Abstract
Background
PCDH8 is a newly-discovered suppressor gene that is frequently inactivated by aberrant methylation in several human cancers, including prostate cancer. The identification of PCDH8 methylation can be used as a potential predictive biomarker. Prostate cancer patients with high Gleason score are considered as being at high risk for tumor recurrence and progression, and adjuvant therapy is often routinely performed in clinical practice. In the present study, we did not measure the methylation of PCDH8 in these patients. The main purpose of the present study was to evaluate the clinical significance of PCDH8 methylation in serum of prostate cancer patients with low Gleason score.
Material/Methods
PCDH8 methylation in serum samples of 117 patients and 47 controls was checked by methylation-specific PCR (MSP). Then, we correlated PCDH8 methylation status with the clinicopathological parameters of prostate cancer patients with low Gleason score and patient outcomes.
Results
We found that PCDH8 was more frequently methylated in serum samples of patients with prostate cancer than in controls. PCDH8 methylation was correlated with advanced clinical stage (P=0.021), higher level of preoperative PSA (P=0.008), and positive lymph node metastasis (P=0.010). Moreover, patients with PCDH8 methylation had worse biochemical recurrence (BCR)-free survival (P<0.001) than patients without. Independent prognostic factors for worse BCR-free survival of prostate cancer patients with low Gleason score were: PCDH8 methylation in serum (Exp (B)=3.147, 95% CI: 1.152–7.961, P=0.007), clinical stage (Exp (B)=2.53, 95% CI: 1.032–4.763, P=0.025) and lymph node status (Exp (B)=1.476, 95% CI: 1.107–4.572, P=0.042).
Conclusions
Our study indicated that PCDH8 methylation in serum occurred frequently in prostate cancer patients and was correlated with risk factors for poor outcome. The methylation of PCDH8 in serum is a potential predictive marker for prostate cancer patients with low Gleason score after surgery.
MeSH Keywords: Biological Markers, DNA Methylation, Prostatic Neoplasms
Background
Prostate cancer is the most commonly diagnosed genital malignant disease in men, with an estimated 220 800 newly diagnosed cases and 27 540 deaths in 2015 in the USA [1]. In addition, the morbidity rate of prostate cancer has recently been increasing in China [2]. Currently, with the use of prostate-specific antigen (PSA) and prostate biopsy for prostate cancer screening, more and more patients can be diagnosed in the early stage and treated by radical prostatectomy [3,4]. However, about 25–50% of prostate cancer patients will have tumor recurrence after surgery, and many will die of the cancer [5,6]. Although current clinicopathological features such as Gleason score, disease stage, and PSA level provide important information, they do not accurately predict an individual patient’s prognosis, and new biomarkers are needed for prognostication [7,8].
Prostate cancer is a heterogeneous disease; genetic and epigenetic mechanisms are involved in the initiation and progression of the disease and may be used as potential biomarkers [9–11]. DNA methylation is the most common epigenetic event in human cancer. Growing evidence indicates that aberrant DNA methylation has potential to improve the accuracy of human cancer prognosis [12–14]. PCDH8, belonging to the cadherin super-family, plays crucial functions in cell adhesion, proliferation, differentiation, and migration [15–18]. Previous reports indicated that PCDH8 functions as a tumor suppressor and is often silenced by aberrant promoter methylation in human cancers, including prostate cancer [15–18]. In our previous study, we confirmed that PCDH8 was often methylated in prostate cancer tissues, and was correlated with malignant behaviors and poor outcome of the disease [19]. However, little is known about the clinical significance of PCDH8 methylation in serum of prostate cancer, especially in low Gleason score cases.
In this study, we investigated the methylation of PCDH8 in serum samples of primary low Gleason score prostate cancer patients and controls, and the relationship between PCDH8 methylation and clinicopathological features was also analyzed, in order to evaluate the prognostic significance of PCDH8 methylation in serum of prostate cancer patients. The relationship between PCDH8 methylation and the BCR-free survival was also analyzed.
Material and Methods
Patients and serum samples
We included a total of 117 prostate cancer patients who underwent radical prostatectomy as primary therapy for clinically localized adenocarcinoma of the prostate at the Third Hospital of Hebei Medical University and Xuzhou Cancer Hospital from 2001 to 2011. All the patients were diagnosed with prostate cancer by prostate biopsy and pathological examination. No patients had received radiotherapy, chemotherapy, or anti-androgen treatment before surgery, and no adjuvant therapy was performed before BCR. Postoperative pathological examination indicated that the Gleason score was less than 7 in all patients.
As normal controls, we included 47 age-matched patients with benign prostatic hyperplasia. The main clinical and pathological features are recorded in Table 1. All the prostate cancer patients were followed up at intervals ranging from 11 to 60 months. BCR was defined as the period between surgery and the testing of 2 successive values of serum PSA level ≥0.2 ng/ml, as previously reported [19]. Peripheral blood samples (10 ml) were collected before surgery. Clotting of serum samples was allowed for 60 min before centrifugation at 1800 g per min for 10 min, and the supernatants were stored at −80°C, as we reported previously [20]. This study was performed according to the Declaration of Helsinki and was approved by the local ethics committee (200101THM003 and 200101XCHxx7). Written informed consent was obtained from each participant.
Table 1.
The clinical and pathological characteristics of patients with prostate cancer (n=117).
| Characteristics | Variables | No (%). |
|---|---|---|
| Age (years) | ≤65 | 62 (53.0%) |
| >65 | 55 (47.0%) | |
| Preoperative PSA (ng/ml) | ≤10 | 40 (34.2%) |
| >10 | 77 (65.8%) | |
| Clinical stage | T1 | 56 (47.9%) |
| T2 | 41 (24.0%) | |
| T3 | 20 (28.1%) | |
| Gleason score | 4 | 29 (24.8%) |
| 5 | 47 (40.2%) | |
| 6 | 41 (35.0%) | |
| Lymph node status | N0 | 98 (83.8%) |
| N1 | 19 (16.2%) | |
| Surgical margin status | Negative | 103 (88.0%) |
| Positive | 14 (12.0%) | |
| Biochemical recurrence | No | 83 (70.9%) |
| Yes | 34 (29.1%) |
DNA extraction and bisulfite treatment
DNA was extracted from 1 ml of the archived serum from each participant, using the QIAmp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. The isolated DNA was modified with bisulfite using the EpiTect Bisulfite Kit (Qiagen, Valencia, CA) and standard protocol, as described previously [20].
Methylation-specific PCR (MSP)
MSP was performed using respective primers for either methylated or unmethylated DNA. Methylated: forward 5′-CGGTTATTGGTTATTCGGTTCC-3′ and reverse 5′-ACGAA CTCTAAAAACGCGCG-3′. Unmethylated: forward 5′-GGTGGT TATTGGTTATTTGGTTT-3′ and reverse 5′-CCAACAAACTCT AAAAACACACA-3′ [15–19]. Amplifications were carried out as we reported previously [15–19].
Statistical analysis
The difference in PCDH8 methylation between prostate cancer patients and controls was checked by Fisher’s exact test. The chi-square test was used to evaluate the correlations between PCDH8 methylation and clinicopathological parameters. For BCR-free survival analysis, Kaplan-Meier survival analysis was used, and the difference in survival was analyzed using the log-rank test. Univariate and multivariate Cox proportional hazard models were used to evaluate the predictive value of PCDH8 methylation in serum. SPSS 13.0 software was used for statistical analyses. P<0.05 was considered as statistically significant.
Results
PCDH8 methylation in serum of prostate cancer patients and controls
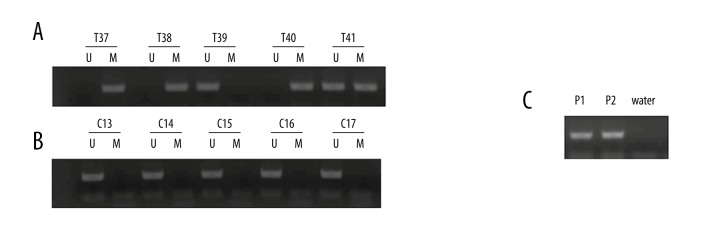
PCDH8 methylation was detected in 49 (41.9%) prostate cancer patients, but no PCDH8 methylation was found in the controls, and the difference between these 2 groups was statistically significant (P<0.001). A representative MSP result is shown in Figure 1.
Figure 1.
Representative MSP results of PCDH8 methylation in serum of patients with prostate cancer and controls. (A) Patients with prostate cancer; T37, 38, 40, and 41 exhibited methylated PCDH8; T39 exhibited unmethylated PCDH8. (B) Controls; C13–17 exhibited unmethylated PCDH8. (C) Positive control for MSP; P1 – Positive methylated control in vitro; P2 – Positive unmethylated control in vitro.
Correlations between PCDH8 Methylation and Clinicopathological Features
We found that PCDH8 methylation was significantly associated with advanced stage (P=0.021), higher level of preoperative PSA (P=0.008), positive lymph node metastasis (P=0.010). No significant association between PCDH8 methylation and age, surgical margin status, or Gleason score was found. The findings are shown in Table 2.
Table 2.
The correlations between PCDH8 methylation in serum and clinicopathologic features of patients with prostate cancer (n=117).
| Features | Variables | No. | M (%) | U (%) | P |
|---|---|---|---|---|---|
| Age (years) | ≤65 | 62 | 24 (38.7) | 38 (61.3) | 0.460 |
| >65 | 55 | 25 (45.5) | 30 (54.5) | ||
| Preoperative PSA (ng/ml) | ≤10 | 40 | 10 (25.0) | 30 (75.0) | 0.008 |
| >10 | 77 | 39 (50.6) | 38 (49.4) | ||
| Clinical stage | T1/T2 | 97 | 36 (37.1) | 61 (62.9) | 0.021 |
| T3 | 20 | 13 (65.0) | 7 (35.0) | ||
| Gleason score | ≤5 | 76 | 30 (39.5) | 46 (60.5) | 0.473 |
| 6 | 41 | 19 (46.3) | 22 (53.7) | ||
| Lymph node status | N0 | 98 | 36 (36.7) | 62 (63.3) | 0.010 |
| N1 | 19 | 13 (68.4) | 6 (31.6) | ||
| Surgical margin status | Negative | 103 | 42 (40.8) | 61 (59.2) | 0.512 |
| Positive | 14 | 7 (50.0) | 7 (50.0) | ||
| Total | 117 | 49 (41.9) | 68 (58.1) |
M – methylation; U – unmethylation.
The predictive value of PCDH8 methylation in serum for prostate cancer
BCR occurred in 34 patients with prostate cancer during the follow-up period. The result indicated that patients with PCDH8 methylation had worse BCR-free survival than in patients without (Figure 2), indicating that PCDH8 methylation may be a risk factor for outcome in patients with prostate cancer. Then, univariate and multivariate Cox regression model analysis was performed. Univariate Cox regression analysis showed that Gleason score, preoperative PSA level, pathologic stage, and protocadherin8 promoter methylation were risk factors for worse BCR-free survival. Subsequently, these risk factors were entered into multivariate analysis, demonstrating that PCDH8 methylation is an independent predictive risk factor for BCR-free survival (Exp (B)=3.147, 95% CI: 1.152–7.961, P=0.007). These findings are summarized in Table 3.
Figure 2.
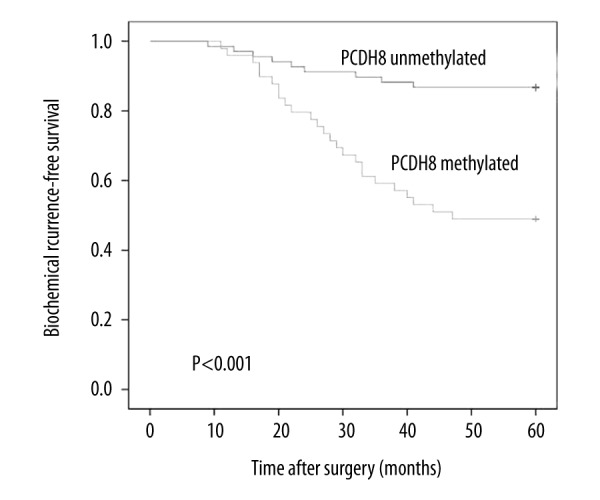
Associations between PCDH8 methylation and BCR-free survival of patients after surgery. Patients with PCDH8 methylated showed significantly shorter BCR-free survival than those with PCDH8 unmethylated (P<0.001, log-rank test).
Table 3.
Prognostic value of PCDH8 methylation in serum for the BCR-free survival in univariate and multivariate analysis.
| Varivale | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Exp (B) | 95% CI | P | Exp (B) | 95% CI | P | |
| Age | 0.869 | 0.684–10.491 | 0.742 | |||
| PSA | 1.537 | 1.074–6.582 | 0.039 | 1.122 | 0.796–5.357 | 0.238 |
| Clinical stage | 3.257 | 1.376–10.427 | 0.005 | 2.523 | 1.032–4.763 | 0.025 |
| Gleason score | 1.365 | 0.692–9.248 | 0.156 | |||
| Lymph node status | 2.482 | 1.418–7.315 | 0.017 | 1.476 | 1.107–4.572 | 0.042 |
| Surgical margin status | 1.137 | 0.844–4.036 | 0.365 | |||
| PCDH8 methylation | 3.864 | 1.462–8.962 | <0.001 | 3.147 | 1.152–7.961 | 0.007 |
Discussion
Prostate cancer is a common malignancy in men. The main challenge in management of prostate cancer in clinical practice is to distinguish aggressive disease from indolent disease [21–24]. Currently, the clinicopathological parameters, such as serum PSA, clinical stage, and Gleason score, are most commonly used as prognostic factors, especially Gleason score. High Gleason score is considered as the main risk factor for poor outcome [3–8]. There is an urgent need to improve the existing tools for accurate prognosis of prostate cancer, due to its heterogeneous property, particularly for patients with low Gleason score, in order to improve treatment strategy and prognosis [3–8]. This is the reason why we choose patients with low Gleason scores as research objects.
DNA methylation, the most frequently studied epigenetic alteration, participates in the occurrence and development of prostate cancer and has the potential to be a reliable prognostic biomarker, which has been demonstrated in previous studies [9–12]. DNA methylation can be checked not only in tumor tissues, but also in the body fluids, such as serum, plasma, urine, and semen. Recent studies demonstrated that DNA methylation in body fluids, especially in serum, has the potential to be a reliable biomarker for the prediction of outcome [9,11,25]. Moreover, detection of DNA methylation in serum is a non-invasive method that can be widely used in clinical practice [26,27]. The detection of DNA methylation in serum can represent the entire tumor character, as cell-free DNA originates from the leaking of tumor cells into circulation or from apoptotic and necrotic cells, which has been shown in previous studies [25–27]. MSP is a widely-used method for the detection of DNA methylation. Advantages of this method are that it is simple and convenient, needs only a short time for analysis, and can obtain results from a small amount of DNA [28]. In our study, we detected PCDH8 methylation in serum samples of prostate cancer patients to find a reliable prognostic factor for low Gleason score patients.
In the present study, our findings indicate that PCDH8 methylation was a frequent event in serum of patients with prostate cancer, and no PCDH8 methylation was found in the controls, suggesting that PCDH8 methylation in serum may be specific for prostate cancer. Subsequently, we evaluated the correlations between PCDH8 methylation and clinicopathological features. We found that PCDH8 methylation was associated with higher level of preoperative PSA and positive lymph node metastasis, which are risk factors for prostate cancer progression [4,7]. The results indicate that PCDH8 methylation in serum may be associated with patient outcome. We analyzed the association between PCDH8 methylation status and Gleason score by dividing the patients into 2 groups according Gleason score (group 1: Gleason score ≤5; group 2: Gleason score=6), and found that PCDH8 methylation did not correlate with high Gleason score. One possible explanation may be that only low Gleason score patients were involved in the current study. The results are similar to those of previous studies in liver cancer and gastric cancer [15,29].
During the follow-up time, 34 patients had BCR. The results of Kaplan-Meier survival analysis and log-rank test suggest that patients with PCDH8 methylation in serum had worse BCR-free survival than in patients without. This result suggests that PCDH8 methylation in serum may be a predictive biomarker for tumor recurrence. To further evaluate the prognostic value of PCDH8 methylation in serum, univariate and multivariate models were used. In the multivariate analysis, a significant correlation was observed between PCDH8 methylation and BCR, suggesting that PCDH8 methylation in serum is a predictive biomarker of poor outcome in patients with low Gleason score, who would previously have been regarded as being at low risk. Therefore, measuring PCDH8 methylation in serum may be helpful in identifying individual patient risk of BCR due to prostate cancer. However, further studies are needed to confirm our findings, as the number of patients in our study was relatively small.
Conclusions
In conclusion, we characterized PCDH8 methylation in serum of prostate cancer patients with low Gleason score and analyzed its relationship to classical clinicopathological parameters and patient outcomes. We found that PCDH8 methylation in serum is an independent risk factor for the BCR of low Gleason score prostate cancer patients after surgery, and can be used as a potential prognostic biomarker. In the future, the methylation status of PCDH8 in serum should be checked before surgery, and for the patients with PCDH8, methylation hormonal therapy should be performed after surgery, so as to prevent tumor recurrence and achieve better prognosis.
Footnotes
Source of support: This study was supported by the Xuzhou Medical Talented Youth Project (No. 2014007), Jiangsu University Clinical Fund (No. JLY20140109), the Jiangsu Province Health and Family Planning Fund (No. Q201514), the Jiangsu Province Peak Talents Project (No. 2014-WSW-066), and the Jiangsu Youth Medical Talent Project (No. QNRC2016395)
Competing of interests
The authors had no conflicts of interest to declare in relation to this article.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Henrique R, Ribeiro FR, Fonseca D, et al. High promoter methylation levels of APC predict poor prognosis in sextant biopsies from prostate cancerpatients. Clin Cancer Res. 2007;13(20):6122–29. doi: 10.1158/1078-0432.CCR-07-1042. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen-Nielsen M, Borre M. Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl Med. 2016;46(6):484–90. doi: 10.1053/j.semnuclmed.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Wan X, Yang S, Huang W, et al. UHRF1 overexpression is involved in cell proliferation and biochemical recurrence in prostate cancer afterradical prostatectomy. J Exp Clin Cancer Res. 2016;35:34. doi: 10.1186/s13046-016-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perera M, Krishnananthan N, Lindner U, et al. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13(11):641–53. doi: 10.1038/nrurol.2016.177. [DOI] [PubMed] [Google Scholar]
- 7.Filella X, Foj L. Prostate cancer detection and prognosis: from prostate specific antigen (PSA) to exosomal biomarkers. Int J Mol Sci. 2016;17(11):E1784. doi: 10.3390/ijms17111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsaur I, Thurn K, Juengel E, et al. Evaluation of TKTL1 as a biomarker in serum of prostate cancer patients. Cent European J Urol. 2016;69(3):247–51. doi: 10.5173/ceju.2016.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu P, Cao Z, Wu S. New progress of epigenetic biomarkers in urological cancer. Dis Markers. 2016;2016:9864047. doi: 10.1155/2016/9864047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferro M, Buonerba C, Terracciano D, et al. Biomarkers in localized prostate cancer. Future Oncol. 2016;12(3):399–411. doi: 10.2217/fon.15.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa-Pinheiro P, Montezuma D, Henrique R, et al. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics. 2015;7(6):1003–15. doi: 10.2217/epi.15.56. [DOI] [PubMed] [Google Scholar]
- 12.Geybels MS, Wright JL, Bibikova M, et al. Epigenetic signature of Gleason score and prostate cancer recurrence after radical prostatectomy. Clin Epigenetics. 2016;8:97. doi: 10.1186/s13148-016-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blute ML, Jr, Damaschke NA, Jarrard DF. The epigenetics of prostate cancer diagnosis and prognosis: Update on clinical applications. Curr Opin Urol. 2015;25(1):83–88. doi: 10.1097/MOU.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strand SH, Orntoft TF, Sorensen KD. Prognostic DNA methylation markers for prostate cancer. Int J Mol Sci. 2014;15(9):16544–76. doi: 10.3390/ijms150916544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Peng Y, Yang F, et al. PCDH8 is frequently inactivated by promoter hypermethylation in liver cancer: Diagnostic and clinical significance. J Cancer. 2016;7(4):446–52. doi: 10.7150/jca.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YL, Wang YL, Fu XL, et al. Aberrant methylation of PCDH8 is a potential prognostic biomarker for patients with clear cell renal cell carcinoma. Med Sci Monit. 2014;20:2380–85. doi: 10.12659/MSM.892433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin YL, Wang YL, Ma JG, et al. Clinical significance of protocadherin 8 (PCDH8) promoter methylation in non-muscle invasive bladder cancer. J Exp Clin Cancer Res. 2014;33:68. doi: 10.1186/s13046-014-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu JS, Koujak S, Nagase S, et al. PCDH8, the human homolog of PAPC, is a candidate tumor suppressor of breast cancer. Oncogene. 2008;27(34):4657–65. doi: 10.1038/onc.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu WB, Gui SL, Lin YL, et al. Promoter methylation of protocadherin8 is an independent prognostic factor for biochemical recurrence of early-stage prostate cancer. Med Sci Monit. 2014;20:2584–89. doi: 10.12659/MSM.893083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin YL, Deng QK, Wang YH, et al. Aberrant protocadherin17 (PCDH17) methylation in serum is a potential predictor for recurrence of early-stage prostate cancer patients after radical prostatectomy. Med Sci Monit. 2015;21:3955–60. doi: 10.12659/MSM.896763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morlacco A, Pan J, Karnes RJ. Risk-prediction tools in prostate cancer: The challenge of tailoring. Asian J Androl. 2016;18(6):952. doi: 10.4103/1008-682X.179526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng QK, Lei YG, Lin YL, et al. Prognostic value of Protocadherin10 (PCDH10) methylation in serum of prostate cancer patients. Med Sci Monit. 2016;22:516–21. doi: 10.12659/MSM.897179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Liu M, Guan Y, et al. Hypoxia-responsive mir-301a and mir-301b promote radioresistance of prostate cancer cellsvia downregulating NDRG2. Med Sci Monit. 2016;22:2126–32. doi: 10.12659/MSM.896832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Yang X, Si T, et al. Clinicopathological and prognostic factors in 106 Prostate cancer patients aged ≤55 years: A single-center study in China. Med Sci Monit. 2016;22:3935–42. doi: 10.12659/MSM.901040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warton K, Mahon KL, Samimi G. Methylated circulating tumor DNA in blood: Power in cancer prognosis and response. Endocr Relat Cancer. 2016;23(3):R157–71. doi: 10.1530/ERC-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellinger J, Müller SC, Dietrich D. Epigenetic biomarkers in the blood of patients with urological malignancies. Expert Rev Mol Diagn. 2015;15(4):505–16. doi: 10.1586/14737159.2015.1019477. [DOI] [PubMed] [Google Scholar]
- 27.How Kit A, Nielsen HM, Tost J. DNA methylation based biomarkers: Practical considerations and applications. Biochimie. 2012;94(11):2314–37. doi: 10.1016/j.biochi.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Hoque MO. DNA methylation changes in prostate cancer: Current developments and future clinical implementation. Expert Rev Mol Diagn. 2009;9(3):243–57. doi: 10.1586/erm.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang D, Zhao W, Liao X, et al. Frequent silencing of protocadherin 8 by promoter methylation, a candidate tumor suppressor for human gastric cancer. Oncol Rep. 2012;28(5):1785–91. doi: 10.3892/or.2012.1997. [DOI] [PubMed] [Google Scholar]