Abstract
Background
Not all patients with spinal cord compression due to cervical spondylotic myelopathy (CSM) have clinical symptoms and signs. The aim of this study was to investigate and compare the imaging findings in asymptomatic and symptomatic patients with CSM with mild to moderate cervical spinal cord compression.
Material/Methods
A retrospective clinical study included 68 patients. Group A (n=30) had no symptoms and signs; group B (n=38) had symptoms and signs of cervical myelopathy. The age, sex, body mass index (BMI), history of steroid treatment, duration of symptoms, number of spondylotic cervical segments, Torg ratio, range of motion (ROM), incidence of cervical segmental instability, overall curvature of the cervical spine, direction of spinal cord compression, and spinal cord magnetic resonance imaging (MRI) signal intensity were compared.
Results
For groups A and B, the Torg ratio was 90.3% and 83.6% (P<0.05), the incidence of cervical segmental instability was 23.3% and 65.8% (P<0.05), and the incidence of a spinal cord high intensity signal was 13.3% and 86.9% (P<0.05). Logistic regression analysis showed myelopathy as a dependent variable, independently associated with cervical segmental instability (OR=5.898, P=0.037), an MRI T2-weighted intramedullary high signal (OR=9.718, P=0.002), and Torg ratio (OR=0.155, P=0.006).
Conclusions
Cervical segmental instability, a high intramedullary signal on T2-weighted MRI, and the Torg ratio had the greatest capacity to distinguish between asymptomatic and symptomatic patients with CSM with mild to moderate cervical spinal cord compression.
MeSH Keywords: Magnetic Resonance Imaging, Spinal Cord Compression, Spinal Cord Diseases, Spine
Background
Patients with cervical spondylotic myelopathy (CSM) do not always have symptoms and signs associated with spinal cord compression. Spinal cord compression can be caused by several factors, including segmental instability and secondary vertebral and cervical spinal joint osteophyte formation resulting from intervertebral disc degeneration and decreased intervertebral space height. However, experimental studies indicate a certain resistance of the spinal tissue to chronic cervical spinal cord compression and also a delay in the development of symptomatic myelopathy [1–3].
Magnetic resonance imaging (MRI) is a valuable diagnostic tool to use before surgical decompression because it allows the visualization, not only of the degree of spinal cord compression but also of intramedullary signal changes [4]. The influence of cord compression on the development of symptomatic myelopathy varies among individuals, and MRI findings significantly overlap between individuals with CSM and those with non-myelopathic compression, resulting in a clinical-radiological mismatch. Bednarik et al. called this phenomenon ‘pre-symptomatic spondylotic cervical cord compression’ [5,6]. Patients with pre-symptomatic spondylotic cervical cord compression lack the typical symptoms and signs of upper motor neuron damage caused by spinal cord compression, apart from neck and shoulder pain, nerve root irritation and limited neck range of movement (ROM). However, it is unclear why a patient with cervical spinal cord compression does not experience symptoms and signs of myelopathy [5–10]. Furthermore, the factors that impact pre-symptomatic spondylotic cervical cord compression and CSM are not clear.
The aim of this study was to investigate and compare the imaging findings in asymptomatic and symptomatic patients with CSM with mild to moderate cervical spinal cord compression.
Material and Methods
Ethical approval
This retrospective clinical study was approved by the Ethics Committee of the Third Hospital of Hebei Medical University in China. Because this was a retrospective study and all the data were collected and analyzed anonymously, individual patient consents to participate in the study were not required. The clinical and imaging methods were carried out in accordance with currently approved guidelines.
Study design and patient population
A retrospective clinical study was performed on the clinical and imaging data from 68 patients with cervical spondylotic myelopathy (CSM) with mild to moderate cervical spinal cord compression. The study included patients with CSM and cervical spinal cord compression of one-third to one-half (mild to moderate), determined according to the ratio calculated by division of the smallest anteroposterior diameter of the cervical cord by the normal anteroposterior diameter, as assessed by magnetic resonance imaging (MRI).
Patients were excluded from the study if they had the following: (1) A Torg ratio (the ratio of the diameter of the cervical canal to the width of the cervical body on lateral view) taken as an indication of cervical stenosis if less than 0.75 [11,12]; (2) vertebral posterior margin osteophyte formation and ossification of the posterior longitudinal ligament; (3) cervical spondylotic radiculopathy with nerve root involvement but no significant compression of the spinal cord; (4) spinal cord posterior compression caused by ligamentum flavum hypertrophy; (5) cervical fractures and cervical instability caused by trauma; (6) cervical spinal tumors; (7) congenital cervical spinal fusion and deformity; (8) a history of cervical spine surgery or other conditions, such as cerebral infarction, cerebral thrombosis, myelitis, or peripheral neuropathy.
This retrospective study included 68 patients with cervical spinal cord compression who were first treated in our hospital from November 2015 to November 2016, including 37 men and 31 women, 38–72 years of age, with an average age of 52.6 years. There were 32 single-segment cases, 22 two-segment cases, and 14 three-segment cases. Clinical history and neurological examination of all patients were conducted by the same physician.
Based on the symptoms and signs of myelopathy, cases were divided into two groups: group A consisted of 30 patients with no symptoms or signs of myelopathy (asymptomatic group); group B consisted of 38 patients with symptoms and signs of myelopathy (symptomatic group). Patients in group B had an average score of 12.6 points, according to the Japanese Orthopaedic Association (JOA) score. All patients had X-ray films taken of the cervical spine in the anteroposterior and lateral positions and the extended and flexed positions. Cervical spine computed tomography (CT) and MRI examinations were also performed.
Assessment of clinical and imaging indices
The following data from the clinical and imaging indices were collected and analyzed.
Clinical indices: age, sex, duration of disease, body mass index (BMI), history of steroid use, and the number of affected segments.
-
Imaging indices:
The Torg ratio, which was unaffected by magnification error, was calculated as the sagittal diameter of the cervical canal divided by the sagittal diameter of the vertebral body on lateral radiographs; the average Torg ratio of C3–C7 was recorded.
The overall range of motion (ROM) of the cervical spine was determined according to the Penning method [13]: the ROM was the angle subtended between straight lines parallel to the C2–C7 vertebral posterior margins on maximum flexion and extension when viewed in lateral cervical X-ray films (Figure 1).
The cervical segmental instability was determined according to the White-Panjabi method [14]: a horizontal intervertebral displacement >3.5 mm was considered to be displacement instability; a difference in rotational angles between two adjacent vertebrae >11° was considered to be rotation instability (Figure 2).
Overall curvature of the cervical spine (C2–C7), the Cobb angle, or the angle between lines drawn parallel to the inferior edge of the C2 vertebral body and the inferior edge of the C7 vertebral body.
Spinal cord MRI signal intensity grading: the intramedullary signals of all patients were divided into three levels based on MRI T2-weighted images, according to the grading method of Yukawa et al. [15]: grade 0 indicated the absence of high intramedullary signals; grade 1 indicated moderate intramedullary signals; and grade 2 indicated that there were high intramedullary signals.
The direction of spinal cord compression: spinal cord compression from the anterior median axis was classified as central type, and spinal cord compression from one side of the anterior median axis was classified as paracentral type (Figures 3, 4).
Figure 1.
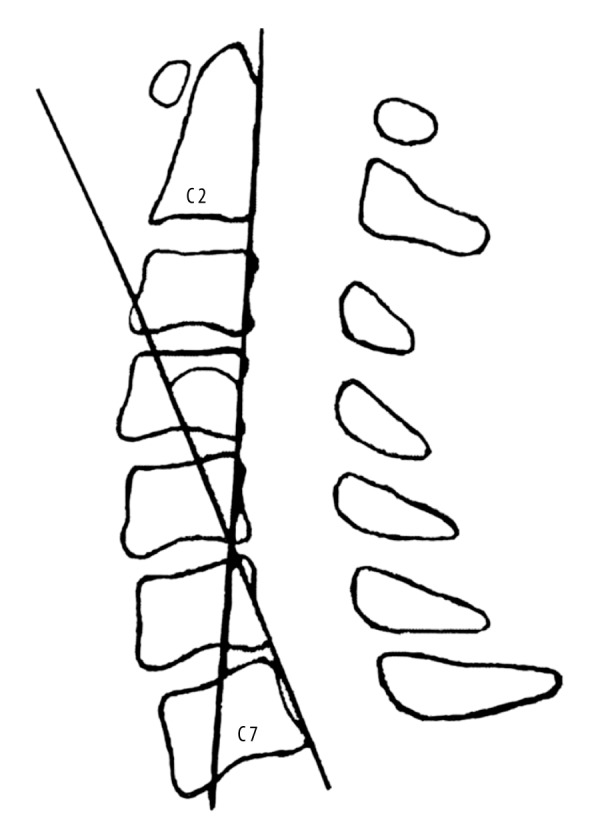
The C2–C7 angle: the angle between the lines parallel to the posterior margin of the C2 and C7 vertebral bodies.
Figure 2.
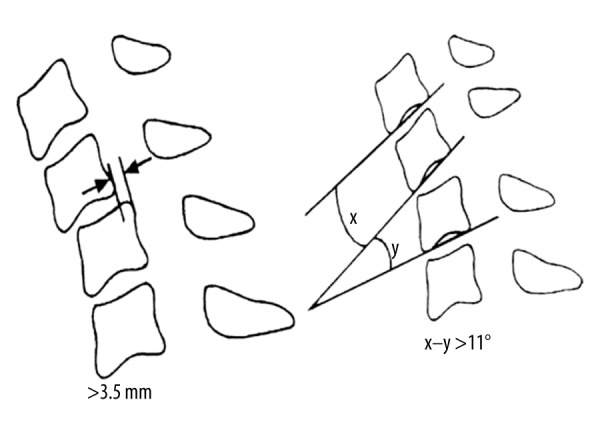
The cervical segmental instability, determined according to the White-Panjabi method. A horizontal intervertebral displacement >3.5 mm was deemed to represent displacement instability. A difference in rotational angle between two adjacent vertebrae >11° was deemed to represent rotation instability (X–Y). X or Y indicates the angle between the lines parallel to the inferior margin of the two adjacent vertebral bodies.
Figure 3.
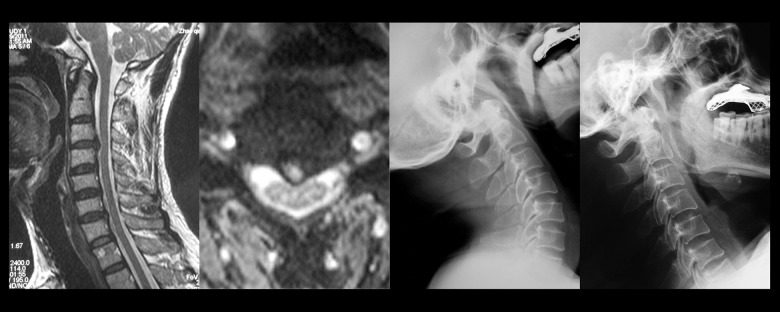
Cervical spine magnetic resonance imaging (MRI) of a 56-year-old male patient who had suffered from neck and back discomfort for 14 months. Clinical examination showed no significant myelopathic signs. The cervical MRI showed mild spinal cord compression in C3–C4 due to central cervical intervertebral disc herniation, without a spinal cord high signal. The flexion and extension cervical spine X-ray revealed no cervical segmental instability.
Figure 4.
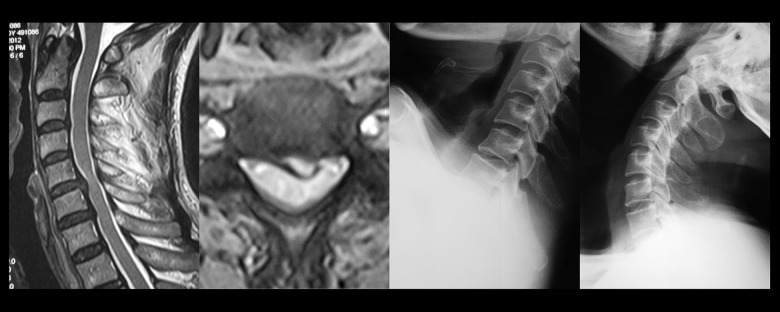
Cervical spine magnetic resonance imaging (MRI) of a 54-year-old male patient who had suffered from neck and back discomfort for two years. This patient had symptoms that became more severe in the previous three months, with numbness of the limbs and unsteady gait. On examination, the patient had hyperreflexia of the patellar tendon, ankle clonus (+), bilateral Hoffmann’s reflex (finger flexor response) (+). The cervical MRI showed spinal cord compression in C5–C6 caused by left-sided cervical intervertebral disc herniation, with a high spinal cord signal at this level. The hyperflexion and hyperextension cervical spine X-ray showed C5–C6 segmental instability.
Statistical analysis
The SPSS 13.0 statistical software package (SPSS Inc., Chicago, Ill, USA) was used for statistical analysis. Normality and variance homogeneity tests were conducted for all measurement data. The t-test for two independent samples was conducted for age, duration of disease, BMI, Torg ratio, ROM, and C2–C7 Cobb angle between the two groups. The χ2 test was conducted for comparisons of sex, use of steroids, number of affected segments, the incidence of cervical segment instability, the direction of spinal cord compression, and the incidence of high intramedullary signals. Dichotomous logistic regression analysis was used to analyze the correlation between observation indexes and symptoms and signs of myelopathy; a P-value of <0.05 was considered to be statistically significant.
Results
Patients in group A were 38–67 years old, with an average age of 52.5±8.9 years. Patients in group B were 38–72 years old, with an average age of 52.6±9.2 years. There were 16 men and 14 women in group A, and 21 men and 17 women in group B. The mean body mass index (BMI) was 26.4±4.0 in group A and 24.5±4.5 in group B. There were nine cases who had a history of steroid use in group A, and 13 cases in group B.
Disease duration in group A was 18–36 months, with an average duration of 27.3±5.5 months, and disease duration in group B was 18–40 months, with an average duration of 28.0±6.5 months. There were 14 single-segment cases, ten two-segment cases and six three-segment cases in group A, and 18 single-segment cases, 12 two-segment cases and eight three-segment cases in group B. Statistical analysis showed that there were no significant differences in age, sex, BMI, steroid use, duration of disease, or number of lesioned segments between the two groups (Table 1).
Table 1.
Comparison of demographic characteristics between asymptomatic (group A) and symptomatic (group B) patients.
| Variables | Group A (n=30) | Group B (n=38) | P-value |
|---|---|---|---|
| Age (years) | 52.5±8.9 | 52.6±9.2 | 0.965 |
| Sex | 0.874 | ||
| Male | 16 | 21 | |
| Female | 14 | 17 | |
| BMI (kg m−2) | 26.4±4.0 | 24.5±4.5 | 0.066 |
| Use of steroid | 0.712 | ||
| Yes | 9 | 13 | |
| No | 21 | 25 | |
| Lesioned segment | 0.987 | ||
| Single-segment | 14 | 18 | |
| Two-segment | 10 | 12 | |
| Three-segment | 6 | 8 | |
| Duration of disease (months) | 27.3±5.5 | 28.0±6.5 | 0.672 |
BMI – body mass index.
The Torg ratio in group A (90.3±5.5%) was significantly increased compared with group B (83.6±4.3%; P<0.05). The overall range of movement (ROM) of the cervical spine was 47.5±9.5° in group A and 44.1±11.5° in group B, with no significant difference between the two groups.
There were seven patients with cervical segmental instability in group A and 25 patients in group B, demonstrating a significantly greater incidence of cervical segmental instability in group B (P<0.05). The C2–C7 Cobb angle was 14.1±9.3° in group A and 14.0±8.8° in group B, which was not significantly different.
Among the patients in group A, 19 showed a central-type spinal cord compression, and 11 showed a paracentral-type spinal cord compression. Within group B, 17 cases showed central-type spinal cord compression, and 21 cases exhibited paracentral-type spinal cord compression, with no significant differences between the two groups.
For the cervical spine T2-weighted MRI intramedullary signal intensity grading, there were 26 patients with a grade of 0, three patients with a grade of 1, and one patient with a grade of 2 in group A. In group B, there were five patients with a grade of 0, 24 patients with a grade of 1, and nine patients with a grade of 2, showing a significant difference between the two groups (P<0.05; Table 2).
Table 2.
Imaging characteristics in asymptomatic (group A) and symptomatic (group B) patients.
| Variables | Group A (n=30) | Group B (n=38) | P-value |
|---|---|---|---|
| Torg ratio (%) | 90.3±5.5 | 83.6±4.3 | 0.000 |
| ROM (°) | 47.5±9.5 | 44.1±11.5 | 0.197 |
| Cervical segmental instability (%) | 0.000 | ||
| Yes | 7 (23.3) | 25 (65.8) | |
| No | 23 (76.7) | 13 (34.2) | |
| C2–C7 Cobb angle (°) | 14.1±9.3 | 14.0±8.8 | 0.976 |
| Spinal cord high signal (%) | 0.000 | ||
| Yes | 4 (13.3) | 33 (86.9) | |
| No | 26 (86.7) | 5 (13.1) | |
| The direction of SCC (%) | 0.127 | ||
| Central type | 19 (63.3) | 17 (44.7) | |
| Paracentral type | 11 (36.7) | 21 (55.3) |
ROM – range of motion; SCC – spinal cord compression.
Logistic regression analysis showed that myelopathy was a dependent variable, and showed an independent association with cervical segmental instability (OR=5.898; P=0.037), T2-weighted MRI intramedullary high signal (OR=9.718; P=0.002) and Torg ratio (OR=0.155; P=0.006) (Table 3).
Table 3.
Predictive power of parameters to distinguish between asymptomatic (group A) and symptomatic (group B) patients.
| Variables | β | Odds ratios (OR) | P-value |
|---|---|---|---|
| Cervical segmental instability | 1.775 | 5.898 | 0.037 |
| Spinal cord intramedullary high signal | 2.274 | 9.718 | 0.002 |
| Torg ratio | −1.866 | 0.155 | 0.006 |
Discussion
Degenerative changes are important features in the pathogenesis of cervical spondylotic myelopathy (CSM). Three clinical symptoms are typical of this condition: axial pain, cervical nerve root pain, and myelopathy. In recent years, magnetic resonance imaging (MRI) has been increasingly used in primary hospitals, resulting in a significant increase in the detection rate of cervical spinal cord compression. MRI can detect intervertebral disc compression from the anterior imaging, but this approach makes it difficult to assess the severity of spinal cord compression.
In 1987, Teresi et al. [16] first reported asymptomatic spinal cord compression in 35 patients who underwent cervical MRI for nasopharyngeal carcinoma. Further studies showed that asymptomatic compression is a common subclinical phenomenon (7.9–27%). However, asymptomatic spinal cord compression cannot be diagnosed as CSM. The diagnosis of CSM should be based mainly on the symptoms and signs of spinal cord compression, combined with characteristic imaging features. CSM can be established only when the consistent symptoms, signs, and imaging findings are present. Pre-symptomatic spondylotic cervical cord compression is not the same as CSM, as there are differences in the clinical features.
The pathogenesis of CSM is complex, and the onset of CSM is believed to be caused by multiple factors. Clinically, some patients with CSM with minimal cervical disc herniation have clinical symptoms that are more severe than patients with a large disc herniation and severe spinal cord compression. Conversely, some patients with large cervical disc herniation and severe spinal cord compression shown by MRI have mild or absent clinical manifestations. Based on these previous observations, in this study, we investigated some of the factors associated with and affecting symptom presentation in patients with mild to moderate cervical spinal cord compression (one-third to one-half), without the developmental cervical spinal stenosis. We aimed to clarify why some patients have signs and symptoms of myelopathy while others do not.
The findings of this study showed that there were no significant differences in age, sex, body mass index (BMI), steroid use, duration of disease or number of affected segments between the asymptomatic and symptomatic patients with spinal cord compression. Age and sex might be associated with morbidity from cervical myelopathy, but this was not conclusive. The longer the spinal cord is compressed, there is likely to be a greater possibility of irreversible spinal cord injury. However, duration of the disease might equate with the duration of cord compression, but in this study, the onset of cord compression could not be judged accurately.
In this study, there was no statistically significant association between the patient’s BMI or previous steroid use and myelopathic symptoms. However, symptomatic patients had an increased cervical spinal canal sagittal diameter. Cervical segmental instability and a high intramedullary signal were closely related to the symptoms and signs of myelopathy. Overall curvature of the cervical spine, ROM, and spinal cord compression direction had no impact on the presence of myelopathic signs and symptoms.
Previous studies have shown that the process of degenerative changes in the cervical spine, including cervical flexion and extension activity, diminish following the emergence of cervical symptoms [17]. These previous findings are supported by the findings of the present study, which showed that the overall range of movement (ROM) of the cervical spine in the two groups was less than in the general population.
Measuring the ROM of a single cervical vertebral segment might have clinical diagnostic value because the overall ROM reflects changes in the whole cervical spine, and sometimes a change in the ROM is caused by instability of a single vertebral segment. Since the 1960s, segmental instability caused by intervertebral joint degeneration of the cervical spine has been known to be an important factor that contributes to the onset of SCM [18]. Therefore, segmental instability may have a pathogenic role in degenerative cervical spinal cord compression.
The findings of this study showed that cervical segmental instability was closely related to the symptoms and signs of myelopathy. Because of cervical degeneration, the overall ROM of the cervical spine decreased. Furthermore, some segments that compensate for the restricted motion might undergo accelerated degeneration of the intervertebral joint, as exceeding the normal range of physiological motion results in cervical instability. The increase in intervertebral activity, including anterior cervical flexion and posterior extension, can cause or aggravate spinal cord compression and contribute to the myelopathic symptoms. It should be noted that as the degenerative process proceeds, the formation of osteophytes around intervertebral discs and facet joints allows the cervical spine to re-acquire stability, but this can also lead to spinal cord and nerve root involvement.
Lee et al. concluded that the high MRI T2-weighted intramedullary signals are caused by spinal cord compression and obstruction to local venous return, which leads to increased venous pressure, venous congestion and elevated intramedullary vascular permeability, and results in spinal cord edema [19]. Ramanauskas et al. have proposed that a high MRI T2-weighted intramedullary signal represents spinal cord edema in the early cord compression period and that spinal cord necrosis and cysts occur in the late cord compression period [20]. In the present study, we found that a high MRI T2-weighted intramedullary signal was significantly associated with myelopathic symptoms and signs in patients with cervical spinal cord compression.
In the present study, the direction of cervical spinal cord compression in both groups was studied, and there was no statistically significant difference between them. However, asymptomatic patients had a high proportion of the central type, while the symptomatic CSM patients had a high proportion of the paracentral type. In the early process of degeneration, disease development is slow, and the spinal cord can obtain compensation or establish a collateral circulation for blood supply. Additionally, local bone absorption and removal of adipose tissue can expand the spinal canal to reduce cord compression and increase blood flow. Because the cervical spinal canal is triangular, the risk of spinal cord damage for patients with paracentral cord compression is high. Furthermore, the pyramidal tract, located laterally, is more vulnerable to compression and produces symptoms and signs of myelopathy. Patients with presymptomatic spondylotic cervical cord compression and nerve root symptoms are more likely to deteriorate to CSM [5,6], which further suggests that spinal cord compression of the paracentral type may be a risk factor for the occurrence of myelopathic symptoms and signs. However, in the present study, the lack of a difference in the spinal cord compression direction between the symptomatic and asymptomatic groups may be a result of the small study sample size.
In this study, logistic regression analysis showed that for patients with mild to moderate cervical spinal cord compression, the occurrence of symptoms and signs of myelopathy was closely associated with cervical spinal segmental stability, a high spinal cord intramedullary signal, and the Torg ratio. Cervical spine instability and a high MRI T2-weighted signal image were associated with an increased probability of myelopathic symptoms and signs, with the high signal having the greatest impact. Conversely, a greater Torg ratio, indicative of a wider sagittal diameter, was associated with a reduced occurrence of symptoms and signs of myelopathy.
There are some limitations of this study. Apart from the small study sample size, only patients with mild to moderate cervical spinal cord compression were included, without patients with severe cervical spinal cord compression. As presymptomatic spondylotic cervical cord compression and CSM are diseases with a complex pathogenesis, a multicenter study with a larger sample size and more prospective studies are needed to clarify the impact of other factors on asymptomatic and symptomatic patients with CSM.
Conclusions
The findings of this study showed that cervical segmental instability, a high intramedullary signal on T2-weighted MRI, and the Torg ratio had the greatest capacity to distinguish between asymptomatic and symptomatic patients with CSM with mild to moderate cervical spinal cord compression.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Kim P, Haisa T, Kawamoto T, et al. Delayed myelopathy induced by chronic compression in the rat spinal cord. Ann Neurol. 2004;55:503–11. doi: 10.1002/ana.20018. [DOI] [PubMed] [Google Scholar]
- 2.Harkey HL, al-Mefty O, Marawi I, et al. Experimental chronic compressive cervical myelopathy: Effects of decompression. J Neurosurg. 1995;83:336–41. doi: 10.3171/jns.1995.83.2.0336. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mefty O, Harkey HL, Marawi I, et al. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993;79:550–61. doi: 10.3171/jns.1993.79.4.0550. [DOI] [PubMed] [Google Scholar]
- 4.Mehalic TF, Pezzuti RT, Applebaum BI. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26:217–26. doi: 10.1097/00006123-199002000-00006. discussion 226–27. [DOI] [PubMed] [Google Scholar]
- 5.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical cord compression. Spine (Phila Pa 1976) 2004;29:2260–69. doi: 10.1097/01.brs.0000142434.02579.84. [DOI] [PubMed] [Google Scholar]
- 6.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondylotic cervical myelopathy: An updated predictive model. Eur Spine J. 2008;17:421–31. doi: 10.1007/s00586-008-0585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovalova I, Kerkovsky M, Kadanka Z, et al. Prevalence and imaging characteristics of nonmyelopathic and myelopathic spondylotic cervical cord compression. Spine (Phila Pa 1976) 2016;41:1908–16. doi: 10.1097/BRS.0000000000001842. [DOI] [PubMed] [Google Scholar]
- 8.Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: Pathophysiology, natural history, and clinical evaluation. J Bone Joint Surg Am. 2002;84-A:1872–81. doi: 10.2106/00004623-200210000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Kovalova I, Bednarik J, Kerkovsky M, et al. Asymptomatic spondylotic cervical cord compression. Cesk Slov Neurol N. 2015;78:24–33. [Google Scholar]
- 10.Kadanka Z, Kerkovsky M, Bednarik J, Jarkovsky J. Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2007;32:2573–77. doi: 10.1097/BRS.0b013e318158cda0. [DOI] [PubMed] [Google Scholar]
- 11.Torg JS, Corcoran TA, Thibault LE, et al. Cervical cord neurapraxia: Classification, pathomechanics, morbidity, and management guidelines. J Neurosurg. 1997;87:843–50. doi: 10.3171/jns.1997.87.6.0843. [DOI] [PubMed] [Google Scholar]
- 12.Yue WM, Tan SB, Tan MH, et al. The Torg-Pavlov ratio in cervical spondylotic myelopathy: A comparative study between patients with cervical spondylotic myelopathy and a non-spondylotic, non-myelopathic population. Spine. 2001;26:1760–64. doi: 10.1097/00007632-200108150-00006. [DOI] [PubMed] [Google Scholar]
- 13.Penning L. Normal movements of the cervical spine. Am J Roentgenol. 1978;130:317–26. doi: 10.2214/ajr.130.2.317. [DOI] [PubMed] [Google Scholar]
- 14.White AA, Panjabi MM. The basic kinematics of the human spine. A review of past and current knowledge. Spine. 1978;3:12–20. doi: 10.1097/00007632-197803000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Yukawa Y, Kato F, Yoshihara H, et al. MR T2 image classification in cervical compression myelopathy: Predictor of surgical outcomes. Spine. 2007;32:1675–78. doi: 10.1097/BRS.0b013e318074d62e. [DOI] [PubMed] [Google Scholar]
- 16.Teresi LM, Lufkin RB, Reicher MA, et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology. 1987;164:83–88. doi: 10.1148/radiology.164.1.3588931. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Wu B, Van Hoof T, et al. Are the standard parameters of cervical spine alignment and range of motion related to age, sex, and cervical disc degeneration. J Neurosurg Spine. 2015;23:274–79. doi: 10.3171/2015.1.SPINE14489. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Liu H, Wang H, Zhou D. Segmental instability in cervical spondylotic myelopathy with severe disc degeneration. Spine. 2006;31:1327–31. doi: 10.1097/01.brs.0000218508.86258.d4. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Koyanagi I, Hida K, et al. Spinal cord edema: Unusual magnetic resonance imaging findings in cervical spondylosis. J Neurosurg. 2003;99:8–13. doi: 10.3171/spi.2003.99.1.0008. [DOI] [PubMed] [Google Scholar]
- 20.Ramanauskas WL, Wilner HI, Metes JJ, et al. MR imaging of compressive myelomalacia. J Comput Assist Tomogr. 1989;13:399–404. doi: 10.1097/00004728-198905000-00005. [DOI] [PubMed] [Google Scholar]