Abstract
The role of neutrophil and lymphocyte counts in blood as prognosis predictors in Community Acquired Pneumonia (CAP) has not been adequately studied. This was a derivation-validation retrospective study in hospitalized patients with CAP and no prior immunosuppression. We evaluated by multivariate analysis the association between neutrophil and lymphocyte counts and mortality risk at 30-days post hospital admission in these patients. The derivation cohort (n = 1550 patients) was recruited in a multi-site study. The validation cohort (n = 2846 patients) was recruited in a single-site study. In the derivation cohort, a sub-group of lymphopenic patients, those with < 724 lymphocytes/mm3, showed a 1.93-fold increment in the risk of mortality, independently of the CURB-65 score, critical illness, and receiving an appropriate antibiotic treatment. In the validation cohort, patients with < 724 lymphocytes/mm3 showed a 1.86-fold increment in the risk of mortality. The addition of 1 point to the CURB-65 score in those patients with < 724 lymphocytes/mm3 improved the performance of this score to identify non-survivors in both cohorts. In conclusion, lymphopenic CAP constitutes a particular immunological phenotype of the disease which is associated with an increased risk of mortality. Assessing lymphocyte counts could contribute to personalized clinical management in CAP.
Keywords: Community, Acquired, Pneumonia, Lymphocyte, Mortality
Highlights
-
•
Lymphopenia is a frequent finding in hospitalized patients with CAP, affecting approximately 50% of the patients.
-
•
Lymphopenic CAP (L-CAP) with < 724 lymphocytes/mm3 is associated with an increase in the risk of mortality at 30 days.
-
•
Assessing lymphocyte counts at hospital admission could contribute to personalized clinical management and treatment in CAP.
Our study reveals the existence of lymphopenia (< 1000 lymphocytes/mm3 in blood) in about half of the patients hospitalized with CAP. Our work identified a subgroup of patients with lymphopenia (those with < 724 lymphocytes/mm3) which constituted a particular immunological phenotype of the disease associated with higher mortality risk. This group could be identified at an early stage using a simple leukogram, a test readily available in hospital laboratories worldwide. In turn, these patients could potentially benefit from specific interventions of prompt aggressive treatment or also of adjuvant therapies with immunomodulators which stimulate lymphocytes.
1. Introduction
Community-acquired pneumonia (CAP) is a serious health problem causing high morbidity and mortality worldwide (Mandell et al., 2007). The rates of patients hospitalized due to CAP are increasing, with 22%–42% of adults needing admission to the hospital. In addition, CAP has an associated mortality of 5%–14% in patients requiring hospitalization. Approximately 5% of the patients hospitalized with CAP require admission to an intensive care unit (ICU). In these severe cases mortality rises to 35% (Lim et al., 2009).
Host individual variability is now recognized as a key factor influencing clinical expression and prognosis of CAP (Leoni and Rello, 2017). Learning from the successes of using precision medicine in the treatment of cancer, identifying individual phenotypes associated with poor outcome in CAP could help to better understand the pathogenesis of this disease and to improve its treatment (Rello and Perez, 2016; Prina et al., 2016; Aliberti et al., 2014). In this regard, Davenport et al., using a transcriptomic analysis, found a gene expression signature (SRS1) which identifies individuals with an immunosuppressed phenotype and higher 14-day mortality within a cohort of patients with sepsis secondary to CAP (Davenport et al., 2016). In addition, new biomarkers such as expression of HLA-DR on monocytes (Zhuang et al., 2015), soluble CD14 (presepsin) (Klouche et al., 2016), interleukins (Menéndez et al., 2009; Andrijevic et al., 2014), procalcitonin (Liu et al., 2016a), and mid-regional pro-ADM (Liu et al., 2016b) could help risk assessment in the early stages of the disease. As an alternative to these new sophisticated (and expensive)“omics” and biomarker-based approaches, a simple leukogram has shown potential to be a tool for classifying patients with CAP or sepsis based on their outcomes. The neutrophil/lymphocyte ratio (NLR) has been proposed as a candidate predictor of mortality for hospitalized CAP patients (Cataudella et al., 2017). Further, we have demonstrated that patients with septic shock who fail to expand circulating neutrophil counts in their blood present an increased risk of mortality (Bermejo-Martín et al., 2014).
The objective of the present study was to evaluate the potential role of neutrophil and lymphocyte counts in blood as biomarkers of mortality risk in patients with CAP and no antecedents of immunosuppression. For this purpose, we performed a derivation-validation retrospective study employing two large cohorts of hospitalized patients with this disease.
2. Materials and Methods
2.1. Study Design
A derivation-validation retrospective study was performed to evaluate the association between neutrophil and lymphocyte counts at hospital admission and the risk of mortality at 30 days following admission, in patients hospitalized with CAP. The derivation cohort comprised patients recruited in the context of a multi-site study while those of the validation cohort were recruited in the context of a single-site study. Only those patients showing complete data for lymphocyte and neutrophil counts in the first 24 h following admission to the hospitaland the variables “admission to the ward or ICU”, and “mortality at 30 days post-admission from hospital” were included in the analysis.
2.2. Patient Selection, Inclusion and Exclusion Criteria
2.2.1. Multi-Site (Derivation) Cohort
The patients included in this cohort had been admitted to 14 Spanish hospitals (NEUMONAC group), other than the Hospital Clinic (Barcelona). Patients were recruited from January 2012 to June 2015. Inclusion criteria were the presence of (assumed) new pulmonary infiltrate shown by chest radiograph and respiratory signs and symptoms compatible with CAP (cough, expectoration, chest pain, dyspnea, fever, among others). Exclusion criteria were as follows: nursing-home patients and immunosuppression status (human immunodeficiency virus-positive, acute leukemias, myelodysplastic syndromes, myelodysplastic and myeloproliferative syndromes, chronic myeloproliferative syndromes, lymphoproliferative syndromes, monoclonal gammopathies, marrow failure syndromes, primary immunodeficiencies and severe chronic neutropenia, solid-organ transplantation, > 14 days of treatment with > 20 mg/day of prednisone or equivalent, and other immunosuppressive drugs).
2.2.2. Single-Site (Validation) Cohort
This comprised consecutive patients admitted to the Hospital Clinic, Barcelona, Spain, between January 2005 and December 2015 with a diagnosis of CAP. Pneumonia was defined as an (assumed) new pulmonary infiltrate found on the hospital admission chest radiograph and symptoms and signs of lower respiratory tract infection. Patients with prior immunosuppression were excluded (for example, patients with neutropenia after chemotherapy or bone marrow transplantation, patients with drug-induced immunosuppression as a result of solid-organ transplantation, corticosteroid (> 10 mg/day) or cytotoxic therapy, and patients with HIV-infection).
2.2.3. Other Definitions
In both studies, acute respiratory distress syndrome (ARDS) was identified in the first 24 h after hospital admission by applying the criteria described in the Berlin definition (ARDS Definition Task Force et al., 2012). The appropriateness of empiric antibiotic treatment was defined according to multidisciplinary guidelines for the management of CAP (Torres et al., 2013). Acute renal failure was defined using the criteria developed by the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group (Bellomo et al., 2004). Septic shock was defined according to the definition proposed by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (Bone et al., 1992).
2.3. Ethics
for the multi-site cohort, the Ethics Committee of the coordinating center approved the study (Code: 2011/0512). For the single-site cohort, the Ethics Committee of the Hospital Clinic of Barcelona approved the study (Code: 2009/5451). Given the observational and retrospective nature of the study, informed consent was waived. This study fulfils the standards indicated by the Declaration of Helsinki.
2.4. Leukocyte, Lymphocyte and Neutrophil Quantification
This measurement was performed on blood collected in ethylenediaminetetraacetic acid tubes by using the automatic analyzers available in each participating hospital at the central laboratories, under standard operative procedures approved for clinical use. Lymphopenia was considered as a total lymphocyte count < 1000/mm3, following the definition proposed in Hematology, 9th ed (Vasu S, Caligiuri MA, 2015).
2.5. Statistical Analysis (Supplementary file 1)
Differences in demographic and clinical characteristics between patient cohorts or sub-groups were assessed using the chi-squared test. The association between lymphocyte, neutrophil counts, C-reactive protein (CRP) levels, and the risk of mortality in the 30 days following hospital admission was evaluated by logistic regression analysis (Hosmer DW et al., 2013). Potential confounding variables were selected by assessing the association between those variables shown in Table 1 with mortality. Variables yielding a p value < 0.1 in the univariate regression analysis were further included in the multivariate analysis as adjusting variables. Final selection of the variables was performed by using the backward stepwise selection method (Likelihood Ratio) (pin < 0.05, pout < 0.10). Lymphocyte, neutrophil, and CRP concentrations were transformed to Naeperian log values in order to reach a normal distribution. Those variables showing a Spearman correlation coefficient > 0.3 with another inclusive variable were excluded from the multivariate analysis (Healey, 2014). The final number for each analysis (excluding the missing values) is shown in Table 1, Table 2, Table 3, Table 4, Table 5. The ability of lymphocyte counts to differentiate survivors from non-survivors was assessed by using the area under the receiver operating characteristic curve analysis (AUC). The cut-off for lymphocyte counts regarding mortality prediction was obtained in the derivation cohort by calculating the optimal operating point (OOP) in the AUC, as previously described (Almansa et al., 2017). The OOP was considered the value for which the point on the curve had the minimum distance to the upper left corner (where sensitivity = 1 and specificity = 1). By Pythagoras' theorem this distance is:
Table 1.
Clinical characteristics of the studied cohorts. Data presented as n (%) or median (interquartile range). Microbiological data were calculated using the number of patients with positive microbiological identification. COPD: Chronic Obstructive Pulmonary Disease; ICU: Intensive care unit, ARDS: Acute Respiratory Distress Syndrome.
| Characteristics | Multi-site (derivation) cohort N = 1550 |
Single-site (validation) cohort N = 2846 |
P value | |||
|---|---|---|---|---|---|---|
| N (%) | N for each variable | N (%) | N for each variable | |||
| Demographic data and treatment | Age (≥ 65 years) | 926 (59.7) | 1550 | 1948 (68.6) | 2838 | < 0.002 |
| Sex male | 962 (62.1) | 1550 | 1733 (60.9) | 2846 | ns | |
| Current smoking | 319 (21.9) | 1456 | 548 (19.5) | 2813 | ns | |
| Alcohol abuse | 131 (9.0) | 1451 | 382 (13.6) | 2805 | < 0.001 | |
| Pneumococcal vaccine | 162 (11.9) | 1352 | 467 (18.7) | 2496 | < 0.001 | |
| Influenza vaccine | 572 (40.1) | 1427 | 1204 (48.1) | 2502 | < 0.001 | |
| Oral steroids | 72 (4.6) | 1550 | 127 (4.5) | 2792 | ns | |
| Inhaled steroids | 364 (23.5) | 1550 | 567 (20.3) | 2788 | 0.015 | |
| Antibiotic last month | 384 (24.8) | 1550 | 663 (24.7) | 2686 | ns | |
| Comorbidity | Cardiac disease | 436 (28.3) | 1538 | 409 (14.5) | 2824 | < 0.001 |
| Chronic renal failure | 138 (9.0) | 1529 | 218 (7.7) | 2817 | ns | |
| Liver disease | 62 (4.1) | 1527 | 141 (5.0) | 2817 | ns | |
| Diabetes mellitus | 337 (22) | 1529 | 636 (22.7) | 2804 | ns | |
| Chronic pulmonary disease (COPD/asthma) | 421 (27.6) | 1525 | 1090 (39.3) | 2771 | < 0.001 | |
| Neurological disease | 254 (16.6) | 1526 | 574 (20.9) | 2750 | 0.001 | |
| Initial severity | Initial ICU admission | 131 (8.5) | 1550 | 586 (20.6) | 2846 | < 0.001 |
| CURB65 (≥ 3) | 219 (14.3) | 1534 | 488 (20.7) | 2355 | < 0.001 | |
| Microbiology | Known microbiology etiology | 702 (45.3) | 1550 | 1161 (40.8) | 2846 | 0.004 |
| Streptococcus pneumoniae | 435 (62.0) | 702 | 481 (41.4) | 1161 | < 0.001 | |
| Legionella pneumophila | 33 (4.7) | 702 | 61 (5.3) | 1161 | ns | |
| Polimicrobial etiology | 82 (11.7) | 702 | 153 (13.2) | 1161 | ns | |
| Virus | 95 (13.5) | 702 | 204 (17.6) | 1161 | 0.021 | |
| Complications and outcomes | Appropriate antibiotic | 1049 (85.8) | 1223 | 2313 (95.8) | 2414 | < 0.001 |
| Acute renal failure | 87 (5.6) | 1550 | 760 (27.2) | 2796 | < 0.001 | |
| Pleural effusion | 95 (6.1) | 1550 | 400 (14.4) | 2772 | < 0.001 | |
| Mechanical ventilation | 95 (6.3) | 1501 | 301 (13.1) | 2305 | < 0.001 | |
| ARDS | 56 (3.6) | 1550 | 123 (4.6) | 2667 | ns | |
| Septic shock | 65 (4.2) | 1550 | 158 (5.7) | 2789 | 0.034 | |
| Mortality at 30 days | 72 (4.6) | 1550 | 234 (8.2) | 2846 | < 0.001 | |
Table 2.
Univariate and multivariate regression analysis for mortality risk in the multi-site (derivation) cohort. OR: Odds Ratio; CI: confidence interval.
| Variable | Univariate |
Multivariate (N = 1209) |
||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p | OR | CI 95% | p | |
| Cardiac disease | 2.10 | 1.30–3.39 | 0.002 | – | – | – |
| Neurological disease | 2.60 | 1.55–4.36 | < 0.001 | – | – | – |
| Chronic renal failure | 3.41 | 1.92–6.06 | < 0.001 | 2.46 | 1.17–5.18 | 0.017 |
| CURB-65 ≥ 3 | 6.35 | 3.89–10.37 | < 0.001 | 4.13 | 2.23–7.65 | < 0.001 |
| Initial ICU admission | 3.39 | 1.88–6.09 | < 0.001 | 2.26 | 1.04–4.90 | 0.039 |
| Apropriate antibiotic treatment | 0.27 | 0.15–0.48 | < 0.001 | 0.26 | 0.14–0.49 | < 0.001 |
| Lymphocytes (cell/mm3) (Ln) | 0.57 | 0.42–0.78 | < 0.001 | 0.66 | 0.44–0.98 | 0.044 |
Table 3.
Univariate and Multivariate regression analysis for mortality risk in the single site (validation) cohort. OR: Odds Ratio; CI: confidence interval.
| Univariate |
Multivariate (N = 1878) |
|||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p | OR | CI 95% | p | |
| Smoking | < 0.001 | 0.059 | ||||
| No smoker | 1 | – | – | 1 | – | – |
| Smoker | 0.39 | 0.25–0.61 | < 0.001 | 0.47 | 0.25–0.88 | 0.019 |
| Ex-smoker | 0.69 | 0.51–0.94 | 0.017 | 0.80 | 0.53–1.21 | 0.29 |
| Cardiac disease | 1.45 | 1.02–2.04 | 0.038 | – | – | – |
| Chronic renal failure | 1.83 | 1.21–2.78 | 0.004 | – | – | – |
| Neurological disease | 3.64 | 2.75–4.82 | < 0.001 | 2.41 | 1.60–3.63 | < 0.001 |
| CURB-65 ≥ 3 | 3.42 | 2.51–4.66 | < 0.001 | 2.85 | 1.92–4.23 | < 0.001 |
| ARDS (complication) | 3.40 | 2.15–5.37 | < 0.001 | 3.29 | 1.78–6.09 | < 0.001 |
| Respiratory virus | 0.44 | 0.21–0.89 | 0.024 | – | – | – |
| Initial ICU admission | 1.54 | 1.14–2.09 | 0.005 | 1.91 | 1.22–2.99 | 0.005 |
| Appropriate antibiotic treatment | 0.34 | 0.20–0.57 | < 0.001 | 0.41 | 0.21–0.80 | 0.009 |
| Lymphocytes (cell/mm3) (Ln) | 0.92 | 0.86–0.98 | 0.008 | 0.89 | 0.81–0.98 | 0.023 |
Table 4.
Multivariate regression analysis to predict mortality risk in the multi-site (derivation) cohort. OR: Odds Ratio; CI: confidence interval.
| Variable | Multivariate analysis (N = 1209) |
||
|---|---|---|---|
| OR | CI 95% | p | |
| Chronic renal failure | 2.47 | 1.18–5.19 | 0.017 |
| CURB-65 ≥ 3 | 3.99 | 2.15–7.40 | 0.000 |
| Initial ICU admission | 2.22 | 1.03–4.80 | 0.043 |
| Apropriate antibiotic treatment | 0.27 | 0.14–0.50 | 0.000 |
| < 724 lymphocyte/mm3 | 1.93 | 1.06–3.51 | 0.031 |
Table 5.
Multivariate regression analysis to predict mortality risk in the single site (validation) cohort. OR: Odds Ratio; CI: confidence interval.
| Multivariate analysis (N = 1878) |
|||
|---|---|---|---|
| OR | CI 95% | p | |
| Smoking | 0.043 | ||
| No smoker | 1 | – | – |
| Smoker | 0.46 | 0.24–0.86 | 0.015 |
| Ex-smoker | 0.77 | 0.51–1.15 | 0.20 |
| Neurological disease | 2.36 | 1.57–3.54 | < 0.001 |
| CURB-65 ≥ 3 | 2.77 | 1.87–4.09 | < 0.001 |
| ARDS (complication) | 3.17 | 1.71–5.89 | < 0.001 |
| Initial ICU admission | 1.72 | 1.10–2.70 | 0.019 |
| Appropriate antibiotic treatment | 0.42 | 0.22–0.81 | 0.010 |
| < 724 lymphocytes/mm3 | 1.86 | 1.28–2.71 | 0.001 |
The ability of this cut-off value to predict 30-day mortality was further evaluated by using multivariate logistic regression analysis in the derivation cohort in a first step and in the validation cohort in a second step. Comparisons between the AUCs were assessed according to the method of DeLong et al. (DeLong et al., 1988). The level of significance was set at 0.05 (2-tailed). All analyses were performed with IBM SPSS Statistics 20.0 (Armonk, New York) and MedCalc 17.8.5 (Ostend, Belgium).
3. Results
3.1. Clinical Characteristics of the Patients (Table 1)
the derivation cohort comprised 1550 patients, and the validation cohort 2846. The proportion of patients older than 65 years was higher in the validation study. In turn, the proportion of those who had received vaccination against S. pneumoniae or influenza virus was higher in the validation study. Prevalence of chronic pulmonary disease and neurological disease was also higher in those patients recruited in the validation cohort. Therefore, the symptoms observed in this cohort were in turn more severe, as evidenced by the increased proportion of patients showing a CURB-65 score ≥ 3, of patients requiring admission to the ICU, and finally of patients showing renal failure, pleural effusion, septic shock, and mechanical ventilation as complications during hospitalization. In agreement with this scenario, the frequency of non-survivors in the validation cohort was almost 2-fold greater than that in the derivation cohort, although the majority of the patients in this cohort had received an appropriate antibiotic treatment. Patients in the derivation cohort more frequently showed an antecedent of cardiac disease, and these patients also showed a higher proportion of pneumococcal CAP cases and a lower proportion of viral CAP within those patients with a positive microbiological identification. The mean length of hospitalization was 6 (4–9) days in the derivation study and 8 days (6–12) in the validation study (median, interquartile range). The percentage of patients showing lymphopenia in each cohort was the same, 52% (818 of 1550 patients in the derivation study and 1499 patients of 2846 patients in the validation study). In the derivation study and validation study, respectively, leukocyte counts were similar in both cohorts, median (interquartile range): leucocytes (cells/mm3): 13,000 (9200–17,790) vs 12,300 (8400–17,300); neutrophils (cells/mm3): 10,823 (7274–15,187) vs 10,126 (6636–14,700); and lymphocytes (cells/mm3): 957 (616–1445) vs 950 (578–1440).
3.2. Analysis of Mortality Risk in the 30 days Following Hospital Admission
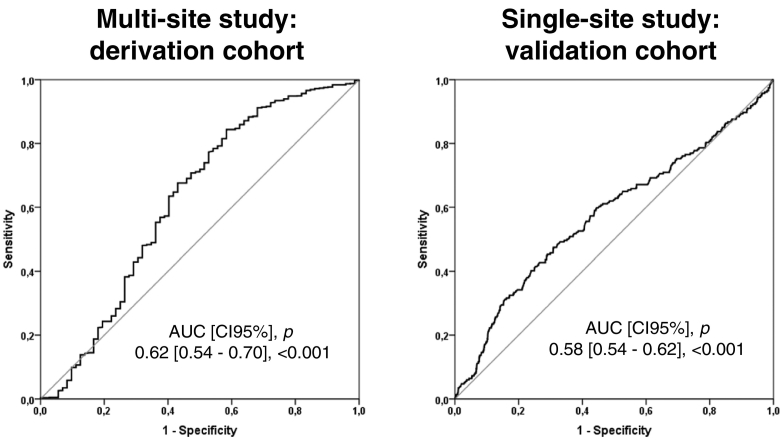
Neutrophil counts were not associated with 30-day mortality in the univariate analysis, neither in the derivation cohort (OR [95%CI], p: 0.75 [0.52–1.09], 0.135), nor in the validation cohort (0.95 [0.89–1.02], 0.170). The same was true for CRP (OR [95%CI], p: 1.13 [0.94–1.36], 0.194 in the derivation cohort, and in the validation cohort (1.02 [0.88–1.17], 0.800). In consequence, these parameters were not evaluated in the multivariate analysis. In contrast, lymphocyte counts were a protective factor against 30-day mortality in both cohorts and in both the univariate and the multivariate analysis (Table 2, Table 3). AUC analysis demonstrated that lymphocyte counts were able to differentiate between survivors and non-survivors at 30 days, although the areas obtained were modest (Fig. 1).
Fig. 1.
AUROC Analysis of Lymphocyte Concentrations in Blood to Predict Survival at 30-Days Post-Admission.The OOP was identified in the derivation cohort as < 724 lymphocytes/mm3. Sensitivity/Specificity for the OOP were 57%/68% in the derivation cohort and 67%/50% in the validation cohort.
3.3. Derivation and Validation of the Lymphocyte Cut-Off Value to Predict 30-day Mortality
AUC analysis in the derivation cohort identified 724 lymphocytes/mm3 as the best cut-off value for identifying non-survivors (Fig. 1). Multivariate analysis demonstrated that presenting with lymphocyte counts below 724 lymphocytes/mm3constituted an independent risk factor of mortality in the derivation cohort (Table 4). The same result was found in the validation cohort (Table 5). In both cohorts, the ORs adjusted by their respective confounding factors were very similar (1.93 in the derivation cohort and 1.86 in the validation cohort).
3.4. Improvement of the Ability of the CURB-65 Score to Predict Mortality by Addition of Lymphocyte Counts
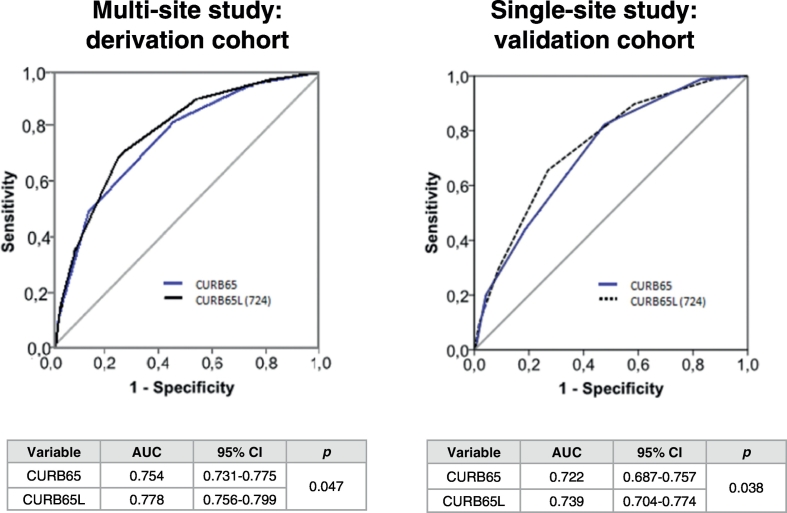
Those patients with lymphocyte counts below 724 lymphocytes/mm3 were given an extra point in the CURB-65 score, to build the “CURB-65L” score (“L” meaning “lymphocyte”). When an AUROC analysis was performed to compare the ability of CURB-65 to predict mortality against that of the new CURB-65L score, the latter yielded a significantly higher AUC in both the derivation and the validation cohorts, based on the results of the DeLong test (Fig. 2).
Fig. 2.
Comparison of AUCs of CURB-65 and CURB-65L to Predict 30-Day Mortality: One extra point was added to the CURB-65 score of those patients with lymphocyte counts below 724 cells/mm3 to build the CURB-65L.
4. Discussion
Our study revealed a surprising finding: 52.8% of the patients with CAP showed lymphopenia at hospital admission (< 1000 lymphocytes/mm3) (Vasu and Caligiuri, 2015), in the absence of antecedents of immunosuppression. Re-examining the data of another large cohort from Marrie and Wu, including 3043 hospitalized patients with CAP and no antecedents of immunosuppression (Marrie and Wu, 2005), a similar prevalence of lymphopenia was observed at hospital admission (48.4%). In our view, the high prevalence and relevance of lymphopenia in CAP has not been sufficiently considered by the medical community. Lymphopenia could be a cause or a consequence of CAP. Impaired production or increased apoptosis of lymphocytes caused by the presence of chronic diseases or critical illness (Marshall et al., 2008), enhanced adhesion to the vascular endothelium, or massive migration of these cells to the lungs could explain the presence of lymphopenia in some patients with this disease.
Interestingly, we identified a sub-group of lymphopenic patients, those with lymphocyte counts below 724 lymphocytes/mm3, which presented a significantly higher risk of 30-day mortality. This subgroup, which represented 33.5% of the patients in the derivation cohort and 35.6% in the validation cohort, accounted for an increased proportion of critically ill patients and of those who developed complications (Supplementary files 2 and 3).
In the work from Marrie and Wu, a lymphocyte count < 1000 cell/mm3 was associated with early but not with late mortality. Nonetheless, there are a number of important differences between this work and ours. The most important one is that Marrie and Wu excluded patients with leukocyte counts < 1000 cells/mm3 and those with critical illness. In addition, they did not evaluate cut-off values below 1000 lymphocytes/mm3. Finally, they studied hospital mortality, not mortality at 30-days post-admission as we did.
Early identification of patients at risk of death is a crucial step in the clinical management of CAP (Aliberti et al., 2014). In this regard, it was particularly interesting to identify the outcomes corresponding to < 724 lymphocytes/mm3. Our results demonstrate that the new CURB-65L score, which considers the presence of lymphopenia below 724 lymphocytes/mm3, outperformed the CURB-65 in the detection of non-survivors. This new CURB-65L score could help to recognize early those CAP patients at risk of a poor outcome. Prompt identification of these patients could contribute to an improvement of their prognosis, because they might benefit from more aggressive treatment strategies. In this regard, evaluation of lymphocyte counts is an inexpensive, widely available test in clinical settings worldwide.
Our results also suggest that lymphocyte counts should be taken into consideration as a potential confusion factor in the design and analysis of future clinical trials evaluating drugs or interventions for the treatment of CAP, because they influence prognosis. In addition, our findings suggest that patients with low lymphocyte counts could be in need of new therapeutic approaches, since the multivariate analysis demonstrated that the presence of low lymphocyte counts conferred an increased risk of mortality independently of receiving an appropriate antibiotic treatment and intensive care. Because of this, adjunctive therapy with drugs inducing expansion of lymphocyte counts or modulating the function of these cells (Han et al., 2015; Shindo et al., 2015; Shindo et al., 2017) could represent an option for these patients to be explored in future clinical trials.
Finally, our results showed that neutrophil counts were not associated with mortality risk in patients with CAP and no prior immunosupression, raising doubts for the potential role of drugs aimed to expand neutrophils in this kind of patients. In fact, a systematic review from Cheng et al. in 2007 concluded that the use of G-CSF as an adjunct to antibiotics was not associated with improved 28-day mortality (Cheng et al., 2007). Therefore, the absence of any significant association between neutrophil counts with mortality suggests that the potential value of the neutrophil to lymphocyte ratio as biomarker of mortality in CAP with no immunosupression should be carefully re-evaluated. Additionally, our work presents evidence that CRP is not a biomarker of 30-day mortality in these patients.
A limitation of our study was its retrospective nature. This precluded using potential confusion factors such as body mass index, nutritional intake markers (albumin or pre-albumin) as adjusting variables for lymphocyte counts in the multivariate analysis. Our study lacked data on lymphocyte subsets. The specific impact of CD4, CD8 T cells, B and NK lymphocytes on mortality should be studied in future prospective studies, as well as that of T-regulatory cells (a specific subset of CD4 + T cells with the role of suppressing potentially deleterious activities of T-helper cells). In addition, the identified cut-off values for total lymphocyte counts should be confirmed and validated in future prospective studies, comparing their prognostic efficacy against that of other emerging markers such as Proadrenomedullin.
In conclusion, lymphocyte counts are an independent biomarker of mortality in hospitalized patients with CAP and no immunosuppression. Patients with lymphopenic CAP (L-CAP) with lymphocyte counts < 724 lymphocytes/mm3 constitute a particular immunological phenotype of the disease involving one-third of the patients which is associated with a 2-fold increase in the risk of mortality at 30 days post hospital admission. Using the CURB-65L could help to better detect patients with poor outcomes. In consequence, assessing lymphocyte counts at hospital admission could contribute to personalized clinical management and treatment in CAP.
Funding Source
The study was funded by Ciber de Enfermedades Respiratorias (CibeRes CB06/06/0028), 2009 Support to Research Groups of Catalonia 911, IDIBAPS, “Convocatoria extraordinaria PII's-SEPAR 2011 ref: 1046/2011”, “Beca SEPAR 2012 ref:145/2012, and “Beca Sociedad valenciana de Neumologia (SVN) 2013”. Catia Cilloriz is a recipient of ERS Short Term Fellowship and Postdoctoral Grant “Strategic plan for research and innovation in health-PERIS 2016-2020”. Raquel Almansa and Jesús F Bermejo are supported by Consejería de Sanidad de Castilla y León-IECSCYL (EMER07050). The institutions supporting this work did not have any role in the in study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Confilcts of Interest
The authors declare no conflicts of interest regarding this submission.
Contributors
JFBM, AT, Rosario M conceived the study and contributed to drafting of the paper. AG, participated in the statistical analysis. CC contributed to the study concept and design and contributed to drafting of the paper. Raúl M participated in the statistical analysis and contributed to drafting of the paper. RA contributed to the study concept and design, participated in the statistical analysis and contributed to drafting of the paper. AC contributed to the study concept and design. The NEUMONAC group participated in the acquisition of data. All authors participated in the analysis or interpretation of data.
Researchers of the NEUMONAC group
Pedro Pablo España. (Hospital de Galdakao, Galdakao); Luis Borderías (Hospital San Jorge, Huesca); Olga Rajas (Hospital La Princesa, Madrid); Jordi Almirall (Hospital de Mataró, Mataró); Rafael Zalacaín (Hospital de Cruces, Bilbao); Montserrat Vendrell (Hospital Josep Trueta, Girona); Salvador Bello (Hospital Miguel Servet, Zaragoza); Isabel Mir (Hospital Son Llàtzer, Palma de Mallorca); Concepción Morales (Hospital Virgen de las Nieves, Granada); Luis Molinos (Hospital Universitario Central de Asturias, Oviedo); Ricard Ferrer (Hospital Mutua Terrasa, Terrasa); Mª Luisa Briones (Hospital Clínico Universitario, Valencia); Rosa Malo (Hospital Puerta de Hierro, Majadahonda).
Acknowledgments
Acknowledgements
We thank the nursing teams of the participant hospitals for their help with sample collection for leukogram analysis through the years.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.09.023.
Appendix A. Supplementary data
Supplementary material
References
- Aliberti S., Brambilla A.M., Chalmers J.D., Cilloniz C., Ramirez J., Bignamini A., Prina E., Polverino E., Tarsia P., Pesci A., Torres A., Blasi F., Cosentini R. Phenotyping community-acquired pneumonia according to the presence of acute respiratory failure and severe sepsis. Respir. Res. 2014;15:27. doi: 10.1186/1465-9921-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almansa R., Ortega A., Ávila-Alonso A., Heredia-Rodríguez M., Martín S., Benavides D., Martín-Fernandez M., Rico L., Aldecoa C., Rico J., López de Cenarruzabeitia I., Beltrán de Heredia J., Gomez-Sanchez E., Aragón M., Andrés C., Calvo D., Andaluz-Ojeda D., Liu P., Blanco-Antona F., Blanco L., Gómez-Herreras J.I., Tamayo E., Bermejo-Martin J.F. Quantification of immune dysregulation by next-generation polymerase chain reaction to improve sepsis diagnosis in surgical patients. Ann. Surg. 2017 doi: 10.1097/SLA.0000000000002406. [DOI] [PubMed] [Google Scholar]
- Andrijevic I., Matijasevic J., Andrijevic L., Kovacevic T., Zaric B. Interleukin-6 and procalcitonin as biomarkers in mortality prediction of hospitalized patients with community acquired pneumonia. Ann. Thorac. Med. 2014;9:162–167. doi: 10.4103/1817-1737.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Bellomo R., Ronco C., Kellum J.A., Mehta R.L., Palevsky P., Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care Lond. Engl. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo-Martín J.F., Tamayo E., Ruiz G., Andaluz-Ojeda D., Herrán-Monge R., Muriel-Bombín A., Fe Muñoz M., Heredia-Rodríguez M., Citores R., Gómez-Herreras J., Blanco J., EXPRESS (Expresión Génica en Sepsis) and GRECIA (Grupo de Estudios y Análisis en Cuidados Intensivos) groups Circulating neutrophil counts and mortality in septic shock. Crit. Care Lond. Engl. 2014;18:407. doi: 10.1186/cc13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone R.C., Balk R.A., Cerra F.B., Dellinger R.P., Fein A.M., Knaus W.A., Schein R.M., Sibbald W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Cataudella E., Giraffa C.M., Di Marca S., Pulvirenti A., Alaimo S., Pisano M., Terranova V., Corriere T., Ronsisvalle M.L., Di Quattro R., Stancanelli B., Giordano M., Vancheri C., Malatino L. Neutrophil-to-lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J. Am. Geriatr. Soc. 2017 doi: 10.1111/jgs.14894. [DOI] [PubMed] [Google Scholar]
- Cheng A.C., Stephens D.P., Currie B.J. Granulocyte-colony stimulating factor (G-CSF) as an adjunct to antibiotics in the treatment of pneumonia in adults. Cochrane Database Syst. Rev. 2007:CD004400. doi: 10.1002/14651858.CD004400.pub3. [DOI] [PubMed] [Google Scholar]
- Davenport E.E., Burnham K.L., Radhakrishnan J., Humburg P., Hutton P., Mills T.C., Rautanen A., Gordon A.C., Garrard C., Hill A.V.S., Hinds C.J., Knight J.C. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir. Med. 2016;4:259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Han D., Shang W., Wang G., Sun L., Zhang Y., Wen H., Xu L. Ulinastatin- and thymosin α1-based immunomodulatory strategy for sepsis: a meta-analysis. Int. Immunopharmacol. 2015;29:377–382. doi: 10.1016/j.intimp.2015.10.026. [DOI] [PubMed] [Google Scholar]
- Healey J.F. 10th ed. Cengage Learning; 2014. Statistics: A Tool for Social Research. [Google Scholar]
- Hosmer D.W., Lemeshow S., Sturdivant R.X. 3rd ed. Wiley; 2013. Applied Logistic Regression. [Google Scholar]
- Klouche K., Cristol J.P., Devin J., Gilles V., Kuster N., Larcher R., Amigues L., Corne P., Jonquet O., Dupuy A.M. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann. Intensive Care. 2016;6:59. doi: 10.1186/s13613-016-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni D., Rello J. Severe community-acquired pneumonia: optimal management. Curr. Opin. Infect. Dis. 2017;30:240–247. doi: 10.1097/QCO.0000000000000349. [DOI] [PubMed] [Google Scholar]
- Lim W.S., Baudouin S.V., George R.C., Hill A.T., Jamieson C., Le Jeune I., Macfarlane J.T., Read R.C., Roberts H.J., Levy M.L., Wani M., Woodhead M.A., Pneumonia Guidelines Committee of the BTS Standards of Care Committee BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl. 3) doi: 10.1136/thx.2009.121434. https://doi.org/10.1136/thx.2009.121434 (iii1-55) [DOI] [PubMed] [Google Scholar]
- Liu D., Su L.-X., Guan W., Xiao K., Xie L.-X. Prognostic value of procalcitonin in pneumonia: a systematic review and meta-analysis. Respirology. 2016;21:280–288. doi: 10.1111/resp.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Xie L., Zhao H., Liu X., Cao J. Prognostic value of mid-regional pro-adrenomedullin (MR-proADM) in patients with community-acquired pneumonia: a systematic review and meta-analysis. BMC Infect. Dis. 2016;16:232. doi: 10.1186/s12879-016-1566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell L.A., Wunderink R.G., Anzueto A., Bartlett J.G., Campbell G.D., Dean N.C., Dowell S.F., File T.M., Musher D.M., Niederman M.S., Torres A., Whitney C.G., Infectious Diseases Society of America, American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007;44(Suppl. 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T.J., Wu L. Factors influencing in-hospital mortality in community-acquired pneumonia: a prospective study of patients not initially admitted to the ICU. Chest. 2005;127:1260–1270. doi: 10.1016/S0012-3692(15)34475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.C., Charbonney E., Gonzalez P.D. The immune system in critical illness. Clin. Chest Med. 2008;29(605–616):vii. doi: 10.1016/j.ccm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Menéndez R., Martínez R., Reyes S., Mensa J., Filella X., Marcos M.A., Martínez A., Esquinas C., Ramirez P., Torres A. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64:587–591. doi: 10.1136/thx.2008.105312. [DOI] [PubMed] [Google Scholar]
- Prina E., Ceccato A., Torres A. New aspects in the management of pneumonia. Crit. Care Lond. Engl. 2016;20:267. doi: 10.1186/s13054-016-1442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rello J., Perez A. Precision medicine for the treatment of severe pneumonia in intensive care. Expert Rev. Respir. Med. 2016;10:297–316. doi: 10.1586/17476348.2016.1144477. [DOI] [PubMed] [Google Scholar]
- Shindo Y., Unsinger J., Burnham C.-A., Green J.M., Hotchkiss R.S. Interleukin-7 and anti-programmed cell death 1 antibody have differing effects to reverse sepsis-induced immunosuppression. Shock Augusta Ga. 2015;43:334–343. doi: 10.1097/SHK.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y., McDonough J.S., Chang K.C., Ramachandra M., Sasikumar P.G., Hotchkiss R.S. Anti-PD-L1 peptide improves survival in sepsis. J. Surg. Res. 2017;208:33–39. doi: 10.1016/j.jss.2016.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A., Barberán J., Falguera M., Menéndez R., Molina J., Olaechea P., Rodríguez A., Grupo de la Guía Multidisciplinar para el Manejo de la Neumonía Adquirida en la Comunidad Multidisciplinary guidelines for the management of community-acquired pneumonia. Med. Clin. (Barc.) 2013;140:223.e1–223.e19. doi: 10.1016/j.medcli.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Vasu S., Caligiuri M.A. McGraw-Hill Education; 2015. Lymphocytosis and Lymphocytopenia, in: Hematology. [Google Scholar]
- Zhuang Y., Li W., Wang H., Peng H., Chen Y., Zhang X., Chen Y., Gao C. Predicting the outcomes of subjects with severe community-acquired pneumonia using monocyte human leukocyte antigen-DR. Respir. Care. 2015;60:1635–1642. doi: 10.4187/respcare.03953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material