Abstract
The third member of the Dickkopf family (DKK-3), also known as reduced expression in immortalized cells (REIC), is a tumor suppressor present in a variety of tumor cells. Regarding the regulation of the Wnt/β-catenin signaling pathway, exogenous DKK-1 and DKK-2 are reported to inhibit Wnt signaling by binding the associated effectors. However, whether exogenous DKK-3 inhibits Wnt signaling remains unclear. A recombinant protein of human full-length DKK-3 was used to investigate the exogenous effects of the protein in vitro in KPK1 human renal cell carcinoma cells. It was demonstrated that the expression of phosphorylated (p-)β-catenin (inactive form as the transcriptional factor) was increased in KPK1 cells treated with the exogenous DKK-3 protein. The levels of non-p-β-catenin (activated form of β-catenin) were consistently decreased. It was revealed that the expression of transcription factor (TCF) 1 and c-Myc, the downstream transcription factors of the Wnt/β-catenin signaling pathway, was inhibited following treatment with DKK-3. A cancer cell viability assay confirmed the anti-proliferative effects of exogenous DKK-3 protein, which was consistent with a suppressed Wnt/β-catenin signaling cascade. In addition, as low-density lipoprotein receptor-related protein 6 (LRP6) is a receptor of DKK-1 and DKK-2 and their interaction on the cell surface inhibits Wnt/β-catenin signaling, it was examined whether the exogenous DKK-3 protein affects LRP6-mediated Wnt/β-catenin signaling. The LRP6 gene was silenced and the effects of DKK-3 on the time course of the upregulation of p-β-catenin expression were subsequently analyzed. Notably, LRP6 depletion elevated the base level of p-β-catenin; however, there was no significant effect on its upregulation course or expression pattern. These findings indicate that exogenous DKK-3 upregulates p-β-catenin and inhibits Wnt/β-catenin signaling in an LRP6-independent manner. Therefore, exogenous DKK-3 protein may inhibit the proliferation of KPK1 cells via inactivating Wnt/β-catenin signaling.
Keywords: third member of the Dickkopf family/reduced expression in immortalized cells, Wnt, β-catenin, kidney cancer, cell proliferation
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults, accounting for ~3% of all adult malignancies (1). It is the seventh most common type of cancer in males and the ninth most common cancer type in females, representing ~90% of all kidney malignancies (2). The incidence and mortality rates of RCC have increased in recent years: 65,150 patients are diagnosed and 13,680 RCC-associated mortalities occur each year (3). Therefore, improving current understanding of the mechanisms underlying RCC progression and developing novel therapeutic approaches for RCC are required.
Wnt signaling serves an important role in disease development processes in adults (4), triggering at least two, perhaps three, pathways that employ Wnt receptors of the frizzled seven transmembrane classes. The first of these is the canonical Wnt/β-catenin pathway (5), in which Wnt3a, a member of the Wnt ligands, binds to a frizzled receptor and the corresponding co-receptor of low-density lipoprotein receptor-related protein 6 (LRP6). This results in the stabilization of cytosolic β-catenin and facilitates its translocation into the nucleus to activate downstream transcription factors (6). The second pathway involved is the planar cell polarity (PCP) pathway, which does not involve β-catenin but recruits small GTPases of the Rho/Cdc42 family to activate c-Jun N-terminal kinase (JNK); in vertebrates, this appears to be triggered by Wnts (7). The final possible pathway, the Wnt/Ca2+ cascade, is still largely controversial and may overlap in part with the PCP pathway (8).
Dickkopf (DKK) genes comprise an evolutionary conserved gene family of four members (DKK 1–4) and exhibit distinct cross-overlapping expression patterns in humans and mice (9). Exogenous DKK 1, 2 and 4 are reported to possess similar functions in suppressing the activity of Wnt/β-catenin (canonical) pathway by binding to the co-receptor LRP5/6 with high affinity (10,11). However, the suppressive role of exogenous DKK-3 in the Wnt/β-catenin signaling cascade has been controversial. DKK-3, the most divergent member of the DKK family in terms of DNA sequence, function and evolution, alternatively known as reduced expression in immortalized cells (REIC), does not appear to bind to LRP5/6 (12) or inhibit the Wnt/β-catenin signaling pathway, as has been reported in numerous studies (9,11,13,14).
The recombinant protein of full length DKK-3 was prepared in the current study in order to investigate the exogenous effects of the protein in vitro in KPK1 human renal cell carcinoma cells. The anti-proliferative effects of the DKK-3 protein were assessed to determine a possible therapeutic use for the protein. To clarify whether exogenous DKK-3 inhibits Wnt/β-catenin signaling, the effects of recombinant DKK-3 protein on the levels of phosphorylated (p-) and non-p-β-catenin in KPK1 cells were investigated. The involvement of the LRP6 transmembrane receptor in the extracellular actions of DKK-3 protein and in the Wnt/β-catenin signaling pathway was also evaluated.
Materials and methods
Cell culture
The KPK1 human renal cell carcinoma cell line was kindly provided by Professor S. Naito (Department of Urology, University of Kyushu, Fukuoka, Japan) (15). The PC3 human prostate cancer cell line and the 211H human malignant pleural biphasic mesothelioma cell line were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), penicillin (100 IU/ml) and streptomycin (100 µg/ml). The cells were cultured at 37°C in an atmosphere containing 5% CO2 and air, and split at a ratio of 1:5 every three days.
Preparation of the recombinant DKK-3 protein
The recombinant protein of human full length DKK-3 was transiently expressed in FreeStyle™ 293-F cells (Thermo Fisher Scientific, Inc.) using Freestyle 293 Expression Medium and the 293Fectin transfection reagent (Thermo Fisher Scientific, Inc.), in accordance with the manufacturer's instructions. Briefly, exponentially growing cells (1×106 cells/ml) with 180 ml medium were prepared in a 500-ml flask. Following transfection with 180 µg each of the expression plasmid DNA and 293Fectin complex, the cells were cultivated using an orbital shaker (125 rpm) at 37°C in the presence of 8% CO2 for four days. The secreted proteins in the culture medium were concentrated using Amicon Ultra-15 Centrifugal Filters (EMD Millipore, Billerica, MA, USA), then purified and stored as previously described (16).
Western blot analysis
The cells were treated with the recombinant DKK-3 protein at a final concentration of 10 µg/ml in the culture medium, and the cell lysate was obtained prior to treatment and at 1, 6, 24, 48 and 72 h after treatment. The cells were collected in ice-cold lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin] for 15 min on ice (17). The cell fragments were removed by centrifugation (21,500 × g) at 4°C for 10 min and the supernatant lysate was immediately stored at −80°C. The protein concentrations were determined using the Pierce BCA protein assay reagent kit (Thermo Fisher Scientific, Inc.). After adding 5X loading buffer [450 mM Tris (pH 6.8), 45% sucrose, 10% β-mercaptoethanol, 15% SDS and bromophenol blue], the extracted cell proteins (10 µg) were separated using a Mini-Protean® TGX™ gels kit (10% 15-well comb; 15 µl; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The proteins on the gel were then transferred onto a PVDF (Thermo Fisher Scientific, Inc.) membrane using a Trans-Blot Turbo Transfer System (Bio-Rad Laboratories, Inc.). Following the transfer, the membrane was blocked in 5% non-fat milk (cat. no. 1706404XTU; Bio-Rad Laboratories, Inc.) diluted with TBST buffer (Bio-Rad Laboratories, Inc.; cat. no. 1610734) and then incubated with the following primary antibodies (all used as supplied) overnight at 4°C: p-β-catenin (cat. no. 9561), non-P-β-catenin (cat. no. 8814), p-glycogen synthase kinase 3 (GSK-3)β (cat. no. 9323), transcription factor 1 (TCF1; cat. no. 2203), c-Myc (cat. no. 9402), Met (cat. no. 4560), low-density lipoprotein receptor-related protein 6 (LRP6; cat. no. 3395), Wnt3a (cat. no. 2721) and β-actin (cat. no. 4967) (all from Cell Signaling Technology, Inc., Danvers, MA, USA). After washing the membrane in TBST buffer three times (5 min each), the membrane was incubated with the anti-rabbit (cat. no. 9670531) or anti-mouse (cat. no. 9471754) secondary antibodies (GE Healthcare Life Sciences, Little Chalfont, UK) with 5% BSA in TBST (1:5,000) buffer for 1 h at room temperature. The protein-antibody complexes were visualized using the enhanced chemiluminescence detection method (ECL Prime Western Blotting Detection Reagent; GE Healthcare Life Sciences) and medical X-ray film.
Cell proliferation assay
KPK1 cells were plated in 96-well plates at a final volume of 100 µl culture medium (1,000 cells/well) at 37°C with 6.5% CO2. Following a 12-h incubation, the cells were treated with DKK-3 protein at final concentrations of 10 or 50 µg/ml in culture medium. For the control treatment, an equal volume of PBS was added. Cell viability was assessed at the indicated days (days 1, 2, 3 and 4) following treatment using a Cell Proliferation kit II (XTT; Roche Diagnostics GmbH, Mannheim, Germany), in accordance with the manufacturer's instructions. The conversion of the orange formazan dye was measured using a microplate reader (model 550; Bio-Rad Laboratories, Inc.) at an absorbance wavelength of 492–690 nm.
Small interfering (si)RNA transfection for LRP6 knockdown
KPK1 cells (2×105 cells per well in 6-well plates) were incubated with 2 ml antibiotic-free culture medium supplemented with 10% FBS for 18–24 h until the cells were 60–80% confluent. The siRNA reagent [LRP6 siRNA (h); #sc-37233; Santa Cruz Biotechnology, Inc., Dallas, TX, USA] and matched control reagent were transfected into the KPK1 cells for 6 h at 37°C in a 5% CO2 incubator. Following transfection, the medium was changed to complete medium and further incubated for 24 h. The cells were then treated with the recombinant DKK-3 protein at a final concentration of 10 µg/ml in culture medium, and the cell lysates were obtained for western blot analysis at the indicated time points.
Statistical analysis
The data are presented as the means ± standard error. Unpaired Student's t-tests were performed to analyze the statistical significance of differences between two groups. Statistical analyses were performed using StatView version 4.5 software (Abacus Concepts, Piscataway, NJ, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Exogenous DKK-3 inhibits Wnt/β-catenin signaling in a time-dependent manner in KPK1 cells
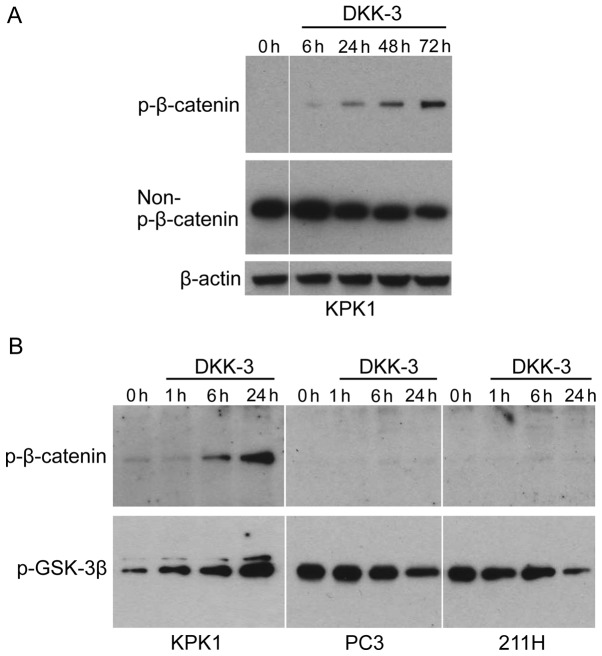
KPK1 cells were treated with the recombinant DKK-3 protein, following which the expression of p-β-catenin (inactive form of β-catenin) and non-p-β-catenin (activated form) was examined by western blot analysis (Fig. 1A). The p-β-catenin was gradually increased in KPK1 cells in a time-dependent manner. The level of non-p-β-catenin was correspondingly reduced, indicating that exogenous DKK-3 protein inhibits Wnt/β-catenin signaling in a time-dependent manner. To further elucidate the inhibitory mechanism in Wnt/β-catenin signaling effected by the exogenous DKK-3 protein, the p-level of glycogen synthase kinase-3β (GSK-3β) was analyzed, which has been demonstrated to phosphorylate β-catenin, thus targeting it for degradation (5,6). In KPK1 cells, the level of p-GSK-3β was gradually increased as the p-β-catenin was increased (Fig. 1B). By contrast, in PC3 and 211H cells the levels of p-β-catenin were low and did not increase during the course of treatment with DKK-3 protein. In these cells, contrary to KPK1 cells, the level of p-GSK-3β was gradually decreased.
Figure 1.
Using recombinant human full-length DKK-3 protein, the exogenous effects of DKK-3 were investigated with respect to Wnt/β-catenin signaling. (A) Cell samples were obtained prior to treatment and at 6, 24, 48 and 72 h after treatment. The expression of p-β-catenin and non-p-β-catenin was examined in KPK1 human renal cell carcinoma cells via western blot analysis. β-actin was used as the loading control. (B) Cell samples were obtained prior to treatment and at 1, 6 and 24 h after treatment. The expression of p-β-catenin and p-GSK-3β was examined in the indicated human cancer cells via western blot analysis. DKK, Dickkopf-related protein; p-, phosphorylated; GSK-3β, glycogen synthase kinase 3β; REIC, reduced expression in immortalized cells.
Exogenous DKK-3 inhibits the downstream targets of Wnt/β-catenin signaling
It was investigated whether DKK-3 protein suppresses the expression of TCF1 and c-Myc, the downstream key effectors of Wnt/β-catenin signaling (Fig. 2). The levels of two key targets, (known as tumor progression factors), which have an important role in the cell cycle, apoptosis and cellular transformation (4,6), were significantly suppressed following treatment. TCF1 expression was markedly reduced during the early phase following DKK-3 treatment and subsequently restored. With regard to c-Myc, the expression level was gradually reduced after treatment. The expression of Wnt3a was also decreased in a time-dependent manner. By contrast, no significant changes were observed in the expression of Met, the level of which was regulated by signaling pathways other than Wnt/β-catenin (18,19). These findings indicate that exogenous DKK-3 protein downregulates the Wnt/β-catenin-targeted molecules of TCF1 and c-Myc.
Figure 2.
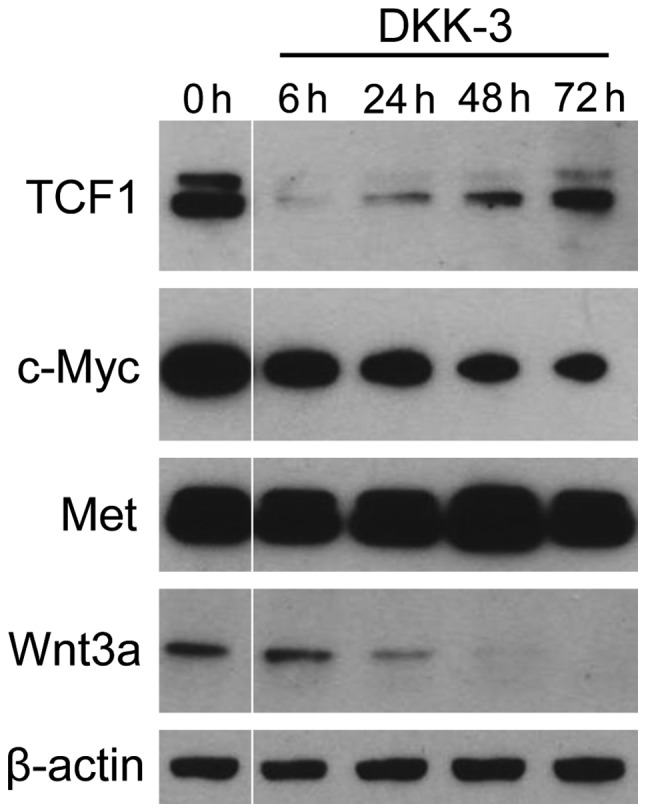
The exogenous effects of DKK-3 protein were investigated in KPK1 cells in terms of the downstream targets of Wnt/β-catenin signaling. Cell samples were obtained prior to treatment and at 6, 24, 48 and 72 h after treatment. The expression of TCF1 and c-Myc was examined via western blot analysis as the key effectors of the signaling pathway. β-actin was used as the loading control. DKK, Dickkopf-related protein; REIC, reduced expression in immortalized cells; TCF1, transcription factor 1.
Exogenous DKK-3 exhibits anti-proliferative effects in KPK1 cells
To examine the anti-proliferative effects of exogenous DKK-3 protein, the viability of KPK1 cells post-treatment was assayed. The viability of cells treated with the final concentrations of 10 or 50 µg/ml DKK-3 protein was evaluated, and a significant inhibition of cell proliferation was observed in each case at day 4 (Fig. 3). Dose-dependency was observed in the inhibitory effects.
Figure 3.
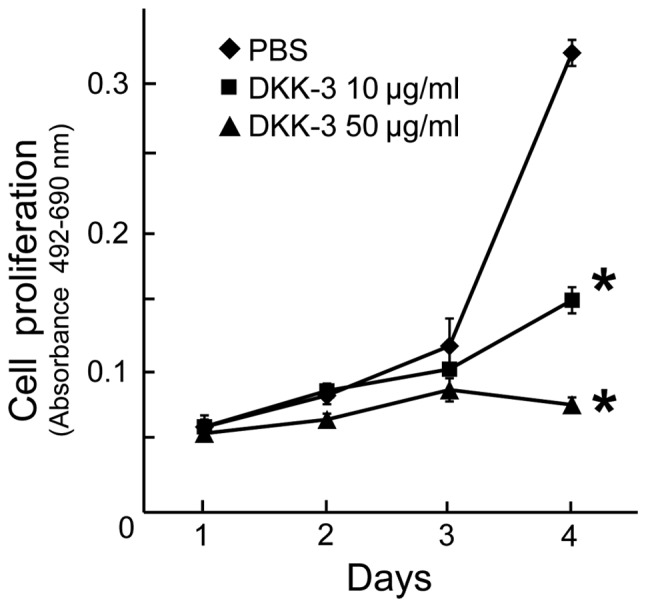
The anti-proliferative effects of exogenous DKK-3 protein were investigated in KPK1 cells using cell viability assay kits. Cell proliferation was determined at the indicated days following treatment. There was a significant difference in each DKK-3 treatment group when compared with the control (*P<0.05). The data were obtained for three experiments. DKK, Dickkopf-related protein; REIC, reduced expression in immortalized cells.
LRP6 knockdown elevated the base level of the Wnt/β-catenin signaling but did not influence the upregulation course or pattern
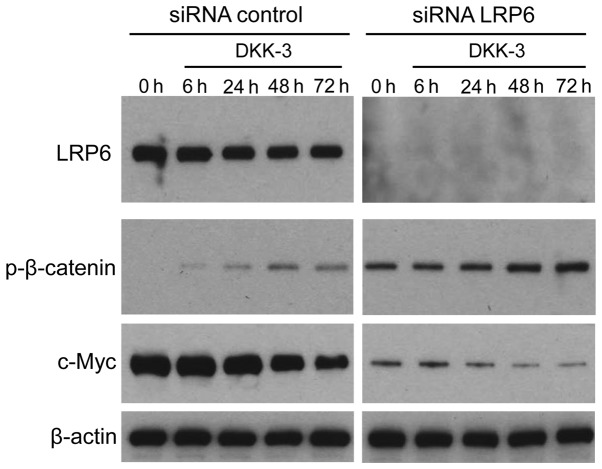
It was investigated whether exogenous DKK-3 protein affects LRP6-mediated Wnt/β-catenin signaling in KPK1 cells. The LRP6 gene was silenced using specific siRNA, subsequently facilitating verification of expression depletion via western blot analysis (Fig. 4). Under a lack of LRP6 expression, the effects of DKK-3 were analyzed with respect to the time course of p-β-catenin and c-Myc expression. However, LRP6 depletion upregulated the basal expression levels of p-β-catenin, thus maintaining its upregulation course and pattern of control. p-β-catenin expression in the knocked LRP6 group was strongly upregulated compared with the un-knocked LRP6. In addition, the basal expression of p-β-catenin was also enhanced by DKK-3 without LRP6. For c-Myc downstream of β-catenin, the base level was attenuated, maintaining its downregulation course and pattern. Depletion of the Wnt pathway receptor LRP6 exerted no significant effects on the extracellular DKK-3-induced course of Wnt/β-catenin signaling, indicating that DKK-3 blocks the signaling in a LRP6-independent manner in KPK1 cells.
Figure 4.
It was evaluated whether exogenous DKK-3 protein affects LRP6-mediated Wnt/β-catenin signaling. Following depletion of LRP6 expression by siRNA, the exogenous effects of DKK-3 were investigated in KPK1 cells in terms of the downstream targets of Wnt signaling. Cell samples were obtained prior to treatment and at 6, 24, 48 and 72 h after treatment. The expression of p-β-catenin and c-Myc was examined via western blot analysis as the key effectors of the signaling pathway. β-actin was used as the loading control. DKK, Dickkopf-related protein; p-, phosphorylated; LRP6, low-density lipoprotein receptor-related protein 6; REIC, reduced expression in immortalized cells; siRNA, small interfering RNA.
Discussion
DKK-3 is a tumor suppressor in a variety of cancer cells; however, the role and mechanism of exogenous DKK-3 protein in the Wnt/β-catenin signaling pathway has remained unclear (9). In addition, it is unclear whether or not exogenous DKK-3 (or DKK-1/2) inhibits Wnt signaling (13). In the present study, it was demonstrated that the levels of p-β-catenin were increased in KPK1 cells treated with exogenous DKK-3 protein. The non-p-β-catenin was consistently decreased, indicating that the DKK-3 protein functions as an inhibitor of Wnt/β-catenin on the cell surface.
Few prior studies have detailed the involvement of extracellular DKK-3 protein in Wnt/β-catenin signaling (9–11). In DKK-3-transfected human glioma cells, a decrease in non-p-β-catenin (activated form of β-catenin) was observed in parallel with an increase in the levels of intra- and extracellular DKK-3 protein (20). The authors suggested that extracellular DKK-3 inhibited the Wnt/β-catenin signaling pathway via binding to transmembrane receptors. The findings of previous studies demonstrating a lack of modulation by extracellular DKK-3 protein on the Wnt/β-catenin pathway (20), as well as the results of the current study, suggest that the role of DKK-3 may be dependent upon the cell type and characteristics.
It was revealed that the expression of TCF1 and c-Myc, the downstream transcription factors of Wnt/β-catenin signaling in cancer transcription cascades (4–6), is downregulated following treatment with recombinant DKK-3 protein. These results not only support the suppressive effects of exogenous DKK-3 protein on Wnt/β-catenin signaling, but also suggest an inhibitory role of DKK-3 in the cell proliferation promoted by TCF1 and c-Myc. Indeed, a cancer cell viability assay confirmed the anti-proliferative effects of exogenous DKK-3 protein in KPK1 cells, as presented in Fig. 3, possibly due to the suppressed levels of TCF1 and c-Myc in the Wnt/β-catenin signaling cascade (21). Notably, the significant downregulation of TCF1 was observed in the early stages (6 h) following treatment, and the expression of c-Myc gradually decreased, as depicted in Fig. 2. As TCF1 is a transcription factor for c-Myc expression (4,5,21), the downregulation of TCF1 likely induced the gradual decrease in c-Myc levels. The protein expression levels of Met were also examined; Met is regulated by transcription factors other than TCF1 (18,19). There were no significant changes recorded in Met expression following treatment with recombinant DKK-3 protein, suggesting that its regulation is independent of exogenous DKK-3-associated modifications of Wnt/β-catenin signaling. With regard to the reduced expression of Wnt3a following DKK-3 treatment, modifications to the activity of β-catenin/TCF1 target genes may have suppressed its transcription (6,21). The downregulation of these molecules may be due to transcriptional suppression via the Wnt/β-catenin signaling pathway effected by exogenous DKK-3 protein.
As LRP6 is a cell-surface receptor for extracellular DKK-1 and DKK-2 proteins, and these interactions inhibit the Wnt/β-catenin signaling pathway (11,14), it was examined whether or not exogenous DKK-3 protein affects the LRP6-mediated Wnt/β-catenin signaling in KPK1 cells. The LRP6 gene was silenced and the effects of DKK-3 on the time course of the upregulation of p-β-catenin expression subsequently analyzed. Notably, LRP6 depletion elevated the basal level of p-β-catenin; however, there was no significant effect on its upregulation course or pattern. With regard to downstream c-Myc, the basal level declined but maintained its downregulation course and pattern. The involvement of extracellular DKK-3 in the Wnt/β-catenin signaling pathway may function as an alternative mechanism rather than as an antagonist blocking LRP6 in KPK1 cells. A previous study reported that DKK-3 interacts with the Wnt pathway receptors Kremen1 (Krm1) and Krm2, and not with LRP6 in biochemical assays (22), it is worth further exploring the modification of Wnt/β-catenin signaling in terms of the interaction of DKK-3 with Krm receptors on the cell surface.
In order to analyze cell types other than KPK1, the exogenous DKK-3-associated modifications of Wnt/β-catenin signaling were examined in PC3 human prostate cancer cells and 211H human malignant mesothelioma cells. Treatment with recombinant DKK-3 protein did not lead to elevation of p-β-catenin levels in PC3 cells or 211H cells. The results indicated that the inhibitory effect of DKK-3 on Wnt/β-catenin signaling could be absent or masked in these cell types, possibly due to cross-talk signaling occurring in untested pathways. The effect of exogenous DKK-3 protein on the level of p-GSK-3β, the possible upstream regulator of p-β-catenin in Wnt/β-catenin signaling, was also investigated. However, the effect on p-GSK-3β levels was contrary between the KPK1 human kidney cancer cells and the other two cell types. Further studies may enable elucidation of the molecular mechanisms by which exogenous DKK-3 protein modifies p-β-catenin levels in the GSK-3β/β-catenin axis.
In conclusion, it was demonstrated for the first time that exogenous treatment with DKK-3 protein exerts anti-proliferative effects in KPK1 cells and inhibits the Wnt/β-catenin signaling pathway in an LRP6-independent manner. Exogenous DKK-3 protein appears to inhibit KPK1 cell proliferation by inactivating Wnt/β-catenin signaling through the upregulation of p-β-catenin. Although the binding partner of DKK-3 on the cell surface remains to be elucidated, these findings assist in clarifying the anti-cancer mechanisms of extracellular DKK-3. The Wnt signaling pathway serves an important role in the carcinogenesis and the progression of renal cell carcinoma (23), and the in vivo tumor-suppressive effects of the recombinant full length DKK-3 protein were previously demonstrated in the RENCa tumor model of murine renal carcinoma (24). Therefore, recombinant DKK-3 protein may be a promising agent for treating renal cell carcinoma.
Acknowledgements
This study was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan, Takeda Science Foundation and Novartis Pharma Research Grants (JSPS KAKENHI, grant nos. JP15H04297, JP15H04974, JP15K10590 and JP16K11004) and the China Scholarship Council (grant no. 201308210161). The authors thank Ms. Fusaka Oonari (Okayama University) for her valuable assistance.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 5.Cadigan KM, Liu YI. Wnt signaling: Complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–260. doi: 10.1016/S1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 8.Kohn AD, Moon RT. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 10.Brott BK, Sokol SY. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol. 2002;22:6100–6110. doi: 10.1128/MCB.22.17.6100-6110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 12.Fujii Y, Hoshino T, Kumon H. Molecular simulation analysis of the structure complex of C2 domains of DKK family members and β-propeller domains of LRP5/6: Explaining why DKK3 does not bind to LRP5/6. Acta Med Okayama. 2014;68:63–78. doi: 10.18926/AMO/52403. [DOI] [PubMed] [Google Scholar]
- 13.Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/S0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 14.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 15.Naito S, Kanamori T, Hisano S, Tanaka K, Momose S, Kamata N. Human renal cell carcinoma: Establishment and characterization of two new cell lines. J Urol. 1982;128:1117–1121. doi: 10.1016/S0022-5347(17)53357-1. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, Kashiwakura Y, Huang P, Ochiai K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH, Kumon H. Immunological aspects of REIC/Dkk-3 in monocyte differentiation and tumor regression. Int J Oncol. 2009;34:657–663. doi: 10.3892/ijo_00000191. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi M, Sadahira T, Ueki H, Kinoshita R, Murata H, Yamamoto KI, Futami J, Nasu Y, Ochiai K, Kumon H, et al. Robust cancer-specific gene expression by a novel cassette with hTERT and CMV promoter elements. Oncol Rep. 2017 Jun 12; doi: 10.3892/or.2017.5710. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 18.Abounader R, Ranganathan S, Kim BY, Nichols C, Laterra J. Signaling pathways in the induction of c-met receptor expression by its ligand scatter factor/hepatocyte growth factor in human glioblastoma. J Neurochem. 2001;76:1497–1508. doi: 10.1046/j.1471-4159.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- 19.Morozov VM, Massoll NA, Vladimirova OV, Maul GG, Ishov AM. Regulation of c-met expression by transcription repressor Daxx. Oncogene. 2008;27:2177–2186. doi: 10.1038/sj.onc.1210865. [DOI] [PubMed] [Google Scholar]
- 20.Mizobuchi Y, Matsuzaki K, Kuwayama K, Kitazato K, Mure H, Kageji T, Nagahiro S. REIC/Dkk-3 induces cell death in human malignant glioma. Neuro Oncol. 2008;10:244–253. doi: 10.1215/15228517-2008-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura RE, Hackam AS. Analysis of Dickkopf3 interactions with Wnt signaling receptors. Growth Factors. 2010;28:232–242. doi: 10.3109/08977191003738832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q, Krause M, Samoylenko A, Vainio S. Wnt signaling in renal cell carcinoma. Cancers (Basel) 2016;8 doi: 10.3390/cancers8060057. pii: E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinoshita R, Watanabe M, Huang P, Li SA, Sakaguchi M, Kumon H, Futami J. The cysteine-rich core domain of REIC/Dkk-3 is critical for its effect on monocyte differentiation and tumor regression. Oncol Rep. 2015;33:2908–2914. doi: 10.3892/or.2015.3885. [DOI] [PubMed] [Google Scholar]