Abstract
Background
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed for depression and anxiety, but their efficacy relative to placebo has been questioned. We aimed to test how manipulation of verbally induced expectancies, central for placebo, influences SSRI treatment outcome and brain activity in patients with social anxiety disorder (SAD).
Methods
We did a randomized clinical trial, within an academic medical center (Uppsala, Sweden), of individuals fulfilling the DSM-IV criteria for SAD, recruited through media advertising. Participants were 18 years or older and randomized in blocks, through a computer-generated sequence by an independent party, to nine weeks of overt or covert treatment with escitalopram (20 mg daily). The overt group received correct treatment information whereas the covert group was treated deceptively with the SSRI described, by the psychiatrist, as active placebo. The treating psychiatrist was necessarily unmasked while the research staff was masked from intervention assignment. Treatment efficacy was assessed primarily with the self-rated Liebowitz Social Anxiety Scale (LSAS-SR), administered at week 0, 1, 3, 6 and 9, also yielding a dichotomous estimate of responder status (clinically significant improvement). Before and at the last week of treatment, brain activity during an emotional face-matching task was assessed with functional magnetic resonance imaging (fMRI) and during fMRI sessions, anticipatory speech anxiety was also assessed with the Spielberger State-Trait Anxiety Inventory - State version (STAI-S). Analyses included all randomized patients with outcome data at posttreatment. This study is registered at ISRCTN, number 98890605.
Findings
Between March 17th 2014 and May 22nd 2015, 47 patients were recruited. One patient in the covert group dropped out after a few days of treatment and did not provide fMRI data, leaving 46 patients with complete outcome data. After nine weeks of treatment, overt (n = 24) as compared to covert (n = 22) SSRI administration yielded significantly better outcome on the LSAS-SR (adjusted difference 21.17, 95% CI 10.69–31.65, p < 0.0001) with more than three times higher response rate (50% vs. 14%; χ2(1) = 6.91, p = 0.009) and twice the effect size (d = 2.24 vs. d = 1.13) from pre-to posttreatment. There was no significant between-group difference on anticipatory speech anxiety (STAI-S), both groups improving with treatment. No serious adverse reactions were recorded. On fMRI outcomes, there was suggestive evidence for a differential neural response to treatment between groups in the posterior cingulate, superior temporal and inferior frontal gyri (all z thresholds exceeding 3.68, p ≤ 0.001). Reduced social anxiety with treatment correlated significantly with enhanced posterior cingulate (z threshold 3.24, p = 0.0006) and attenuated amygdala (z threshold 2.70, p = 0.003) activity.
Interpretation
The clinical and neural effects of escitalopram were markedly influenced by verbal suggestions. This points to a pronounced placebo component in SSRI-treatment of SAD and favors a biopsychosocial over a biomedical explanatory model for SSRI efficacy.
Funding resources
The Swedish Research Council for Working Life and Social Research (grant 2011-1368), the Swedish Research Council (grant 421-2013-1366), Riksbankens Jubileumsfond – the Swedish Foundation for Humanities and Social Sciences (grant P13-1270:1).
Keywords: Expectancies, SSRI, Social anxiety disorder, Placebo effect, Neuroimaging, fMRI
Highlights
-
•
Overt surpassed covert SSRI treatment with doubled effect size and tripled response rate on the main social anxiety outcome.
-
•
Overt vs. covert SSRI treatment yielded different neural changes in brain areas involved in emotion-cognition interactions.
-
•
This study suggests that the presentation of a treatment may be as important as the treatment itself.
Using truthful or deceiving verbal instructions, we tested how expectancies influence SSRI efficacy in social anxiety disorder. The number of responders was more than three times higher after open administration of escitalopram 20 mg compared to covert administration of the drug presented as “active placebo” in a cover story. Correct vs. incorrect information about the SSRI also yielded different neural changes in brain areas involved in emotion-cognition interactions. The benefit of SSRI medication seems to be highly affected by psychological factors like positive expectancies traditionally associated with placebo. Our results favor a biopsychosocial over a biomedical explanatory model for SSRI efficacy.
1. Introduction
Selective serotonin reuptake inhibitors (SSRIs) are one of the most commonly prescribed psychotropic medications but the clinical significance of this class of drugs has been widely debated in the field of depression (Bschor and Kilarski, 2016; Khan and Brown, 2015) and anxiety (Roest et al., 2015; Sugarman et al., 2014). In depression, this debate has been fueled by meta-analytic studies failing to demonstrate a clinically meaningful advantage of SSRIs over placebo (Kirsch et al., 2002) although superior SSRI (> placebo) effects have been noted, e.g. in severely depressed patients (Bschor and Kilarski, 2016; Khan and Brown, 2015) and when limiting statistical analyses to depressed mood as a single-item measure (Hieronymus et al., 2015). It has even been argued that improvement attributed to antidepressants in double-blind trials could reflect an enhanced placebo response, i.e., beneficial outcome unrelated to the specific/active properties of the treatment itself, due to treatment expectations induced by perceived side effects (Moncrieff et al., 2004). Indeed, previous research in anxiety (Colloca et al., 2004), depression (Chen et al., 2011; Rutherford et al., 2016) and pain (Bingel et al., 2011; Colloca et al., 2004) supports that the patients' expectancies and beliefs can have a profound influence on therapeutic outcomes. However, with regard to the SSRI vs. placebo minimal difference debate, conclusions have been based mostly on meta-analytic (Kirsch et al., 2002) and correlational studies (Chen et al., 2011), hampering causal inference.
Here, we tested how verbally induced expectancies, a crucial placebo mechanism, influence SSRI efficacy in patients with social anxiety disorder (SAD). Treatment studies of SAD have noted response rates of about 50–60% for SSRIs like escitalopram and 40% for placebo (Baldwin et al., 2016). In a previous neuroimaging trial of SAD we found striking similarities between SSRI and placebo responders both regarding clinical effects and altered anxiety-related brain activity (Faria et al., 2012, Faria et al., 2014). However, previous studies could not fully infer about the influence of expectancies on SSRI treatment outcome because expectancies have not been measured and experimentally manipulated. Thus, in the present study, we experimentally addressed the question raised by meta-analytic studies i.e., to what extent the clinical effects of SSRIs can be manipulated by verbal suggestions. Patients with SAD were treated with equivalent doses of escitalopram (20 mg daily) for 9 weeks, but only one group was correctly informed about the treatment received and its effectiveness. By use of a credible cover story, the other group was led to believe that they were treated with an “active placebo” (neurokinin-1 antagonist) that lacked specific anxiolytic properties, but was expected to induce side effects similar to the SSRI.
Thus, the aim was to assess the clinical efficacy of escitalopram with and without expectations of improvement, i.e. overt vs. covert SSRI treatment. To evaluate effects also on objective brain parameters, neural reactivity to a disorder-relevant emotional face-matching task (Hariri et al., 2002; Gingnell et al., 2016) was assessed before and at the last week of treatment with functional magnetic resonance imaging (fMRI). Based on reports of a strong placebo effect in SAD treatment (Baldwin et al., 2016; Faria et al., 2012, Faria et al., 2014) and the association between SAD symptoms and amygdala reactivity (Faria et al., 2012, Faria et al., 2014; Furmark et al., 2008; Gingnell et al., 2016), we expected larger clinical improvement and greater attenuation of amygdala responsivity in the overt as compared to covert SSRI group.
2. Materials and Methods
2.1. Study Design and Participants
This was an investigator-initiated, randomized, clinical trial on escitalopram with manipulation of verbal instructions i.e., expectancies, conducted in an academic medical centre (Uppsala, Sweden). Patients with SAD were randomized into two treatment arms but all received equal doses of 20 mg escitalopram per day for nine weeks (10 mg the first week). However, whereas patients in the overt treatment arm were correctly informed about the treatment received, patients in the covert arm were deceived with a cover story (see procedure, expectancy manipulation).
The Regional Ethical Review Board, Uppsala and the Medical Products Agency in Sweden approved the study which complied with the standards established by the Declaration of Helsinki. All participants were provided with verbal and written instructions about the study procedure and objectives, comparing escitalopram and “active placebo”. The stated purpose of evaluating active placebo was to acquire knowledge about its neural effects, for later use as an improved control treatment in clinical trials. All participants gave written informed consent prior to inclusion. Participants in the covert group were debriefed by the study psychiatrist at unblinding. For ethical reasons, all participants were also offered an internet-based cognitive behavioral therapy (CBT) program (Andersson et al., 2006) for free after initial treatment.
Participants (aged 18 years and older) were recruited through newspaper advertisements and public billboards. During the initial screening, the Social Phobia Screening Questionnaire (SPSQ) (Furmark et al., 1999) and the Montgomery Åsberg Depression Rating Scale (MADRS-S) (Montgomery and Asberg, 1979) were administered online. Participants passing initial screening were interviewed using the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998), the SAD section of the Structured Clinical Interview for DSM-IV (SCID-I) (First et al., 1997), and underwent a medical check-up. All participants had to meet the DSM-IV (American Psychiatric Association, 2000) criteria for SAD as the primary diagnosis. Exclusion criteria were: contraindications for MR, age < 18 or > 65 years, presence of severe somatic disease or serious psychiatric disorder such as psychosis or severe major depression, treatment for any psychiatric disorder (ongoing or terminated within three months), pregnancy, menopause, and drug or alcohol abuse/dependency.
2.2. Randomization and Masking
Randomization, stratified by sex and age, was determined by a computerized random-number generator in blocks of two by an independent third party (APL, Stockholm, Sweden). The treating psychiatrist of the study (K.W.) assigned participants to the trial, after baseline measurements and consent, and allocation to intervention was implemented by use of a numbered list. Randomization codes were kept secret, in a sealed opaque envelope, at the psychiatrist's clinic until study completion. APL, Stockholm, Sweden, prepared standard escitalopram tablets for the overt group, and unmarked capsules for the covert group, both containing escitalopram 20 mg (Cipralex, H. Lundbeck AB, Helsingborg, Sweden) which were placed in containers marked with “Active placebo/Escitalopram 20 mg” (10 mg first week). Due to the nature of the investigation, the treating psychiatrist (K.W.) was not blinded while the research staff was blinded to the intervention allocation after randomization.
To minimize experimenter influence, the primary outcome measure i.e., the Liebowitz Social Anxiety Scale self-rate version (LSAS-SR) (Fresco et al., 2001) with automated scoring, was filled out online at home and participants were instructed not to reveal their allocated treatment to assessors. After the final assessments, before unblinding, all personnel in the study completed a short written questionnaire with the following (yes/no) questions: 1. During the study, did you know treatment allocation for any of the participants? (If yes, for how many?); 2. Did any of the participants disclose information of any type that revealed their allocated treatment to you? According to the questionnaire, none of the personnel in the trial, beside the non-blinded psychiatrist (K.W.), reported knowledge about group allocation for any participant.
2.3. General Procedure
After initial screening, baseline measurements and consent, two fMRI scanning sessions with an emotional face-matching task were scheduled for each participant, one before and one at the end, during the last week, of treatment. At the end of each fMRI-session, participants were exposed to a public speaking behavioral test in which they gave a two minute speech on a freely chosen topic in front of a silent audience (5–8 persons) – see Gingnell et al., 2016. Anticipatory anxiety was assessed with the Spielberger State-Trait Anxiety Inventory - State version (STAI-S) (Spielberger et al., 1970) before the speech. All participants were informed, prior to signing the informed consent, that they were requested to hold a speech after each fMRI-session. Before and during week 1, 3, 6, and 9 of treatment, participants filled out the primary outcome LSAS-SR (Fresco et al., 2001) online at home.
After the posttreatment fMRI scanning session, participants revisited the psychiatrist for a complementary clinical evaluation. Approximately 6 months after this visit, the participants in the covert group were contacted again by the psychiatrist and the cover story was revealed (see Supplement). After treatment, and at unblinding, participants were offered further contact with the psychiatrist at own cost and access to a free internet CBT program (Andersson et al., 2006). Efficacy of the internet CBT program offered to all participants after initial treatment was not assessed due to low numbers of completers.
2.4. Expectancy Manipulation
After the first fMRI session, an experienced psychiatrist (K.W.) assessed additional baseline clinical symptom severity (see Supplement), and the participants were handed their supply of daily escitalopram 20 mg (10 mg first week) with separate verbal instructions for the overt and covert arm. The overt group was correctly informed about the SSRI treatment and the expected improvement, whereas the covert group were told that they would receive the active placebo, i.e. a non-functioning neurokinin-1 antagonist (GW597599) likely to induce side effects similar to escitalopram but out of which no symptom-improvement could be expected (see the Supplementary appendix for the full written and verbal information to participants). Participants revisited the psychiatrist's clinic after one week and were then handed their supply of the medication for the remainder of the study period. Compliance was assessed by serum drug concentrations at the posttreatment fMRI.
2.5. Functional Magnetic Resonance Imaging
MR imaging was performed using a Philips Achieva 3.0 T whole body MR-scanner (Philips Medical Systems, Best, The Netherlands) equipped with an 8-channel head-coil. Five of the participants (three in the overt and two in the covert group) were scanned after an upgrade of the scanner software and for those participants a 32-channel head coil was used. Participants were positioned supine in the scanner. An anatomical T1-weighted image (echo time(TE) = 15 ms; repetition time (TR) = 5700 ms; inversion time = 400 ms; field of view = 230 × 230 mm2; voxel size = 0.8 × 1.0 × 2.0 mm3; 60 contiguous slices) and a blood‑oxygenation-level dependent (BOLD) echo planar imaging (EPI) sequence (TE = 35 ms; TR = 3000 ms; flip angle = 90°, acquisition matrix = 76 × 77, voxel size = 3.0 × 3.0 × 3.0 mm3, gap = 1 mm, 30 axial slices) was acquired. Visual stimuli were presented through goggles (Visual System, NordicNeuroLab, Bergen, Norway) using E-prime (Psychology Software Tools, Sharpsburg, PA, USA).
2.6. fMRI Paradigm
The paradigm included matching of fearful or angry facial expressions and geometrical shapes (Hariri et al., 2002). In each trial, a target face or shape was displayed at the top of the screen and, by pressing a button with their left or right index finger, the participants indicated which one of two lower images displayed the same emotion or shape as the target (see Supplement, Fig. S1). Face and shape trials were presented in blocks of 6, in which images were presented for 4 s, interspaced with a fixation cross (2s for shape trials and a random duration of 2, 4, or 6s for face trials). The expressed emotion or shape of the target varied from trial to trial, and each face block had an equal mix of emotions as well as sex of the faces. Accuracy and reaction times were recorded but there were no significant group differences on these measures. The fMRI session included four additional scans not reported in the present study.
2.7. Primary Outcome Measures
The main clinical outcome measure was the continuous LSAS-SR (Fresco et al., 2001) (assessed online 5 times), which was also used to determine response status according to the criteria for clinically significant improvement (Jacobson and Truax, 1991). Responders exhibited a reliable change index > 1.96 and a posttreatment total score < 39, falling within 2 SD from the mean of the normal population (Fresco et al., 2001). Further, anticipatory speech anxiety was assessed after each fMRI-session with the STAI-S (Spielberger et al., 1970), after three minutes of preparation.
2.8. Secondary Outcome Measures
Before and after the full treatment period, five secondary outcome measures were also administered online: the Social Interaction Anxiety Scale (SIAS) (Mattick and Clarke, 1998), Social Phobia Scale (SPS) (Mattick and Clarke, 1998), MADRS-S (Montgomery and Asberg, 1979), Beck Anxiety Inventory (BAI) (Beck et al., 1988), and the Quality of Life Inventory (QOLI) (Frisch et al., 1992).
2.9. Credibility Ratings
Before the first fMRI-session and after nine weeks of treatment, participants rated their treatment beliefs both about escitalopram and “active placebo”. Questions, asked before the first scanning session, i.e., prior to randomization, included how logical the treatment seemed; how sure the patients were that they would be improved in symptomatology by the treatment; how strongly they would recommend the treatment to a friend in a similar situation; how they thought that the treatment would affect other types of anxiety disorders; how much improvement they would expect themselves if given the described treatment. All questions were rated between 0 and 10, 0 indicating “not at all” and 10 “very much”. The results of these questions were used to create a summed credibility score (0–50) for each treatment.
At posttreatment, participants answered two questions regarding the perceived value of having been part of the study, one open and one with fixed rating steps (1 = not valuable, 2 = slightly valuable or neutral, 3 = rather valuable, 4 = very valuable). They were also asked how sure they were that the treatment they had been assigned to was effective for SAD and how strongly they would recommend the treatment to a friend with similar symptoms, both rated between 0 and 10, 0 indicating “not at all” and 10 “very much”. In addition to this they responded to an open question whether their view of the effectiveness of the treatment they had received had changed during the study period (recoded into 1 = more negative, 2 = slightly more negative, 3 = none, 4 = slightly more positive, 5 = more positive).
Credibility/expectancy assessments were not performed continuously during treatment to avoid causing suspicion regarding the cover story. Participants in the covert group were also asked to rate their beliefs at the follow-up psychiatric appointment when the cover story was revealed (see the Supplement). Ratings were not available from 4 individuals.
2.10. fMRI Outcome
Estimates of blood-oxygen-level-dependent (BOLD) reactivity was obtained for each participant and session, by contrasting emotional faces against geometrical shapes. Change in this reactivity with treatment was then calculated for each participant and compared across groups (see below).
2.11. Power Calculations
Assessing the effect of verbally-induced expectations on SSRI-treatment is a novel approach but power calculations based on previous placebo controlled SSRI trials (Faria et al., 2012, Faria et al., 2014; Gingnell et al., 2016) assumed a difference between treatment arms in mean ± SD LSAS-scores of 11.4 ± 11.7. Given α = 0.05 and n = 24 per group, the study had 80% power to detect a difference between treatments.
2.12. Analyses of Behavioral Data
Demographic and pretreatment clinical data were compared between groups by t-tests, Mann-Whitney U tests or Chi-square tests using IBM SPSS Statistics 20. Clinical treatment effects were evaluated using repeated measure MANOVA/ANOVA with t-test for follow-up analyses. For 3 (1%) missing LSAS-SR scores (n = 1 week3, n = 2 week 6) the last observation was carried forward. The standardized mean difference (Cohen's d pre-post) was also calculated for each group. For behavioral and demographic analyses, a p-value of < 0.05 was considered significant. Statistical analyses were based on participants with complete outcome data, thereby excluding one early drop-out (male) in the covert group. A data monitoring delegate oversaw the study.
2.13. Analyses of Imaging Data
The fMRI-data were analysed in MATLAB (MathWorks, Natick, MA, USA) using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Each participant's BOLD EPI images were realigned to the mean image of each session, slice timing corrected to the middle slice of each volume, co-registered with the anatomical scan and normalized to Montreal Neurological Institute (MNI) standard space using parameters obtained from unified segmentation of the anatomical image. Finally, smoothing was performed using an 8 mm Gaussian kernel (full width, half maximum). The BOLD signal in each voxel was high-pass filtered with 128 s, regressed on the stimulus function (boxcar, onsets and durations of face- and shape-stimuli), six movement parameters obtained from the realignment step, and convolved with the canonical hemodynamic response function provided by SPM.
Individual difference images, representing changes in BOLD reactivity (emotional faces vs. shapes), were calculated by subtracting the pretreatment from the posttreatment contrast map, and used in second level group comparisons.
Between-groups t-tests were used to analyse the neural reactivity-changes with treatment. Comparisons in the automated anatomical labeling library (aal) region of interest (ROI) for bilateral amygdala were assessed at p < 0.05 FWE-corrected for multiple comparisons. In addition to this, we used an exploratory whole brain approach with p < 0.001 and a cluster extent of ≥ 10 voxels. Follow-up analyses of associations between symptom improvement and altered BOLD reactivity in the amygdala and the observed clusters from the exploratory whole brain analysis, were assessed in the whole sample by including LSAS-SR change scores in regression analyses at p < 0.05. Follow-up analyses were also performed to assess group differences using psychophysiological interaction (PPI) analyses of amygdala connectivity with time-series fMRI data extracted from obtained peak voxels entered as a regressor together with task (faces vs. shapes) and the interaction between the two. For all fMRI-analyses time point of scanning (before/after upgrade) was used as a covariate. Spatial localizations are reported in Montreal Neurological Institute (MNI) coordinates.
3. Results
3.1. Participants
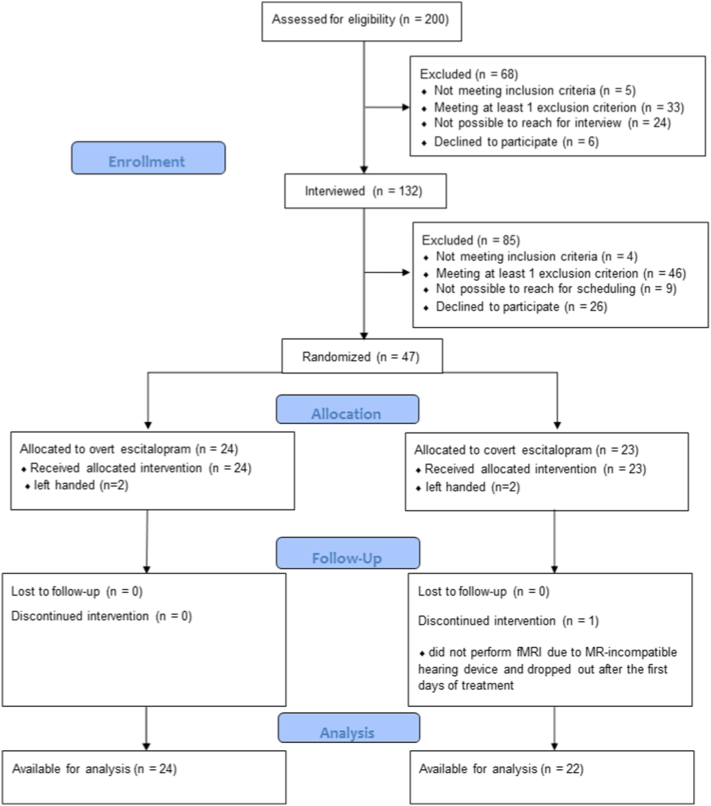
Between March 17, 2014, and May 22nd 2015, a total of 47 patients with SAD (mean ± SD age 31.8 ± 10.2 years; 18 women) were recruited and randomly assigned either to overt (n = 24) or covert (n = 23) SSRI treatment. One male participant assigned to the covert group did not provide fMRI-data and dropped out of treatment after a few days, limiting the statistical evaluation to 46 patients with complete outcome data. See Fig. 1 for the-trial profile and Table 1 for descriptive characteristics.
Fig. 1.
Trial profile.
Table 1.
Descriptive characteristics.
| Overt SSRI (n = 24) |
Covert SSRI (n = 22) |
Statistic (df) |
p | |
|---|---|---|---|---|
| Age, years, mean (sd) | 31.0 (10.6) | 32.0 (9.7) | t(44) = 0.33 | 0.74 |
| Sex, men, n (%) | 15 (62.5) | 13 (59.1) | χ2(1) = 0.06 | 0.81 |
| Civil status, single, n (%) | 12 (50) | 7 (31.8) | χ2(1) = 1.57 | 0.21 |
| Education > 12 years, n (%) | 12 (50) | 7 (31.8) | χ2(1) = 1.57 | 0.21 |
| Comorbidity, n (%) | 9 (37.5) | 13 (59.1) | χ2(1) = 2.14 | 0.14 |
| GAD | 1 (4.2) | 4 (18.2) | ||
| Panic disorder | – | 1 (4.5) | ||
| Agoraphobia | 6 (25) | 5 (22.7) | ||
| OCD, mild | 1 (4.2) | – | ||
| Depression, mild | 5 (20.8) | 5 (22.7) | ||
| Dysthymia | 2 (8.3) | 2 (9.1) | ||
| Earlier psychological treatment, n (%) | 10 (41.7) | 9 (40.9) | χ2(1) = 0.00 | 0.96 |
| Earlier psychotropic medication, n (%) | 4 (16.7) | 7 (31.8) | χ2(1) = 1.45 | 0.23 |
| SSRIs | 4 (16.7) | 6 (27.3) | ||
| Venlafaxine | – | 1 (4.5) |
GAD = generalized anxiety disorder, OCD = obsessive compulsive disorder, SSRI = selective serotonin reuptake inhibitor.
3.2. Pretreatment Evaluation
There were no significant differences between the overt and covert SSRI group before treatment on baseline demographics, disease characteristics (Table 1), or on any primary or secondary outcome measure (Table 2). Before randomization, significantly higher (t(42) = 6.58, p < 0.0001) credibility ratings were given for SSRI treatment (M ± SD 29.77 ± 7.07) compared with “active placebo” according to the cover story (M ± SD 19.53 ± 9.29), see Fig. 2 (top left). The overt and covert groups did not differ in their initial (pre-randomization) ratings of the two treatments (p > 0.38).
Table 2.
Clinical variables before in comparison to after treatment.
| Overt SSRI (n = 24) |
Covert SSRI (n = 22) |
F(1, 44) (between) | p-Value (between) | Partial η2 (between) | |
|---|---|---|---|---|---|
| Liebowitz Social Anxiety Scale self-reporta | 16.58 | <0.0001 | 0.27 | ||
| Pre (SD) | 83.71 (20.13) | 81.45 (17.82) | |||
| Post (SD | 41.08 (17.91) | 60.00 (20.17) | |||
| Paired t-test, p-Value | 10.71, p < 0.0001 | 6.58, p < 0.0001 | |||
| Cohen's d/SD changeb (within) | 2.24/19.50 | 1.13/15.30 | |||
| STAI-S Anticipatory anxietya | 0.04 | 0. 84 | 0.001 | ||
| Pre (SD) | 58.21 (9.01) | 60.91 (7.83) | |||
| Post (SD) | 48.42 (12.02) | 51.73 (11.74) | |||
| Paired t-test, p-value | 4.79, p < 0.0001 | 4.03, p = 0.001 | |||
| Cohen's d/SD changeb (within) | 0.92/10.01 | 0.93/10.69 | |||
| Social Phobia Scale | 2.97 | 0.09 | 0.06 | ||
| Pre (SD) | 39.25 (14.10) | 39.23 (14.47) | |||
| Post (SD) | 22.75 (15.07) | 28.91 (13.13) | |||
| Paired t-test, p-value | 7.48, p < 0.0001 | 3.59, p = 0.002 | |||
| Cohen's d/SD changeb (within) | 1.12/10.81 | 0.75/13.48 | |||
| Social Interaction Anxiety Scale | 6.91 | 0.01 | 0.14 | ||
| Pre (SD) | 54.92 (13.82) | 54.36 (10.92) | |||
| Post (SD) | 36.08 (15.97) | 46.00 (13.11) | |||
| Paired t-test, p-value | 6.77, p < 0.0001 | 2.94, p = 0.008 | |||
| Cohen's d/SD changeb (within) | 1.26/13.62 | 0.70/13.35 | |||
| Montgomery Åsberg Depression Rating Scale self-report | 5.40 | 0.02 | 0.11 | ||
| Pre (SD) | 17.04 (7.23) | 14.91 (7. 60) | |||
| Post (SD) | 7.38 (5.75) | 10.27 (5.28) | |||
| Paired t-test, p-value | 6.09, p < 0.0001 | 3.19, p = 0.004 | |||
| Cohen's d/SD changeb (within) | 1. 47/7.78 | 0.70/6.82 | |||
| Beck Anxiety Inventory | 1.18 | 0.28 | 0.03 | ||
| Pre (SD) | 19.92 (8.66) | 19.18 (7. 82) | |||
| Post (SD) | 9.25 (6.94) | 11.18 (5.58) | |||
| Paired t-test, p-value | 7.83, p < 0.0001 | 3.84, p = 0.001 | |||
| Cohen's d/SD changeb (within) | 1.36/6.68 | 1.18/9.78 | |||
| Quality Of Life Inventory | 8.37 | 0.006 | 0.16 | ||
| Pre (SD) | 0. 66 (1.62) | 0.87 (1.34) | |||
| Post (SD) | 2.01 (1.25) | 1.10 (1.27) | |||
| Paired t-test, p-value | 4.39, p = 0.0002 | 1.03, p = 0.32 | |||
| Cohen's d/SD changeb (within) | 0.89/1.50 | 0.15/1.05 |
SSRI: escitalopram, STAI-S: Spielberger State-Trait Anxiety Inventory State version.
Primary outcomes.
Standard deviation for change score pre-post.
Fig. 2.
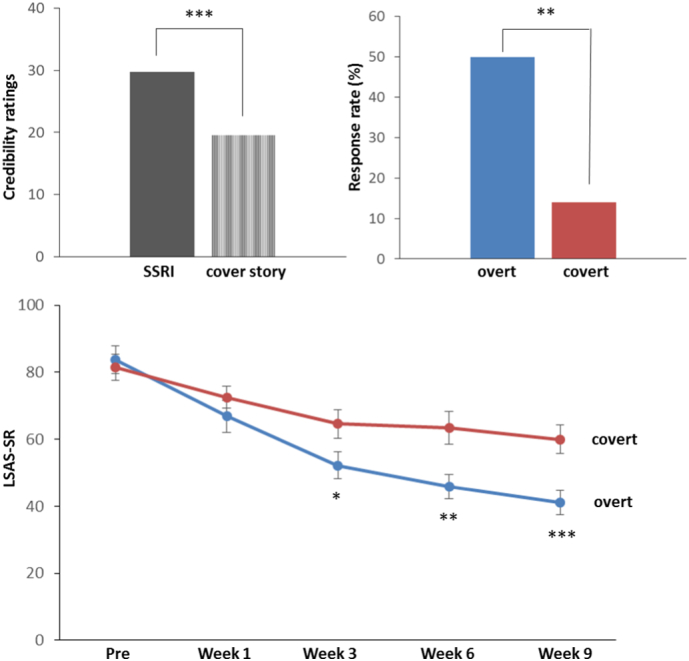
Top left: Initial credibility ratings for SSRI (escitalopram) and “active placebo” described as a neurokinin-1 antagonist in the cover story before randomization (50 = maximal credibility); Top right: Percentage of individuals meeting the criteria for clinically significant improvement on the Liebowitz Social Anxiety Scale (LSAS-SR); Lower panel: Time course of treatment response on the LSAS-SR. *) p < 0.05, **)p < 0.01, ***)p < 0.005.
3.3. Primary Clinical Outcomes
Online assessments of LSAS-SR, yielded a significant Group × Time interaction (F(4,176) = 9.16, p < 0.0001, ƞ2 = 0.17) in the repeated measures ANOVA, supporting larger improvement in the overt as compared to the covert SSRI arm, with a significant between-group difference emerging at week 3 (t(44) = 2.23, p = 0.03) – see Fig. 2 (lower panel). Accordingly, after treatment there were significantly more responders, i.e. individuals meeting the criteria for clinically significant improvement on the LSAS-SR, in the overt (12/24, 50%) than in the covert (3/22, 14%) SSRI arm (χ2(1) = 6.91, p = 0.009), Fig. 2 (top right). For LSAS-SR, the (pre-post) effect size was d = 2.24 in the overt vs. d = 1.13 in the covert group. Anticipatory speech anxiety (STAI-S) ratings before the behavioral test did not change differently (pre-post) in the two groups, both improving with treatment (Table 2).
3.4. Secondary Clinical Outcomes
With regard to secondary clinical measures, both groups improved significantly from pre- to posttreatment on all measures with the exception of quality of life in the covert arm (Table 2). There was a significant Group × Time interaction indicating better treatment outcome in the overt as compared to covert SSRI arm on measures of social interaction anxiety, depression and quality of life (Table 2). Repeated measures MANOVA of all primary and secondary measures indicated a significant Group × Time (pre/post) interaction (Wilk's λ = 0.62, F(7,38) = 3.30, p = 0.008, ƞ2 = 0.38) in favor of overt SSRI.
3.5. Treatment Satisfaction
After nine weeks of treatment, participants in both groups were equally content with having participated in the study (M ± SD: overt = 3.33 ± 0.82; covert = 2.96 ± 0.70; U = 205, p = 0.09), but participants in the overt group were more likely to believe that their received treatment was good for SAD (M ± SD: overt = 6.96 ± 2.71; covert = 5.22 ± 2.87; U = 161, p = 0.01), more likely to recommend their treatment to a friend (M ± SD: overt = 7.25 ± 2.83; covert = 5.27 ± 3.38; U = 170, p = 0.04), and more prone to report a change towards a more positive attitude to their treatment during the course of the study (M ± SD: overt = 3.67 ± 1.09; covert = 3.09 ± 0.75; U = 183, p = 0.03) in comparison to the covert group.
3.6. Blood Serum Analyses
Blood serum analyses of escitalopram concentrations at the posttreatment fMRI indicated that all patients had taken their medication as intended (median [25th–75th percentile] 74.0 [48.75–119.75] nmol/l). At posttreatment, there were no significant differences between the overt vs. covert SSRI arm with regard to concentrations of escitalopram (t(44) = 1.62, p = 0.11), desmethylescitalopram (t(44) = 0.50, p = 0.62) or didesmethylescitalopram (t(44) = 0.27, p = 0.79).
3.7. Adverse Events
The total number of recordered adverse events did not differ between the groups (t(44) = 1.40, p = 0.17), while there was a tendency (t(44) = 1.90, p = 0.06) for more adverse events that were deemed to be drug related in the overt (M ± SD 3.39 ± 2.62) as compared to the covert (2.22 ± 1.38) SSRI group. The most common adverse events were nausea, tiredness, headache, sleep- and sex-related problems. Events were generally mild to moderate, usually transient, and all were resolved at posttreatment.
3.8. Additional Measures
See the Supplement regarding participants' reactions to the reveal of the cover story, the psychiatrist's complementary evaluation, and comparisons of covert SSRI treatment with waiting-list and CBT data.
3.9. fMRI: Overt vs. Covert SSRI Administration
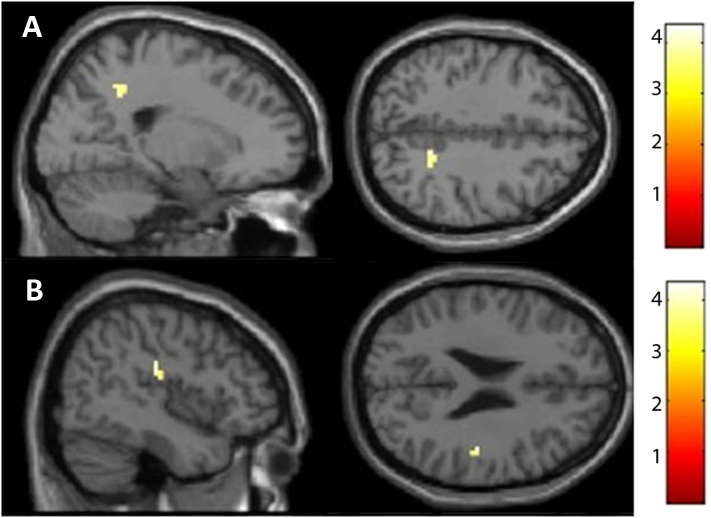
Amygdala ROI analysis did not yield significant between-group results while an exploratory whole-brain analysis indicated a differential neural treatment response with relatively increased (pre to post) reactivity to the emotional faces in the overt SSRI arm, and decreased reactivity in the covert group, in the bilateral posterior cingulate cortex, left mid temporal gyrus and left inferior frontal gyrus (Table 3, Fig. 3). There were no significant group differences in the above regions before treatment.
Table 3.
Brain regions exhibiting altered neural reactivity as a function of overt (n = 24) or covert (n = 22) SSRI administration.
| Contrast |
MNI coordinate |
Cluster sizea | Z value | p value | ||
|---|---|---|---|---|---|---|
| Brain region | x | y | z | |||
| Overt > covert SSRI | ||||||
| R posterior cingulate gyrus (BA23) | 9 | − 28 | 28 | 3780 | 4.58 | < 0.0001 |
| L mid temporal gyrus (BA39) | − 30 | − 58 | 25 | 513 | 3.93 | < 0.0001 |
| L posterior cingulate gyrus (BA31) | − 21 | − 28 | 34 | 351 | 3.83 | < 0.0001 |
| L inferior frontal gyrus (BA46) | − 36 | 35 | 1 | 270 | 3.69 | 0.0001 |
| Covert > overt SSRI | ||||||
| ns | ||||||
Whole brain search, p ≤ 0.001 k > 10; SSRI = selective serotonin reuptake inhibitor (escitalopram); R = right, L = left; BA = Brodmann area.
Volume in mm3, voxel size: 27 mm3.
Fig. 3.
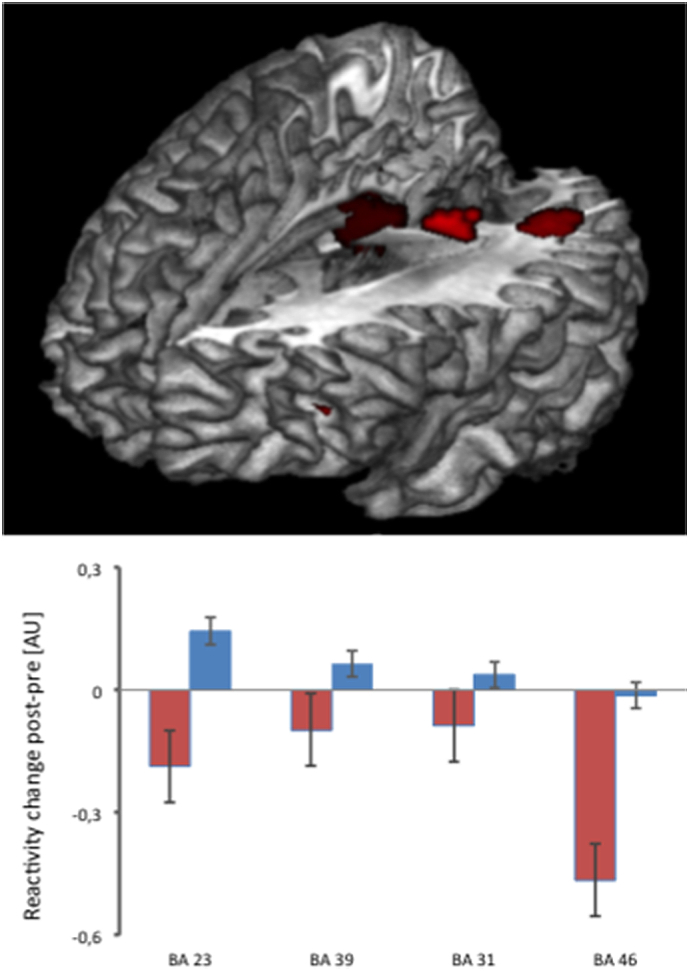
Top panel: Relatively increased neural reactivity after overt as compared to covert treatment with escitalopram in the bilateral posterior cingulate cortex as well as the left mid temporal and inferior frontal gyri, measured during the emotional face-matching paradigm. Lower panel: Interaction plots illustrating the neural changes in the four implicated cortical regions from pre to posttreatment in the overt (blue bars) as compared to the covert (red bars) group. Error bars reflect the standard error of the mean. AU refers to arbitrary units and BA refers to Brodmann area.
3.10. Brain-behavior Correlations
Follow-up analysis revealed that decreased social anxiety, as measured with LSAS-SR from pre- to posttreatment, was associated with reduced activation of the right amygdala (x = 33, y = − 1, z = − 29; Z = 2.70, k = 972 mm3, p = 0.003 - see Fig. 4, top panel) and increased activation of the posterior cingulate/precuneus region (x = − 18, y = − 31, z = 34, Z = 3.24, k = 216 mm3, p = 0.0006; x = 12, y = − 46, z = 16, Z = 3.10, k = 2133 mm3, p = 0.001; x = − 30, y = − 58, z = 28, Z = 2.85, k = 486 mm3; p = 0.002 - see Fig. 4, lower panel).
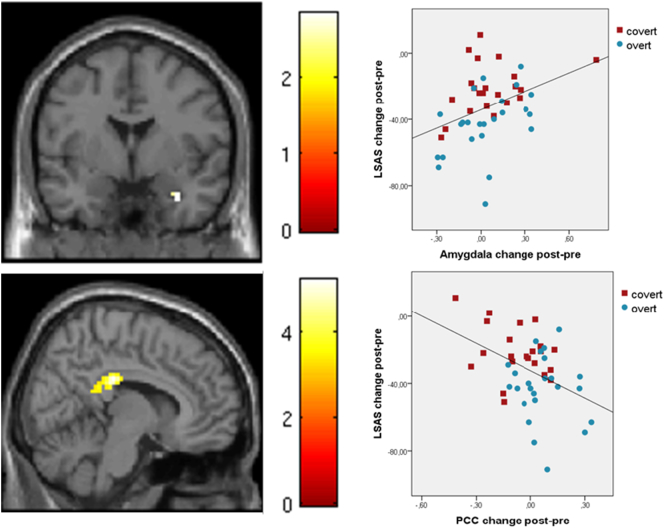
Fig. 4.
Top panel: Positive correlation between reduced amygdala reactivity and reduced scores on the Liebowitz social anxiety scale (LSAS-SR) with treatment. Lower panel: Negative correlation between increased reactivity in the posterior cingulate cortex (PCC) and reduced scores on LSAS-SR with treatment. Color bars represent T-scores, brighter colors indicating stronger correlations. Blue circles indicate overt and red squares covert treatment with escitalopram.
3.11. Psychophysiological Interaction Analysis
Based on the correlation between change in amygdala reactivity and symptom improvement (LSAS-SR) with treatment, psychophysiological interaction (PPI) follow-up analysis of group differences in amygdala connectivity was conducted with time-series fMRI data extracted from the obtained peak voxel (33, − 1, − 29) entered as a regressor together with task (faces vs. shapes) and the interaction between the two. The covert as compared to overt group, exhibited increased connectivity after treatment between the amygdala and right dorsal posterior cingulate cortex (x = 21, y = − 49, z = 40; Z = 3.62; 144 mm3, p < 0.00001) and right insula (x = 45, y = − 22, z = 25; Z = 3.94; 144 mm3, p < 0.00001) – see Fig. 5.
Fig. 5.
Psychophysiological interaction analysis showing increased connectivity between the amygdala and A) the right dorsal posterior cingulate cortex and B) the right insula, in the covert as compared to the overt group. Color bars represent T-scores, brighter colors indicating higher values.
4. Discussion
The present study demonstrates that verbal instructions influence SSRI anxiolytic outcome and associated brain parameters. In contrast to previous studies (e.g., Chen et al., 2011), we used a randomized design with truthful vs. deceiving instructions to estimate the contribution of verbally induced expectancies to SSRI efficacy in SAD. Better clinical outcome was noted in the overt as compared to the covert SSRI group on the primary LSAS-SR measure, with a doubled (pre-post) effect size, more than tripled response rate (50% vs 14%), greater treatment satisfaction after 9 weeks, and superior improvement according to multivariate analysis of all primary and secondary outcome measures. Thus, the instructions given while prescribing SSRIs make a significant clinical difference. This is in accordance with previous literature showing that administering a treatment covertly is not as efficient as an open administration (Colloca et al., 2004; Bingel et al., 2011). For post-operative anxiety, covert administration of benzodiazepines has been reported to be ineffective, and experimental studies in pain have also shown that the needed doses to achieve satisfactory clinical outcomes are much higher when drugs are administered covertly (Colloca et al., 2004; Bingel et al., 2011). In fact, verbally-induced negative treatment expectancies may even abolish opioid-induced analgesia (Bingel et al., 2011).
In SAD, we have previously demonstrated highly similar changes in brain activity and functional couplings for SSRI and placebo responders (Faria et al., 2012, Faria et al., 2014), suggesting that expectancies of improvement could play an important role in SSRI treatment. Here, we show that overt administration of an SSRI is considerably more effective on the LSAS-SR, in comparison to covert administration suggesting that the efficacy of SSRIs may be highly dependent on psychological effects like positive expectancies, traditionally associated with placebo response. However, the groups improved equally on anticipatory anxiety before the public speaking challenge, and escitalopram administered covertly, when compared to arms from a previous RCT, was superior to a waiting-list condition and comparable to Internet-based CBT (see Supplement). While this must be interpreted cautiously in the absence of randomized comparisons, it is plausible that (covert) escitalopram possesses at least moderate anxiolytic properties that need to be augmented with psychosocially induced expectancies to reach full clinical potential. Overall, our results favor a biopsychosocial over a pure biomedical explanatory model for SSRI efficacy in SAD.
The clinical effect was accompanied by a differential neural response to treatment as measured with BOLD-fMRI during an emotional face-matching task. Although we did not observe significantly altered amygdala reactivity between groups, attenuated amygdala activity with treatment correlated significantly with reduced social anxiety, consistent with our previous studies (Faria et al., 2012, Faria et al., 2014; Gingnell et al., 2016). After covert as compared to overt SSRI administration, the amygdala was more functionally coupled with other regions involved in emotion processing, including the insula and dorsal posterior cingulate cortex. Consistently, experimental studies suggest that the coupling between the amygdala and other nodes of the emotion processing network is stronger when fear memories are intact as compared to attenuated following disrupted reconsolidation (Agren et al., 2012). The most pronounced difference between groups in the present study was observed in the posterior cingulate cortex (Table 3) and for the whole sample, increased reactivity within this region correlated with reduced social anxiety (Fig. 4). In depression, the posterior cingulate has been shown to be more activated after treatment both in SSRI- and placebo responders (Mayberg et al., 2002) and neuroplastic changes within the posterior cingulate have been reported after prolonged escitalopram/citalopram intake in healthy volunteers (Kraus et al., 2014). Together with the adjacent precuneus, the posterior cingulate is a central node of the default mode network where altered neural activity has been associated with learning, memory, reward, and task engagement (Pearson et al., 2011). The posterior cingulate is also consistently activated by emotional stimuli and suggested to be a region in which cognition and emotion interact (Maddock, 1999). Thus, this may be a brain area where cognitive expectancies exert their effect on anxiety. However, elucidation of the mechanisms involved would require additional research e.g., with use of MRI-tasks that specifically manipulate expectancies together with mediation analyses.
There has been a sharp increase in neuroimaging studies of placebo in recent years (Benedetti, 2014), but very few have used deception to separate placebo from the drug effect or vice versa. Positron emission tomography studies have noted large additional effects of drug (methylphenidate) expectancy on brain glucose metabolism in cocaine abusers (Volkow et al., 2003) and healthy volunteers (Volkow et al., 2006). Similarly, fMRI trials of pain analgesia have demonstrated that expectancies, when combined with active treatment, yield substantial additional reduction in reported pain concomitantly with neurofunctional changes in pain processing brain regions (Bingel et al., 2011; Schenk et al., 2014; however, see Atlas et al., 2012). But to our knowledge, the present neuroimaging trial is the first deception-placebo study of SSRIs and of prolonged treatment in patients.
Because the overt SSRI treatment yielded significantly higher initial credibility ratings, none of the participants reported doubts about the cover story, blood serum analyses showed equal compliance in both groups, and a differential clinical effect was obtained, we conclude that the verbal manipulation was successful and the study integrity well maintained. Among the limitations it should be noted, however, that the sample size was modest, imposing limits on statistical power. Also, long-term follow-up assessments of relapse rates were not possible to conduct. Moreover, we could not evaluate a group treated with placebo described as escitalopram and an overt placebo arm, i.e. the remaining two cells of the balanced placebo design (Ross et al., 1962). Thus, both drug and expectancies were not manipulated in our study and therefore we could not properly test whether drug and placebo effects are truly additive (Rutherford and Roose, 2013; Atlas et al., 2012).
In conclusion, the present study demonstrates that the anxiolytic effect of escitalopram in SAD is highly sensitive to expectancies, as indicated by behavioral and neuroimaging evidence. Our study shows that verbal suggestions can have a profound influence on the anxiolytic effect of SSRIs. Thus, the presentation of a treatment may be as important as the treatment itself.
Funding Sources
Financial support was provided from the Swedish Research Council for Working Life and Social Research, the Swedish Research Council, Riksbankens Jubileumsfond – the Swedish Foundation for Humanities and Social Sciences (TF) and International Post-doc grant 437-2014-6767 (VF). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflicts of Interest
We declare no competing interests.
Author Contributions
Design of study (VF, MG, IA, MF, TF). Collection of data (VF, MG, JMH, OH, AF, SH, KW, IA, JE, KNTM, PC, GA, MR, EML, TF). Data analysis (VF, MG, AF, TF). Drafting of manuscript (VF, MG, MF, TF). All authors contributed to and have approved of the final manuscript. All authors are accountable for all aspects of the work.
Acknowledgments
Acknowledgments
We thank Jörgen Rosén, Fredrik Åhs, Johannes Björkstrand, Thomas Ågren, Hanna Wallberg, Henrik Annerstedt and Nimo Farah for their valuable assistance with data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.09.031.
Appendix A. Supplementary methods, results and appendix
Supplementary material
References
- Agren T., Engman J., Frick A., Bjorkstrand J., Larsson E.M., Furmark T., Fredrikson M. Disruption of reconsolidation erases a fear memory trace in human amygdala. Science. 2012;337:1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Fourth edition. American Psychiatric Publishing, Inc.; 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. [Google Scholar]
- Andersson G., Carlbring P., Holmström A., Sparthan E., Furmark T., Nilsson-Ihrfelt E., Buhrman M., Ekselius L. Internet-based self-help with therapist feedback and in vivo group exposure for social phobia: a randomized controlled trial. J. Consult. Clin. Psychol. 2006;74:677–686. doi: 10.1037/0022-006X.74.4.677. [DOI] [PubMed] [Google Scholar]
- Atlas L.Y., Whittington R.A., Lindquist M.A., Wielgosz J., Sonty N., Wager T.D. Dissociable influences of opiates and expectations on pain. J. Neurosci. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D.S., Asakura S., Koyama T., Hayano T., Hagino A., Reines E., Larsen K. Efficacy of escitalopram in the treatment of social anxiety disorder: a meta-analysis versus placebo. Eur. Neuropsychopharmacol. 2016;26:1062–1069. doi: 10.1016/j.euroneuro.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. 2014;84:623–637. doi: 10.1016/j.neuron.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Bingel U., Wanigasekera V., Wiech K., Mhuircheartaigh R., Lee M.C., Ploner M., Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Bschor T., Kilarski L.L. Are antidepressants effective? A debate on their efficacy for the treatment of major depression in adults. Expert. Rev. Neurother. 2016;16:367–374. doi: 10.1586/14737175.2016.1155985. [DOI] [PubMed] [Google Scholar]
- Chen J.A., Papakostas G.I., Youn S.J., Baer L., Clain A.J., Fava M., Mischoulon D. Association between patient beliefs regarding assigned treatment and clinical response: reanalysis of data from hypericum depression trial study group. J. Clin. Psychiatry. 2011;72:1669–1676. doi: 10.4088/JCP.10m06453. [DOI] [PubMed] [Google Scholar]
- Colloca L., Lopiano L., Lanotte M., Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet. 2004;3:679–684. doi: 10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- Faria V., Appel L., Ahs F., Linnman C., Pissiota A., Frans O., Bani M., Bettica P., Pich E.M., Jacobsson E., Wahlstedt K., Fredrikson M., Furmark T. Amygdala subregions tied to SSRI and placebo response in patients with social anxiety disorder. Neuropsychopharmacology. 2012;37:2222–2232. doi: 10.1038/npp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria V., Ahs F., Appel L., Linnman C., Bani M., Bettica P., Pich E.M., Wahlstedt K., Fredrikson M., Furmark T. Amygdala-frontal couplings characterizing SSRI and placebo response in social anxiety disorder. Int. J. Neuropsychopharmacol. 2014;17:1149–1157. doi: 10.1017/S1461145714000352. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research. New York State Psychiatric Institute; 1997. Structured clinical interview for DSM-IV axis I disorders - non-patient edition. [Google Scholar]
- Fresco D.M., Coles M.E., Heimberg R.G., Liebowitz M.R., Hami S., Stein M.B., Goetz D. The Liebowitz social anxiety scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol. Med. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Frisch M.B., Cornell J., Villanueva M., Retzlaff P.J. Clinical validation of the Quality of Life Inventory. A measure of life satisfaction for use in treatment planning and outcome assessment. Psychol. Assess. 1992;4:92–101. [Google Scholar]
- Furmark T., Tillfors M., Everz P.O., Marteinsdottir I., Gefvert O., Fredrikson M. Social phobia in the general population: prevalence and sociodemographic profile. Soc. Psychiatry Psychiatric Epidemiol. 1999;34:416–424. doi: 10.1007/s001270050163. [DOI] [PubMed] [Google Scholar]
- Furmark T., Appel L., Henningsson S., Ahs F., Faria V., Linnman C., Pissiota A., Frans O., Bani M., Bettica P., Pich E.M., Jacobsson E., Wahlstedt K., Oreland L., Langstrom B., Eriksson E., Fredrikson M. A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. J. Neurosci. 2008;28:13066–13074. doi: 10.1523/JNEUROSCI.2534-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingnell M., Frick A., Engman J., Alaie I., Bjorkstrand J., Faria V., Carlbring P., Andersson G., Reis M., Larsson E.M., Wahlstedt K., Fredrikson M., Furmark T. Combining escitalopram and cognitive-behavioural therapy for social anxiety disorder: randomised controlled fMRI trial. Br. J. Psychiatry. 2016;209:229–235. doi: 10.1192/bjp.bp.115.175794. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Kolachana B., Fera F., Goldman D., Egan M.F., Weinberger D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hieronymus F., Emilsson J.F., Nilsson S., Eriksson E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol. Psychiatry. 2015;21:523–530. doi: 10.1038/mp.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson N.S., Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Khan A., Brown W.A. Antidepressants versus placebo in major depression: an overview. World Psychiatry. 2015;14:294–300. doi: 10.1002/wps.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I., Moore T., Scoboria A., Nichols S. The emperor's new drugs: an analysis of antidepressant medication data submitted to the U.S. Food and Drug Administration. Prev Treat. 2002;5 (article 23) [Google Scholar]
- Kraus C., Ganger S., Losak J., Hahn A., Savli M., Kranz G.S., Baldinger P., Windischberger C., Kasper S., Lanzenberger R. Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. NeuroImage. 2014;84:236–244. doi: 10.1016/j.neuroimage.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Maddock R.J. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Mattick R.P., Clarke J.C. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav. Res. Ther. 1998;36:455–470. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Silva J.A., Brannan S.K., Tekell J.L., Mahurin R.K., McGinnis S., Jerabek P.A. The functional neuroanatomy of the placebo effect. Am. J. Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- Moncrieff J., Wessely S., Hardy R. Active placebos versus antidepressants for depression. Cochrane Database Syst. Rev. 2004;1 doi: 10.1002/14651858.CD003012.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Pearson J.M., Heilbronner S.R., Barack D.L., Hayden B.Y., Platt M.L. Posterior cingulate cortex: adapting behavior to a changing world. Trends. Cogn. Sci. 2011;15:143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest A.M., de Jonge P., Williams C.D., de Vries Y.A., Schoevers R.A., Turner E.H. Reporting bias in clinical trials investigating the efficacy of second-generation antidepressants in the treatment of anxiety disorders: a report of 2 meta-analyses. JAMA Psychiatry. 2015;72:500–510. doi: 10.1001/jamapsychiatry.2015.15. [DOI] [PubMed] [Google Scholar]
- Ross S., Krugman A.D., Lyerly S.B., Clyde D.J. Drugs and placebos: a model design. Psychol. Rep. 1962;10:383–392. [Google Scholar]
- Rutherford B.R., Roose S.P. A model of placebo response in antidepressant clinical trials. Am. J. Psychiatry. 2013;170:723–733. doi: 10.1176/appi.ajp.2012.12040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford B.R., Wall M.M., Brown P.J., Choo T.-H., Wager T.D., Peterson B.S., Chung S., Kirsch I., Roose S.P. Patient expectancy as a mediator of placebo effects in antidepressant clinical trials. Am. J. Psychiatry. 2016;174:135–142. doi: 10.1176/appi.ajp.2016.16020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk L.A., Sprenger C., Geuter S., Buchel C. Expectation requires treatment to boost pain relief: an fMRI study. Pain. 2014;155:150–157. doi: 10.1016/j.pain.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorin P., Janavs J., Weiller E., Herqueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. 1970. Manual for the State-trait Anxiety Inventory. [Google Scholar]
- Sugarman M.A., Loree A.M., Baltes B.B., Grekin E.R., Kirsch I. The efficacy of paroxetine and placebo in treating anxiety and depression: a meta-analysis of change on the Hamilton Rating Scales. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Ma Y., Fowler J.S., Zhu W., Maynard L., Telang F., Vaska P., Ding Y.S., Wong C., Swanson J.M. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J. Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Ma Y., Fowler J.S., Wong C., Jayne M., Telang F., Swanson J.M. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. NeuroImage. 2006;32:1782–1792. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material