Abstract
Background
To explore the effect of hypoxic preconditioning on the JAK/STAT3 signaling pathway and its effect on trophoblast cell viability and angiogenesis in preeclampsia (PE).
Material/Methods
Placental tissues from normal pregnant women and PE patients were collected to detect the expression levels of JAK and STAT3. Trophoblast cells separated from the PE patients were assigned to 4 groups. The expression levels of phosphorylated p-JAK and p-STAT3 were measured by Western blot. Cell viability, colony-forming ability, and cell apoptosis were assessed. The levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF) were determined by enzyme-linked immunosorbent assay (ELISA).
Results
The expression levels of JAK and STAT3 were higher in the placental tissues of PE patients than in those of normal pregnant women. Compared with the blank group, in the hypoxia group the expression levels of p-JAK and p-STAT3 were increased, cell viability was promoted, the number of colonies was increased, cell apoptosis was inhibited, and the levels of VEGF, bFGF, and HGF were all elevated. However, in comparison with the hypoxia group, the expression levels of p-JAK and p-STAT3 were reduced, the cell viability was inhibited, the colonies were decreased, the levels of VEGF, bFGF, and HGF were all decreased, and cell apoptosis was promoted in the hypoxia + si-JAK group.
Conclusions
These findings indicate that hypoxic preconditioning may contribute to activation of the JAK/STAT3 signaling pathway, thus promoting trophoblast cell viability and angiogenesis in PE.
MeSH Keywords: Cell Hypoxia, MAP Kinase Signaling System, Pre-Eclampsia
Background
PE is a pregnancy-specific syndrome clinically characterized by hypertension and proteinuria, which occurs during a woman’s gestation period [1]. PE is one of the leading causes of maternal and neonatal morbidity and mortality in the world, affecting 2% to 8% of pregnancies [2,3]. PE exerts impairs functioning of the kidneys, liver, and central nervous system, and causes symptoms such as headaches and cerebral or visual disturbances. The etiology remains poorly understood [4]. Because delivery of the placenta and fetus is the only effective way to resolve hypertension, the placenta plays an important part in PE progression, and the factors or signals from placental ischemia or damaged placental blood flow are involved in the relationship of the placenta with maternal vascular dysfunction [5]. Abnormal lipid metabolism is related to the pathogenesis, and acute atherosis has been identified in uteroplacental beds in PE [2].
PE may be caused by impaired invasion of trophoblasts, which is mediated by a complicated network of cells, regulators, growth factors, cytokines, and signaling pathways [1,6]. At the early stage of pregnancy, trophoblast cells differentiate into extravillous trophoblasts that highly invade the maternal decidua to the inner myometrium and migrate along the spiral arteries, contributing to placenta development and pregnancy [7]. Trophoblasts incompletely remodel uterine spiral arteries, reducing maternal blood flow to the placenta, and trophoblast differentiation along the signaling pathway, causing uterine invasion, is abnormal at a molecular level [8].
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is constantly activated in various tumors and regulates the proliferation, growth, differentiation, migration, and invasion of cancer cells [9]. JAK phosphorylates STAT for binding DNA and regulation of gene transcription, and activated JAK plays a key role in the specificity of cytokine function [10]. STAT3, one of 7 members of the STAT family of proteins that participate in normal cellular responses to cytokines and growth factors as transcription factors, is persistently activated in human malignancies [11,12]. Constitutive STAT3 activation promotes tumor cell proliferation and accelerates tumorigenesis [13,14]. Tumor hypoxia has been found to promote the metastasis of non-small cell lung cancer (NSCLC) by ERK signaling, and hypoxia inducible factor-1α (HIF-1α) triggered by hypoxic environment results in the growth of tumor vasculature [15]. Therefore, the purpose of this study was to analyze the effect of hypoxic preconditioning on the JAK/STAT3 signaling pathway and its effect on trophoblast cell viability and angiogenesis in PE.
Material and Methods
Study subjects
Placental tissues were obtained from 42 normal pregnant women (average age, 26.8±3.2 years) and 42 PE patients (average age, 27.1±3.6 years) in The Third Affiliated Hospital, Sun Yat-sen University from April 2015 to August 2016. PE patients were included if they were clinically diagnosed with PE and were without pregnancy complications such as hypertension. PE patients were excluded if they had cardiac disease and other diseases, and if they had heart, lung, liver, or kidney dysfunction. The study was approved and supervised by the Ethics Committee of The Third Affiliated Hospital, Sun Yat-sen University and informed consent was obtained from each subject.
Cell separation, culture, and purification
Sterile placental tissues in late pregnancy were collected. Maternal villus tissues were cut from the placenta near the umbilical cord. The villus tissues were repeatedly washed with normal saline, cut into paste, digested with trypsin, and filtered. The filtrate was added into a centrifuge tube containing fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and the supernatant was discarded after centrifugation, and then resuspended and precipitated by Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, USA) with serum. The undigested tissues were digested 3 times for cell suspension. Percoll solution with a higher density (3 ml) was added into the centrifuge tube (50 ml) and then Percoll solution with a lower density (3 ml) was added slowly. The cell suspension was centrifuged and the cells were sucked with a pipette into the centrifuge tube (50 ml). The cells were diluted with DMEM (1: 4) and centrifuged for removal of the supernatant, then this step was repeated. MDME medium containing 20% FBS, 20 000 U/ml penicillin, and 20 000 U/ml streptomycin (Hyclone, Logan, UT, USA) was used for suspension and precipitation. The cell suspension density was adjusted to 5~6×105 cells/ml and the cells were cultured in an incubator with 5% CO2 at 37°C.
Identification of trophoblast cells by immunohistochemistry performed to detect the expression of cytokeratin-7 and vimentin
The cells were seeded into a 6-well plate with slides for culture in DMEM containing 20% FBS and adhered to the slides after 24 h. After 24 h, the slides were washed with phosphate-buffered saline (PBS) 3 times, fixed with cold acetone for 20 min, and cleaned with PBS 3 times for 5 min each time. The slides were treated with 0.3% H2O2 for 20 min at room temperature, then incubated in a wet box with PBS and 25% FBS at room temperature for 20 min, and the medium was removed gently. Mouse anti-human cytokeratin-7 and vimentin monoclonal antibodies were added into the wet box at 37°C for 60 min. PBS was used to wash slides 3 times for 5 min each time. The secondary antibody labeled with biotin was added into the wet box for 60 min at 37°C and the samples were rinsed with PBS 3 times for 5 min. The fresh DAB solution was supplemented for staining for 15 min at room temperature. The positive cells with tan cytoplasm were observed under a microscope. The cells were rinsed with water to terminate the reaction. Next, the cells were restained with hematoxylin, dehydrated by gradient ethanol, made transparent with xylene, mounted with neutral balsam, and observed under the microscope.
Cell grouping and treatment
The trophoblast cells identified in the logarithmic growth phase were collected and divided into 4 groups: (1) the blank group (cultured in normoxia); (2) the hypoxia group (cultured in a hypoxia incubator with 1% oxygen, 5% CO2, and nitrogen for 48 h); (3) the hypoxia + si-negative control (si-NC) group (transfected with si-NC, the negative control of si-JAK, in a hypoxia incubator for 48 h); and (4) the hypoxia + si-JAK group (transfected with si-JAK in a hypoxia incubator for 48 h). The vectors of si-JAK and si-NC were designed and constructed by Shanghai GenePharma Co., Ltd. (Shanghai, China). According to the instructions of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), the trophoblast cells were transfected for 48 h and used for the subsequent experiment of detecting cell viability and angiogenesis.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA was extracted from the placental tissues and the trophoblast cells of each group by Trizol according to manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA) and the purity of total RNA was tested by ultraviolet spectroscopy and formaldehyde-denaturing gel electrophoresis. The RNA (1 μg) was converted into cDNA using AMV reverse transcriptase. The primers for the qRT-PCR assay was designed and synthetized by Invitrogen (Carlsbad, CA, USA) (Table 1), using glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) as the internal reference. The conditions were pre-denaturation at 94°C for 40 s, denaturation at 94°C for 40 s, annealing at 60°C for 40 s and extension at 72°C for 1 min, with 40 cycles, and then extension at 72°C for 10 min. The products were evaluated by use of Opticon Monitor 3 software (Bio-Rad, Hercules, CA, USA) after agarose gel electrophoresis. The thresholds were manually selected at the lowest point of each logarithmic amplification curve to obtain the threshold value (Ct). The relative quantification was calculated with the use of the 2−ΔΔCt method, which presented the ratio of target gene expressions between the experiment group and control group. ΔΔCt was calculated as [Ct (target gene) – Ct (reference gene)]experiment group – [Ct (target gene) – Ct (reference gene)]control group. The experiment was performed in triplicate to obtain the average.
Table 1.
The primer sequence for qRT-PCR.
| Gene | Sequence |
|---|---|
| JAK | F: 5′-TGGGAA ATCTGCTACAATGG -3′ |
| R: 5′- ATGATGGCTCGGAAGAAAGG -3′ | |
| STAT3 | F: 5′-ATGGGTTTCATCAGCAAGGAG-3′ |
| R: 5′- GGGAATGTCAGGGTAGAGGTAGAC-3′ | |
| GAPDH | F: 5′- CTAAGGCCAACCGTGAAAAG-3′ |
| R: 5′- ACCAGAGGCATACAGGGACA-3′ |
qRT-PCR – quantitative real-time polymerase chain reaction.
Western blotting
The proteins were extracted from the placental tissues and trophoblast cells of each group, and the protein concentration was determined by use of the BCA protein assay kit (Wuhan Boster Biological Technology Ltd., Wuhan, China). The extracted proteins were added with the sample buffer (30 μg in each well) and boiled at 95°C for 10 min. The proteins were separated with 10% polyacrylamide gel (Wuhan Boster Biological Technology Ltd., Wuhan, China) electrophoresis with a voltage of 80 v to 120 v. The proteins were transferred via a wet-transfer process at 100 mv for 45–70 min to polyvinylidene fluoride (PVDF) membrane. Then, the membranes were blocked with 5% bovine serum albumin (BSA) at room temperature for 1 h, and incubated overnight at 4°C with the addition of rabbit anti-mouse JAK, STAT3, phosphorylated (p)-JAK and p-STAT3 primary antibodies (1: 1000) (Cell Signaling Technology, Beverly, MA, USA), and β-actin primary antibody (1: 3000) (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). After fully cleaning by Tris-buffered saline containing Tween-20 (TBST) (3 times/5 min), the membranes were incubated with the secondary antibody (Shanghai Miaotong Biological Technology Co., Ltd, Shanghai, China) at room temperature for 1 h. After cleaning membranes (3 times/5 min), the chemiluminescence reagent was used to develop using a Gel Doc EZ Imager (Bio-Rad, Hercules, CA, USA). β-actin was used as the internal reference. The gray values of target bands were analyzed using Image J software. The experiment was performed in triplicate to obtain the average.
MTT assay
The cells were diluted and seeded into a 96-well plate with 5×104 cells per well (6 wells in each group). When the cell confluence reached 80%, the cells were treated as the above grouping. After reoxygenation, the cells were supplemented with 20 μl MTT (Sigma, Missouri, USA) and incubated at 37°C for 4 h. MTT solution was discarded before 150 μl dimethyl sulfoxide (Sigma, Missouri, USA) was added to each well and shaken for 10 min. The absorbance (OD) in each well was measured at 490 nm with a microplate reader. The experiment was performed in triplicate to obtain the average OD. The survival rate of cells was calculated as (OD in the experiment group - OD in the blank group)/OD in the blank group.
Colony formation assay
Trophoblast cells in logarithmic growth phase were seeded into a 6-well plate and cultured with 2 ml medium in each well. The cells were scattered evenly after shaking the plate gently. The medium was removed when the clones were seen in the plate. The cells were washed by PBS 3 times and fixed with methanol for 15 min. After the methanol was sucked out, the cells were cleaned with PBS twice and 0.5% crystal violet was added for 10 min. The cells were washed with distilled water to remove the staining solution and air-dried at room temperature, then the clone cells were counted.
Flow cytometry
Trophoblast cells in logarithmic growth phase were digested, mixed, suspended, and collected after centrifugation, with the removal of the supernatant. The cells were washed with precooled PBS at 4°C and centrifuged at 1000 rpm for 5 min, which was repeated twice. The cell density was adjusted to 106 cells/ml and the precooled PBS (200 μl) was used to wash the cells before centrifugation. The cells were resuspended in 100 μl binding buffer and mixed with 2 μl Annexin-V-FITC (20 μg/ml) on ice in the dark for 15 min. Next, we added with 300 μl PBS and 1 μl PI (50 μg/ml) to the cells and put the mixture into a flow cytometer. The cells in the left upper quadrant were mechanically damaged cells, those in the right upper quadrant were apoptotic cells or necrotic cells, those in the left lower quadrant were negative normal cells, and those in the right lower quadrant were early apoptotic cells.
Enzyme-linked immunosorbent assay (ELISA)
The supernatant was collected and the particles were removed by centrifugation. The samples were stored at −20°C without repeated freezing and thawing. The levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF) were detected by ELISA (R&D Systems, Minneapolis, MN, USA). The blank wells were used for zero. The OD in each well was measured at 450 nm with a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The experiment was performed in triplicate to obtain the average.
Statistical analysis
All data were analyzed by SPSS 21.0 (SPSS, Inc., Chicago, IL, USA). Measurement data are expressed as mean ± standard deviation (SD). The least significant difference (LSD) test was used for pairwise comparisons. Multiple groups were compared by the one-way analysis of variance (ANOVA). The measurement data with normal distribution were compared by t test between 2 groups. P<0.05 was considered as statistically significant.
Results
Expression levels of JAK and STAT3 in placental tissues of PE patients
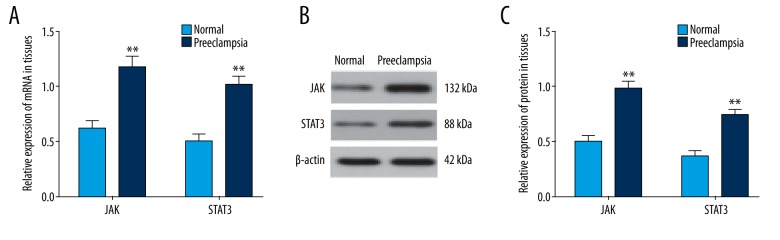
The qRT-PCR and Western blotting showed that compared with the normal pregnant women, the mRNA and protein expression levels of JAK and STAT3 were elevated in the placental tissues of PE patients (all P<0.01), indicating that the JAK/STAT3 signaling pathway was associated with PE (Figure 1).
Figure 1.
The expression levels of JAK and STAT3 in placental tissues of PE patients and normal pregnant women. (A) The qRT-PCR was performed to determine the mRNA levels of JAK and STAT3; (B) the gray values of JAK and STAT3 protein bands; (C) the protein levels of JAK and STAT3 in tissues; PE – preeclampsia; qRT-PCR – quantitative real-time polymerase chain reaction; ** P<0.01 compared with the normal pregnant women.
The morphology of trophoblast cells
The separated trophoblast cells were observed as mononuclear cells, mainly under an inverted microscope. The cells started to be adhered after 3 h, and were mostly adhered after 18 h, and adhered totally after 24 h, with various forms.
Identification of trophoblast cells
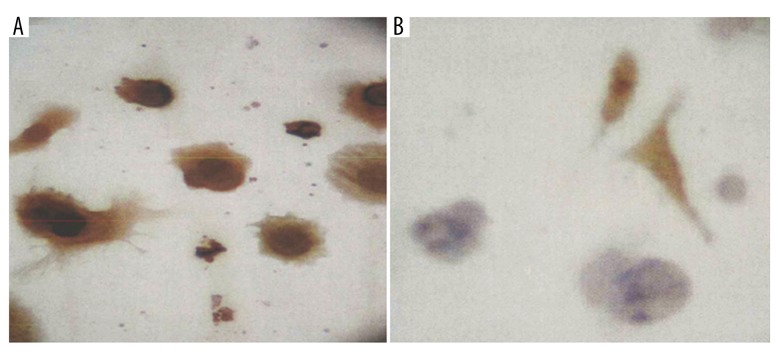
Tan particles were seen in the cytoplasm of positive cells. Immunohistochemistry indicated that the keratin presented positive and vimentin presented negative in the trophoblast cells, while the keratin presented negative and vimentin presented positive in the vascular endothelial cells and interstitial cells. The percentage of cells with positive keratin was over 90% (Figure 2).
Figure 2.
Trophoblast cells identified by immunohistochemistry (×400). (A) The trophoblast cells presented positive keratin and negative vimentin; (B) the vascular endothelial and interstitial cells presented negative keratin and positive vimentin.
Expressions of p-JAK and p-STAT3 in trophoblast cells among the blank, hypoxia, hypoxia + si-NC, and hypoxia + si-JAK groups
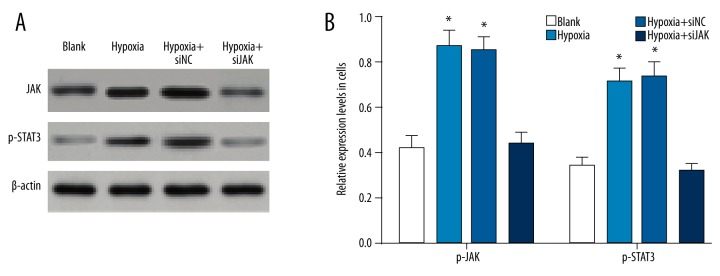
The Western blotting assay suggested that the expression levels of p-JAK and p-STAT3 in the trophoblast cells were increased in the hypoxia group and hypoxia + si-NC group compared to the blank group (all P<0.01), while there was no significant difference in the p-JAK and p-STAT3 expression levels between the hypoxia group and blank group (all P>0.05) (Figure 3). The results indicate that hypoxia preconditioning upregulated the p-JAK and p-STAT3 expression levels in the trophoblast cells and activated the JAK/STAT3 signaling pathway.
Figure 3.
The expression levels of p-JAK and p-STAT3 in trophoblast cells by Western blotting among the blank, hypoxia, hypoxia + si-NC, and hypoxia + si-JAK groups. (A) The gray values of p-JAK and p-STAT3 protein bands in trophoblast cells; (B) the expression levels of p-JAK and p-STAT3 in trophoblast cells; NC – negative control; * P<0.05 compared with the blank group.
Hypoxic preconditioning promoted trophoblast cell viability by activation of the JAK/STAT3 signaling pathway
As shown in Figure 4, cell viability rose during 48 h in the blank and hypoxia + si-JAK groups, and cell viability had no significant change in the blank and hypoxia + si-JAK groups at the same time point (all P>0.05). However, cell viability in the hypoxia group was significantly higher than that in the blank group and hypoxia + si-JAK group at the same time point (all P<0.05), suggesting that hypoxia-induced activation of JAK/STAT3 signaling pathway promoted the viability of trophoblast cells in PE. The hypoxia and hypoxia + si-NC groups showed no significant difference in cell viability at the same time point (all P>0.05).
Figure 4.
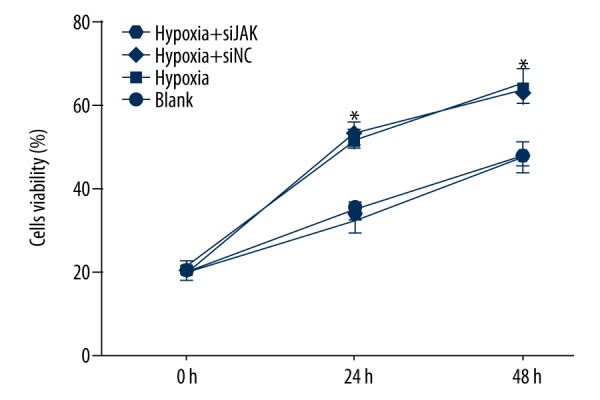
The trophoblast cell viability among the blank, hypoxia, hypoxia + si-NC, and hypoxia + si-JAK groups by the MTT assay. NC – negative control; * P<0.05 compared with the blank group at 0, 24, and 48 h.
Hypoxic preconditioning promoted colon-forming abilities of trophoblast cells by activation of the JAK/STAT3 signaling pathway
In Figure 5, the clone numbers were similar in the hypoxia and hypoxia + si-NC groups (all P>0.05). In comparison to the blank group, the clone numbers were obvious increased in the hypoxia and hypoxia + si-NC groups (all P<0.05), demonstrating that hypoxia contributed to the colony formation of the trophoblast cells. However, the colony formation of trophoblast cells in the hypoxia + si-JAK group was similar to that in the blank group (all P>0.05).
Figure 5.
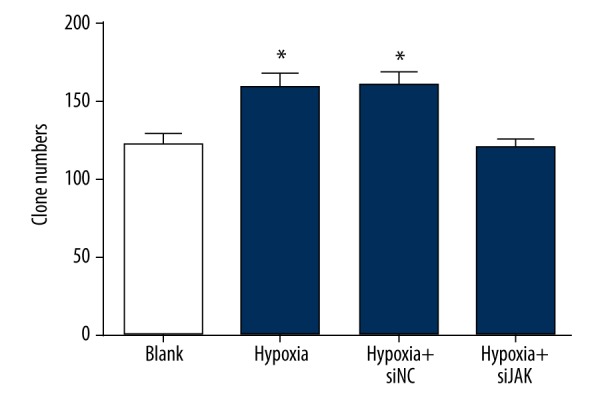
The number of colonies of trophoblast cells among the blank, hypoxia, hypoxia + si-NC, and hypoxia + si-JAK groups by colony formation assay. NC – negative control; P<0.05 compared with the blank group.
Hypoxic preconditioning inhibited trophoblast cell apoptosis by activation of the JAK/STAT3 signaling pathway
The apoptosis rate of trophoblast cells was decreased in the hypoxia group and hypoxia + si-NC group than that in the blank group (all P<0.05) (Figure 6). Compared with the hypoxia group, the apoptosis rate in the hypoxia + si-JAK group was the same as in the blank group (all P>0.05). The results indicate that hypoxia-induced activation of the JAK/STAT3 signaling pathway inhibited the apoptosis of trophoblast cells.
Figure 6.
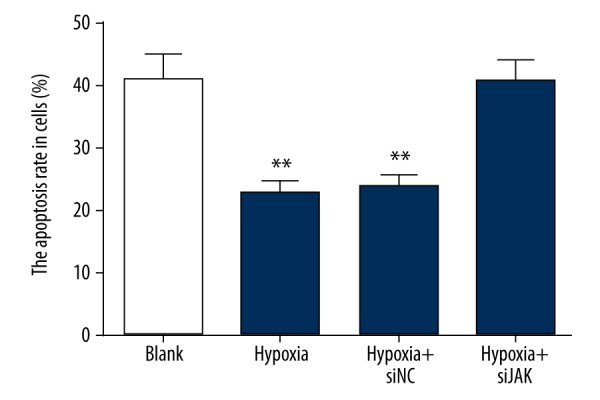
Trophoblast cell apoptosis among the blank, hypoxia, hypoxia + si-NC, and hypoxia + si-JAK groups by flow cytometry. NC – negative control; P<0.05 compared with the blank group.
The levels of VEGF, bFGF, and HGF in trophoblast cells among the blank, hypoxia, hypoxia + si-NC, and hypoxia + si-JAK groups
ELISA results showed that the levels of angiogenesis-related factors VEGF, bFGF, and HGF significantly increased in the hypoxia group and hypoxia + si-NC group compared to the blank group (all P<0.05), suggesting that hypoxia led to the angiogenesis of trophoblast cells. No significant difference was found in the levels of VEGF, bFGF, and HGF between the hypoxia + si-JAK group and blank group, and between the hypoxia group and hypoxia + si-NC group (all P>0.05) (Table 2).
Table 2.
Levels of angiogenesis-related factors in trophoblast cells among four groups.
| Group | VEGF | bFGF | HGF |
|---|---|---|---|
| Blank group | 538.42±46.03 | 16.35±1.47 | 616.54±48.02 |
| Hypoxia group | 697.79±45.14* | 27.32±2.16* | 789.53±58.31* |
| Hypoxia + si-NC group | 695.26±45.52* | 28.18±2.05* | 787.18±57.84* |
| Hypoxia + si-JAK group | 541.38±45.73 | 15.64±1.52 | 614.29±48.25 |
VEGF – vascular endothelial growth factor; bFGF – basic fibroblast growth factor; HGF – hepatocyte growth factor; NC – negative control;
P<0.05 compared with the blank group of the same factor.
Discussion
PE is related to shallow trophoblast invasion and insufficient spiral artery remodeling, which generally results in placental hypoxia that eventually causes the maternal manifestations of the disease [16,17]. An increasing number of studies have discovered that placenta tissue hypoxia is one of the important pathologic characteristics of PE [18,19]. The present study concludes that hypoxic preconditioning activates the JAK/STAT3 signaling pathway, and promotes the viability of trophoblast cells and angiogenesis, while it inhibits the cell apoptosis, in PE.
Initially, the results showed that the placental tissues of PE patients had higher expressions of JAK and STAT3 compared with the normal pregnant women. The JAK/STAT3 signaling pathway has been confirmed as the most important signaling pathway in maintaining the pluripotency of embryonic stem cells [20]. Activation of the JAK/STAT signaling pathways in trophoblast cells can lead to the progression of PE [21]. STATs are tyrosine phosphorylated in response to cytokines, hormones, or growth factors as transcription factors, which regulate gene transcription associated with cell growth, inflammation, and immune responses [22]. STAT3, as a member of the STATs family, is able to transmit signals from the cell surface to the nucleus in the activation of growth and cytokines factors, which can improve the survival rate of tumor cells [23]. Additionally, it has been shown that STAT3 can contribute to carcinogenesis and tumor progression by increasing gene expression [24]. Similarly, Bollrath et al. proved that STAT3/p-STAT3 is correlated with tumor progression leading to cancer, and the high expressions of STAT3 and p-STAT3 exists in many kinds of cancers [25]. Accumulating evidence demonstrates that activation of the JAK/STAT3 signaling pathway by growth factors or cytokines plays an important role in the growth and progression of tumors [26,27], which is consistent with our results.
The expressions of p-JAK and p-STAT3 in the trophoblast cells were higher in the hypoxia group and hypoxia + si-NC group than in the blank group, which proves that hypoxia can activate the JAK/STAT3 signaling pathway. A previous study reported that hypoxic-conditioned media infusion increased STAT3 phosphorylation and SOCS3 expression [28]. SOCS3 protein can negatively regulate the JAK/STAT signaling pathway, and is also a regulator of other signaling pathways via cross-talk [29]. Activated STAT3 induces the expression of SOCS3, which in turn terminates the JAK/STAT signaling cascade, thus forming a negative-feedback loop that converts the cells into a basal state [30]. The phosphorylation of STAT3 activation is induced by JAK2, and phosphorylated STAT3 dimers are transformed into the nucleus to induce the expression of genes regulated by STAT, which is associated with the proliferation, differentiation, and survival of cells [31]. The JAK/STAT3 signaling pathway has 2 major phosphorylated sites – the Tyr705 phosphorylated site and the Ser727 phosphorylated site8 – and the Tyr705 phosphorylated site activated by JAK and Src is essential for excitation of STAT3 [32]. Recently, de Groot et al. discovered that hypoxia can induce STAT3 during the initiation of antiangiogenic therapy [33].
In addition, we found that hypoxic preconditioning promotes colony formation and cell viability and increases the expression of angiogenesis-related factors such as VEGF, bFGF, and HGF, while inhibiting the apoptosis of trophoblast cells in PE. Previous studies show that hypoxia appears to play a key role in the development of preeclampsia [34,35]. Hypoxia is involved in the regulation of differentiation, proliferation, migration, invasion, and apoptosis of trophoblast cells [36,37]. Due to the shallow reconstruction of maternal blood vessels by trophoblasts, it is widely believed that the oxygen tension at the feto-maternal interface in PE is much lower [38]. VEGF is a signal protein that stimulates vasculogenesis and angiogenesis and restores the oxygen supply to tissues when blood circulation is inadequate, and its secretion is induced in trophoblast cells [19,39]. VEGF expression is regulated by STAT3, whose expression in the setting of failed antiangiogenic therapy may be secondary to the induction of VEGF independent angiogenic pathways [40].
Conclusions
In conclusion, the present study provides a new insight that hypoxia activates the JAK/STAT3 signaling pathway, thus contributing to an increase in trophoblast cell viability and in angiogenesis, and to a reduction in cell apoptosis in PE. The limitations of this study are that it was designed using cells in culture and used an in vitro hypoxia model. Further investigations are needed to investigate the changes of the JAK/STAT3 signaling pathway in placental tissues treated by hypoxic preconditioning in a mouse PE model.
Footnotes
Source of support: Our study was supported by the Guangzhou Science and Technology Plan project, Major Project of Collaborative Innovation, item number: 201704020170
Conflict of interests
None.
References
- 1.Liu J, Song G, Lin X, et al. Upregulated unique long 16 binding protein 1 detected in preeclamptic placenta affects human extravillous trophoblast cell line (HTR-8/SVneo) invasion by modulating the function of uterine natural killer cells. Exp Ther Med. 2017;13(4):1447–55. doi: 10.3892/etm.2017.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mistry HD, Kurlak LO, Mansour YT, et al. Increased maternal and fetal cholesterol efflux capacity and placental CYP27A1 expression in pre-eclampsia. J Lipid Res. 2017;58(6):1186–95. doi: 10.1194/jlr.M071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen M, Smith GN, Rodger M, et al. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS One. 2017;12(4):e0175914. doi: 10.1371/journal.pone.0175914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollegaard B, Lykke JA, Boomsma JJ. Time from pre-eclampsia diagnosis to delivery affects future health prospects of children. Evol Med Public Health. 2017;2017:53–66. doi: 10.1093/emph/eox004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Q, Tang J, Li N, et al. A novel mechanism of angiotensin II-regulated placental vascular tone in the development of hypertension in preeclampsia. Oncotarget. 2017;8(19):30734–41. doi: 10.18632/oncotarget.15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z, Li S, Fan W, Ma Q. Transforming growth factor beta1 promotes invasion of human JEG-3 trophoblast cells via TGF-beta/Smad3 signaling pathway. Oncotarget. 2017;8(20):33560–70. doi: 10.18632/oncotarget.16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eksteen M, Heide G, Tiller H, et al. Anti-human platelet antigen (HPA)-1a antibodies may affect trophoblast functions crucial for placental development: A laboratory study using an in vitro model. Reprod Biol Endocrinol. 2017;15(1):28. doi: 10.1186/s12958-017-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gormley M, Ona K, Kapidzic M, et al. Preeclampsia: Novel insights from global RNA profiling of trophoblast subpopulations. Am J Obstet Gynecol. :2017. doi: 10.1016/j.ajog.2017.03.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Ouyang J, Pan X, Lin H, et al. GKN2 increases apoptosis, reduces the proliferation and invasion ability of gastric cancer cells through down-regulating the JAK/STAT signaling pathway. Am J Transl Res. 2017;9(2):803–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Dela Pena-Ponce MG, Rodriguez-Nieves J, Bernhardt J, et al. Increasing JAK/STAT signaling function of infant CD4+ T cells during the first year of life. Front Pediatr. 2017;5:15. doi: 10.3389/fped.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kundumani-Sridharan V, Van Quyen D, Subramani J, et al. Novel interactions between NFATc1 (Nuclear Factor of Activated T cells c1) and STAT-3 (Signal Transducer and Activator of Transcription-3) mediate G protein-coupled receptor agonist, thrombin-induced biphasic expression of cyclin D1, with first phase influencing cell migration and second phase directing cell proliferation. J Biol Chem. 2012;287(27):22463–82. doi: 10.1074/jbc.M112.362996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong AL, Soo RA, Tan DS, et al. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann Oncol. 2015;26(5):998–1005. doi: 10.1093/annonc/mdv026. [DOI] [PubMed] [Google Scholar]
- 13.Kowshik J, Baba AB, Giri H, et al. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS One. 2014;9(10):e109114. doi: 10.1371/journal.pone.0109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee A, Khuda-Bukhsh AR. Quercetin down-regulates IL-6/STAT-3 signals to induce mitochondrial-mediated apoptosis in a nonsmall- cell lung-cancer cell line, A549. J Pharmacopuncture. 2015;18(1):19–26. doi: 10.3831/KPI.2015.18.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YJ, Shyu WC, Chang CW, et al. Tumor hypoxia regulates Forkhead Box C1 to promote lung cancer progression. Theranostics. 2017;7(5):1177–91. doi: 10.7150/thno.17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Xiao D, Hu Y, et al. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension. 2013;62(3):599–607. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Q, Wu G, Li JX. Effect of hypoxia on expression of placental trophoblast cells SATB1 and beta-catenin and its correlation with the pathogenesis of preeclampsia. Asian Pac J Trop Med. 2016;9(6):567–71. doi: 10.1016/j.apjtm.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Huppertz B. Oxygenation of the placenta and its role in pre-eclampsia. Pregnancy Hypertens. 2014;4(3):244–45. doi: 10.1016/j.preghy.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Iriyama T, Wang W, Parchim NF, et al. Hypoxia-independent upregulation of placental hypoxia inducible factor-1alpha gene expression contributes to the pathogenesis of preeclampsia. Hypertension. 2015;65(6):1307–15. doi: 10.1161/HYPERTENSIONAHA.115.05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Sang H, Zhang K, et al. Stat3 phosphorylation is required for embryonic stem cells ground state maintenance in 2i culture media. Oncotarget. 2017;8(19):31227–37. doi: 10.18632/oncotarget.16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin N, Zhang H, Luo X, et al. IL-27 activates human trophoblasts to express IP-10 and IL-6: implications in the immunopathophysiology of preeclampsia. Mediators Inflamm. 2014;2014:926875. doi: 10.1155/2014/926875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George EM, Arany I. Induction of heme oxygenase-1 shifts the balance from proinjury to prosurvival in the placentas of pregnant rats with reduced uterine perfusion pressure. Am J Physiol Regul Integr Comp Physiol. 2012;302(5):R620–26. doi: 10.1152/ajpregu.00617.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gritsko T, Williams A, Turkson J, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12(1):11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 24.Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA. 2007;104(18):7391–96. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Liao Q, Zeng Z, Guo X, et al. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene. 2014;33(16):2098–109. doi: 10.1038/onc.2013.161. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Tao P, Zhou Q, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8(13):20741–50. doi: 10.18632/oncotarget.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic conditioned medium from human adipose-derived stem cells promotes mouse liver regeneration through JAK/STAT3 signaling. Stem Cells Transl Med. 2016;5(6):816–25. doi: 10.5966/sctm.2015-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19(4):414–22. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babon JJ, Varghese LN, Nicola NA. Inhibition of IL-6 family cytokines by SOCS3. Semin Immunol. 2014;26(1):13–19. doi: 10.1016/j.smim.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jo HA, Kim JY, Yang SH, et al. The role of local IL6/JAK2/STAT3 signaling in high glucose-induced podocyte hypertrophy. Kidney Res Clin Pract. 2016;35(4):212–18. doi: 10.1016/j.krcp.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le J, Zhang DY, Zhao Y, et al. ITF promotes migration of intestinal epithelial cells through crosstalk between the ERK and JAK/STAT3 pathways. Sci Rep. 2016;6:33014. doi: 10.1038/srep33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Groot J, Liang J, Kong LY, et al. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget. 2012;3(9):1036–48. doi: 10.18632/oncotarget.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaiman D, Calicchio R, Miralles F. Landscape of transcriptional deregulations in the preeclamptic placenta. PLoS One. 2013;8(6):e65498. doi: 10.1371/journal.pone.0065498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moslehi R, Mills JL, Signore C, et al. Integrative transcriptome analysis reveals dysregulation of canonical cancer molecular pathways in placenta leading to preeclampsia. Sci Rep. 2013;3:2407. doi: 10.1038/srep02407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appel S, Ankerne J, Appel J, et al. CNN3 regulates trophoblast invasion and is upregulated by hypoxia in BeWo cells. PLoS One. 2014;9(7):e103216. doi: 10.1371/journal.pone.0103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang R, Cai J, Zhu Z, et al. Hypoxic trophoblast HMGB1 induces endothelial cell hyperpermeability via the TRL-4/caveolin-1 pathway. J Immunol. 2014;193(10):5000–12. doi: 10.4049/jimmunol.1303445. [DOI] [PubMed] [Google Scholar]
- 38.Luo R, Wang Y, Xu P, et al. Hypoxia-inducible miR-210 contributes to preeclampsia via targeting thrombospondin type I domain containing 7A. Sci Rep. 2016;6:19588. doi: 10.1038/srep19588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer BF, Clegg DJ. Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol. 2014;397(1–2):51–58. doi: 10.1016/j.mce.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Briggs J, Park S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24(36):5552–60. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]