Abstract
Background
Stroke is characterized by an asymmetrical gait pattern that causes poor stability and reduces overall activity levels. The aim of this study was to investigate the effect of whole-body vibration combined with treadmill training (WBV-TT) on walking performance in patients with chronic stroke.
Material/Methods
Thirty ambulatory chronic stroke patients were randomly allocated to the WBV-TT group or the treadmill training (TT) group. The participants in the WBV-TT group performed 6 types of exercises on a vibrating platform for 4.5 minutes and then walked on the treadmill for 20 minutes. The participants in the TT group conducted the same exercise on a platform without vibration and then walked on the treadmill in the same manner. The vibration lasted for 45 seconds in each exercise, and the intervention was performed 3 times weekly for 6 weeks. The treadmill walking speed was gradually increased by 5% in both groups. The outcome measures included the temporospatial parameter of gait (GAITRite®) and 6-minute walk test.
Results
The WBV-TT group showed significant improvements in walking performance with respect to walking speed, cadence, step length, stride length, single-limb support, double-limb support, and 6-minute walk test compared with baseline (p<0.05). Significant improvements were also seen in walking speed, step length, stride length, and double-limb support compared with the TT group (p<0.05).
Conclusions
These findings indicate that WBV-TT is more effective than TT for improving walking performance of patients with chronic stroke.
MeSH Keywords: Rehabilitation, Stroke, Vibration, Walking
Background
Patients with stroke have significant changes in their gait pattern, such as decreased walking speed, asymmetrical walking, and reduced endurance due to damage to their motor functions, including decreases in voluntary muscle strength of the affected side, muscle atrophy, and spasticity [1,2]. Walking ability is an important part of evaluating functional status, and independent walking is one of the major objectives of stroke rehabilitation [3]. In patients with chronic stroke, most of the time in physical treatment is spent on gait training. After a first attack, even if patients are able to walk, it does not mean they can engage in functional walking, which requires endurance [4,5]. The aerobic capacity in terms of cardiopulmonary endurance is closely related to physical activity, including walking performance [5]. Cardiopulmonary endurance, after stroke, was found to be reduced by 50–70% compared to sedentary individuals [6]. These reductions accelerate muscle weakness and impair the walking performance of patients with stroke [5]. However, aerobic or cardiopulmonary endurance are often overlooked. Patients and caregivers are interested in preventing complications through functional improvement of the cardiovascular system, but the early clinical approach is difficult.
Several studies have shown that treadmill training improves the walking ability of stroke patients [7,8]. According to motor learning theory, the best learning occurs when practicing a task matched with performance requiring retention or transfer condition. The integration of motor and sensory stimulation during training accelerates motor learning by nerve specificity [9]. Treadmill training is called task-specific training; it is a training method matched with performance because the subject actually walks. Therefore, treadmill training with high retention can be effectively used for motor learning. A previous study reported that 12-week high-intensity aerobic treadmill exercise significantly improved walking speed (0.13 m/s) and the 6-minute walk test (6MWT, 53m) results in patients with chronic stroke [10]. However, further research is needed on programs that can more effectively improve the gait function of patients with stroke.
Recently, the treadmill speed has been considered as an important factor to improve the walking ability of stroke patients. The traditional treadmill training speed that has been accepted universally in patients with stroke is slow. Fast walking on the treadmill has been reported to improve gait parameters significantly compared to walking slowly [8]. Despite the widespread use of treadmill training, it has limitations in using compensation strategies rather than recovery of normal kinetic symmetry [11]. Therefore, stimulation that induces sensory messages from cutaneous or muscle proprioceptive receptors is needed to promote the use of the affected side instead of excessive use of the less affected side.
Whole-body vibration (WBV) might be a useful intervention to stimulate various receptors in stroke patients. WBV oscillates in the vertical plane at a particular frequency and amplitude. These platform motions cause the movement of joints and stretching of the muscles. Tonic vibration reflexes induced by WBV affect the proprioceptive systems [12]. Rittweger et al. (2003) stated that a low vibration frequency (<20 Hz) induces muscular relaxation, while a medium vibration frequency (>50 Hz) causes muscle soreness or hematomas in untrained subjects [13]. Another study reported that the vastus lateralis was more activated at 30 Hz than at 40 or 50 Hz [14], and a systematic review study showed that positive muscle training effects occur at frequencies of 20 to 45 Hz [15]. These findings suggest the possibility that WBV with 20 to 30 Hz might be a beneficial training therapy for patients with stroke. However, the effects of WBV exercise in subjects with neurologic deficits are still unclear. Only a few studies have evaluated the effect of WBV training on patients with chronic stroke [16–18].
Based on the above evidence, we proposed whole-body vibration combined with treadmill training (WBV-TT) as a therapeutic strategy for hemiparetic gait. Therefore, this study investigated whether WBV-TT can improve the walking function of patients with stroke sufficiently to allow them to lead independent lives. We hypothesized that WBV-TT would improve walking performance in patients with chronic stroke.
Material and Methods
Participants and procedure
This study was designed as an assessor-blind randomized controlled trial. Forty participants were enrolled by the therapists through convenience sampling from an advertisement. Individuals who presented with a gait deviation after a first stroke (>6 months), could walk for more than 30 seconds at >0.8 km/h, with Mini-Mental status examination (MMSE) >21 points, and could understand the nature of the intervention and perform the protocol independently participated in the study.
The exclusion criteria were subjects who had participated in a similar experiment for the last 6 months, had fractures, infectious diseases, cardiac pacemakers, vestibular disorders, cerebellar diseases, visual and auditory problems, walking disability due to orthopedic problems, chronic pain, or contracture in the lower extremity joints. In total, 30 subjects who met the eligibility criteria were included and they were randomly allocated to the WBV-TT or TT group. The other 10 subjects were excluded due to: not meeting the inclusion criteria (n=5), femur fracture (n=1), neurological disorders (n=2), or an orthopedic problem (n=2). Randomization was performed using sealed envelopes containing a paper marked with “O” or “X”. The participants were evaluated at baseline and after the intervention period of 6 weeks, and 3 skilled testers were instructed on how to prevent accidents during walking assessment. Testers were blinded to which intervention each patient had received.
All subjects were instructed about the contents of this study with verbal and written information and were permitted to refuse participation at any time during the experiment. In order to minimize any possible risk, the intervention was conducted under the supervision of a physical therapist. This study was carried out with the approval of the Research Ethics Committee of Sahmyook University.
In the WBV-TT group, participants performed the WBV exercises for 6 weeks, 3 times a week for 4.5 minutes per session, and then treadmill training was carried out for 20 minutes. In the TT group, participants performed the same exercises on the platform without vibration, and then treadmill training was conducted. The exercise time to perform each exercise was the same in the 2 groups. Two subjects in the WBV-TT group dropped out because one subject refused the training and the other subject was discharged from the hospital. Two subjects in the TT group did not attend the post-test session (Figure 1).
Figure 1.
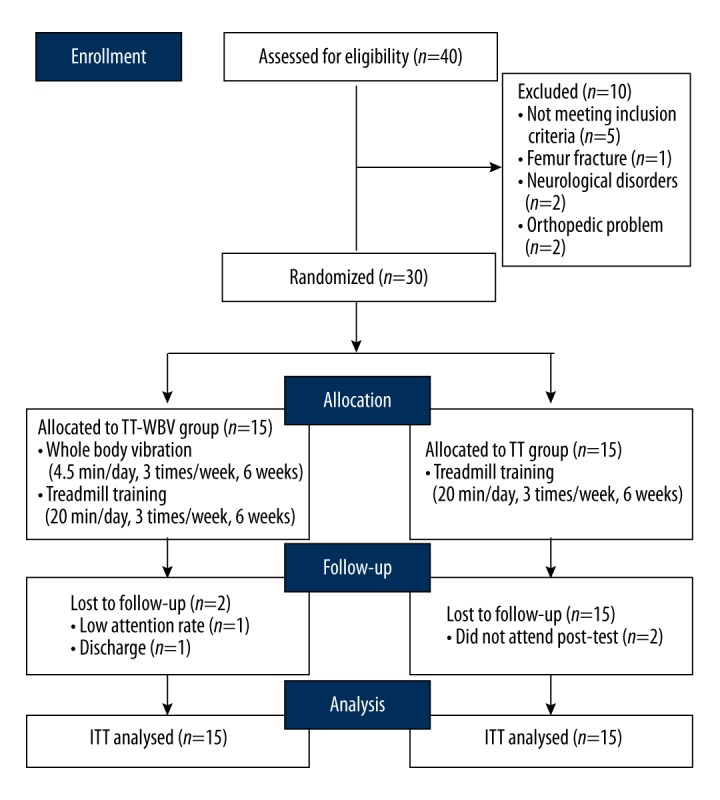
Experimental flow chart. A total of 40 subjects were enrolled and 30 subjects who matched the eligibility criteria were included. They were randomly allocated to 2 groups: the WBV-TT group (n=15) and the TT (n=15) group. Two subjects dropped out in each group and were analyzed based on intention-to-treat principles.
Intervention
A side-alternating vibrator (Galileo 2000, Novotec, Germany, 2011) was used and safety guidelines were explained to the participants before the experiment. WBV-TT was performed based on a previous study that had performed the WBV stimulation with a maximum frequency of 30 Hz and amplitude of 3 mm for 45 seconds and 4 sessions for post-acute stroke [16]. Before conducting the main exercise, the subjects performed stretching exercises for 15 minutes. While standing on the vibration platform, subjects placed their feet parallel to the axis. The participants performed the exercises while lightly holding a support bar always located in front of them. The WBV-TT was performed 3 times a week (4.5 minutes per session) for 6 weeks. Each session included 6 exercises and each exercise was conducted for 45 seconds. A break time of 1 minute was given between the exercises [16]. The frequency was increased gradually by 5 Hz every 2 weeks, from 20 Hz to 30 Hz, because many researchers have demonstrated its therapeutic effect [15,18]. The exercise program was composed of left and right weight shifts (weight shift to the affected side as much as possible), squats (knee joint 45° flexion), anteroposterior weight shift exercises (lifting and lowering the heel), forward lunges (flexion of the affected leg forward), one-leg standing (lifting the affected and less affected leg alternately), and deep squats (knee joint 90° flexion) (Table 1).
Table 1.
Whole body vibration.
| Section (weeks) | Frequency (Hz) | Amplitude (mm) | Break time (min) | Duration | Exercise program |
|---|---|---|---|---|---|
| Warming up exercise | 15 min | Gentle stretching | |||
|
| |||||
| 1–2 | 20 | 3 | 1 | 45 s/1 set × 6set/day | 1. Left and right weight shifts : weight shift to the affected side as much as possible 2. Squats : knee joint 45° flexion |
|
| |||||
| 3–4 | 25 | 3 | 1 | 45 s/1 set × 6 set/day | 3. Anterioposterior weight shift exercises : lifting and lowering the heel 4. Forward lunges : flexion of the affected leg forward |
|
| |||||
| 5–6 | 30 | 3 | 1 | 45 s/1 set × 6 set/day | 5. One leg standing : lifting the affected and less affected leg alternately 6. Deep squats : knee joint 90° flexion |
A treadmill (Track Star, Incheon, Korea, 2011) was used for gait training. All participants started at a 0% slope and a minimum speed of 0.8 km/h, and were fitted with a no-body-weight support harness for safety and allowed to hold a support bar if necessary. Based on the studies of Lau and Mak (2011), the participants defined their maximum walking speed on the first day of every week and it was increased gradually by 5% during the walk [19]. They walked for 20 minutes and were constantly observed by a physical therapist while walking on the treadmill.
Outcome measures
All participants were assessed at baseline and after the intervention. The walking function of the subjects was measured with GAITRite and the 6MWT. GAITRite (GaitRite, CIR systems Inc., USA, 2008) evaluates temporal parameters such as velocity, cadence, time of swing phase and stance phase, and spatial parameters such as stride length, step length, single-limb support% cycle, and double-limb support% cycle, when the subjects were walking on the electronic carpet. For measurement, the subjects were instructed to stand 3 m away from the electronic carpet and walk at a comfortable walking speed across the carpet, stopping after walking 3 m past the electronic carpet. Measurements were repeated 3 times with a 3-minute break between measurements to minimize bias due to muscle fatigue. The average value of the 3 measurements was calculated and recorded.
Before starting the 6MWT, we monitored the cardiovascular conditions of the subjects by measuring the manual heart rate and blood pressure. There was no warm-up exercise before the test and the total distance was measured after the subjects walked along a 20-meter track with marked sticks at intervals of 1 meter, safely yet as fast as possible for 6 minutes. They were allowed to adjust their walking pace and take break times by themselves according to each individual’s ability.
Statistical analysis
The sample size was estimated with G power 3.1 software. The effect size (Cohen’s d) was set to 1.20, alpha level to 0.05, and power to 80% through an a priori power analysis. It was calculated to allocate at least 13 subjects to each group. The general characteristics of the participants are presented as mean ± standard deviation for continuous variables or frequencies for categorical variables using SPSS version 19.0 (IBM, Chicago, IL). The normality test, using the Kolmogorov-Smirnov test, was carried out on the collected data. A chi-square test and independent-samples t test were conducted to analyze the baseline of homogeneity. An intention-to-treat analysis was performed to investigate the effects of the intervention. A two-way repeated-measures ANOVA based on group (WBV-TT, TT) and over time (baseline, pre-test) as within-subject factors was applied for the outcomes of WBV-TT on walking performance. A post hoc test was performed using Scheffe’s method. The partial eta squared was provided as the effect size in ANOVA. The significance level was set to a two-tailed 0.05 for all analyses.
Results
The general characteristics of the WBV-TT and TT groups are presented in Table 2. No significant differences between the groups were found in sex, age, height, weight, type of stroke, paralyzed side, duration, or MMSE points.
Table 2.
General characteristics of the subjects.
| WBV-TT group (n=15) | TT group (n=15) | χ2/t(p) | |
|---|---|---|---|
| Sex (male/female) | 8/7 | 11/4 | 1.292 (0.256) |
| Affected type (infarction/hemorrhage) | 9/6 | 10/5 | 0.144 (0.705) |
| Paretic side (right/left) | 8/7 | 5/10 | 1.222 (0.269) |
| Age (year) | 51.93±8.35 | 53.67±7.38 | −0.602 (0.552) |
| Body weight (kg) | 62.50±8.90 | 66.40±6.63 | −1.361 (0.184) |
| Height (cm) | 166.47±7.38 | 169.27±5.92 | −1.146 (0.261) |
| Duration (month) | 25.13±9.25 | 22.53±10.27 | 0.728 (0.472) |
| MMSE (score) | 26.13±3.04 | 26.07±3.15 | 0.059 (0.953) |
WBV-TT – whole body vibration combined with treadmill training; TT – treadmill training. Values are presented as mean ± standard deviation.
After 6 weeks of training, the WBV-TT group showed significant improvement in walking performance compared to the TT group. Walking speed and cadence and the temporal parameter in walking performance were significantly improved in the WBV-TT group, whereas only walking speed was significantly improved in the TT group when compared to pre-values in each group (p<0.05). The degree of improvement of walking speed in the WBV-TT group was significantly larger than that of the TT group (p<0.05). In the spatial parameters, step length, stride length, single-limb support, and double-limb support were significantly improved in the WBV-TT group compared to their pre-values (p<0.05). The TT group showed significant improvement only in step length of the less affected side and stride length (p<0.05) (Table 3). In the WBV-TT group, step length (affected side and less affected side), stride length, and double-limb support all showed a significantly larger improvement than in the TT group (p<0.05).
Table 3.
Temporospatial variables between the WBV-TT and TT groups.
| Variables | WBV-TT group | TT group | F | p | Effect size (η2) | ||
|---|---|---|---|---|---|---|---|
| Baseline (n=15) | Post-test (n=15) | Baseline (n=15) | Post-test (n=15) | ||||
| Temporal variable | |||||||
| Walking speed (cm/s) | 51.62±25.61 | 67.43±30.77** | 53.07±22.04 | 58.07±25.22* | 8.906 | 0.006 | 0.241 |
| Cadence (step/min) | 84.33±23.01 | 92.54±24.09* | 89.16±19.53 | 92.46±18.52 | 2.933 | 0.098 | 0.095 |
| Spatial variable | |||||||
| Step length (cm) | |||||||
| Affected side | 35.50±13.05 | 42.78±14.70** | 38.25±8.84 | 39.26±10.37 | 14.255 | 0.001 | 0.337 |
| Less affected side | 35.47±12.46 | 42.87±13.45* | 32.10±10.83 | 34.29±12.05* | 7.273 | 0.012 | 0.206 |
| Stride length (cm) | 71.22±24.02 | 85.79±26.73** | 70.49±17.89 | 73.44±20.96* | 13.053 | 0.001 | 0.318 |
| Single limb support (%) | |||||||
| Affected side | 23.02±7.46 | 25.89±6.73* | 23.90±5.87 | 24.31±5.20 | 3.330 | 0.079 | 0.106 |
| Less affected side | 33.28±8.03 | 36.29±5.10* | 33.58±6.37 | 33.98±5.66 | 4.032 | 0.054 | 0.126 |
| Double limbs support (%) | 43.38±14.16 | 37.51±10.77* | 42.38±10.61 | 41.84±9.19 | 7.784 | 0.009 | 0.218 |
Values are presented as mean ±SD. WBV-TT – whole body vibration combined with treadmill training; TT – treadmill training.
p<0.05;
p<0.001 present significant difference between baseline and post-test.
In the 6MWT, both groups were significantly improved as compared with pre-intervention (p<0.05), by 32.95 m and 28.46 m in the WBV-TT group and the TT group, respectively. However, there was no significant difference in 6 MWT between the 2 groups (Figure 2).
Figure 2.
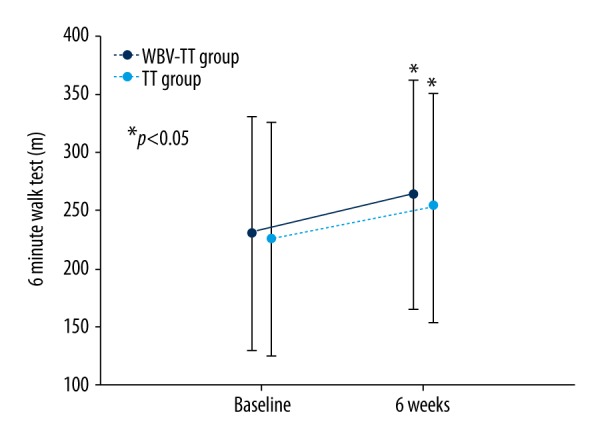
Change in 6-meter walk test results between the WBV-TT and TT groups. * p<0.05 a significant difference between baseline and post-test.
Discussion
The present study demonstrated that both WBV-TT and TT improve the walking performance of patients with chronic stroke. In particular, WBV-TT effectively enhanced the improvement of walking speed, step length, stride length, and double-limb support during walking in patients with stroke. It should be noted that an effective exercise program was used in this study for walking performance, which applied WBV stimulation to 6 exercises, while van Nes et al. (2006) focused solely on balance stimulated using WBV. Although this study included only 18 sessions, WBV-TT might be a high-intensity exercise that can improve the walking performance of patients with chronic stroke.
WBV provides a mechanical vibration stimulus. The participants stand on a vibration platform that provides repeated concentric and eccentric contraction of skeletal muscles due to the vibratory stimulation [20]. The WBV stimulates the somatosensory system of the primary and secondary afferent fibers [12], and causes excitation of the spinal reflex [21]. Most studies applying WBV stimulation to patients with stroke have reported a significant improvement of motor function and activities of daily living using a fixed frequency and amplitude [16,22]. However, a long duration of stimulus causes cumulative fatigue and reduces the fire rate of the motor unit [13]. This is a phenomenon caused by excessive adaptation of the nerve roots. Therefore, some studies reported that walking function improves when applying gradual changes of vibration intensity to stroke subjects in order to prevent adaptation [23]. Therefore, this study also applied short durations of vibration and increased the frequency gradually from 20 to 30 Hz over a period of 2 weeks. The participants also experienced weight-bearing on the affected side through one-leg standing conditions, which may apply proprioceptive stimulation intensively to the affected side. The WBV in this study used side-alternating vibration that could evoke rotational movements around the hip and lumbo-sacral joints [15]. These rotational movements are controlled with the help of ankle evertors, which are important for lateral ankle stability [24]. Improved lateral ankle stability might increase single-limb support to the affected side. Stroke might deteriorate the ability of the spinal cord to utilize load-related sensory signals appropriately, which results in abnormal extensor activity during each stance [25]. WBV may promote knee extensor through proper sensory facilitation of the proprioceptive receptors [26,27]. Single-limb support and double-limb support are important temporal parameters for the gait stability of patients with stroke. The less affected side is lifted off the ground and the affected side remains on the ground to support the body upright during the single-limb support phase of walking. Increased single-limb support means improving the stability of the affected leg, leading to shorter -limb support and longer swing phase period on the contralateral side [28]. This might produce an increase in step length and stride length.
In walking parameters, improvement of walking speed brings about functional changes, such as improved quality of life and social adaptation, after discharge of patients with stroke. However, the effect of WBV on walking performance is controversial [29]. A previous study suggested that it takes a long time to develop neuromuscular coordination, muscular adaptation, and a new walking pattern after WBV exercise [17]. In order to shorten the duration of rehabilitation, we added treadmill training to WBV. After the intervention, the participants with a walking speed of 67.4 cm/s in the WBV-TT group were classified as more than the least-limited community walkers [30]. They could be independent in local stores and all moderate community activities without assistance. Chan et al. (2012) carried out WBV at a frequency of 12 Hz and amplitude of 4 mm for 20 minutes for stroke subjects and reported a significant increase in walking speed as a result. In the WBV-TT group of this study, the stance phase stability of the affected and less affected side was improved because of an increase in single-limb support and a decrease in double-limb support after the program, making walking closer to normal gait kinematics. Because of the improved single-limb stability, cadence was significantly increased and the swing phase of the other leg was increased.
This study began by setting the peak speed at a point at which the patients with stroke could walk during treadmill training, and increased it gradually up to 5%, and eventually 6MWT was improved significantly in both groups. 6MWT is a clinical test for cardiopulmonary fitness and walking endurance. Treadmill training at a peak speed for 20 minutes is an intensive aerobic exercise that can increase peak oxygen uptake [10]. High-load and repetitive training can improve endurance [10]. In addition, repetitive treadmill training activates the central pattern generator and promotes the use of the hip flexor [19]. The participants were allowed to experience overload by increasing the speed gradually. The central pattern generator is activated by sensory input produced during treadmill training, which cause rhythmic muscle activation of the affected side of patients with stroke [31]. Based on this, we found that exercise tolerance and walking endurance increased. The lack of a significant difference in 6MWT between the 2 groups suggests that cardiopulmonary endurance might be affected by the TT rather than the WBV.
This study has several limitations. It is difficult for a subject to use WBV-TT without the assistance of a therapist. For gradually increasing the speed, someone else needs to press the button. That is why we recommend this program should only be applied in a clinical setting. In addition, the subject needs to have basic walking ability, bring able to walk on a treadmill at a speed of at least 0.8 km/h. The results of this study may be difficult to generalize because of its small sample size. Additional practical functional tests for walking and activities of daily living are needed in subsequent related research.
Conclusions
The present study demonstrated that WBV-TT might be a more intensive and effective training program than TT to improve the walking performance of patients with chronic stroke. There is still a lack of scientific evidence to support the efficacy of WBV-TT for stroke survivors. Future studies will need to use a larger sample size and apply sensors or biofeedback to identify precisely whether weight-bearing improves on the affected side.
Footnotes
Source of support: This research was supported by Sahmyook University, the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B03933986), and the Convergence Technology Development Program for Bionic Arms through the NRF funded by the Ministry of Science, ICT, and Future Planning (NRF-2014M3C1B2048632)
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86:1552–56. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Patten C, Kothari DH, et al. Gait deviations associated with post-stroke hemiparesis: improvement during treadmill walking using weight support, speed, support stiffness, and handrail hold. Gait Posture. 2005;22:57–62. doi: 10.1016/j.gaitpost.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Taylor-Piliae RE, Latt LD, Hepworth JT, et al. Predictors of gait velocity among community-dwelling stroke survivors. Gait Posture. 2012;35:395–99. doi: 10.1016/j.gaitpost.2011.10.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng JJ, Chu KS, Dawson AS, et al. Functional walk tests in individuals with stroke: Relation to perceived exertion and myocardial exertion. Stroke. 2002;33:756–61. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 5.Kelly JO, Kilbreath SL, Davis GM, et al. Cardiorespiratory fitness and walking ability in subacute stroke patients. Arch Phys Med Rehabil. 2003;84:1780–85. doi: 10.1016/s0003-9993(03)00376-9. [DOI] [PubMed] [Google Scholar]
- 6.Pang MY, Eng JJ, Dawson AS, et al. The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: A meta-analysis. Clin Rehabil. 2006;20:97–111. doi: 10.1191/0269215506cr926oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ada L, Dean CM, Hall JM, et al. A treadmill and overground walking program improves walking in persons residing in the community after stroke: A placebo-controlled, randomized trial. Arch Phys Med Rehabil. 2003;84:1486–91. doi: 10.1016/s0003-9993(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: Effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–91. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt RA, Lee TD. Motor control and learning. Champaign, IL: Human Kinetics; 2011. [Google Scholar]
- 10.Globas C, Becker C, Cerny J, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: A randomized control trial. Neurorehabil Neural Repair. 2012;26:85–95. doi: 10.1177/1545968311418675. [DOI] [PubMed] [Google Scholar]
- 11.Combs SA, Dugan EL, Ozimek EN, et al. Effects of body-weight supported treadmill training on kinetic symmetry in persons with chronic stroke. Clin Biomech (Bristol, Avon) 2012;27:887–92. doi: 10.1016/j.clinbiomech.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Cardinale M, Rittweger J. Vibration exercise makes your muscles and bones stronger: Fact or fiction? J Br Menopause Soc. 2006;12:12–18. doi: 10.1258/136218006775997261. [DOI] [PubMed] [Google Scholar]
- 13.Rittweger J, Mutschelknauss M, Felsenberg D. Acute changes in neuromuscular excitability after exhaustive whole body vibration exercise as compared to exhaustion by squatting exercise. Clin Physiol Funct Imaging. 2003;23:81–86. doi: 10.1046/j.1475-097x.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- 14.Cardinale M, Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. J Strength Cond Res. 2003;17:621–24. doi: 10.1519/1533-4287(2003)017<0621:eaovlm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Rittweger J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur J Appl Physiol. 2010;108:877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 16.van Nes IJ, Latour H, Schils F, et al. Long-term effects of 6-week whole-body vibration on balance recovery and activities of daily living in the postacute phase of stroke: A randomized, controlled trial. Stroke. 2006;37:2331–35. doi: 10.1161/01.STR.0000236494.62957.f3. [DOI] [PubMed] [Google Scholar]
- 17.Chan KS, Liu CW, Chen TW, et al. Effects of a single session of whole body vibration on ankle plantarflexion spasticity and gait performance in patients with chronic stroke: A randomized controlled trial. Clin Rehabil. 2012;26:1087–95. doi: 10.1177/0269215512446314. [DOI] [PubMed] [Google Scholar]
- 18.Pang MY, Lau RW, Yip SP. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: A randomized controlled trial. Eur J Phys Rehabil Med. 2013;49:439–50. [PubMed] [Google Scholar]
- 19.Lau KW, Mak MK. Speed-dependent treadmill training is effective to improve gait and balance performance in patients with sub-acute stroke. J Rehabil Med. 2011;43:709–13. doi: 10.2340/16501977-0838. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale M, Wakeling J. Whole body vibration exercise: Are vibrations good for you? Br J Sports Med. 2005;39:585–89. doi: 10.1136/bjsm.2005.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebedev MA, Poliakov AV. [Analysis of the interference electromyogram of human soleus muscle after exposure to vibration]. Neirofiziologiia. 1991;23:57–65. [in Russian] [PubMed] [Google Scholar]
- 22.Merkert J, Butz S, Nieczaj R, et al. Combined whole body vibration and balance training using Vibrosphere(R): improvement of trunk stability, muscle tone, and postural control in stroke patients during early geriatric rehabilitation. Z Gerontol Geriatr. 2011;44:256–61. doi: 10.1007/s00391-011-0170-9. [DOI] [PubMed] [Google Scholar]
- 23.Silva A, Silva A, Dias M, et al. Whole body vibration training for lower limb motor function among stroke patients. Int J Ther Rehabil. 2013;20:260–66. [Google Scholar]
- 24.Spiliopoulou SI, Amiridis IG, Tsigganos G, et al. Side-alternating vibration training for balance and ankle muscle strength in untrained women. J Athl Train. 2013;48:590–600. doi: 10.4085/1062-6050-48.4.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinkensmeyer D. How to retrain movement after neurologic injury: How to retrain movement after neurologic injury: A computational rationale for incorporating robot (or therapist) assistance. Proceedings of the 25th Annual International Conference of the IEEE; 2003 Sept 17–21; Cancún, Mexico. IEEE; 2003. [Google Scholar]
- 26.Liao LR, Ng GY, Jones AY, et al. Effects of vibration intensity, exercise, and motor impairment on leg muscle activity induced by whole-body vibration in people with stroke. Phys Ther. 2015;95:1617–27. doi: 10.2522/ptj.20140507. [DOI] [PubMed] [Google Scholar]
- 27.Rees SS, Murphy AJ, Watsford ML. Effects of whole-body vibration exercise on lower-extremity muscle strength and power in an older population: A randomized clinical trial. Phys Ther. 2008;88:462–70. doi: 10.2522/ptj.20070027. [DOI] [PubMed] [Google Scholar]
- 28.Lamontagne A, Richards CL, Malouin F. Coactivation during gait as an adaptive behavior after stroke. J Electromyogr Kinesiol. 2000;10:407–15. doi: 10.1016/s1050-6411(00)00028-6. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg J, Carlsson J. The effects of whole-body vibration training on gait and walking ability – a systematic review comparing two quality indexes. Physiother Theory Pract. 2012;28:485–98. doi: 10.3109/09593985.2011.641670. [DOI] [PubMed] [Google Scholar]
- 30.Perry J, Garrett M, Gronley JK, et al. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–89. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 31.Van de Crommert HW, Mulder T, Duysens J. Neural control of locomotion: Sensory control of the central pattern generator and its relation to treadmill training. Gait Posture. 1998;7:251–63. doi: 10.1016/s0966-6362(98)00010-1. [DOI] [PubMed] [Google Scholar]


