembolic events including pulmonary embolism, deep-vein thrombosis, subclavian vein thrombosis, and vena cava thrombosis were observed in 2% of patients with HR+, HER2– advanced breast cancer who received palbociclib plus fulvestrant in PALOMA-3 [53]. Grade 4 pulmonary embolism was reported in 5% of postmenopausal patients with ER+, HER2– advanced breast cancer treated with palbociclib plus letrozole in the PALOMA-1 study [48]. One case of Grade 3 pulmonary embolism was reported as a dose-limiting toxicity in the first-in-human trial of single-agent ribociclib therapy [34]. In the MONALEESA-2 trial, two cases of pulmonary embolism were reported as serious adverse events in patients treated with ribociclib in combination with letrozole [14]. Throughout the course of palbociclib treatment, patients should be monitored for signs and symptoms of a pulmonary embolism including shortness of breath, hypoxia, chest pain, rapid breathing, or rapid heart rate [38]. Additionally, patients should be encouraged to report fainting episodes, light-headedness, or sweating [66]. As even large emboli can be asymptomatic, patients who experience fainting episodes, light-headedness, or hypotension should be considered being at high risk [67]. The clinical signs of pulmonary embolism are non-specific, and as such health care providers should confirm the diagnosis with computed tomography angiography (CTA) of the pulmonary arteries; alternatively, a ventilation perfusion scan can be performed if CTA is contraindicated [67].
4.5. Cardiac Toxicity
Uncomplicated prolongation of the QT interval has been associated with palbociclib and ribociclib treatment in a dose-dependent manner. A positive linear correlation exists between palbociclib concentration at 125 mg and QT interval.19 At the recommended ribociclib dose of 600 mg/day in the first-in-man, single-agent study in patients with advanced solid tumors or lymphomas, all instances of QT corrected using Frederica’s formula (QTcF) prolongation were Grade 1/2 in severity; Grade 3/4 QTcF prolongation was only observed at the doses of at least 900 mg/day [34]. QTcF prolongation to >480 ms was experienced by 3.3% of patients treated with ribociclib plus letrozole in the MONALEESA-2 trial (Grade 2, n=10 [3%]; Grade 3, n=1 [0.3%]), with most changes occurring in the first four-week cycle of study treatment [14]. QTcF prolongation was limited in MONALEESA-2 by proactive dose interruption or reduction [14]. To reduce the risk of QT prolongation, patients eligible to receive CDK4/6 inhibitor-based therapy are subject to stringent criteria in relation to cardiac status, and electrocardiograms should be performed to assess baseline QTcF [37, 68]. Any concomitant medication administered to the patient for side-effect management should be carefully reviewed to avoid the co-administration of medications known to increase the risk of QTcF prolongation [37, 68]. The QTc interval may also need to be regularly monitored with CDK4/6 inhibitor treatment [37]. Although the majority of QTcF prolongation cases associated with CDK4/6 inhibitor therapy are asymptomatic [34], patients should be encouraged to report any symptoms associated with QTcF prolongation such as palpitations or fainting episodes [69]. Due care should be taken to monitor patients who are experiencing vomiting and/or diarrhea, as the risk of QTcF prolongation also increases if the levels of potassium or sodium in the blood fall below the normal limits [69].
5. PATIENT AWARENESS
Patients awareness and education is a key component of thorough side-effect monitoring. A lack of knowledge of the major side effects and the requirement for regular monitoring can affect a patient’s ability to fully comply with treatment plans. For many side effects, the early implementation of appropriate side-effect management can reduce both symptom severity and the length of patient discomfort. Patients should be made aware of the need for the prompt reporting of key symptoms, including those listed in Table 4. This can be encouraged by the use of telephone calls or e-mail prompts between visits to the clinic, which has been demonstrated to reduce patients visits to the emergency room, and lengthen the duration of treatment in patients receiving chemotherapy [70]. However, patients can be reluctant to notify their health care provider of any side effects due to a fear that their therapy may be interrupted as a result [71]. In the case of neutropenia, recent results from the PALOMA-3 trial demonstrated that dose modifications did not have an adverse effect on treatment efficacy [53]. Transparent communication between the health care provider and the patient must be maintained for the patient to develop a balanced view of the seriousness of any side effects compared with their perceived loss-of-treatment benefit. As CDK4/6 inhibitors are home-based, oral therapy, patients play a key role in dosage and side-effect management. The importance of patient education regarding major side effects should not be underestimated.
CONCLUSION
The addition of CDK4/6 inhibitors to endocrine therapy has been shown to improve efficacy in patients with HR+, HER2- advanced breast cancer, and CDK4/6 inhibitor-based therapies are becoming standard-of-care in this patient population. Both palbociclib and ribociclib have demonstrated improved PFS in combination with endocrine therapy compared with endocrine therapy alone. There are currently no proven biomarkers other than ER positivity known to affect CDK4/6 inhibitor response or resistance. Within this class of CDK4/6 inhibitors, palbociclib, ribociclib, and abemaciclib exhibit different in vitro and pharmacokinetic properties. In particular, the differences between the agents in IC50 values for different kinases and ratios of CDK4:CDK6 inhibition may be clinically relevant, influencing the main toxicities of each individual drug. Although the incidence and severity of side effects associated with palbociclib, ribociclib, and abemaciclib differ, the general toxicities associated with each CDK4/6 inhibitor are similar, including bone marrow suppression with resulting neutropenia and gastrointestinal toxicities. Unlike chemotherapy-associated neutropenia, due to mechanistic differences between cytotoxic agents and CDK4/6 inhibitors, CDK4/6 inhibitor-associated neutropenia is rapidly reversible following dose modifications and reductions with a low incidence of neutropenic fever. The key to the success of CDK4/6 inhibitors in the clinic lies in effective monitoring and management of the associated side effects. In particular, timely clinical assessments and prompt reporting of symptoms are crucial to minimize the severity of side effects, and decrease the frequency of dose delays and treatment interruptions.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
The authors thank Jenny Winstanley PhD for medical editorial assistance with this manuscript.
Footnotes
Kim, S.; Loo, A.; Chopra, R.; Caponigro, G.; Huang, A.; Vora, S.; Parasuraman, S.; Howard, S.; Keen, N.; Sellers, W.; Brain, C. LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6–Reactivating Rb in cancer. Mol. Cancer Ther., 2013, 12(11), Abstract PR02 (Oral presentation).
Dickler, M.; Tolaney, S.; Rugo, H.; Cortes, J.; Diéras, V.; Patt, D.A.; Wildiers, H.; Frenzel, M.; Koustenis, A.; Baselga, J. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J. Clin. Oncol., 2016, 34(Suppl), Abstract 510 (Oral presentation).
Dhuria, S.V.; Siddani, R.; Kosecki, C.M.; Germa, C.; Mondal, S. A phase I food-effect study of the ribociclib (LEE011) drug-in-capsule (DiC) formulation in healthy subjects. J. Clin. Oncol., 2015, 33(Suppl), Abstract e13577.
Shapiro, G.; Rosen, L.S.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Tolaney, S.M.; Beeram, M.; Rasco, DW.; Kulanthaivel, P.; Li, Q.; Hu, T.; Cronier, D.; Chan, E.M.; Flaherty, K.; Wen, P.Y.; Patnaik, A. A first-in-human phase I study of the CDK4/6 inhibitor, LY2835219, for patients with advanced cancer. J. Clin. Oncol., 2013, 31(Suppl), Abstract 2500.
Lallena, M.; Boehnke, K.; Torres, R.; Hermoso, A.; Amat, J.; Calsina, B.; De Dios, A.; Buchanan, S.; Du, J.; Beckmann, R.P.; Gong, X.; Mcnulty, A. In-vitro characterization of abemaciclib pharmacology in ER+ breast cancer cell lines. Cancer Res., 2015, 75(Suppl 15), Abstract 3101.
O’Brien, N.A.; Di Tomaso, E.D.; Ayala, R.; Tong, L.; Issakhanian, S.; Linnartz, R.; Finn, R.S.; Hirawat, S.; Slamon, D.J. In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer. Cancer Res., 2014, 74(Suppl 19), Abstract 4756.
O’Brien, N.; Conklin, D.; Luo, T.; Kalous, O.; von Euw, E.; Hurvitz, S.; Beckmann, R.P.; Mockbee, C.; Slamon, D.J. Preclinical activity of abemaciclib as a single agent or in combination with anti-mitotic or targeted therapies for breast cancer. Cancer Res., 2016, 76(Suppl 14), Abstract 2828.
Parasuraman, S.; Caponigro, G.; Loo, A.; Cao, Z.; Kim, S.; Issa, I.; Matano, A.; Di Tomaso, E.; Infante, J.R.; Cassier, P.; Hirawat, S. LEE011, a potent and selective CDK4/6 inhibitor, under preclinical and clinical investigation. 12th International Congress on Targeted Anticancer Therapies, Washington, DC, March 5-7, 2014, Oral presentation O4.4.
Dickler, M.; Tolaney, S.; Rugo, H.; Cortes, J.; Diéras, V.; Patt, D.A.; Wildiers, H.; Frenzel, M.; Koustenis, A.; Baselga, J. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J. Clin. Oncol., 2016, 34(Suppl), Abstract 510 (Oral presentation).
O’Brien, N.A.; Di Tomaso, E.D.; Ayala, R.; Tong, L.; Issakhanian, S.; Linnartz, R.; Finn, R.S.; Hirawat, S.; Slamon, D.J. In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer. Cancer Res., 2014, 74(Suppl 19), Abstract 4756.
Parasuraman, S.; Caponigro, G.; Loo, A.; Cao, Z.; Kim, S.; Issa, I.; Matano, A.; Di Tomaso, E.; Infante, J.R.; Cassier, P.; Hirawat, S. LEE011, a potent and selective CDK4/6 inhibitor, under preclinical and clinical investigation. 12th International Congress on Targeted Anticancer Therapies, Washington, DC, March 5-7, 2014, Oral presentation O4.4.
Torres, R.; Calsina, B.; Hermoso, A.; Baquero, C.; Mur, C.; Boehnke, K.; Amat, J.; De Dios, A.; Gong, X.; Buchanan, S.; Beckmann, R.P.; Lallena, M.J. Characterization of the mechanism of action for abemaciclib with antiestrogen combined therapy in human breast cancer cell lines. Cancer Res., 2016, 76(Suppl 14), Abstract 2836.
Dickler, M.; Tolaney, S.; Rugo, H.; Cortes, J.; Diéras, V.; Patt, D.A.; Wildiers, H.; Frenzel, M.; Koustenis, A.; Baselga, J. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J. Clin. Oncol., 2016, 34(Suppl), Abstract 510 (Oral presentation).
DeMichele, A.; Clark, A.; Heitjan, D.; Randolph, S.; Gallagher, M.; Lal, P.; Feldman, M.D.; Zhang, P.J.; Schnader, A.; Zafman, K.; Domchek, S.M.; Gogineni, K.; Keefe, S.M.; Fox, K.R.; O’Dwyer, P.J. A phase II trial of an oral CDK 4/6 inhibitor, PD0332991, in advanced breast cancer. J. Clin. Oncol., 2013, 31(Suppl), Abstract 519.
Dickler, M.; Tolaney, S.; Rugo, H.; Cortes, J.; Diéras, V.; Patt, D.A.; Wildiers, H.; Frenzel, M.; Koustenis, A.; Baselga, J. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J. Clin. Oncol., 2016, 34(Suppl), Abstract 510 (Oral presentation).
Dickler, M.; Tolaney, S.; Rugo, H.; Cortes, J.; Diéras, V.; Patt, D.A.; Wildiers, H.; Frenzel, M.; Koustenis, A.; Baselga, J. MONARCH 1: Results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J. Clin. Oncol., 2016, 34(Suppl), Abstract 510 (Oral presentation).
Ma, C.X.; Gao, F.; Northfelt, D.; Goetz, M.; Forero, A.; Naughton, M.; Ademuyiwa, F.; Suresh, R.; Anderson, K.S.; Margenthaler, J.; Aft, R.; Hobday, T.; Moynihan, T.; Gillanders, W.; Cyr, A.; Eberlein, T.J.; Hieken, T.; Krontiras, H.; Hoog, J.; Han, J.; Guo, Z.; Vij, K.; Mardis, E.; Al-Kateb, H.; Sanati, S.; Ellis, M.J. A phase II trial of neoadjuvant palbociclib, a cyclin-dependent kinase (CDK) 4/6 inhibitor, in combination with anastrozole for clinical stage 2 or 3 estrogen receptor positive HER2 negative (ER+HER2-) breast cancer (BC). Cancer Res., 2016, 76, Abstract S6-05 (Oral presentation).
Tolaney, S.M.; Beeram, M.; Beck, J.T.; Conlin A.K.; Dees, E.C.; Dickler, M.N. et al. A phase Ib study of abemaciclib with therapies for metastatic breast cancer. J. Clin. Oncol., 2015, 33(Suppl), Abstract 522.
Zheng, J.; Amantea, M.; Wang, D. Effect of palbociclib concentration on heart rate-corrected QT interval in patients with cancer. Cancer Res., 2015, 75(Suppl 9), Abstract P5-19-16.
CONFLICT OF INTEREST
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. SLS has no conflict of interest. KLB reports research grants to her institution from Novartis and consulting fees from Novartis, Pfizer and Lilly during the conduct of the study. DLT has received speaking engagement honorarium from Tesaro.
REFERENCES
- 1.International Agency for Research on Cancer 2012 http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- 2.American Cancer Society Cancer facts & figures. 2016 http://www.cancer.org/research/cancerfactsstatistics/index
- 3.Hosford S.R., Miller T.W. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharm. Genomics Pers. Med. 2014;7:203–215. doi: 10.2147/PGPM.S52762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborne C., Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu. Rev. Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platet N., Cathiard A., Gleizes M., Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit. Rev. Oncol. Hematol. 2004;51(1):55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.De Marchi T., Foekens J.A., Umar A., Martens J.W. Endocrine therapy resistance in estrogen receptor (ER)-positive breast cancer. Drug Discov. Today. 2016;21(7):1181–1188. doi: 10.1016/j.drudis.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Rugo H.S., Rumble R., Macrae E., Barton D., Connolly H., Dickler M., Fallowfield L., Fowble B., Ingle J.N., Jahanzeb M., Johnston S.R., Korde L.A., Khatcheressian J.L., Mehta R.S., Muss H.B., Burstein H.J. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016;34(25):3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology. 2016 http://www.nccn.org
- 9.Cardoso F., Costa A., Norton L., Senkus E., Aapro M., Andre F., Barrios C.H., Bergh J., Biganzoli L., Blackwell K.L., Cardoso M.J., Cufer T., El Saghir N., Fallowfield L., Fenech D., Francis P., Gelmon K., Giordano S.H., Gligorov J., Goldhirsch A., Harbeck N., Houssami N., Hudis C., Kaufman B., Krop I., Kyriakides S., Lin U.N., Mayer M., Merjaver S.D., Nordström E.B., Pagani O., Partridge A., Penault-Llorca F., Piccart M.J., Rugo H., Sledge G., Thomssen C., Van’t Veer L., Vorobiof D., Vrieling C., West N., Xu B., Winer E., European School of Oncology European Society of Medical Oncology. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489–502. doi: 10.1016/j.breast.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 10.García-Becerra R., Santos N., Díaz L., Camacho J. Mechanisms of resistance to endocrine therapy in breast cancer: Focus on signaling pathways, miRNAs and genetically based resistance. Int. J. Mol. Sci. 2013;14(1):108–145. doi: 10.3390/ijms14010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukas J., Bartkova J., Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D–cyclin-dependent kinase–pRb–controlled G1 checkpoint. Mol. Cell. Biol. 1996;16(12):6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finn R., Martin M., Rugo H., Jones S., Im S., Gelmon K., Harbeck N., Lipatov O.N., Walshe J.M., Moulder S.L., Gauthier E.R., Lu D., Randolph S., Diéras V., Slamon D.J. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., Iwata H., Harbeck N., Zhang K., Theall K.P., Jiang Y., Bartlett C.H., Koehler M., Slamon D. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi G., Stemmer S., Burris H., Yap Y., Sonke G., Paluch-Shimon S., Campone M., Blackwell K.L., André F., Winer E.P., Janni W., Verma S., Conte P., Arteaga C.L., Cameron D.A., Petrakova K., Hart L.L., Villanueva C., Chan A., Jakobsen E., Nusch A., Burdaeva O., Grischke E.M., Alba E., Wist E., Marschner N., Favret A.M., Yardley D., Bachelot T., Tseng L.M., Blau S., Xuan F., Souami F., Miller M., Germa C., Hirawat S., O’Shaughnessy J. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 15.Malumbres M., Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 16.Williams G.H., Stoeber K. The cell cycle and cancer. J. Pathol. 2012;226(2):352–364. doi: 10.1002/path.3022. [DOI] [PubMed] [Google Scholar]
- 17.Johnson A., Skotheim J.M. Start and the restriction point. Curr. Opin. Cell Biol. 2013;25(6):717–723. doi: 10.1016/j.ceb.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacinti C., Giordano A. RB and cell cycle progression. Oncogene. 2006;25(38):5220–5227. doi: 10.1038/sj.onc.1209615. [DOI] [PubMed] [Google Scholar]
- 19.Caldon C.E., Daly R.J., Sutherland R.L., Musgrove E.A. Cell cycle control in breast cancer cells. J. Cell. Biochem. 2006;97(2):261–274. doi: 10.1002/jcb.20690. [DOI] [PubMed] [Google Scholar]
- 20.Lange C.A., Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocr. Relat. Cancer. 2011;18(4):C19–C24. doi: 10.1530/ERC-11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi Y.L., Anders L. Signaling through cyclin D-dependent kinases. Oncogene. 2014;33(15):1890–1903. doi: 10.1038/onc.2013.137. [DOI] [PubMed] [Google Scholar]
- 22.Musgrove E.A., Caldon C.E., Barraclough J., Stone A., Sutherland R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer. 2011;11(8):558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 23.Knudsen E.S., Wang J.Y. Targeting the RB-pathway in cancer therapy. Clin. Cancer Res. 2010;16(4):1094–1099. doi: 10.1158/1078-0432.CCR-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C.X., Ellis M.J. The Cancer Genome Atlas: clinical applications for breast cancer. Oncology. 2013;27(12):1263–1269. [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geradts J., Wilson P.A. High frequency of aberrant p16(INK4A) expression in human breast cancer. Am. J. Pathol. 1996;149(1):15–20. [PMC free article] [PubMed] [Google Scholar]
- 27.Thangavel C., Dean J.L., Ertel A., Knudsen K.E., Aldaz C.M., Witkiewicz A.K., Clarke R., Knudsen E.S. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr. Relat. Cancer. 2011;18(3):333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller T.W., Balko J.M., Fox E.M., Ghazoui Z., Dunbier A., Anderson H., Dowsett M., Jiang A., Smith R.A., Maira S.M., Manning H.C., González-Angulo A.M., Mills G.B., Higham C., Chanthaphaychith S., Kuba M.G., Miller W.R., Shyr Y., Arteaga C.L. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1(4):338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadoo K.A., Gucalp A., Traina T.A. Palbociclib: an evidence-based review of its potential in the treatment of breast cancer. Breast Cancer (Dove Med. Press) 2014;6:123–133. doi: 10.2147/BCTT.S46725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry D.W., Harvey P.J., Keller P.R., Elliott W.L., Meade M., Trachet E., Albassam M., Zheng X., Leopold W.R., Pryer N.K., Toogood P.L. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 2004;3(11):1427–1438. [PubMed] [Google Scholar]
- 31.Gelbert L.M., Cai S., Lin X., Sanchez-Martinez C., Del Prado M., Lallena M.J., Torres R., Ajamie R.T., Wishart G.N., Flack R.S., Neubauer B.L., Young J., Chan E.M., Iversen P., Cronier D., Kreklau E., de Dios A. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/ independent anti-tumor activities alone/in combination with gemcitabine. Invest. New Drugs. 2014;32(5):825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asghar U., Witkiewicz A.K., Turner N.C., Knudsen E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015;14(2):130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton E., Infante J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Infante J., Cassier P., Gerecitano J., Witteveen P., Chugh R., Ribrag V., Chakraborty A., Matano A., Dobson J.R., Crystal A.S., Parasuraman S., Shapiro G.I. A phase I study of the cyclin-dependent kinase 4/6 inhibitor ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2016;22(23):5696–5705. doi: 10.1158/1078-0432.CCR-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMichele A., Clark A.S., Tan K.S., Heitjan D.F., Gramlich K., Gallagher M., Lal P., Feldman M., Zhang P., Colameco C., Lewis D., Langer M., Goodman N., Domchek S., Gogineni K., Rosen M., Fox K., O’Dwyer P. CDK4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin. Cancer Res. 2015;21(5):995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- 36.Scheicher R., Hoelbl-Kovacic A., Bellutti F., Tigan A.S., Prchal-Murphy M., Heller G., Schneckenleithner C., Salazar-Roa M., Zöchbauer-Müller S., Zuber J., Malumbres M., Kollmann K., Sexl V. CDK6 as a key regulator of hematopoietic and leukemic stem cell activation. Blood. 2015;125(1):90–101. doi: 10.1182/blood-2014-06-584417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidula N., Rugo H.S. Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin. Breast Cancer. 2016;16(1):8–17. doi: 10.1016/j.clbc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 38. http://labeling. pfizer.com/ShowLabeling.aspx?id=2191
- 39.Patnaik A., Rosen L., Tolaney S., Tolcher A., Goldman J., Gandhi L., Papadopoulos K.P., Beeram M., Rasco D.W., Hilton J.F., Nasir A., Beckmann R.P., Schade A.E., Fulford A.D., Nguyen T.S., Martinez R., Kulanthaivel P., Li L.Q., Frenzel M., Cronier D.M., Chan E.M., Flaherty K.T. Wen PY3, Shapiro GI7. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6(7):740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz G.K., LoRusso P.M., Dickson M.A., Randolph S.S., Shaik M.N., Wilner K.D., Courtney R., O’Dwyer P.J. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br. J. Cancer. 2011;104(12):1862–1868. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flaherty K.T., Lorusso P.M., Demichele A., Abramson V.G., Courtney R., Randolph S.S., Shaik M., Wilner K.D., O’Dwyer P.J., Schwartz G.K. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin. Cancer Res. 2012;18(2):568–576. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 42.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02630693
- 43.Clinicaltrials.gov https:// clinicaltrials.gov/ct2/show/NCT02732119
- 44.Finn R.S., Dering J., Conklin D., Kalous O., Cohen D.J., Desai A.J., Ginther C., Atefi M., Chen I., Fowst C., Los G., Slamon D.J. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02792725
- 46.Gampenrider S., Rinnerthaler G., Greil R. CDK4/6 inhibition in luminal breast cancer. Memo. 2016;9:76–81. doi: 10.1007/s12254-016-0268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalvai M., Bystricky K. Cell cycle and anti-estrogen effects synergize to regulate cell proliferation and ER target gene expression. PLoS One. 2010;5(6):e11011. doi: 10.1371/journal.pone.0011011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finn R.S., Crown J.P., Lang I., Boer K., Bondarenko I.M., Kulyk S.O., Ettl J., Patel R., Pinter T., Schmidt M., Shparyk Y., Thummala A.R., Voytko N.L., Fowst C., Huang X., Kim S.T., Randolph S., Slamon D.J. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 49. https://www.novartis.com/news/media-releases/novartis-kisqalir-ribociclib-lee011-receives-fda-approval-first-line-treatment
- 50. https://investor.lilly.com/releasedetail.cfm?releaseid=935735
- 51.Finn R.S., Aleshin A., Slamon D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18(1):17. doi: 10.1186/s13058-015-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartsch R., Fitzal F., Hubalek M., Knauer M., Untch M. The role of CDK4/6 inhibitors in breast cancer treatment. Breast Care (Basel) 2015;10:340–343. [Google Scholar]
- 53.Verma S., Bartlett C., Schnell P., DeMichele A., Loi S., Ro J., Colleoni M., Iwata H., Harbeck N., Cristofanilli M., Zhang K., Thiele A., Turner N.C., Rugo H.S. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21(10):1165–1175. doi: 10.1634/theoncologist.2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fontanella C., Bolzonello S., Lederer B., Aprile G. Management of breast cancer patients with chemotherapy-induced neutropenia or febrile neutropenia. Breast Care (Basel) 2014;9(4):239–245. doi: 10.1159/000366466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Naurois J., Novitzky-Basso I., Gill M., Marti F., Cullen M.H., Roila F., ESMO Guidelines Working Group Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2010;21(Suppl. 5):v252–v256. doi: 10.1093/annonc/mdq196. [DOI] [PubMed] [Google Scholar]
- 56.Hu W., Sung T., Jessen B., Thibault S., Finkelstein M., Khan N.K., Sacaan A.I. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin. Cancer Res. 2015;22(8):2000–2008. doi: 10.1158/1078-0432.CCR-15-1421. [DOI] [PubMed] [Google Scholar]
- 57.Johnson S., Torrice C., Bell J., Monahan K., Jiang Q., Wang Y., Ramsey M.R., Jin J., Wong K.K., Su L., Zhou D., Sharpless N.E. Mitigation of hematologic radiation toxicitiy in mice through pharmacological quiescence induced by CDK4/6 inhibition. J. Clin. Invest. 2010;120(7):2528–2536. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Comprehensive Cancer Network 2016 http://www.nccn.org
- 59.American Cancer Society Infections in People With Cancer. http://www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/infectionsinpeoplewithcancer/infections inpeoplewithcancer/
- 60.Benson A.B., Ajani J., Catalano R., Engelking C., Kornblau S., Martenson J., McCallum R., Mitchell E.P., O’Dorisio T.M., Vokes E.E., Wadler S. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 2004;22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 61.Maroun J., Anthony L., Blais N., Burkes R., Dowden S., Dranitsaris G., Samson B., Shah A., Thirlwell M.P., Vincent M.D., Wong R. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr. Oncol. 2007;14:13–20. doi: 10.3747/co.2007.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vuppalanchi R., Saxena R., Maria A., Storniolo V., Chalasani N. Pseudocirrhosis and liver failure in patients with metastatic breast cancer after treatment with palbociclib. Hepatology. 2016;65(5):1762–1764. doi: 10.1002/hep.28720. [DOI] [PubMed] [Google Scholar]
- 63.Björnsson E. Review article: Drug-induced liver injury in clinical practice. Aliment. Pharmacol. Ther. 2010;32(1):3–13. doi: 10.1111/j.1365-2036.2010.04320.x. [DOI] [PubMed] [Google Scholar]
- 64.Mayo Clinic Toxic Hepatitis. http://www.mayoclinic.org/diseases-conditions/toxic-hepatitis/basics/symptoms/con-20026939
- 65.Khorana A., Francis C., Culakova E., Kuderer N., Lyman G. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007;5(3):632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 66.National Heart, Lung, and Blood Institute What is pulmonary embolism? https://www.nhlbi.nih.gov/health/health-topics/topics/
- 67.Limbrey R., Howard L. Developments in the management and treatment of pulmonary embolism. Eur. Respir. Rev. 2015;24(137):484–497. doi: 10.1183/16000617.00006614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curigliano G., Gómez Pardo P., Meric-Bernstam F., Conte P., Lolkema M., Beck J., Bardia A., Martínez García M., Penault-Llorca F., Dhuria S., Tang Z., Solovieff N., Miller M., Di Tomaso E., Hurvitz S.A. Ribociclib plus letrozole in early breast cancer: A presurgical, window-of-opportunity study. Breast. 2016;28:191–198. doi: 10.1016/j.breast.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 69.National Heart, Lung, and Blood Institute What causes long QT syndrome? https://www.nhlbi.nih.gov/health/health-topics/topics/qt
- 70.Basch E., Deal A., Kris M., Scher H., Hudis C., Sabbatini P., Rogak L., Bennett A.V., Dueck A.C., Atkinson T.M., Chou J.F., Dulko D., Sit L., Barz A., Novotny P., Fruscione M., Sloan J.A., Schrag D. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J. Clin. Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Semple D. Nursing strategies for patients on oral chemotherapy. Oncology. 2001;15(Suppl. 2):37–39. [PubMed] [Google Scholar]
- 72.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT022974
- 73.Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT01740427
- 74.ClinicalTrials.gov https:// clinicaltrials. gov/ct2/show/NCT01942135
- 75.ClinicalTrials.gov https://clinicaltrials. gov/ct2/ show/
- 76.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01958021
- 77.ClinicalTrials.gov https://clinicaltrials.gov/ ct2/show/NCT02278120
- 78.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT0242-26
- 79.ClinicalTrials.gov https://clinicaltrials.gov/ ct2/show/NCT02246621
- 80.ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02107703
- 81. https://investor.lilly.com/releasedetail.cfm?Release
- 82.American Cancer Society Caring for the patient with cancer at home: a guide for patients and families. http://www.cancer.org/ acs/groups/cid/documents/webcontent/002818-pdf.pdf
- 83.Al-Khatib S.M., LaPointe N., Kramer J., Califf R. What clinicians should know about the QT interval. JAMA. 2003;289(16):2120–2127. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 84.Kaplowitz N. Drug-induced liver injury. Clin. Infect. Dis. 2004;38:S44–S48. doi: 10.1086/381446. [DOI] [PubMed] [Google Scholar]