Abstract
Introduction:
There have been attempts to alter the prognosis of severe spinal cord injury in different centers, but none of which have reliably altered the outcome. Some trials use stem cells (SCs) that produced widely differing results. We hereby add our experience in our center of a surgical reconstruction of the damaged spinal cord using a mixture of SCs and Platelet-Rich Protein (PRP) with fibrin coated as a biological scaffold.
Materials and Methods:
Four cases of severely damaged spinal cord have been operated for neurolysis and reconstruction of the spinal cord using SCs and platelet-rich protein (PRP) with fibrin coated harvested from the peripheral circulation of the patient. PRP serves to maintain the position of the SCs. One milliliter suspension contains an average of 2.8 × 106 of autologous hematopoietic SCs. Patients were intraoperatively monitored by somatosensory evoked potential, motor evoked potentials, and delta wave. They are clinically followed postoperatively and electromyogram was repeated every 2 weeks. Magnetic resonance imaging (MRI) was repeated regularly. The patients are followed up for a period between 2 and 3 years.
Results:
One patient demonstrated motor and objective sensory improvement (P = 0.05), two other patients reported subjective sensory improvement, and the fourth one remained without any improvement (P = 0.1). None of these patients demonstrated any sign of deterioration or complication either on the surgery or on implanting of the SCs. MRI clearly proved that the inserted biological scaffold remained in place of reconstruction.
Conclusion:
SCs may play a role in restoring spinal cord functions. However, the unsolved problems of the use of SCs and related ethical issues should be addressed.
Keywords: Biological scaffold, hematopoietic autologous stem cells, plasma-rich protein, spinal cord injury
Introduction
The prognosis for patients with severe spinal cord damage is unfavorable. There have been many attempts to alter this prognosis with trials from different centers, but none of which have reliably altered the outcome.[1,2,3,4,5,6,7,8,9] In the past few years, it has been found that transplantation of stem cells (SCs) from a variety of sources plays a positive part in the revival of neural tissue in cases of spinal cord injury (SCI).[10,11,12] In SCI, the main purpose of applying SC transplantation is to restore impaired neuronal tissue and achieve better neurological function.[65,66] The effectiveness of SC therapy in the context of animal experiments has received great recognitions.[1,14,15,16,17] The safety of this therapy has also been partially ascertained.[4] The fact that in few clinical trials,[5,6,13] neurological function was observed to be partially restored, has generated optimism about its role in SCI. Based on current research conducted on the mechanism of SC transplantation, there are indications that transplanted cells have survivability in the injured area, and they ramify into different forms of nerve cells, including neurons, astrocytes, and oligodendrocytes.[7,18] Furthermore, they also play a part in the revival of axons and remyelination,[19,20] neovascularization,[21,22,23] neurotrophic impact[15,24,25,26,27] as well as regulation of local inflammation and directed migration of endogenous neural SCs.[28] It has been proven in research studies[8,10,12,29,30,31,32,33,34,35,36,37] that autologous bone marrow mesenchymal SCs (BM-MSCs) therapy in the case of traumatic SCI sufferers is safe in the long term. Unlike embryonic SCs, it is not ethically contentious to use autologous BM-MSCs. Moreover, given its autologous source, there is no cause for concern about immune rejection.[6] Our study also involved the use of autologous SC but derived from the peripheral circulation rather than directly from BM. We used autologous hematopoietic SCs (HSCs) and PRP to avoid any possible alloimmune or sensitization reactions to the patient which might occur after usage of SCs or PRP from different sources. Besides, easy harvesting of autologous SCs will facilitate the application of this spinal cord repair method to many candidate patients. In addition, from our experience, the HSCs are multipotent cells which have the potential capacity to differentiate into many other types of cells. This latter function of HSCs might help in our opinion in regeneration and repair of the damaged spinal cord cells.
We have performed a clinical trial with the purpose of establishing how safe and potentially effective the autologous HSCs transplantation is. In this, we employed platelet-rich protein (PRP)[39,40] as a biological scaffold to hold the SCs in situ. PRP is a bioaggregate that is composed of growth factors, including transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and basic fibroblast growth factor (bFGF).
Clinical trial design
A team was formed at King Fahd University Hospital, Al Khobar, Saudi Arabia, to investigate the study of the cases of severe SCI and the treatment option of using SCs.
The team is composed of neurosurgeons, a neurophysiologist and neurologist, SCs specialist, and independent team (internal medicine) to closely examine and monitor the patient's progress before and after surgery. A protocol was written and submitted to the hospital administration for approval which was obtained on November 26, 2012.
The decision was made, not to propose the treatment to any patient, but to consider the cases who request this type of treatment.
Patient selection
Each case was discussed within the treating team and with the hospital administration for approval.
Inclusion criteria
Age: <60 years old
Gender: Both male and female
Clinical evidence of severe SCI: The patient may be quadriplegic or paraplegic, with loss of sphincter control, and a clear sensory level. The patient must have exhausted every available methods of treatment with no improvement
Radiological evidence of the injury: Well documented by magnetic resonance imaging (MRI)
Spontaneous recovery: The patient's condition did not show any significant improvement since the trauma
Comorbidities: No concomitant systemic disease
The patient and his/her family should request the surgery and sign informed consent and attend several meetings to discuss this option of treatment.
Exclusion criteria
Evidence for clinical (motor and sensory) and/or electromyography (EMG) improvement in spinal cord functions since the original onset of SCI
Minimal neurological impairment
Major impairment of respiratory, cardiac, hepatic, or other systemic functions
Bed sores.
Four patients in the trial included 3 males and 1 female, aged 21–51 years, treated in between the period of February–December 2013, and were followed up for 2–3 years. The period since their injury ranged from 4 to 180 months. Neurological levels of enrolled patients were between C6 and T12.
Ethical considerations
To follow the ethical code, the following steps were taken:
The protocol to start the project of SCs therapy for severely injured spinal cord patients was submitted to the hospital board for approval. The approval was obtained for each case
All of the patients enrolled in the study requested the treatment. No one was offered the treatment without requesting it
Potential cases were studied carefully, and the selected cases were submitted case-by-case to the hospital authorities for approval
Meetings were conducted between the treating team and every patient and their families to explain all the facts related to this method of treatment
The patient or a family member signed a special consent to show that they understand that the method was experimental and they accepted the consequences or side effects due to the procedure
On the night before surgery, the head of the team (the first author of this paper) went to the patient to ask him/her if he would like to change his/her opinion and cancel the case.
Surgical techniques, isolation, preparation, and transplantation of stem cells
Isolation
After signing informed consent, G-CSF (Neupogen®-FDA approved) was given to the patient as a daily subcutaneous injection (10 μg/kg body weight) for 5 days to increase the SCs number in the blood stream. It stimulates the BM to produce a large number of hematopoietic and progenitor SCs and mobilizes them into the peripheral blood stream afterward. At the 5th day after treatment (day of the operation), blood extraction was done and the mononuclear cell-rich leukapheresis product was extracted out of the sample using COBE® Spectra Apheresis System MNC which used continuous flow centrifugal technology. Extraction of CD34+ cells (hematopoietic progenitor cells) collected from peripheral blood cells was carried out using a Haemonetics MCS + cell separator machine and the final product of SCs was about 60 ml in volume. A trained nurse was present for taking care of the patients during the procedure to ensure their comfort and safety. The SCs were collected while the patients were in the operation room just immediately before spinal cord repair surgery.
The bag of collected SCs was left for 4 h to settle down. The buffy coat rich in SCs was obvious in the interface between plasma and red blood cells. Twenty milliliters of the supernatant plasma was withdrawn and discarded to further enrich the percentage of SCs in the final product bag. After proper mixing, 4 ml of the final SCs product was withdrawn using a sterile syringe.
Preparation
The mainstay of this study is the preparation of platelet-rich protein (PRP) and coated fibrin to be used at the site of SCI with the SCs. Its purpose is to provide a three-dimensional (3D) scaffold capable of giving structural support and releasing endogenous growth factors which may enhance the function of the SCs and more importantly, to permanently patch the defected area. PRP is a bioaggregate that is composed of growth factors, including TGF-β, PDGF, VEGF, IGF, and bFGF.[36,47] It was prepared by extracting 20 ml of autologous peripheral blood from the third opened vein. It was transported to a Vivostat Machine (which is commercially available) for double centrifugation to separate the PRP aliquot from platelet-poor plasma and red blood cells. Afterward, PRP was prepared with fibrinogen to form a fibrin gel by adding calcium and thrombin. PRP was prepared in OR by aspirating 4 ml blood and processed in FACSCalibur flow cytometry machine (Atlanta, USA) to take 2.8 × 106 in 1 ml and placed in the injured spine.
Flow cytometric CD34+ HSC enumeration
Viable peripheral blood CD34+ cell counts were performed using a single-platform method [Figure 3] using a FACSCalibur flow cytometer (BD, San Jose, CA). In brief, two samples were withdrawn from the final SCs’ product bag. One sample was used for a complete blood count and the second one for immunophenotyping of the CD34+ cell count. A volume of 100 μL of SCs’ bag was incubated with 10 μL of CD45 FITC (clone J33; Immunotech, Marseille, France), 10 μL of CD34 PE (Immunotech clone 581), and 10 μL of 7AAD (Immunotech) for 15 min at room temperature in the dark. Red cells were lysed with ammonium chloride (Immunotech) for 10 min. An equal volume of well-mixed Flow-Count fluorospheres (Beckman Coulter, Fullerton, CA, USA) of known concentration was added, and data were acquired on the flow cytometer without washing. The collection bag CD34+ cell counts were calculated and reported. The CD-45dim (total leukocyte count) of the product bag was 68.1 × 103/μL. The CD34+ cell count was 0.48% of the total leukocyte count.
Figure 3.
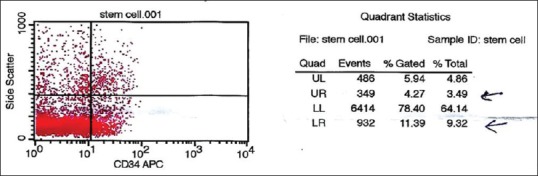
Number of successfully obtained stem cells 2.8 × 106/ml
A 1 ml of SC products was implanted into the spinal cord lesion; afterward, it contained an average 2.8 × 106 CD34+ SC. According to our knowledge, there is no published consensus to date on the optimum dose of CD34+ SCs to be used for repair of the spinal cord injuries. However, in our trial, we adjusted the CD34+ cells’ concentration to 2.8 × 106/ml at the final HSC product bag. In our hands, the length and size of SCI lesions allowed for implanting only 1 ml of the SCs’ product, followed by the addition of autologous PRP.
Transplantation
In the operating room, there were 6 teams working simultaneously and in coordination:
Team to harvest SCs
Team to prepare autologous fibrin sealant and platelet-rich protein
Anesthesia team
Well-trained nurse and technician team
Intraoperative neurophysiological monitoring team
The neurosurgical team.
Under general anesthesia, the patients were positioned in prone position and the level of severely damaged spinal cord was identified. A standard limited one-level laminectomy was performed at the selected level of interest. The dura was opened under microscope. The injured area of spinal cord was carefully dissected and meticulous neurolysis was performed. Most of scar tissues were removed and the injured part of the spinal cord was reconstructed to conform, as closely as possible, to normal shape of spinal cord. The prepared SCs in operating room were introduced to the central canal (1 ml contains 2.8 × 106 cells in average, the number of cells varied from case to case). After that, 1 ml of prepared PRP was sprayed over the SCs in the central canal to form a scaffold to keep the SCs functioning in that area.[1] The spray technique was used to cover the whole operating area and the SCs in 3D fashion. The dura was closed and the wound was closed in layers. Patients who had not been intubated before surgery were extubated immediately after the surgery and transferred to the regular ward.
Intraoperative neuromonitor
To monitor the structural integrity and function of the spinal cord during the procedure of transplantation, somatosensory evoked potentials were used because of their capability of being used under general anesthesia. Motor potentials were evoked with transcranial electrical stimulation of the motor cortex of the brain. Electrical stimulation was then performed with rectangular constant current impulses of 500 μs duration and intensities between 15 and 200 mA. Individual stimuli elicit D-waves [Figure 9], which can be recorded directly from the spinal cord caudal to the site of surgery. Motor evoked potentials can be recorded and detected from either spinal cord or muscles. The potential was recorded from the caudal spinal cord through an epidural electrode and consists of a bi- or tri-phasic sharp discharge (direct or D-wave), followed by a series of polyphasic waves (indirect or I-wave).
Figure 9.
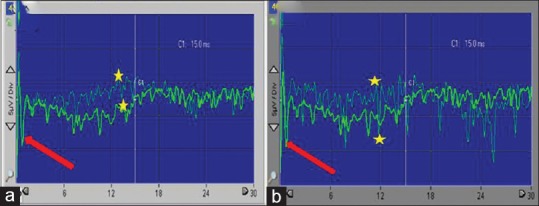
D-wave recorded from the spinal epidural space intraoperative. Notice the baseline (thick red arrows). Notice the D-wave amplitude differences (yellow stars). (a) Before stem cell implantation. (b) After stem cell implantation
Illustrative cases
Case 1
A 21-year-old male was a victim of road traffic accident (RTA). On admission, he exhibited paradoxical breathing, hypotension, low pulse rate, and low oxygen saturation. There was no evidence of head injury, pupils were normal, Glasgow Coma Scale was 15/15, cranial nerves were intact, and computed tomography (CT) brain was normal. He exhibited no movement in his lower limbs, and the power grade was assessed as 0. In the upper limbs, the only movement he was able to achieve was in the shoulders assessed as power grade 3. The sensory level was above the nipples. CT and MRI of cervical spine demonstrated ptosis of C6 over C7 with possible cord transection. Figure 1a–e showed preoperative MRI (sagittal and axial cuts) showing severe spinal cord damaged, indicated by arrow at the level of C6–C7.
Figure 1.
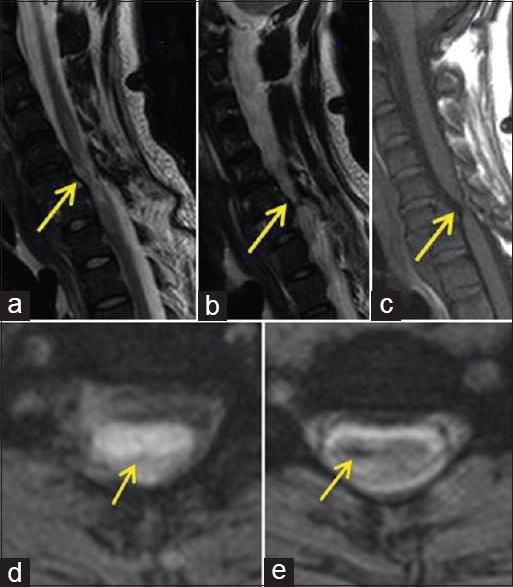
(a-e) Preoperative magnetic resonance imaging of Case 1 (sagittal and axial cuts) showed severe spinal cord damaged, which was indicated by arrow at the level of C6–C7
Two days after admission, the patient underwent anterior decompression and fixation of C6–C7 by plates and screws. Following the operation, there was no evidence of improvement and the patient remained intubated and quadriplegic, receiving a tracheostomy 15 days later. After a further 10 months, with no improvement, the patient underwent reconstruction of the spinal cord with SC and PRP. Figure 2a–c showed intraoperative reconstruction of the spinal cord at level C6–C7 and administration of SCs and PRP forming biological scaffold (1 ml contains 2.8 × 106 Cells) as seen in Figure 3.
Figure 2.
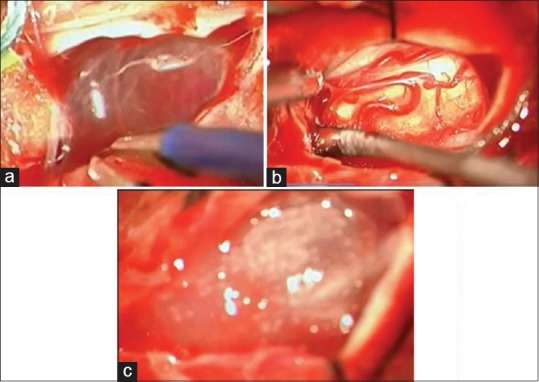
(a) Intraoperative view showed subdural thick scars and arachnoid cyst. (b) The spinal cord is reconstructed after the dissection. (c) The platelet-rich fibrin + stem cells in place at the end of surgery
Follow-up
The patient tolerated the procedure well. Six days following surgery, he was extubated and was breathing spontaneously for the first time since the RTA. Ten days later, he began to talk. Elbows, hands, and left lower limb gradually gained sensation. Movement was evident in both upper limbs. The patient reported sensation in both lower limbs 20 days postoperatively but inconsistent in the right lower limb. At 15 days further, movement was evident the left leg. Three months postsurgery, he was able to move both upper limbs and the left lower limb and gained pin-prick (PP) sensation in his upper limbs, body, and left lower limb. Sensation in the right lower limb was left inconsistent. Cervical MRI showed the biological scaffold remained in place, Figure 4.
Figure 4.
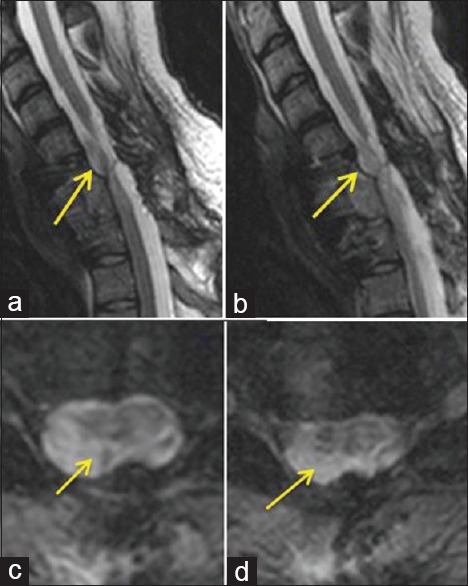
(a-d) Three-month postoperative showed the reconstruction of the spinal cord and the biological PRP scaffold in place (indicated by arrows)
Attempts for assisted standing at 6 months were unsuccessful. However, at 10 months, he succeeded and has progressed to being able to take a few steps.
Electromyogram (EMG) follow-up showed improvement both in motor and sensory functions.
Case 2
A 51-year-old woman presented with a 15-year history of dense paraplegia and uncontrolled sphincter, following RTA. Spinal cord transection at level D7–D8 and D11–D12 was demonstrated by physical examination and MRI. Figure 5a–c showed transection of the spinal cord at level D10-D11. The patient was operated and scar tissues was removed and the pia was scrapped out, dissected away and the spinal cord was reconstructed. 2.8x106 /ml of autologous hematopoietic stem cells were prepared and injected in the reconstructed central canal, Figure 6. PRP were injected over the stem cells to keep the cells in place in the reconstructed spinal canal Figure 7.
Figure 5.
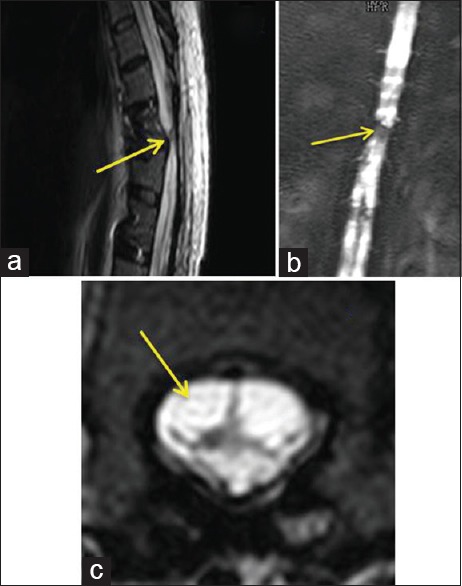
(a) Magnetic resonance imaging - sagittal cuts of the dorsal spine of case 2 showed transection of the spinal cord at the level of D10–D11. (b) Magnetic resonance imaging myelogram showed spinal cord transaction at the level of D10–D11. (c) Magnetic resonance imaging - axial cuts of D10 demonstrated severely damaged and transected spinal cord
Figure 6.
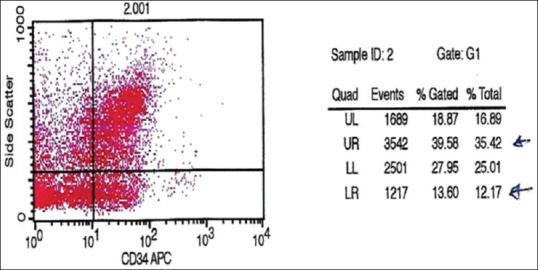
Number of cells 2.8 × 106/ml (cytometric analysis)
Figure 7.
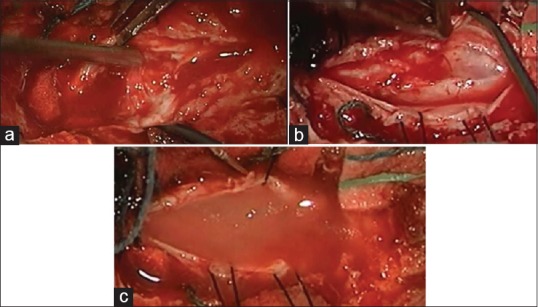
(a) Scars and scattered pieces of the spinal cord as seen under surgical microscope at the beginning of surgery. (b) Reconstruction of the spinal cord. (c) At the end of surgery, PRP (biological scaffold) + stem cells in place, inside, and surrounds the damaged part of the spinal cord
Follow-up
The patient tolerated the procedure very well. Two weeks later, the patient stated that she started to feel some sensation in the lower limbs, which is not convincing. Twelve months postoperatively, no movement was seen; PP and touch, vibration, and position sensation were not consistent. Despite the clinical findings, the patient said that she could feel that she had legs for the first time in 15 years. EMG follow-up showed no marked changes following surgery. Figure 8 showed the biological scaffold remained in place.
Figure 8.
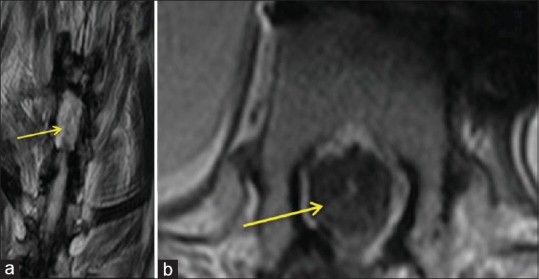
(a) Postoperative magnetic resonance imaging cuts showed reconstruction of the spinal cord and PRP scaffold + stem cells 6 months later after surgery. (b) Postoperative magnetic resonance imaging cuts showed reconstruction of the spinal cord and PRP scaffold + stem cells 6 months later after surgery (axial cuts)
Statistical evaluation
Data are presented as mean ± standard deviation values. To compare data, we evaluated for statistical significance using two-way ANOVA with a post hoc Tukey t-test. In all analyses, a P < 0.05 was considered statistically significant.
Results
Flow cytometric analysis
Two samples were withdrawn from the final SCs’ product bag. One sample was used for a complete blood count and the second one for immunophenotyping of the CD34+ cell count. A FACSCalibur flow cytometry machine (Atlanta, USA) was used to assess the number of the CD34+ count in relation to the CD-45dim count. The CD-45dim (total leukocyte count) of the product bag was 68.1 × 103/μl. The CD34+ cell count was 0.48% of the total leukocyte count. Regarding the 4 ml of SC products implanted into the spinal cord lesion afterward, it contained an average total of 2.8 × 106 CD34+ SCs with a concentration of approximately 700 × 103/ml.
Clinical outcome
The follow-up of these patients revealed several facts:
None of the patients developed any complication of the surgery or use of SCs for over 2–3 years. The patient's condition never deteriorated in any means
All patients showed a significant improvement in their psychological status as they felt that they have tried all that is possible and had learned to accept their status
Only one patient, the youngest one operated within 10 months showed marked motor and sensory improving
One patient claimed gaining of sensation
Two patients did not show any improvement at all
Delta waves seemed to be very important intraoperative monitor
PRP proved to be very successful to keep SCs in the chosen place and functioned as biological scaffold avoiding possible complications and side effects of using artificial scaffolds.
Table 1 American Spinal Injury Association scores of the cases involved before the intervention can be shown. In the treatment group, changes in ASIA Impairment Scale grade were observed in three patients with an improvement from A to B in two patients and from A to C in one patient as shown in the Table 2 at 6 months period.
Table 1.
American Spinal Injury Association scores of the cases involved before the intervention
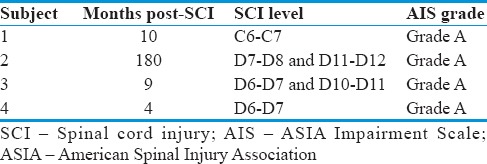
Table 2.
Comparison of American Spinal Injury Association scores before and after the intervention (6 months period)

Electrophysiological findings
Intraoperative neurophysiologic monitoring (IONM) was used to detect early neurophysiological deterioration before being irreversible. It is uncommon to use IONM to detect early improvement in neurophysiological function; however, in our case, we did. We detected obvious changes in the amplitude of D-waves before and after SC implantation as can be shown in Figure 9. There was an approximately 50% increase in amplitude of the D-wave after SC implantation and closing the spinal cord dura.
Discussion
Although laboratory results for the potential use of SCs in differing CNS problems seemed to be promising, patient trials at different centers have produced widely differing results.[1,65] There are serious scientific and ethical issues faced when transferring such successful laboratory experiments to clinical patient care. These problems include no general consensus about the use of SCs; no agreement about the number of cells that should be used; the method to obtain SCs varies greatly from center to center; the method of implantation or use is not agreed upon; the ideal time for use and the outcome of the techniques. Therefore, it is very important to report and record every trial and make the details and results of such trials available for world neuroscience researchers and neurosurgeons.
We present a novel method for transplanting SCs into severely damaged spinal cords. The concepts of our methods are careful and meticulous microdissection of the injured spinal area to remove any scar or necrotic tissue; use of the remaining, possibly functioning parts of the damaged spinal cord to reconstruct a spinal cord; use of hematopoietic autologous SCs, simultaneously with autologous platelet-rich protein (PRP) and fibrin sealant, both prepared on site, to possibly restore the functions of the spinal cord.
PRP has been used recently for treatment of diabetic foot and improves fat grafting, plastic surgery, dental surgery, and other fields.[39,40,41,42,43,44,45,46]
As far as we know, we are the first to use PRP and fibrin sealant along with autologous SCs to treat severe SCI. The principles of use are for reconstruction of the spinal cord and to function as autologous biological 3-D scaffold to contain and maintain the implanted SCs. The fibrin sealant and PRP may enhance the functions of SCs as PRP has the capability to release endogenous growth factors.[44,45,46] Platelet-rich protein is rich in growth factor including TGF-β, PDGF, VEGF, IGF, and bFGF.[40,44,46] PRP is considered as a bioaggregate of growth factors.[39,40]
A SCI is complex, involving different kinds of damage to different types of cells.[47] The environment of the spinal cord changes drastically during the first few weeks after injury (immune cells flow in, toxic substances are released, a scar is formed). A combination of therapies is needed, acting at the appropriate time and on the correct targets.[67,68] Studies in animals have shown that a transplantation of SCs or stem cell-derived cells may contribute to spinal cord repair by replacing the nerve cells that have died as a result of the injury; generating new supporting cells that will reform the insulating nerve sheath (myelin) and act as a bridge across the injury to stimulate regrowth of damaged axons; protecting the cells at the injury site from further damage by releasing protective substances such as growth factors and soaking up toxins such as free radicals, when introduced into the spinal cord shortly after injury; and preventing spread of the injury by suppressing the damaging inflammation that can occur after injury.[69]
Neuroscientists worldwide have not reached a consensus to answer dozens of questions on the use of SCs in clinical practice. We feel that our study is able to answer some of these questions, specifically.
The method to obtain active stem cells
Pluripotent SCs from BM or peripheral blood, the latter SCs have been used in our current study, may have therapeutic promise for SCI.[48,49] Although still debated,[31] these particular adult SCs have been shown to differentiate into bone, fat, tendon, and cartilage cells.[50] It has been published that these cells can also transdifferentiate in vitro into liver,[51] skeletal,[28,52] and cardiac muscle[22,45] cells and into central nervous system cells.[12,53,54,55] Many medical fields are exploring MSCs, for instance, for repair of the heart after myocardial infarction,[56,57] osteogenesis imperfecta in orthopedics,[58,59] organogenesis in internal medicine,[60,61] intervertebral disk disease in neurosurgery,[62,63] and stroke/neurodegenerative diseases in neurology.[54,64]
How to control and direct stem cells to function in the right place?
Combining the implant with PRP and a fibrin patch produces the desired result (fixing the SC in place) and function as biological scaffold.
How to monitor the procedure intraoperatively?
IONM is used to detect early neurophysiological deterioration before being irreversible. It is uncommon to use IONM to detect early improvement in neurophysiological function; however, in our case, we did it. We detected obvious changes in the amplitude of D-waves before and after SC implantation [Figure 9]. There was approximately 50% increase in the D-wave amplitude after SC implantation and closing the spinal cord dura.
What is the suggested technique for implantation?
Our study demonstrates that resection of necrotic tissue at the site with direct implantation can produce a successful outcome. Of the four patients enrolled in the study, one showed marked improvement following the procedure.
Many questions remained unanswered in our study. These include the number of cells required (dose); how to enhance the differentiation of SCs into neural cells; how to cross the scar and promote penetration and communication with the healthy parts of the spinal cord; how to avoid the immunological rejection of SCs; what is the timescale for achieving optimum results; how to best monitor the patient postoperatively; and what are the possible complications and side effects.
Ethical dilemma
Addressing and discussing the ethical use of SCs in the treatment of paraplegic or quadriplegic patients due to severe SCI are not simple. It involves many issues to be covered and addressed, including the perspective of the patient, the patient's family, the neurosurgeon, and the neuroscientist. Media coverage of SC use and possible litigation arising from it should also be addressed.
The basic and fundamental elements of bioethics are autonomy, beneficence, nonmaleficence, justice, dignity, truthfulness, and honesty. All these elements should be considered when discussing the ethical dilemma of using SCs in the management of severely injured spinal cord patients.
Conclusion
Our experience steps forward in the long road for proper use of SCs transplant.
Achieved points:
Ability to mobilize SCs and obtain large number of cells from the peripheral circulation of the same patient
Ability to patch using PRP as a biological scaffold to reconstruct (anatomical reconstruction) the severely damaged spinal cord at different level
Ability to keep the SCs functioning in the chosen site
Clinical results still premature to judge; however, no harm whatever was inflected of any patient.
There are several issues need to be solved such as: 1. To prove that SCs may restore the functions of damaged spinal cord. 2. What is the optimum number of cells needed to restore the functions. 3. To learn about the course of recovery after severe spinal cord injury. 4. The value and precision of intra operative monitors and post operative monitors.
We recommend multicenter cooperation and collaboration to solve the previous mentioned problems. Focused meetings between neurosurgeons and neuroscientists should be encouraged and welcomed to answer the above-mentioned questions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Guest JD, Hiester ED, Bunge RP. Demyelination and schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–93. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 3.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 4.Deda H, Inci MC, Kürekçi AE, Kayihan K, Ozgün E, Ustünsoy GE, et al. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy. 2008;10:565–74. doi: 10.1080/14653240802241797. [DOI] [PubMed] [Google Scholar]
- 5.Curt A, Van Hedel HJ, Klaus D, Dietz V EM-SCI Study Group. Recovery from a spinal cord injury: Significance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25:677–85. doi: 10.1089/neu.2007.0468. [DOI] [PubMed] [Google Scholar]
- 6.Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, et al. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transplant. 2008;17:1277–93. doi: 10.3727/096368908787648074. [DOI] [PubMed] [Google Scholar]
- 7.Tator CH. Review of treatment trials in human spinal cord injury: Issues, difficulties, and recommendations. Neurosurgery. 2006;59:957–82. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
- 8.Moviglia GA, Varela G, Brizuela JA, Moviglia Brandolino MT, Farina P, Etchegaray G, et al. Case report on the clinical results of a combined cellular therapy for chronic spinal cord injured patients. Spinal Cord. 2009;47:499–503. doi: 10.1038/sc.2008.164. [DOI] [PubMed] [Google Scholar]
- 9.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 10.Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15:675–87. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 11.Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114:935–9. doi: 10.1016/j.clineuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy. 2009;11:897–911. doi: 10.3109/14653240903253857. [DOI] [PubMed] [Google Scholar]
- 13.Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV. Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. 2011;25:516–22. doi: 10.3109/02688697.2010.550658. [DOI] [PubMed] [Google Scholar]
- 14.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann Acad Sci. 2001;938:221–9. doi: 10.1111/j.1749-6632.2001.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 15.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–19. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 16.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–82. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–26. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 18.Alberti E, Los M, García R, Fraga JL, Serrano T, Hernández E, et al. Prolonged survival and expression of neural markers by bone marrow-derived stem cells transplanted into brain lesions. Med Sci Monit. 2009;15:BR47–54. [PubMed] [Google Scholar]
- 19.Peru RL, Mandrycky N, Nait-Oumesmar B, Lu QR. Paving the axonal highway: From stem cells to myelin repair. Stem Cell Rev. 2008;4:304–18. doi: 10.1007/s12015-008-9043-z. [DOI] [PubMed] [Google Scholar]
- 20.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–23. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;41(Suppl 1):94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oz Oyar E, Kardes O, Korkmaz A, Omeroglu S. Effects of vascular endothelial growth factor on ischemic spinal cord injury caused by aortic cross-clamping in rabbits. J Surg Res. 2009;151:94–9. doi: 10.1016/j.jss.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki H, Ishikawa M, Tanaka N, Nakanishi K, Kamei N, Asahara T, et al. Administration of human peripheral blood-derived CD133+ cells accelerates functional recovery in a rat spinal cord injury model. Spine (Phila Pa 1976) 2009;34:249–54. doi: 10.1097/BRS.0b013e3181913cde. [DOI] [PubMed] [Google Scholar]
- 24.Alexanian AR, Maiman DJ, Kurpad SN, Gennarelli TA. In vitro and in vivo characterization of neurally modified mesenchymal stem cells induced by epigenetic modifiers and neural stem cell environment. Stem Cells Dev. 2008;17:1123–30. doi: 10.1089/scd.2007.0212. [DOI] [PubMed] [Google Scholar]
- 25.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, et al. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–38. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xuan AG, Long DH, Gu HG, Yang DD, Hong LP, Leng SL. BDNF improves the effects of neural stem cells on the rat model of alzheimer's disease with unilateral lesion of fimbria-fornix. Neurosci Lett. 2008;440:331–5. doi: 10.1016/j.neulet.2008.05.107. [DOI] [PubMed] [Google Scholar]
- 27.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–41. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 28.Walker TL, White A, Black DM, Wallace RH, Sah P, Bartlett PF. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J Neurosci. 2008;28:5240–7. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 30.Carlson KB, Singh P, Feaster MM, Ramnarain A, Pavlides C, Chen ZL, et al. Mesenchymal stem cells facilitate axon sorting, myelination, and functional recovery in paralyzed mice deficient in Schwann cell-derived laminin. Glia. 2011;59:267–77. doi: 10.1002/glia.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. doi: 10.1126/science.297.5585.1299. [DOI] [PubMed] [Google Scholar]
- 32.Cho KJ, Trzaska KA, Greco SJ, McArdle J, Wang FS, Ye JH, et al. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1 alpha. Stem Cells. 2005;23:383–91. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- 33.Corti S, Locatelli F, Donadoni C, Strazzer S, Salani S, Del Bo R, et al. Neuroectodermal and microglial differentiation of bone marrow cells in the mouse spinal cord and sensory ganglia. J Neurosci Res. 2002;70:721–33. doi: 10.1002/jnr.10455. [DOI] [PubMed] [Google Scholar]
- 34.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–27. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 35.Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H, et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010;24:702–8. doi: 10.1177/1545968310369801. [DOI] [PubMed] [Google Scholar]
- 36.Kitchel SH, Wang MY, Lauryssen CL. Techniques for aspirating bone marrow for use in spinal surgery. Neurosurgery. 2005;57(4 Suppl):286–9. doi: 10.1227/01.neu.0000176412.17360.95. [DOI] [PubMed] [Google Scholar]
- 37.Lennon DP, Haynesworth SE, Young RG, Dennis JE, Caplan AI. A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow-derived mesenchymal stem cells. Exp Cell Res. 1995;219:211–22. doi: 10.1006/excr.1995.1221. [DOI] [PubMed] [Google Scholar]
- 38.Richardson SM, Curran JM, Chen R, Vaughan-Thomas A, Hunt JA, Freemont AJ, et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27:4069–78. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Modarressi A. Platlet rich plasma (PRP) improves fat grafting outcomes. World J Plast Surg. 2013;2:6–13. [PMC free article] [PubMed] [Google Scholar]
- 40.Picard F, Hersant B, Bosc R, Meningaud JP. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: A review and a proposal for a new standard care. Wound Repair Regen. 2015;23:638–43. doi: 10.1111/wrr.12317. [DOI] [PubMed] [Google Scholar]
- 41.McDonald JW, Becker D, Holekamp TF, Howard M, Liu S, Lu A, et al. Repair of the injured spinal cord and the potential of embryonic stem cell transplantation. J Neurotrauma. 2004;21:383–93. doi: 10.1089/089771504323004539. [DOI] [PubMed] [Google Scholar]
- 42.Del Fabbro M, Lolato A, Bucchi C, Taschieri S, Weinstein RL. Autologous platelet concentrates for pulp and dentin regeneration: A literature review of animal studies. J Endod. 2016;42:250–7. doi: 10.1016/j.joen.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Elnehrawy NY, Ibrahim ZA, Eltoukhy AM, Nagy HM. Assessment of the efficacy and safety of single platelet-rich plasma injection on different types and grades of facial wrinkles. J Cosmet Dermatol. 2017;16:103–11. doi: 10.1111/jocd.12258. [DOI] [PubMed] [Google Scholar]
- 44.Stambolsky C, Rodríguez-Benítez S, Gutiérrez-Pérez JL, Torres-Lagares D, Martín-González J, Segura-Egea JJ. Histologic characterization of regenerated tissues after pulp revascularization of immature dog teeth with apical periodontitis using tri-antibiotic paste and platelet-rich plasma. Arch Oral Biol. 2016;71:122–8. doi: 10.1016/j.archoralbio.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, et al. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2015;10:CD008548. doi: 10.1002/14651858.CD008548.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;10:CD006899. doi: 10.1002/14651858.CD006899.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nandoe Tewarie RD, Hurtado A, Levi AD, Grotenhuis JA, Oudega M. Bone marrow stromal cells for repair of the spinal cord: Towards clinical application. Cell Transplant. 2006;15:563–77. doi: 10.3727/000000006783981602. [DOI] [PubMed] [Google Scholar]
- 49.Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24:835–45. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- 50.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 51.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–70. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 53.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro . J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 55.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 56.Chamberlain JR, Schwarze U, Wang PR, Hirata RK, Hankenson KD, Pace JM, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 57.Olsson F, Denham M, Cole TJ, Hooper SB, Mollard R. Deriving respiratory cell types from stem cells. Curr Stem Cell Res Ther. 2007;2:197–208. doi: 10.2174/157488807781696203. [DOI] [PubMed] [Google Scholar]
- 58.Masaka T, Miyazaki M, Du G, Hardjo M, Sakaguchi M, Takaishi M, et al. Derivation of hepato-pancreatic intermediate progenitor cells from a clonal mesenchymal stem cell line of rat bone marrow origin. Int J Mol Med. 2008;22:447–52. [PubMed] [Google Scholar]
- 59.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al. Transplantation of mesenchymal stem cells embedded in atelocollagen gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–41. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 60.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–45. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 61.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: Potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) 2005;30:2379–87. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 62.Helm GA, Gazit Z. Future uses of mesenchymal stem cells in spine surgery. Neurosurg Focus. 2005;19:E13. doi: 10.3171/foc.2005.19.6.14. [DOI] [PubMed] [Google Scholar]
- 63.Lee J, Kuroda S, Shichinohe H, Ikeda J, Seki T, Hida K, et al. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology. 2003;23:169–80. doi: 10.1046/j.1440-1789.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 64.Sugaya K. Possible use of autologous stem cell therapies for Alzheimer's disease. Curr Alzheimer Res. 2005;2:367–76. doi: 10.2174/1567205054367919. [DOI] [PubMed] [Google Scholar]
- 65.Khazaei M, Ahuja CS, Fehlings MG. Induced pluripotent stem cells for traumatic spinal cord injury. Front Cell Dev Biol. 2017;4:152. doi: 10.3389/fcell.2016.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang YH, Chen J, Zhou J, Nong F, Lv JH, Liu J. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. 2017;13:203–7. doi: 10.3892/etm.2016.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: A comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013;185:485–92. doi: 10.1503/cmaj.121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014;13:1241–56. doi: 10.1016/S1474-4422(14)70144-9. [DOI] [PubMed] [Google Scholar]


