Abstract
Persistent infection with Helicobacter pylori may contribute to the carcinogenesis of gastric cancer through modulating local prostaglandin E2 (PGE2) levels. Cyclooxygenase-2 (COX-2) and 15-hydroxy prostaglandin dehydrogenase (15-PGDH) are two key enzymes that regulate PGE2 synthesis and inactivation, respectively. The present study was designed to investigate the expression of COX-2 and 15-PGDH in gastric cancer specimens (n=66) in comparison to that of control specimens (n=70) and, furthermore, to semi-quantitatively assess the level of COX-2 and 15-PGDH mRNA and protein in tissues with or without H. pylori infection by reverse transcription-polymerase chain reaction and immunohistochemistry, respectively. It was revealed that COX-2 was expressed in almost all gastric cancer specimens infected with H. pylori (32 out of 33 specimens), but it was also expressed in 2/3 gastric cancers without H. pylori infection (22 out of 33 specimens). By contrast, COX-2 was expressed in <1/6 control subjects regardless of H. pylori infection. Furthermore, 15-PGDH was expressed in control samples but significantly downregulated in gastric cancer specimens. H. pylori infection resulted in slight inhibition of 15-PGDH in control subjects, but significant inhibition of 15-PGDH mRNA expression and protein synthesis in the gastric cancer specimens. These findings indicated that COX-2 and 15-PGDH, the two enzymes that regulate PGE2 levels, were significantly altered in gastric cancer, and that H. pylori may contribute to gastric carcinogenesis through modulating COX-2 and 15-PGDH mRNA expression and protein synthesis.
Keywords: Helicobacter pylori, carcinogenesis, gastric cancer, prostaglandin E2 levels, cyclooxygenase-2, 15-hydroxy prostaglandin dehydrogenase
Introduction
Helicobacter pylori is a gram-negative, spiral-shaped bacterium present in the human stomach. It is estimated that H. pylori infects ~50% of the world's population, and it is a risk factor not only for the pathogenesis of chronic gastritis and peptic ulcer disease (1), but has also been classified as a carcinogen by the World Health Organization (2). Chronic infection with H. pylori results in prolonged inflammation which is mediated by inflammatory mediators, including cytokines and prostaglandin E2 (PGE2) (3). In this regard, upregulation of the inducible type of cyclooxygenase-2 (COX-2) (4) and elevated levels of pro-inflammatory cytokines (1) have been observed in the gastric mucosa of patients with H. pylori infection.
COX-2 is a key rate-limiting and inducible enzyme responsible for the formation of prostanoids and thromboxanes (5). PGE2 is the main prostanoid generated from arachidonic acid, catalyzed by COX-2, and it exerts physiological as well as pathological effects through prostaglandin receptors (6,7). Elevation of PGE2 has been observed in multiple types of cancer and is associated with tumor growth and angiogenesis (7,8). PGE2 levels are regulated not only by its synthetic enzyme, COX-2, and microsomal prostaglandin synthase (mPGES), but also by its degrading enzyme, 15-hydroxy prostaglandin dehydrogenase (15-PGDH) (9). 15-PGDH is the key enzyme that inactivates PGE2 by catalyzing the oxidation of its 15 (S)-hydroxy group, which results in the formation of inactive 15-keto metabolites (10). Consistent with its function of regulating the local concentration of PGE2, studies have demonstrated that 15-PGDH is expressed in normal colonic epithelial cells, but transcription of 15-PGDH mRNA is lost in the majority of colon cancers (10–12).
Although the incidence of gastric cancer is declining, it remains the fourth most common cancer and the second leading cause of cancer-associated mortality globally (13). Multiple factors may be involved in the carcinogenesis of gastric cancer, including genetics and environmental factors. H. pylori is one of the most important environmental risk factors for gastric malignancies (13,14). Persistent inflammation in response to H. pylori infection is hypothesized to contribute to tumor cell proliferation and metastasis, and to affect survival (15,16). While the molecular mechanisms of gastric tumors remain poorly understood, modulation of COX-2 expression and 15-PGDH mRNA, and the resultant elevated local PGE2 levels, may be responsible for H. pylori-associated carcinogenesis. Therefore, prostaglandins, particularly PGE2, may be involved in maintaining gastric mucosal integrity through regulating gastric mucosal blood flow, kinetics of epithelial cells, synthesis of mucus and inhibition of gastric acid secretion (17). By contrast, PGE2 transactivates epidermal growth factor receptor and initializes mitogenic signal transduction in gastric epithelial and colon cancer cells (18), by which mechanism PGE2 may trigger carcinogenesis and proliferation of gastrointestinal cancers.
In the present study, the expression of COX-2 and 15-PGDH was investigated in gastric cancer specimens in comparison with normal tissues, and the levels of these enzymes were analyzed by grouping the samples with or without H. pylori infection.
Materials and methods
Study population
The present study enrolled a total of 66 patients who had received surgical resection of gastric cancer at the Second Affiliated Hospital of Tianjin Medical University (Tianjin, China) between January 2011 and August 2012, in order to study the potential involvement of H. pylori in the development of gastric cancer. Of these patients, 38 (57.6%) were male; the average age was 65.8 (range, 35–85) years; 23 patients were tumor node metastasis (TNM) stage I and II; 43 were TNM stage III and IV (19); and 44 patients had lymph node metastasis. In comparison, 70 patients who had no atrophic gastritis (as evidenced by gastric tissue biopsies) were also enrolled into the present study. Of these patients 32 patients were male, with an average age of 39.7 (range, 20–60) years. All the enrolled patients had no history of using non-steroid anti-inflammation drugs, antibiotics within 4 weeks of the study, anti-acid drugs or bismuth. Written consent was obtained from all patients and the study protocol was approved by the Ethics Committee of The Tianjin Medical University (Tianjin, China).
Determination of H. pylori infection
H. pylori infection was determined prior to surgery by the 14C-urea breath test (3).
Tissue specimen collection and immunohistochemistry
The tissues obtained via surgical resection from cancer patients and via biopsy from control subjects were fixed with 10% formalin, paraffin-embedded and sectioned (4 µm). The slides were deparaffinized with xylene and then gradually rehydrated with 100, 95, 85 and 75% ethanol followed by water. Following antigen retrieval with 10 mM citrate buffer (pH 6.0) at 96°C for 20 min and non-specific blocking with normal goat serum (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) at room temperature for 1 h, the tissue slides were allowed to react with monoclonal anti-COX-2 (cat no. 160112; dilution, 1:100) and anti-15-PGDH (cat no. 160615; dilution 1:200) (both from Cayman Chemical, Ann Arbor, MI, USA) overnight at 4°C. Following incubation with horseradish peroxidase-conjugated second antibody (1:1,000; cat no. ZB-2301; ZSGB-Bio Co., Ltd., Beijing, China) at room temperature for 20 min, the slides were developed with 3,3′-diaminobenzidine solution (ZSGB-Bio Co., Ltd.), followed by counterstaining with hematoxylin. Samples were examined under a light microscope (BX41; Olympus Corporation, Tokyo, Japan) and ≥5 random fields were captured.
All slides were reviewed and scored by one gastrointestinal pathologist. For 15-PGDH, the number of cells with positive 15-PGDH was scored under 5 high power field (magnification, ×400) as follows: 0, <5% cells positively stained; 1, 5–25% cells positively stained; 2, 25–50% cells positively stained; 3, 50–75% cells positively stained; and 4, >75% cells positively stained. COX-2 staining >10% was considered to be a positive sample of COX-2 expression. 15-PGDH was semi-quantitatively scored by counting positive cell number as well as intensity of positive staining as follows: 1, light brown; 2, brown; 3, dark brown. Total 15-PGDH positive staining score was calculated as follows: Score by positive cell number × score intensity of positive staining.
Reverse transcription-polymerase chain reaction (RT-PCR)
Expression of 15-PGDH mRNA was determined by RT-PCR. Briefly, total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and quantified using a spectrometer. Sequences of the primers were as follows: 15-PGDH forward, 5′-GCTGACCAGCAACAACTGAGA-3′ and reverse, 5′-CTGGACAAATGGCATTCAGTC-3′; and β-actin forward, 5′-CTGGGACGACATGGAGAAAA-3′ and reverse, 5′-AAGGAAGGCTGGAAGAGTGC-3′. Briefly, cDNA was synthesized using 1 µg total RNA with the RT kit (Aoke Biology Research Co., Ltd., Beijing, China). PCR was then performed using a commercially available kit (Aoke Biology Research Co., Ltd.). RT-PCR was performed according to the manufacturer's protocol (Aoke Biology Research Co., Ltd.). The thermocycling conditions performed were as follows: 95°C for 5 min, 94°C for 30 sec, 57°C for 30 sec, and 72°C for 30 sec; total 30 cycles followed by 72°C for 10 min.
Statistical analysis
Data were analyzed by SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Quantitative data was expressed as the mean ± standard deviation. Paired data was analyzed by Student's t-test. Event occurrence was examined by the χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical features of patients with or without H. pylori infection
Of the 66 patients with gastric cancer, 33 (50%) were infected with H. pylori, while 38 (54.3%) of the 70 control subjects had H. pylori infection. No significant difference was observed in age and gender between the patients with or without H. pylori infection (P>0.05; Table I).
Table I.
Characteristics of the control patients and patients with gastric cancer with or without H. pylori infection.
| H. pylori-positive, n | H. pylori-negative, n | |||||
|---|---|---|---|---|---|---|
| GC | Con | GC | Con | χ2 (GC/Con) | P-value | |
| Age (years) | 0.32/0.23 | >0.05 | ||||
| <60 | 11 | 28 | 12 | 24 | ||
| >60 | 22 | 10 | 21 | 8 | ||
| Sex | 0.29/0.03 | >0.05 | ||||
| Male | 21 | 17 | 17 | 15 | ||
| Female | 12 | 21 | 16 | 17 | ||
H. pylori, Helicobacter pylori; GC, gastric cancer; Con, control.
Comparison of COX-2 positivity in patients with gastric cancer with or without H. pylori infection
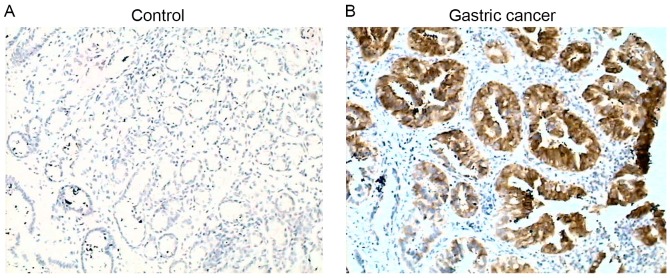
COX-2 protein was detectable in 14.3% (10/70) of control subjects, while it was detectable in 81.8% (54/66) of patients with gastric cancer, and was significantly increased compared with the control (P<0.01; Fig. 1). Of the 66 patients with gastric cancer, 33 were positive and 33 were negative for H. pylori infection. COX-2 was positively expressed in 32/33 (97%) patients with H. pylori infection, while it was positively expressed in 22/33 (66.7%) patients without H. pylori infection (P<0.01; Table II).
Figure 1.
Cyclooxygenase-2 protein expression in (A) normal and (B) gastric cancer specimens, as detected by immunostaining. Images presented are representative of n=38 (control) and n=33 (gastric cancer). Magnification, ×100.
Table II.
Scoring of 15-PGDH protein and mRNA levels and COX-2 protein levels in control vs. gastric cancer with or without H. pylori infection.
| H. pylori positive | H. pylori negative | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | N | 15-PGDH Protein | 15-PGDH mRNA | COX-2 +/− | N | 15-PGDH Protein | 15-PGDH mRNA | COX-2 +/− | P-valuea |
| Gastric cancer | 33 | 1.10±0.47 | 0.68±0.31 | 32/1 | 33 | 2.10±0.78 | 1.09±0.51 | 22/11 | <0.01 |
| Control | 38 | 10.02±1.24 | 2.72±1.20 | 6/32 | 32 | 11.54±1.40 | 3.03±1.32 | 4/28 | >0.05 |
| P-valueb | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
Comparison between H. pylori-positive and negative samples
comparison between control and gastric cancer samples. 15-PDGH, 15-hydroxy prostaglandin dehydrogenase; COX-2, cyclooxygenase-2; H. pylori, Helicobacter pylori.
In addition, COX-2 protein levels were significantly increased in patients with gastric cancer with lymphatic metastasis [100% (23/23) patients with, and 76.2% (16/21) without H. pylori infection; Table III] compared with patients without lymphatic metastasis [80% (8/10) patients with, and 58.3% (7/12) without H. pylori infection; P<0.01; Table III].
Table III.
Scoring of 15-PGDH protein and mRNA levels and COX-2 protein levels in gastric cancer with or without LM and H. pylori infection.
| H. pylori-positive, n | H. pylori-negative, n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metastasis | Total | 15-PGDH protein | 15-PGDH mRNA | COX-2 +/− | Total | 15-PGDH protein | 15-PGDH mRNA | COX-2 +/− | P-valuea |
| With LM | 23 | 0.46±0.19 | 0.18±0.07 | 23/0 | 21 | 0.90±0.40 | 0.44±0.20 | 16/5 | <0.01 |
| Without LM | 10 | 1.12±0.50 | 0.56±0.23 | 8/2 | 12 | 1.78±0.80 | 1.02±0.45 | 7/5 | <0.05 |
| P-valueb | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
Comparison between H. pylori-positive and -negative samples
comparison between with LM vs. without LM 15-hydroxy prostaglandin dehydrogenase; COX-2, cyclooxygenase-2; LM lymphatic metastasis; H. pylori, Helicobacter pylori.
However, patients had a similar positive rate of COX-2 expression in stage III–IV gastric cancer [100% (22/22) patients with H. pylori infection and 66.7% (14/21) without H. pylori infection; Table IV] compared with that in stage I–II gastric cancer [91% (10/11) patients with H. pylori infection and 66.7% (8/12) without H. pylori infection; Table IV].
Table IV.
Scoring of 15-PGDH protein and mRNA levels and COX-2 protein levels in gastric cancer at different stages with or without H. pylori infection.
| H. pylori-positive, n | H. pylori-negative, n | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer stage | Total | 15-PGDH protein | 15-PGDH mRNA | COX-2 +/− | Total | 15-PGDH protein | 15-PGDH mRNA | COX-2 +/− | P-valuea |
| I–II | 11 | 1.79±083 | 0.69±0.26 | 10/1 | 12 | 2.47±1.10 | 1.07±0.46 | 8/4 | <0.05 |
| III–IV | 22 | 0.81±0.36 | 0.27±0.11 | 22/0 | 21 | 1.32±0.53 | 0.42±0.17 | 14/7 | <0.05 |
| P-valueb | <0.01 | <0.01 | <0.01 | <0.01 | |||||
Comparison between H. pylori-positive and -negative samples
comparison between stage I–II and stage III–IV samples. 15-hydroxy prostaglandin dehydrogenase; COX-2, cyclooxygenase-2; H. pylori, Helicobacter pylori.
Comparison of 15-PGDH mRNA and protein expression in patients with gastric cancer with or without H. pylori infection
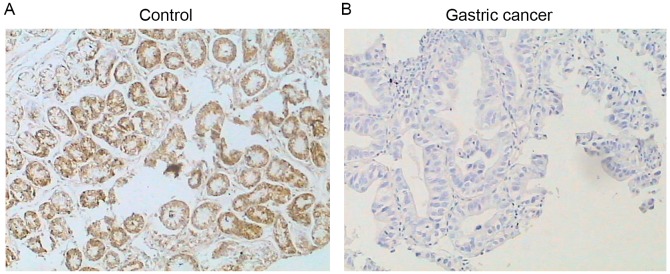
15-PGDH protein was detectable by immunostaining in the control subjects (Fig. 2), and no difference was observed in the protein level of 15-PGDH in the control subjects with (10.02±1.24) or without (11.54±1.40) H. pylori infection (P>0.05; Table II). Similarly, no difference was observed in the expression of 15-PGDH mRNA between control subjects with (2.72±1.20) or without (3.03±1.32) H. pylori infection (P>0.05; Table II). However, expression levels of mRNA and protein of 15-PGDH in the patients with gastric cancer were significantly reduced compared with normal subjects (P<0.01; Figs. 2 and 3). In addition, patients with H. pylori infection had a more significant decrease in 15-PGDH mRNA (0.68±0.31) and protein (1.10±0.47) compared with patients without the bacterial infection (1.09±0.51, mRNA; 2.10±0.78, protein; P<0.01; Table II).
Figure 2.
Protein expression of 15-hydroxy prostaglandin dehydrogenase in (A) normal and (B) gastric cancer specimens, as detected by immunostaining. Images presented are representative of n=38 (control) and n=33 (gastric cancer). Magnification, ×100.
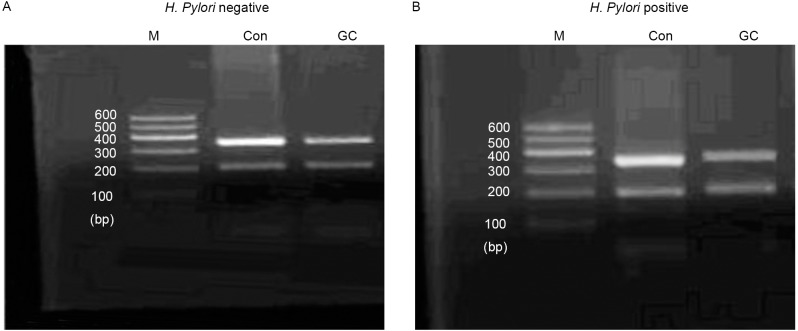
Figure 3.
Expression of 15-hydroxy prostaglandin dehydrogenase mRNA in normal patients and patients with gastric cancer (A) with or (B) without H. pylori infection, assessed by reverse transcription polymerase chain reaction. Data presented is representative of 3 separate experiments from each sample. M, DNA marker; Con, control; GC, gastric cancer; H. pylori, Helicobacter pylori.
Expression levels of 15-PGDH mRNA and protein were lowest in the patients with lymphatic metastasis and bacterial infection (0.18±0.07 for mRNA and 0.46±0.19 for protein; Table III), and were significantly lower compared with patients without lymphatic metastasis but with bacterial infection (0.56±0.23, mRNA; 1.12±0.50, protein; P<0.01; Table III) or patients with lymphatic metastasis but without bacterial infection (0.44±0.20, mRNA; 0.90±0.40, protein; P<0.01; Table III). Expression of 15-PGDH mRNA and protein was highest in the patients without lymphatic metastasis and negative infection of H. pylori (1.02±0.45, mRNA; 1.78±0.80, protein; P<0.01; Table III), which were significantly different from the other three groups (P<0.01; Table III).
Similarly, late stage patients with H. pylori infection had the lowest mRNA and protein levels of 15-PGDH (0.27±0.11, mRNA; 0.81±0.36, protein), which were significantly lower compared with stage I–II patients with bacterial infection (0.69±0.26, mRNA; 1.79±0.83, protein; P<0.01; Table IV) or stage III–IV patients without bacterial infection (0.42±0.17, mRNA; 1.32±0.53, protein; P<0.01; Table IV). Expression of 15-PGDH mRNA and protein was highest in the stage I–II patients without bacterial infection (1.07±0.46, mRNA; 2.47±1.10, protein) and were significantly increased compared with the other three groups (P<0.05; Table IV).
Discussion
Chronic inflammation is involved in the development of cancer. Bacterial infection of the gastrointestinal tract may trigger inflammation through elevating PGE2. COX-2 and 15-PGDH are two crucial enzymes that synthesize and degrade PGE2, respectively. In order to investigate the expression of COX-2 and 15-PGDH and its potential association with H. pylori infection in gastric cancer, 33 patients with gastric cancer with H. pylori infection and 33 patients with gastric cancer without the bacterial infection were enrolled in the present study. COX-2 protein was detectable by immunohistochemistry in almost all the gastric cancer samples infected with H. pylori, and by contrast it was detectable in <2/3 of the patients without H. pylori infection. In contrast to gastric cancer samples, COX-2 was positively expressed in <1/6 of control subjects regardless of H. pylori infection. However, the PGE2 degrading enzyme, 15-PGDH, was expressed in control samples, but its expression was markedly suppressed in gastric cancer samples. H. pylori infection resulted in slight inhibition of 15-PGDH in control subjects, but significant inhibition of 15-PGDH mRNA levels and protein synthesis in patients with gastric cancer. These findings indicated that enzymes that regulate PGE2 levels were significantly altered in gastric cancer, and that H. pylori may be involved in modulating the synthesis of COX-2 and 15-PGDH.
Previous studies have indicated that 15-PGDH acts as a tumor suppressor in multiple types of tumors, including breast, lung, colon, thyroid and pancreatic cancers (12,20–23). Song et al (24) reported that 15-PGDH was not expressed in 70.1% of gastric cancer specimens. In addition, Ryu et al (11) reported that 15-PGDH was suppressed in patients with mild gastritis who were positive for H. pylori infection. Consistently, the present study demonstrated that expression of 15-PGDH mRNA and protein was significantly suppressed in gastric cancer samples compared with control samples, and infection with H. pylori resulted in additional downregulation of 15-PGDH mRNA and protein levels. These findings indicated that H. pylori infection may trigger carcinogenesis of stomach cancer by elevating PGE2 levels through modulating expression of 15-PGDH.
Previous studies have also demonstrated that gastric tumors may be induced by activated macrophages in transgenic mice overexpressing COX-2 and the PGE2-converting enzyme microsomal prostaglandin E synthesis (25), and that COX-2 was induced by H. pylori infection (26,27). A significant upregulation of COX-2 was also previously identified in >70% of gastric cancer specimens, and this was reversed when H. pylori was eliminated (28). The present study revealed that COX-2 was expressed in >80% (54/66) gastric cancer specimens, and the patients with H. pylori infection had a significantly increased positive rate of COX-2 expression compared with patients without H. pylori infection, indicating that the activation of prostaglandin synthesis may mediate H. pylori-induced gastric cancer.
PGE2 levels are controlled not only by PGE2 synthesis through inducible enzymes, including COX-2 and mPGES-1/2, but also by the PGE2 inactivating enzyme 15-PGDH. Notably, the present analysis of gastric cancer specimens and control subjects revealed that H. pylori infection was associated with not only significant downregulation of 15-PGDH, but also upregulation of COX-2. In the present study, upregulation of COX-2 and suppression of 15-PGDH was demonstrated, indicating that elevated PGE2 levels in the pathogenesis of gastric cancer are a consequence of not only increased synthesis through upregulating COX-2 but also reduced degradation by suppressing 15-PGDH. Therefore, in addition to antibiotics for eradication of H. pylori infection, medicines targeting PGE2 levels through inhibiting COX-2 or stimulating 15-PGDH may be an effective strategy. In this regard, drugs that decrease the risk of colon carcinogenesis by inhibiting COX-2 have been tested in clinical trials, however, these drugs are problematic due to their unfavorable cardiovascular side effects (29). Thus, a strategy of combining H. pylori eradication and expression of 15-PGDH in the gastrointestinal tract may be an effective way to prevent gastric carcinogenesis.
Taken together, the present study demonstrated that COX-2 was expressed in almost all gastric cancers infected with H. pylori, but it was also expressed in <2/3 of gastric cancers without H. pylori infection. In addition, COX-2 was positively expressed in <1/6 of control subjects regardless of H. pylori infection. By contrast, 15-PGDH was expressed in control samples and it was markedly downregulated in gastric cancer samples. H. pylori infection resulted in slight inhibition of 15-PGDH in control subjects, but significant inhibition of 15-PGDH mRNA expression and protein synthesis in the patients with gastric cancer. These findings indicated that COX-2, which elevates PGE2 levels and 15-PGDH, which decreases PGE2 levels, are significantly altered in gastric cancer, and that H. pylori may modulate COX-2 and 15-PGDH mRNA expression and protein synthesis.
References
- 1.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: A new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. doi: 10.1053/j.gastro.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Liu N, Wu Q, Wang Y, Sui H, Liu X, Zhou N, Zhou L, Wang Y, Ye N, Fu X, et al. Helicobacter pylori promotes VEGF expression via the p38 MAPKmediated COX2PGE2 pathway in MKN45 cells. Mol Med Rep. 2014;10:2123–2129. doi: 10.3892/mmr.2014.2458. [DOI] [PubMed] [Google Scholar]
- 4.Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/S0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 5.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 6.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 7.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 8.Pai R, Szabo IL, Soreghan BA, Atay S, Kawanaka H, Tarnawski AS. PGE(2) stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochem Biophys Res Commun. 2001;286:923–928. doi: 10.1006/bbrc.2001.5494. [DOI] [PubMed] [Google Scholar]
- 9.Tai HH. Prostaglandin catabolic enzymes as tumor suppressors. Cancer Metastasis Rev. 2011;30:409–417. doi: 10.1007/s10555-011-9314-z. [DOI] [PubMed] [Google Scholar]
- 10.Na HK, Park JM, Lee HG, Lee HN, Myung SJ, Surh YJ. 15-Hydroxyprostaglandin dehydrogenase as a novel molecular target for cancer chemoprevention and therapy. Biochem Pharmacol. 2011;82:1352–1360. doi: 10.1016/j.bcp.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Ryu YM, Myung SJ, Park YS, Yang DH, Song HJ, Jeong JY, Lee SM, Song M, Kim DH, Lee HJ, et al. Inhibition of 15-hydroxyprostaglandin dehydrogenase by Helicobacter pylori in human gastric carcinogenesis. Cancer Prev Res (Phila) 2013;6:349–359. doi: 10.1158/1940-6207.CAPR-12-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myung SJ, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitas F. Twenty five years since the first prospective study by Forman et al (1991) on Helicobacter pylori and stomach cancer risk. Cancer Epidemiol. 2016;41:159–164. doi: 10.1016/j.canep.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wu WK, Gallo RL, Fang EF, Hu W, Ling TK, Shen J, Chan RL, Lu L, Luo XM, et al. Critical role of antimicrobial peptide cathelicidin for controlling Helicobacter pylori survival and infection. J Immunol. 2016;196:1799–1809. doi: 10.4049/jimmunol.1500021. [DOI] [PubMed] [Google Scholar]
- 15.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 16.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 17.Tanigawa T, Watanabe T, Hamaguchi M, Sasaki E, Tominaga K, Fujiwara Y, Oshitani N, Matsumoto T, Higuchi K, Arakawa T. Anti-inflammatory effect of two isoforms of COX in H. pylori-induced gastritis in mice: Possible involvement of PGE2. Am J Physiol Gastrointest Liver Physiol. 2004;286:G148–G156. doi: 10.1152/ajpgi.00137.2003. [DOI] [PubMed] [Google Scholar]
- 18.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: A novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 19.Society of Stomach Cancer of Chinese Anti-Cancer Association, corp-author; Society of Pathology of Chinese Anti-Cancer Association; Chinese Society of Clinical Oncology, corp-author. Chinese expert consensus on the molecular-targeted therapy for HER-2-positive advanced gastric cancer. Zhonghua Zhong Liu Za Zhi. 2013;35:315–319. doi: 10.3760/cma.j.issn.0253-3766.2013.04.017. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 20.Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26:65–72. doi: 10.1093/carcin/bgh277. [DOI] [PubMed] [Google Scholar]
- 21.Wolf I, O'Kelly J, Rubinek T, Tong M, Nguyen A, Lin BT, Tai HH, Karlan BY, Koeffler HP. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006;66:7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 22.Pham H, Chen M, Li A, King J, Angst E, Dawson DW, Park J, Reber HA, Hines OJ, Eibl G. Loss of 15-hydroxyprostaglandin dehydrogenase increases prostaglandin E2 in pancreatic tumors. Pancreas. 2010;39:332–339. doi: 10.1097/MPA.0b013e3181baecbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng-Rogenski S, Gee J, Ignatoski KW, Kunju LP, Bucheit A, Kintner HJ, Morris D, Tallman C, Evron J, Wood CG, et al. Loss of 15-hydroxyprostaglandin dehydrogenase expression contributes to bladder cancer progression. Am J Pathol. 2010;176:1462–1468. doi: 10.2353/ajpath.2010.090875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song HJ, Myung SJ, Kim IW, et al. 15-hydroxyprostaglandin dehydrogenase is downregulated and exhibits tumor suppressor activity in gastric cancer. Cancer Invest. 2011;29:257–265. doi: 10.3109/07357907.2011.568562. [DOI] [PubMed] [Google Scholar]
- 25.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669–1678. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sierra JC, Hobbs S, Chaturvedi R, Yan F, Wilson KT, Peek RM, Jr, Polk DB. Induction of COX-2 expression by Helicobacter pylori is mediated by activation of epidermal growth factor receptor in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;305:G196–G203. doi: 10.1152/ajpgi.00495.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slomiany BL, Slomiany A. Induction in gastric mucosal prostaglandin and nitric oxide by Helicobacter pylori is dependent on MAPK/ERK-mediated activation of IKK-beta and cPLA2: Modulatory effect of ghrelin. Inflammopharmacology. 2013;21:241–251. doi: 10.1007/s10787-013-0169-5. [DOI] [PubMed] [Google Scholar]
- 28.Sun WH, Yu Q, Shen H, Ou XL, Cao DZ, Yu T, Qian C, Zhu F, Sun YL, Fu XL, Su H. Roles of Helicobacter pylori infection and cyclooxygenase-2 expression in gastric carcinogenesis. World J Gastroenterol. 2004;10:2809–2813. doi: 10.3748/wjg.v10.i19.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]