Abstract
Background
In a placebo-controlled trial of the peptide-based therapeutic HIV-1 p24Gag vaccine candidate Vacc-4x, participants on combination antiretroviral therapy (cART) received six immunizations over 18 weeks, followed by analytical treatment interruption (ATI) between weeks 28 and 52. Cell-mediated immune responses were investigated as predictors of Vacc-4x effect (VE) on viral load (VL) and CD4 count during ATI.
Methods
All analyses of week 28 responses and fold-changes relative to baseline considered per-protocol participants (Vacc-4x:placebo = 72:32) resuming cART after week 40. Linear regression models with interaction tests were used. VE was estimated as the Vacc-4x–placebo difference in log10-transformed VL (VEVL) or CD4 count (VECD4).
Findings
A lower fold-change of CD4+ T-cell proliferation was associated with VECD4 at week 48 (p = 0.036, multiplicity adjusted q = 0.036) and week 52 (p = 0.040, q = 0.080). A higher fold-change of IFN-γ in proliferation supernatants was associated with VEVL at week 44 (p = 0.047, q = 0.07). A higher fold-change of TNF-α was associated with VEVL at week 44 (p = 0.045, q = 0.070), week 48 (p = 0.028, q = 0.070), and week 52 (p = 0.037, q = 0.074). A higher fold-change of IL-6 was associated with VEVL at week 48 (p = 0.017, q = 0.036). TNF-α levels (> median) were associated with VECD4 at week 48 (p = 0.009, q = 0.009).
Interpretation
These exploratory analyses highlight the potential value of investigating biomarkers in T-cell proliferation supernatants for VE in clinical studies.
Keywords: Analytical treatment interruption (ATI), HIV, Therapeutic vaccine, Viral load, Immune predictors, CD4
Highlights
-
•
Ex vivo CD4+ T-cell proliferation was predictive of Vacc-4x effect.
-
•
IFN-γ, TNF-α and IL-6 secretion in T-cell proliferation supernatants were predictive of Vacc-4x effect.
-
•
Such immune predictors could be utilized to mitigate risks associated with cART interruption towards HIV cure.
No immune correlates or predictors of therapeutic vaccine effect (i.e. a reduction in viral load compared to placebo on treatment interruption) for human immunodeficiency virus (HIV)-1 are known. We investigated a broad array of cytokines/chemokines produced in T-cell proliferation supernatants from a placebo-controlled clinical study of a therapeutic HIV vaccine. Although such supernatants do not provide cell type-specific readouts, the cytokines/chemokines studied included T-helper (Th)1, Th2, growth factor, immuno-modulatory and pro-inflammatory functions. Specifically, we found that, IFN-γ, TNF-α and IL-6 secretion correlated with vaccine effect, suggesting such supernatants could represent important sample material not previously considered for the identification of immune markers of vaccine effect.
1. Introduction
Vacc-4x is a peptide-based therapeutic human immunodeficiency virus (HIV)-1 vaccine candidate, that aims to induce cell-mediated immune responses to conserved regions of p24Gag. Vacc-4x is administered intradermally for uptake by dendritic cells in the presence of granulocyte-macrophage colony stimulating factor (GM-CSF). Dendritic cells are potent antigen presenting cells that have the capacity for cross presentation allowing for epitope presentation on human leukocyte antigen (HLA) classes I and II. Following peptide uptake and intracellular proteolytic processing within dendritic cells, presentation of conserved epitopes on HLA class II stimulates CD4+ T cells. Moreover, presentation of epitopes on HLA class I stimulates CD8+ T cells, which can then kill infected cells presenting these conserved epitopes. Since sustained cytolytic effects of CD8+ T-cells require immune help from CD4+ T-cells, Vacc-4x carries both HLA class I and class II epitopes. Effective cell-mediated immune responses that can control and/or reduce virus levels, may maintain CD4+ T-cell counts and/or slow their decline during analytical treatment interruption (ATI).
Vacc-4x was shown to be safe and immunogenic in a randomized, double-blind, placebo-controlled phase 2 clinical study enrolling 137 HIV-1-infected participants who were virologically suppressed on combination antiretroviral therapy (cART) (Pollard et al., 2014). Six immunizations were given between week 1 (baseline) and week 18 while participants continued cART. At week 28 eligible participants underwent ATI and were removed from cART for up to six months until week 52. Although no differences between the treatment groups for the pre-specified efficacy endpoints (cART resumption and changes in CD4 count change during ATI) were observed, there was a significant reduction in viral load (VL) set-point (Pollard et al., 2014) and better preserved CD4 counts (Huang et al., 2016a) in the Vacc-4x group compared to placebo. In addition, both groups demonstrated increases from baseline in HIV-1-specific IFN-γ-secreting peripheral blood mononuclear cells (PBMCs) against p24 antigens by Enzyme-linked ImmunoSpot (ELISPOT) assay at week 52; however, only in the Vacc-4x group was this increase associated with a statistically significant reduction in VL set-point compared to the placebo group during ATI (Pollard et al., 2014). This finding suggests the quality of cell-mediated immune responses (CMIs) during ATI may have differed between the two treatment groups and that specific CMIs elicited by Vacc-4x contributed to the beneficial Vacc-4x effect (VE) on VL and CD4 count.
The objective of this study was to follow up on the prior observations by systematically assessing CMIs at week 28 relative to baseline (wk28/wk1) or on their own (week 28) as predictors of VE during ATI. We hypothesized that T-cell immunity in terms of the magnitude and/or the presence (yes or no) of immune biomarkers (with or without reference to baseline values), especially those related to immune stimulation, T-cell proliferation and inflammation would predict the effect of Vacc-4x (vs. placebo) on controlling VL and/or maintaining CD4 count during ATI. To address these hypotheses, we considered immune biomarkers including interferon-gamma (IFN-γ) secreting T-cells measured by ELISPOT, CD4+ and CD8+ and Total (CD3+) T-cell proliferation measured by a carboxyfluorescein succinimidyl ester (CFSE) assay, and cytokines released from T-cell proliferation supernatants ex vivo measured by the Luminex assay.
2. Materials and Methods
2.1. Study Procedures
The Vacc-4x study took place between July 2008 and June 2010 in Spain, Italy, the USA, the UK, and Germany (Pollard et al., 2014). Participants aged 18–55 years and virologically-suppressed on cART prior to enrollment were randomized to receive four weekly Vacc-4x or placebo priming immunizations and two boosting immunizations at weeks 16 and 18, followed by ATI by week 28. During ATI, participants returned to cART if their CD4 count reached 350 cells/μl or fell by 50%. Pre-ART VL and CD4 count were also available for most participants. All participants in the study had provided informed consent, and the study protocol had been approved by all participating regional ethics committees. Full inclusion criteria can be found at www.clinicaltrials.gov under the identifier NCT00659789.
2.2. Immunological and Safety Measurements
CMIs to p24 antigens were measured at weeks 1 and 28 in PBMCs using ELISPOT and T-cell proliferation assays [CFSE staining]. Cytokine and chemokine concentrations were assessed in proliferation supernatants using a Bioplex 200 Luminex machine (Bio-Rad, Hercules, CA). A total of 22 continuous and 30 binary immune variables based on immune responses measured at week 28 relative to baseline (wk28/wk1 fold-change) or on their own (week 28) were evaluated.
Safety measurements, e.g. lactose dehydrogenase (LDH), white blood cell (WBC) and anti-C5/gp41732–744 antibody levels which target a region on HIV-1 envelope glycoporteins, were also determined at baseline and over time. Antibodies to the 5th constant (C5) domain of HIV-1 envelope glycoproteins and a part of the transmembrane glycoprotein gp41, (gp41732–744), were tested because they have been shown to be associated with moderate viral load and slower disease progression, suggesting they may have an impact upon HIV-associated immune activation (Sørensen et al., 2017).
2.2.1. Peptides and Protein Antigens
Peptides were synthesized at Schafer-N (Copenhagen, Denmark). All peptides used for in vitro immunological analyses were first re-suspended in DMSO and then water. Recombinant p24 core protein, HTLV IIIB was obtained from Bioprocess Pty Ltd. Staphylococcal enterotoxin B (SEB) (200 ng/mL) and media containing DMSO concentrations equivalent to those in the peptide pool wells were used as positive and negative controls, respectively. The p24 antigens used in these analyses were overlapping 15-mer peptides offset by 2 amino acids corresponding to the region on p24 covered by Vacc-4x. In addition, full length recombinant p24 protein clade B was used.
2.2.2. Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
T-cell responses were evaluated from PBMCs prepared at each participating site. After collection, all PBMCs were frozen at − 80 °C and shipped to the central laboratory at CHUV, Switzerland within 21 days for storage in liquid nitrogen. The same batch of fetal bovine serum (FBS) was used for all sites to reduce inter-laboratory variation. All sites preparing PBMCs were accredited prior to the study start and their ability to prepare PBMCs with recovery > 70% and viability > 80% on thawing was confirmed (Boaz et al., 2009).
Ex vivo interferon-γ (IFN-γ) secretion was examined in thawed PBMCs following stimulation with p24 antigens using an ELISPOT assay, T-cell proliferation was examined using a CFSE assay as described in Pollard et al. (2014), and cytokine/chemokine production was assessed in proliferation supernatants using a Luminex assay at weeks 1 and 28.
2.2.3. ELISPOT Assay Criteria
ELISPOT assays were considered valid if the mean of triplicate wells did not exceed 50 spot forming units (SFUs)/106 PBMCs in the negative control (medium) and > 500 SFUs/106 PBMCs in the positive control (SEB). Three ELISPOT assay immune variables were assessed as predictors of vaccine effect: fold-change of the mean SFU in the stimulated over the negative control as well as a continuous biomarker, fold-change of the mean SFU above or below the median value, and the protocol–defined response positivity as dichotomized variables. Assay responses were defined positive if the mean SFU in stimulated was four times or greater than the negative control and at least 55 SFUs per 106 PBMCs (Bart et al., 2008; Harari et al., 2008).
2.2.4. T-Cell Proliferation Assays (CFSE)
T-cell proliferation was assessed ex vivo in CD4+ and CD8+ T-cells, as well as in the total T-cell population (CD3+), using a CFSE assay (Cellerai et al., 2010; Perreau et al., 2011). Staphylococcal enterotoxin B (SEB) stimulation and medium alone served as positive and negative controls, respectively (Harari et al., 2008). Three CFSE assay immune variables were assessed as predictors of vaccine effect: fold-change of percent CFSE low dividing cells (CFSELo) in the stimulated over the negative control wells as a continuous biomarker, fold-change of percent CFSElo above or below the median value, and the protocol-defined response positivity as dichotomized variables. Assay responses were defined as positive if the percentage CFSElo dividing cells reactive to p24 antigens was three times or greater than the percentage CFSE low dividing cells in the negative (medium) control (Harari et al., 2008).
2.2.5. Cytokine Measurements by Luminex
In addition, supernatants from proliferation assays were frozen for subsequent Luminex assays. Upon thawing, the concentrations of 27 chemokines/cytokines (pg/mL levels) in the thawed supernatants were determined upon recombinant p24 stimulation using the Luminex platform (BioRad Inc.; Hercules, CA). Among these markers, the following had an appropriate dynamic range and were evaluated as predictors of vaccine effect: IFN-γ, tumor necrosis factor (TNF)-α, T-helper (Th)1, interleukin (IL)-14, IL-13, and Th2; the immune modulators IL-10 and IL-17; and the inflammatory markers IL-6 and macrophage inflammatory protein (MIP)-1β. The remaining 20 chemokines/cytokines were excluded from further analysis due to lack of variation across individuals: IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-7, IL-8, IL-9, IL-12p70, IL-15, Eotaxin, fibroblast growth factor (FGF)-basic, granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), interferon gamma-induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1, MIP-1α, platelet derived growth factor (PDGF)-β, regulated on activation, normal T-cell expressed and secreted (RANTES), and vascular endothelial growth factor (VEGF). For each immune marker, two immune variables were assessed as predictors of vaccine effect: fold-change of concentration in the stimulated over the negative control as a continuous biomarker, and the fold-change above or below the median.
2.3. Statistical Analyses
All analyses were conducted in the subset of per-protocol (PP) participants who did not resume cART until after week 40 (Vacc-4x n = 72; placebo n = 32) (Fig. 1, Fig. S1) (Pollard et al., 2014). This time point was at least 12 weeks after cART interruption; thus, participants had experienced peak viremia rebound by then (Kutzler and Jacobsen, 2008). The VL and CD4 count endpoints were log10-transformed VL and CD4 count at weeks 44, 48, and 52 during ATI, with and without subtracting log10-transformed pre-ART VL and CD4 count, respectively. VE on VL (VEVL) and CD4 count (VECD4) were defined as the mean difference of VL and CD4 count endpoints, respectively, between the Vacc-4x and placebo groups. A positive (or negative) immune predictor indicates a positive (or negative) association between immune response levels and Vacc-4x benefit. Vacc-4x benefit is indicated by having a lower VL or a higher CD4 count in the Vacc-4x group compared with placebo.
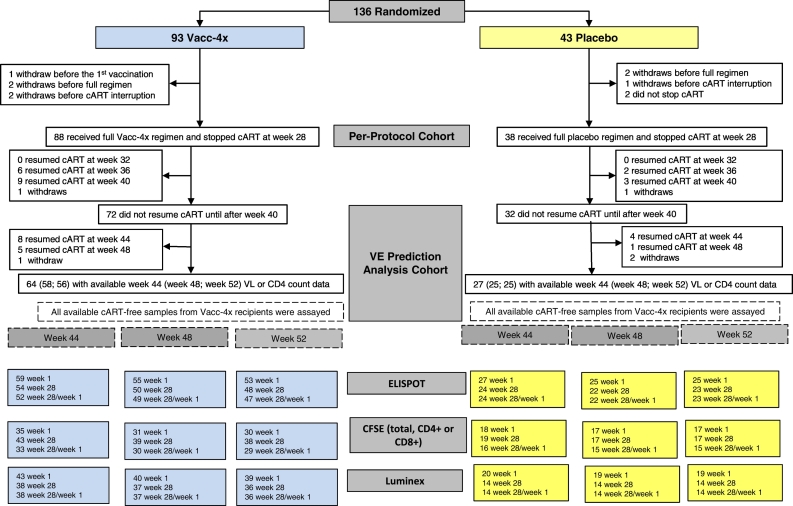
Fig. 1.
CONSORT diagram showing the availability of immunological samples for the vaccine effect prediction analysis of the phase 2 Vacc-4x clinical study.
For each VL and CD4 count endpoint, univariate and multivariable linear regression models of observed complete-case data were used to identify potential predictors of VE. Missing data due to insufficient specimens were assumed to be missing completely at random. Wald tests were used to evaluate statistical interactions between each immune variable predictor and treatment assignment. All reported results are from multiple regression models adjusted for at least one of the following baseline confounding variables identified as predictors of the VL or CD4 count endpoint in the Vacc-4x or placebo group: preART VL or CD4, median-dichotomized LDH (for VL endpoints), continuous LDH (for preART-adjusted VL endpoints), median-dichotomized WBC (for preART-adjusted VL endpoints), anti-C5/gp41732–744 antibodies, LDH or WBC (for CD4 endpoints), or dichotomized WBC or anti-C5/gp41732–744 (for preART-adjusted CD4 endpoints). Sex at birth was also considered in the analyses of the Vacc-4x group or the combined Vacc-4x and placebo groups. However, due to the sparsity of female participants, especially in the placebo group, analyses did not adjust for sex at birth when models were unstable. When considering what baseline covariates might contribute to the analysis of immune responses as predictors of vaccine effect (our study objective), we first examined all available baseline demographic and clinical factors as potential predictors of VL and/or CD4 count during ATI within each treatment group as previously described (Huang et al., 2016a). Specifically, we considered gender (male or female), age (both continuous and dichotomized), country (Germany, Spain, US, or UK and Italy combined), time on cART (continuous and dichotomized), time since HIV diagnosis (continuous and dichotomized), CD4 nadir (continuous and dichotomized), preART VL (continuous and dichotomized), preART CD4 count (continuous and dichotomized), as well as host genetics class I HLA type. Among these baseline factors, together with safety factors considered in a separate analysis (Huang et al., 2016b), age, gender, preART VL, LDH and WBC were identified as significant predictors of VL and/or CD4 count during ATI, which were subsequently included in the analyses of vaccine effect predictors reported here. Other baseline factors were found to be only marginally or not predictive and hence were not included in further analysis.
Q-values were calculated to account for multiple comparisons within the analysis of each endpoint and predictor type (continuous or categorical) (Benjamini and Hochberg, 1995). Multiple testing adjustments for interaction tests were applied across immune variables that were identified as a significant predictor in either the individual or combined treatment groups. Predictors with associated unadjusted p-value < 0.05 and q-value < 0.1 were considered strong evidence of signals for hypothesis-generating purposes. Longitudinal data analyses of VL and CD4 count endpoints over time between week 36 and week 52 were also performed. Analyses were performed using R (version 3.2.1) (R Core Team, 2015).
3. Results
3.1. Descriptive Summary of the Study Data
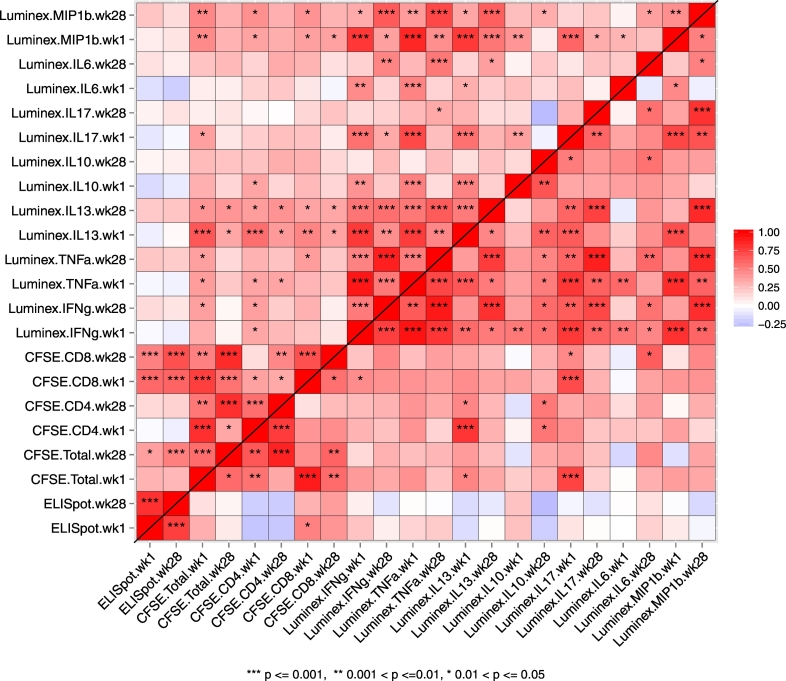
In the PP cohort, week 1 baseline immune responses were generally highly correlated with week 28 immune responses of the same biomarker (Fig. 2). Therefore, we considered the fold-changes of week 28 immune responses relative to week 1 as potential predictors of VEVL and VECD4, to control for pre-existing immune responses in this HIV-1-infected study population. We also observed positive correlations at week 28 across assays between ELISPOT responses and CFSE CD8+ or total T-cell (CD3+) responses in the Vacc-4x group but not the placebo group. This suggests that Vacc-4x immunizations may have impacted not only the magnitude but also the coordination of immune responses, consistent with observations for other vaccines (e.g., Chung et al., 2015).
Fig. 2.
Correlations between week 1 and week 28 immune responses in the Vacc-4x group (upper diagonal) and placebo group (lower diagonal). The direction and strength of the Spearman correlation coefficients are represented in color grade, where dark red indicates a perfect positive correlation and dark blue indicates a perfect negative correlation. The significance of the correlation is reported as: p < 0.05 = *; p < 0.01 = **; and p < 0.001 = ***.
Sufficient dynamic ranges were observed for both the wk1/wk28 fold-change (Fig. 3, Fig. 4) and week 28 immune variables (Figs. S2 and S3), supporting the planned regression analyses. Statistical power was dampened by up to 48% missing data in some assays (Fig. 1), mainly caused by challenges of obtaining sufficient specimens from all study participants.
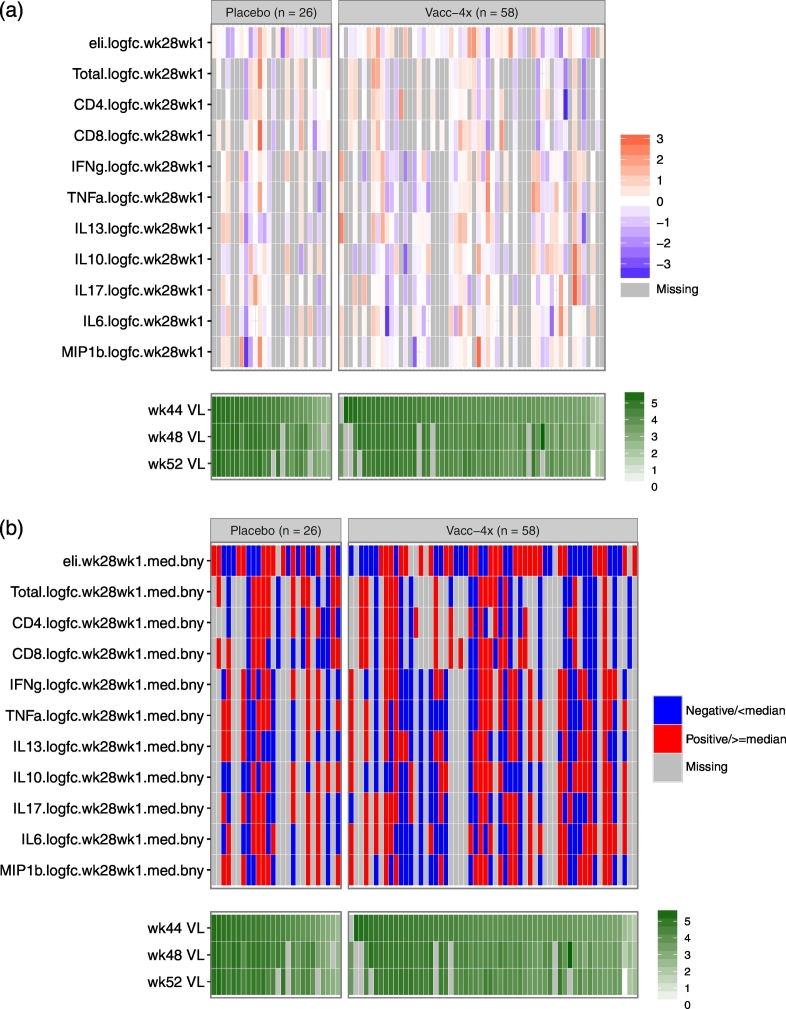
Fig. 3.
Distributions of immune response variables (wk28/wk1) in relation to Weeks 44, 48, and 52 VL values in the placebo and Vacc-4x groups. Each cell indicates the magnitude of each variable (row) for each placebo or Vacc-4x recipient (column) in color grade, where darker red indicates a higher level and darker blue indicates a lower level. Gray shading indicates missing data. The columns are ordered by Week 52 VL, separately within the placebo and Vacc-4x groups. Panel (a) shows mean-centered magnitude of each measurement in standard deviation scale for each continuous marker in relation to VL (log10-scale); Panel (b) shows dichotomized status of each measurement based on median-cutoff (indicated with a suffix “med.bny”) or positivity (indicated with a suffix “pos”) in relation to VL (log10-scale).
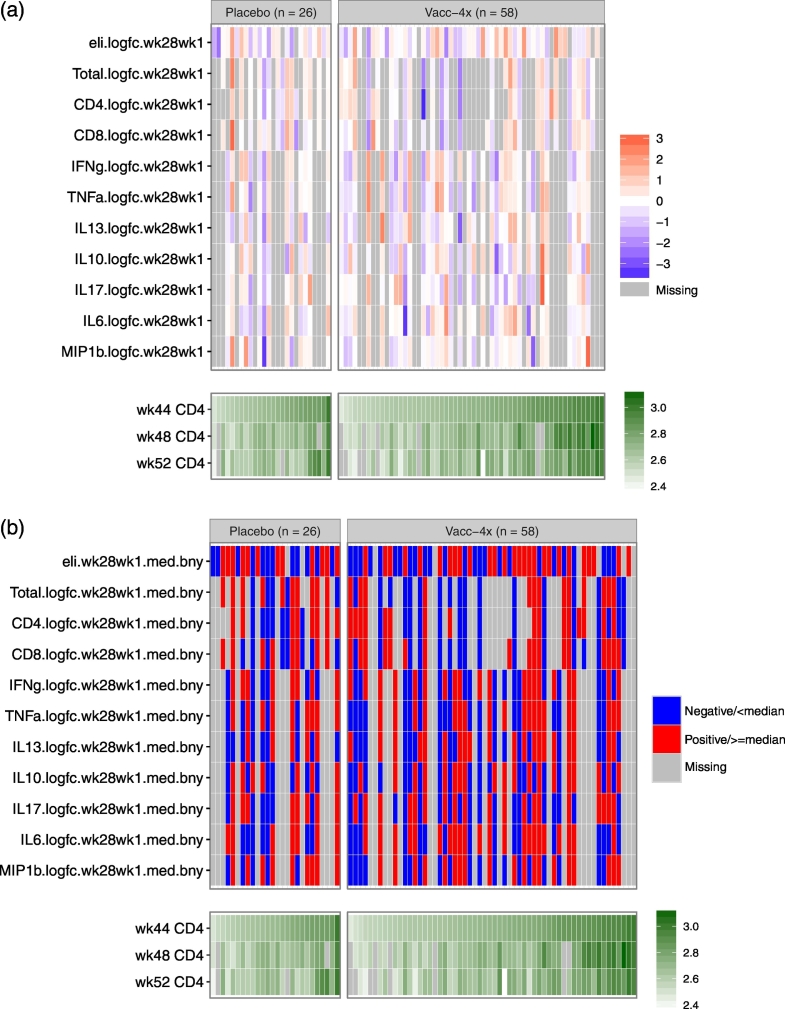
Fig. 4.
Distributions of immune response variables (wk28/wk1) in relation to Weeks 44, 48, and 52 CD4 count values in the placebo and Vacc-4x groups. Each cell indicates the magnitude of each measurement (row) for each placebo or Vacc-4x recipient (column) in color grade, where darker red indicates a higher level and darker blue indicates a lower level. Gray shading indicates missing data. The columns are ordered by Week 52 CD4 count, separately within the placebo and Vacc-4x groups. Panel (a) shows mean-centered magnitude of each measurement in standard deviation scale for each continuous marker in relation to CD4 count (log10-scale); Panel (b) shows dichotomized status of each measurement based on median-cutoff (indicated with a suffix “med.bny”) or positivity (indicated with a suffix “pos”) in relation to CD4 count (log10-scale).
Among 22 wk28/wk1 fold-change immune variables, one measured in PBMCs by the CFSE assay was identified as a negative predictor of VECD4 at week 44, 48, and/or 52 and three in proliferation supernatants were identified as positive predictors of VEVL (Table 1). These results are detailed below.
Table 1.
Summary of wk28/wk1 immunological predictors of Vacc-4x effect.
| Assay | Biomarker | Endpoint | Groupa | Effectb (95% CI) | Interaction p (q) value |
|---|---|---|---|---|---|
| Luminex | IFN-γ | Week 44 VL | 25%-ile | 0.444 (− 0.265, 1.154) | 0.047 (0.070) |
| 75%-ile | − 0.376 (− 0.921, 0.169) | ||||
| TNF-α | Week 44 VL | 25%-ile | 0.223 (− 0.326, 0.772) | 0.045 (0.070) | |
| 75%-ile | − 0.288 (− 0.806, 0.231) | ||||
| Week 48 VL | 25%-ile | 0.349 (− 0.239, 0.936) | 0.028 (0.077) | ||
| 75%-ile | − 0.246 (− 0.801, 0.308) | ||||
| Week 52 VL | 25%-ile | 0.347 (− 0.370, 1.064) | 0.037 (0.074) | ||
| 75%-ile | − 0.324 (− 0.995, 0.347) | ||||
| IL-6 binary | Week 48 VL | IL6 < median | 0.572 (− 0.122, 1.267) | 0.017 (0.052) | |
| IL6 ≥ median | − 0.576 (− 1.254, 0.101) | ||||
| CFSE | CD4+ binary | Week 48 CD4 | CD4+ < median | 0.141 (0.013, 0.269) | 0.036 (0.036) |
| CD4+ ≥ median | − 0.062 (− 0.203, 0.079) | ||||
| Week 52 CD4 | CD4+ < median | 0.123 (0.002, 0.244) | 0.040 (0.080) | ||
| CD4+ ≥ median | − 0.065 (− 0.193, 0.063) |
Group: for continuous markers, Group indicates the 25th and 75th percentiles of the identified marker; for binary markers, Group indicates the dichotomized subgroups based on the identified marker.
Effect: the effect was estimated as the log10 difference between Vacc-4x and placebo in the given Group. P-values (< 0.05) and q values (< 0.1) were considered significant.
3.2. CD4 T-Cell Proliferation as a Predictor of Vaccine Effect
We found that lower wk28/wk1 fold-change of CD4+ T-cell proliferation was significantly associated with better preserved week 48 and 52 CD4 counts in the Vacc-4x group compared to the placebo group (week 48: interaction p = 0.036, q = 0.036; week 52: interaction p = 0.040, q = 0.080) (Table 1, Fig. 5). For week 48, an estimated VECD4 of 0.14 (95% CI: 0.01, 0.27) log10 increase was observed in the mean CD4 count among Vacc-4x vs. placebo recipients whose wk28/wk1 CD4+ CFSE levels were below the median, and an estimated VECD4 of 0.06 (95% CI:− 0.20, 0.08) log10 decrease otherwise. For week 52, an estimated VECD4 of 0.12 (95% CI: 0.002, 0.24) log10 increase was observed in the mean CD4 count among Vacc-4x vs. placebo recipients whose wk28/wk1 CD4+ CFSE levels were below the median, and an estimated VECD4 of 0.07 (95% CI: − 0.19, 0.06) log10 decrease otherwise (Table 1). Similar patterns were observed for week 44 VECD4, though not statistically significant (Fig. 5). However, higher week 28 CD4 T-cell proliferation (without adjusting for week 1 levels) was associated with better preserved CD4 counts in the Vacc-4x group compared to the placebo group at week 52 (Fig. S4; Table S1). This finding suggests that baseline CD4+ T-cell proliferation levels may infer the immune capacity of an HIV-infected participant and play an important role in predicting VECD4.
Fig. 5.
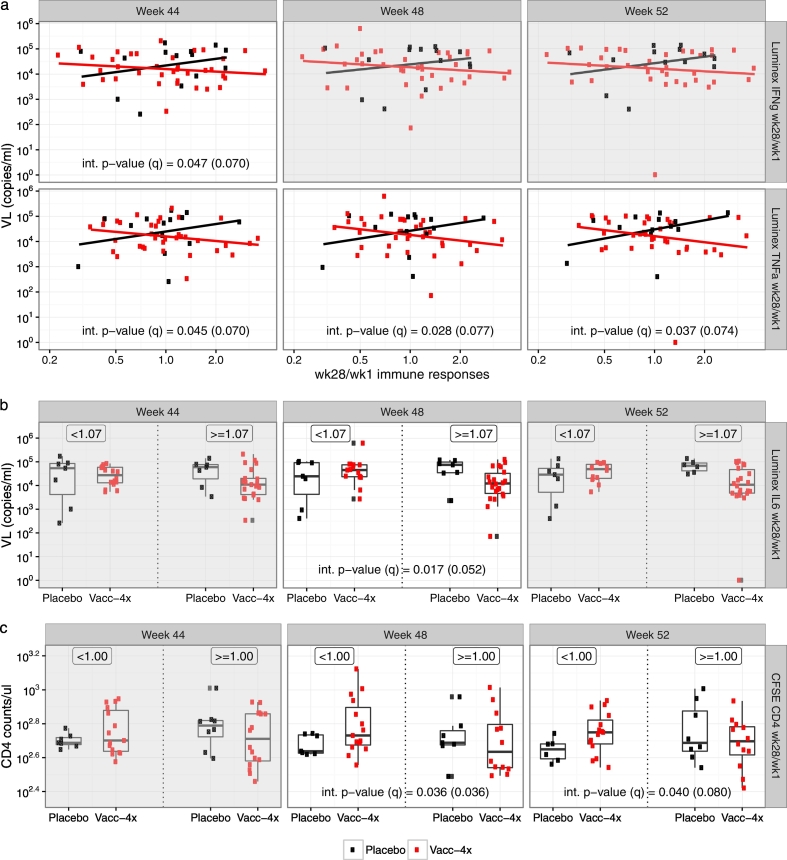
Distributions of VL or CD4 count by identified wk28/wk1 immunological predictors of vaccine effect. Panel (a) shows VL vs. wk28/wk1 Luminex IFN-γ and TNF-α; Panel (b) shows the distribution of VL by treatment group for wk28/wk1 Luminex IL-6 < and ≥ median (1.07 log10); Panel (c) shows the distribution of CD4 count by treatment group for wk28/wk1 CFSE CD4+ < and ≥ median (1.0 log10).
3.3. IFN-γ Secretion in T-Cell Proliferation Supernatants as a Predictor of Vaccine Effect
We also found that higher wk28/wk1 fold-change of IFN-γ secretion in proliferation supernatants was associated with reduced week 44 VL in the Vacc-4x group vs. the placebo group (interaction p = 0.047, q = 0.07) (Fig. 5, Table 1), with an estimated VEVL of 0.44 (95% CI: − 0.27, 1.15) log10 increase in the mean VL among Vacc-4x vs. placebo recipients at the 25% percentile level, and an estimated VEVL of 0.38 (95% CI: -0.92, 0.17) log10 decrease at the 75th percentile level of the wk28/wk1 IFN-γ secretion level (Table 1). Similar patterns were observed for week 48 and 52 VEVL, though not statistically significant, possibly due to small sample sizes (Fig. 5). However, higher week 28 IFN-γ secretion levels were associated with increased week 44 VL (Table S1 and Fig. S5). This further emphasizes the important role of baseline immune capacity.
3.4. TNF-α Secretion in T-Cell Proliferation Supernatants as a Predictor of Vaccine Effect
In addition, we found that higher wk28/wk1 fold-change of TNF-α secretion in proliferation supernatants was significantly associated with reduced VL at all three time-points post week 40 in the Vacc-4x group compared to the placebo group (Fig. 5) (week44: interaction p = 0.045, q = 0.070; week48: p = 0.028, q = 0.077; week52: p = 0.037, q = 0.074) (Table 1). At week 44, an estimated VEVL of 0.22 (95% CI:− 0.33, 0.77) log10 increase was observed in the mean VL among Vacc-4x vs. placebo recipients at the 25th percentile level, and an estimated VEVL of 0.29 (95% CI:− 0.81, 0.23) log10 decrease at the 75th percentile of the wk28/wk1 TNF-α secretion levels; at week 48, an estimated VEVL of 0.35 (95% CI:− 0.24, 0.94) log10 increase was observed in the mean VL among Vacc-4x vs. placebo recipients at the 25th percentile level, and an estimated VEVL of 0.25 (95% CI:− 0.8, 0.31) log10 decrease at the 75th percentile of the wk28/wk1 TNF-α secretion levels; at week 52, an estimated VEVL of 0.35 (95% CI:− 0.37,1.06) log10 increase was observed in the mean VL among Vacc-4x vs. placebo recipients at the 25th percentile level, and an estimated VEVL of 0.32 (95% CI:− 1.0, 0.35) log10 decrease at the 75th percentile of the wk28/wk1 TNF-α secretion level (Table 1). Furthermore, wk28/wk1 TNF-α levels above the median were associated with improved preART-adjusted week 48 CD4 count (interaction p = 0.009; q = 0.009) (Table S2, Fig. S6), and week 28 TNF-α levels were associated with preART-adjusted week 52 CD4 counts (interaction p = 0.024; q = 0.096) (Fig. S7; Table S3) between the Vacc-4x and placebo groups. These results suggest a qualitative difference in response between the Vacc-4x and placebo groups since increasing IFN-γ and TNF-α levels were not associated with lower VL or better preserved CD4 count in the placebo group (data not shown).
3.5. IL-6 Secretion in T-Cell Proliferation Supernatants as a Predictor of Vaccine Effect
Lastly, we found that higher wk28/wk1 fold-change of IL-6 secretion in the proliferation supernatants was associated with reduced VL at week 48 in the Vacc-4x group compared to the placebo group (interaction p = 0.017, q = 0.036), with an estimated VEVL of 0.57 (95% CI: − 0.12, 1.27) log10 increase in the mean VL among Vacc-4x vs. placebo recipients at the 25% percentile level, and an estimated VEVL of 0.58 (95% CI: − 1.26, 0.10) log10 decrease at the 75th percentile level of the wk28/wk1 IL-6 secretion levels. Similar patterns were also observed for VEVL at weeks 44 and 52, though not statistically significant (Fig. 5).
Among 30 week 28 immune variables, besides CD4+ T-cell proliferation levels and IFN-γ, TNF-α mentioned above, IL-13 secretion, IL-17 secretion, and total T-cell proliferation levels were identified as negative predictors of VEVL and/or VECD4 (Table S1), whereas MIP-1β was identified as a positive predictor of VECD4 (Table S1; Fig. S4). Furthermore, increased MIP-1β secretion levels were associated with increased preART-adjusted VECD4 (Fig. S7, Table S3). However, this finding was heavily influenced by a few data points and should be interpreted with caution.
4. Discussion
One objective of therapeutic HIV-1 vaccination is to facilitate durable immune control of VL in the absence of cART, enabling improved preservation of CD4 counts (Pantaleo et al., 1993). Besides HLA class I alleles and CCR5 mutations, no immune biomarkers have been reported to be associated with either the natural control of HIV-1 VL (e.g. in elite controllers) or intervention-induced (e.g. therapeutic vaccination) control of HIV-1 VL. The Vacc-4x phase 2 study was one of the largest placebo-controlled clinical trials of a therapeutic HIV-1 vaccine and included up to 6 months ATI, permitting establishment of a VL set-point. As previously reported, there was no difference in the time to viral rebound between Vacc-4x and placebo groups, although the viral load set point was significantly lower for the Vacc-4x group compared to placebo, and compared to their own preART levels. These findings suggest that Vacc-4x immunization yielded potential vaccine benefit through improved cell-mediated immune responses to HIV compared to the placebo group (Pollard et al., 2014). The Vacc-4x phase 2 study therefore provides a unique opportunity to carry out analyses to determine prospective immune correlates of Vacc-4x effect.
Prior analysis of safety parameters in the Vacc-4x phase 2 study revealed that lower post-vaccination C-reactive protein concentrations in serum and higher anti-C5/gp41732–744 antibody levels were associated with VE in Vacc-4x participants (Huang et al., 2016b), suggesting that low immune activation and/or inflammation may facilitate attaining vaccine effect. It was therefore of further interest to determine the effect of CMIs on VE, including cytokine production from T-cell proliferation supernatants that are not usually addressed in clinical trials of HIV-1 therapeutic or preventative vaccines.
Baseline anti-HIV immune response variability is a particular challenge in the field of therapeutic HIV-1 vaccines because the varying nature and magnitude of baseline immune responses to HIV-1 may influence the ability to effectively detect vaccine-induced immunity. We found that ELISPOT and CFSE responses at baseline were highly correlated with week 28 responses. This finding suggests that the immune systems of individuals with higher baseline immune responses while still on cART may be more responsive to immune stimulation by vaccination than those who had lower baseline immune responses. Similar findings have been made regarding the presence of baseline delayed type hypersensitivity (DTH) responses in an earlier Vacc-4x clinical study (Nyhus et al., 2006). This observation may have important implications for future therapeutic and preventative vaccine studies of potential correlates of vaccine effect, because strong predictors of post-vaccination biomarkers will greatly increase the statistical efficiency of such evaluations (Follman, 2006; Gilbert et al., 2014).
Vacc-4x aims to induce CD8+ T-cell-mediated killing of infected cells, however, this was not measured directly in this study because such assays were less developed at the time the study was conducted. T-cell proliferation is nevertheless considered a central parameter for measuring the immunogenicity of interventions aimed at inducing cell-mediated immunity and ultimately killing of infected cells. T-cell proliferation is a complex process and immune markers associated with T-cell proliferation leading to CD8-mediated killing are not fully understood. We therefore assessed cytokines released during ex vivo T-cell proliferation to determine whether it was possible to identify potential biomarkers for T-cell proliferation associated with the production of CD8+ T-cells that could lead to vaccine-associated reductions in viral load.
Several positive week28/wk1 and week 28 predictors of vaccine effect were identified. Regarding PBMC biomarkers, we found that higher week 28 CD4+ T-cell proliferation but lower wk28/wk1 fold-changes were associated with Vacc-4x benefit in terms of better preserved CD4 counts in the Vacc-4x group vs. the placebo group during ATI. This suggests that vaccine-matched T-cell responses at week 28 may be more focused towards conserved (vulnerable) regions of HIV-1 p24, hence providing benefit in maintaining CD4 counts in the Vacc-4x group as compared to the placebo group. Meanwhile, lower wk28/wk1 fold-changes may be a consequence of higher baseline response levels and the relative difference among participants being greater at baseline than week 28. Another possibility could be the potential effect of Vacc-4x immunization on reducing HIV reservoirs in peripheral blood as has been reported in more recent studies (Rockstroh et al., 2015; Leth et al., 2016). This could theoretically have resulted in lower viral burden in PBMCs by week 28 and T-cell proliferative responses that did not lead to a fold-rise from baseline.
Regarding T-cell proliferation supernatant biomarkers, we found that higher wk28/wk1 TNF-α fold-changes were associated with Vacc-4x benefit in terms of lower VL and better preserved pre-ART adjusted CD4 counts. Such positive correlation was also observed for week 28 TNF-α and preART-adjusted CD4 counts. Moreover, higher wk28/wk1 IFN-γ fold-changes were associated with Vacc-4x benefit in terms of lower VL. Both TNF-α and IFN-γ are established Th1 cytokines that activate effector functions of macrophages, neutrophils and CD8+ T-cells to kill cells infected with intracellular pathogens. Ex vivo IFN-γ production in PBMCs following antigen stimulation (e.g. ELISPOT) has been used as a standard measure of vaccine immunogenicity. However, it is now clear that cytokines induced by vaccination rarely act alone, but rather in concert which complicates identification of immune correlates. For example, measurements of IFN-γ alone have been insufficient to predict efficacy for both tuberculosis (Mittrücker et al., 2007) and HIV (Buchbinder et al., 2008). Indeed, in the STEP study of a preventive adenovirus 5-vectored HIV vaccine, cell-mediated immune responses as measured by IFN-γ ELISPOT, did not correlate with protection, rather, more participants in the vaccinated group became infected (Buchbinder et al., 2008). Subsequent efforts have focused on the induction of polyfunctional T-cells producing more than one cytokine on antigen stimulation as measured by intracellular cytokine staining (ICS) (Burgers et al., 2009; Corey et al., 2009). Recently, alternate statistical methods have been developed to address polyfunctional T-cell responses, such as COMPASS (Lin et al., 2015). This approach revealed strong inverse correlations between CD8+ T-cell responses and HIV-1 infection risk in the efficacy study of another preventive DNA/rAd5 vaccine (Janes et al., 2017). The T-cell responses were based on intracellular cytokine staining of T-cells producing IFN-γ, IL-2 and/or TNF-α.
Our findings nevertheless support the concept that induction of IFN-γ and TNF-α cytokines during ex vivo T-cell proliferation, which involves longer term culture of cells than for ELISPOT and ICS, may be associated with virus control and/or preservation of CD4 count during ATI. As such, cytokine analysis of T-cell proliferation supernatants could emphasize the breadth of cytokines and chemokines released on antigen stimulation and thereby serve to complement ELISPOT and ICS analyses that detect cytokine production from individual cells following short term culture.
We also found that higher wk28/wk1 fold-changes of IL-6 in proliferation supernatants were associated with VE. Interestingly, serum IL-6 was associated with chronic immune activation and adverse events during ATI in the SMART study, where participants underwent treatment interruption in the absence of any therapeutic intervention (El Sadr et al., 2006). In the Vacc-4x study, serum IL-6 levels remained within normal range (Pollard et al., 2014). Our findings could therefore suggest that transient IL-6 released during T-cell proliferation responses is associated with virus control, but that persistent (serum-detectable) IL-6 induction drives HIV replication and disease progression, particularly in the absence of cART. Higher levels of week 28 IL-17 in the placebo group were associated with lower CD4 counts at week 44; the converse was true for the Vacc-4x group. This is consistent with prior observations where higher plasma levels of IL-17 were associated with lower CD4 counts (< 200 cells/mm3) in HIV-1-positive volunteers (Saing et al., 2016). Higher week 28 IL-13 (proinflammatory cytokine) levels above the median were also associated with higher VL at week 48 in the Vacc-4x group vs. the placebo group. Taken together, these findings highlight the complexity of cytokine responses in vaccine studies where induction of some cytokines (e.g. IL-6, IFN-γ and TNF-α) in T-cell proliferation supernatants is associated with Vacc-4x effect whereas induction of other cytokines (e.g. IL-13 and IL-17) did not. These responses sometimes conflict with reported cytokine levels measured in serum or plasma and related to HIV pathogenesis, perhaps because the in vivo measurements may be complicated by underlying immune activation. These observations also emphasize the importance of including placebo controls in clinical studies evaluating therapeutic vaccines.
ELISPOT and CFSE are functional assays that measure ex vivo immune responses to antigen stimulation and can be compared between pre- and post-vaccination time-points. The CFSE positivity criteria were stringent compared to other clinical studies (Danta et al., 2008), which may have influenced the extent of positive proliferation assay responses obtained. Of note, the p24 antigens used in this study corresponded only to the conserved regions in the vaccine, from which there has been little reported immune escape following Vacc-4x immunization (Kran et al., 2010). The Luminex assay analyzed CFSE supernatants following stimulation with full-length recombinant p24, and could therefore also have included responses to p24 epitopes outside the conserved regions in Vacc-4x.
Limitations of this study include 1) limited sample size for well-powered assessment of immune biomarkers as correlates of the observed vaccine effect. The small number of female participants also hindered assessment of the impact of gender. 2) The identified immune biomarkers were only assessed as predictors of vaccine effect, not as biomarkers in the causal pathway of the observed vaccine effect. Future research is needed to determine the clinical implications of these biomarkers. 3) Given the small sample size and the large number of potential biomarkers, we only assessed each biomarker univariately. Future research could examine biomarker interplay and their collective influence on vaccine effect. 4) Limited specimen availability from all time-points precluded more comprehensive characterization of vaccine-induced immune responses (e.g. T-cell exhaustion markers, and ICS analysis).
Nevertheless, to our knowledge, previous studies on HIV immune correlates of protection are predominately in the field of preventative HIV vaccines, where baseline responses are absent (Thakur et al., 2012). In our analyses, we presented results with and without accounting for baseline responses, highlighting their unique role in predicting VE in the context of therapeutic HIV vaccines. Furthermore, much focus of current research remains on the induction of humoral responses, with emphasis on broadly neutralizing antibodies. On the other hand, therapeutic vaccines have largely focused on the induction of cell-mediated immune responses. Immunogenicity following therapeutic vaccination has been described with reductions in viral load (Vardas et al., 2012; Garcia et al., 2013; Pollard et al., 2014), as well as with no or limited effects on the rate of viral rebound or control of viral load (Jacobson et al., 2016; Thompson et al., 2016). No immune correlates have yet been clearly defined from these generally small studies. Perera et al. (2016) evaluated one HIV-1 elite controller, who had received a p24 therapeutic vaccine in 1993. Responses associated with virus control included high fold-changes in plasma levels of fractalkine, Interferon-inducible T-cell alpha chemoattractant (I-TAC), insulin-like-growth factor binding protein (IGFBP)-2, and MIP-1α. It is also not clear whether the correlates for virus control (i.e. reducing viral load set point) are going to be the same as those for delaying viral rebound. Our findings based on one of the largest therapeutic HIV vaccine trials emphasize the importance of baseline-adjusted correlation analyses of immunological markers, and the potential value of assessing cytokine production in T-cell proliferation supernatants harvested from ex vivo antigen stimulation in placebo-controlled clinical trials.
Acknowledgments
Acknowledgements
All participating investigators are gratefully acknowledged for their contributions to this study as well as all the study participants. The authors thank Lindsay Carpp for editorial assistance on the final draft of the manuscript.
Funding Source
This work was funded by Bionor Pharma AS. Bionor Pharma AS had a role in the study design, data collection, analysis, interpretation and writing of the report. The statistical analysis present was carried out at the Fred Hutchinson Cancer Research Center, USA. The corresponding author had full access to all study data and had the final responsibility for the decision to submit for publication.
Conflicts of Interest
MÖ and MS are employees of Bionor Pharma AS. MT and AOH were employees of Bionor Pharma AS during the study. RP and JR are members of Bionor Pharma AS clinical advisory board. The remaining authors have no conflicts of interest to report.
Author Contributions
YH, LZ, and BS carried out statistical analysis; GP and GT carried out ELISPOT and proliferation analyses. MT and AOH carried out cytokine analysis from proliferation supernatants. RP and JR were principal investigators for the study in the USA and Europe, respectively. MÖ and MS coordinated the work, and MS and YH had main responsibility for manuscript writing. All co-authors were involved in editing and commenting on the manuscript.
Footnotes
Supplementary figures and tables to this article can be found online at https://doi.org/10.1016/j.ebiom.2017.09.028.
Contributor Information
Yunda Huang, Email: yunda@scharp.org.
Giuseppe Pantaleo, Email: Giuseppe.Pantaleo@chuv.ch.
Gonzalo Tapia, Email: Gonzalo.Tapia@chuv.ch.
Brittany Sanchez, Email: bprigmore@scharp.org.
Lily Zhang, Email: yzhang2@scharp.org.
Monica Trondsen, Email: monica.trondsen@gmail.com.
Arnt-Ove Hovden, Email: arntovehovden@gmail.com.
Richard Pollard, Email: rbpollard@ucdavis.edu.
Jürgen Rockstroh, Email: Juergen.Rockstroh@ukb.uni-bonn.de.
Mats Ökvist, Email: mo@bionorpharma.com.
Maja A. Sommerfelt, Email: ms@bionorpharma.com.
Appendix A. Supplementary data
Supplementary figures and tables
References
- Bart P.-A., Goodall R., Barber T., Harari A., Guimaraes-Walker A., Khonkarly M., Sheppard N.C., Bangala Y., Frachette M.J., Wagner R., Liljeström P., Kraehenbuhl J.P., Girard M., Goudsmit J., Esteban M., Heeney J., Sattentau Q., McCormack S., Babiker A., Pantaleo G., Weber J., EuroVacc Consortium EV01: A phase I trial in healthy HIV negative volunteers to evaluate a clade C HIV vaccine, NYVAC-C undertaken by the EuroVacc Consortium. Vaccine. 2008;26:3153–3161. doi: 10.1016/j.vaccine.2008.03.083. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Boaz M.J., Hayes P., Tarragona T., Seamons L., Cooper A., Birungi J., Kitandwe P., Semaganda A., Kaleebu P., Stevens G., Anzala O., Farah B., Ogola S., Indangasi J., Mhlanga P., Van Eeden M., Thakar M., Pujari A., Mishra S., Goonetilleke N., Moore S., Mahmoud A., Sathyamoorthy P., Mahalingam J., Narayanan P.R., Ramanathan V.D., Cox J.H., Dally L., Gill D.K., Gilmour J. Concordant proficiency in measurement of T-cell immunity in human immunodeficiency virus vaccine clinical trials by peripheral blood mononuclear cell and enzyme-linked immunospot assays in laboratories from three continents. Clin. Vaccine Immunol. 2009;16:147–155. doi: 10.1128/CVI.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R., Li D., Gilbert P.B., Lama J.R., Marmor M., Del Rio C., McElrath M.J., Casimiro D.R., Gottesdiener K.M., Chodakewitz J.A., Corey L., Robertson M.N., Step Study Protocol Team Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the step study): a double-blind, randomized, placebo-controlled test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers W.A., Chege G.K., Müller T.L., van Harmelen J.H., Khoury G., Shephard E.G., Gray C.M., Williamson C., Williamson A.L. Broad, high-magnitude and multifunctional CD4 + and CD8 + T-cell responses elicited by a DNA and modified vaccinia Ankara vaccine containing human immunodeficiency virus type 1 subtype C genes in baboons. J. Gen. Virol. 2009;90:468–480. doi: 10.1099/vir.0.004614-0. [DOI] [PubMed] [Google Scholar]
- Cellerai C., Perreau M., Rozot V., Bellutti Enders F., Pantaleo G., Harari A. Proliferation capacity and cytotoxic activity are mediated by functionally and phenotypically distinct virus-specific CD8 T cells defined by interleukin-7Ra (CD127) and perforin expression. J. Virol. 2010;84:3868–3878. doi: 10.1128/JVI.02565-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A.W., Kumar M.P., Arnold K.B., Yu W.H., Schoen M.K., Dunphy L.J., Suscovich T.J., Frahm N., Linde C., Mahan A.E., Hoffner M., Streeck H., Ackerman M.E., McElrath M.J., Schuitemaker H., Pau M.G., Baden L.R., Kim J.H., Michael N.L., Barouch D.H., Lauffenburger D.A., Alter G. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell. 2015;163:988–998. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., McElrath J., Kublin J.G. Post STEP modifications for research on HIV vaccines. AIDS. 2009;23:3–8. doi: 10.1097/QAD.0b013e32830e6d6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danta M., Semmo N., Fabris P., Brown D., Pybus O.G., Sabin C.A., Bhagani S., Emery V.C., Dusheiko G.M., Klenerman P. Impact of HIV on host-virus interactions during early hepatitis C virus infection. J. Infect. Dis. 2008;197:1558–1566. doi: 10.1086/587843. [DOI] [PubMed] [Google Scholar]
- El Sadr W.M., Lundgren J.D., Neaton J.D., Gordin F., Abrams D., Arduino R.C., Babiker A., Burman W., Clumeck N., Cohen C.J., Cohn D., Cooper D., Darbyshire J., Emery S., Fätkenheuer G., Gazzard B., Grund B., Hoy J., Klingman K., Losso M., Markowitz N., Neuhaus J., Phillips A., Rappoport C., Strategies for Management of Antiretroviral Therapy (SMART) Study Group CD4 + count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- Follman D. Augmented designs to assess immune response in vaccine trials. Biometrics. 2006;62:1161–1169. doi: 10.1111/j.1541-0420.2006.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia F., Climent N., Guardo C., Gil C., León A., Autran B., Lifson J.D., Martínez-Picado J., Dalmau J., Clotet B., Gatell J.M., Plana M., Gallart T. DCV2/MANON07-ORVACS Study Group. 2013. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci. Transl. Med. 2013;5:166ra2. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- Gilbert P.B., Gabriel E.E., Miao X., Li X., Su S.C., Parrino J., Chan I.S. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J. Infect. Dis. 2014;210:1573–1581. doi: 10.1093/infdis/jiu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A., Bart P.-A., Stöhr W., Tapia G., Garcia M., Medjitna-Rais E., Burnet S., Cellerai C., Erlwein O., Barber T., Moog C., Liljestrom P., Wagner R., Wolf H., Kraehenbuhl J.P., Esteban M., Heeney J., Frachette M.J., Tartaglia J., McCormack S., Babiker A., Weber J., Pantaleo G. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable polyfunctional, and long lasting T-cell responses. J. Exp. Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Sanchez B., Zhang L., Ökvist M., Sommerfelt M.A. Keystone Symposium HIV Vaccines 21st-24th March Abstract no: 2019. 2016. Immunological predictors of vaccine efficacy on viral load and CD4 count in a Phase 2 therapeutic HIV vaccine clinical trial. [Google Scholar]
- Huang Y., Zhang L., Jolliffe D., Hovden A.O., Ökvist M., Pantaleo G., Sommerfelt M.A. A case for preART-adjusted endpoints in HIV therapeutic vaccine trials. Vaccine. 2016;34:1282–1288. doi: 10.1016/j.vaccine.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Jacobson J.M., Routy J.P., Welles S., DeBenedette M., Tcherepanova I., Angel J.B., Asmuth D.M., Stein D.K., Baril J.G., McKellar M., Margolis D.M., Trottier B., Wood K., Nicolette C. Dendritic cell immunotherapy for HIV-1 infection using autologous HIV-1 RNA: a randomized, double-blind, placebo controlled clinical trial. J. Acquir. Immune Defic. Syndr. 2016;72(1):31–38. doi: 10.1097/QAI.0000000000000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes H.E., Cohen K.W., Frahm N., De Rosa S.C., Sanchez B., Hural J., Magaret C.A., Karuna S., Bentley C., Gottardo R., Finak G., Grove D., Shen M., Graham B.S., Koup R.A., Mulligan M.J., Koblin B., Buchbinder S.P., Keefer M.C., Adams E., Anude C., Corey L., Sobieszczyk M., Hammer S.M., Gilbert P.B., McElrath M.J. Higher T-cell responses induced by DNA/rAd5 HIV-1 preventative vaccine are associated with lower HIV-1 infection risk in an efficacy trial. J. Infect. Dis. 2017;215:1376–1385. doi: 10.1093/infdis/jix086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kran A.-M.B., Jonassen T.Ø., Sommerfelt M.A., Løvgården L., Sørensen B., Kvale D. Low frequency of amino acid alterations following therapeutic immunization with HIV-1 Gag p24-like peptides. AIDS. 2010;24:2609–2618. doi: 10.1097/QAD.0b013e32833e502b. [DOI] [PubMed] [Google Scholar]
- Kutzler M.A., Jacobsen J.M. Treatment interruption as a tool to measure changes in immunologic response to HIV-1. Curr. Opin. HIV AIDS. 2008;3:131–135. doi: 10.1097/COH.0b013e3282f54cde. [DOI] [PubMed] [Google Scholar]
- Leth S., Schleimann M.H., Nissen S.K., Højen J.F., Olesen R., Graversen M.E., Jørgensen S., Kjær A.S., Denton P.W., Mørk A., Sommerfelt M.A., Krogsgaard K., Østergaard L., Rasmussen T.A., Tolstrup M., Søgaard O.S. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3(10):e463–72. doi: 10.1016/S2352-3018(16)30055-8. [DOI] [PubMed] [Google Scholar]
- Lin L., Finak G., Ushey K., Seshadri C., Hawn T.R., Frahm N., Scriba T.J., Mahomed H., Hanekom W., Bart P.A., Pantaleo G., Tomaras G.D., Rerks-Ngarm S., Kaewkungwal J., Nitayaphan S., Pitisuttithum P., Michael N.L., Kim J.H., Robb M.L., O'Connell R.J., Karasavvas N., Gilbert P.C., De Rosa S., McElrath M.J., Gottardo R. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat. Biotechnol. 2015;33(6):610–616. doi: 10.1038/nbt.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrücker H.W., Steinhoff U., Köhler A., Krause M., Lazar D., Mex P., Miekley D., Kaufmann S.H. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2007;104(30):12434–12449. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus J.N., Kran A.-M.B., Sommerfelt M.A., Baksaas I., Sørensen B., Kvale D. Multiple antigen concentrations in delayed-type hypersensitivity (DTH) and response diversity during and after immunization with a peptide-based HIV-1 immunotherapy candidate Vacc-4x. Vaccine. 2006;24:1543–1550. doi: 10.1016/j.vaccine.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Fauci A.S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N. Engl. J. Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Perera, S.S., Wang, B., Damian, A., Dyer, W., Zhou, L., Conceicao, V., Saksena, N.K. 2016. Retrospective proteomic analysis of cellular immune responses and protective correlates of p24 vaccination in an HIV elite controller using antibody arrays. Microarrays (Basel) 5(2) (pii: E14). https://doi.org/10.3390/microarrays5020014. [DOI] [PMC free article] [PubMed]
- Perreau M., Welles H.C., Harari A., Hall O., Martin R., Maillard M., Dorta G., Bart P.A., Kremer E.J., Tartaglia J., Wagner R., Esteban M., Levy Y., Pantaleo G. DNA/NYVAC vaccine regimen induces HIV-specific CD4 and CD8 T-cell responses in intestinal mucosa. J. Virol. 2011;85:9854–9862. doi: 10.1128/JVI.00788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard R.B., Rockstroh J.K., Pantaleo G., Asmuth D.M., Peters B., Lazzarin A., Garcia F., Ellefsen K., Podzamczer D., van Lunzen J., Arastéh K., Schürmann D., Clotet B., Hardy W.D., Mitsuyasu R., Moyle G., Plettenberg A., Fisher M., Fätkenheuer G., Fischl M., Taiwo B., Baksaas I., Jolliffe D., Persson S., Jelmert O., Hovden A.O., Sommerfelt M.A., Wendel-Hansen V., Sørensen B. Safety and efficacy of the peptide-based therapeutic vaccine for HIV-1, Vacc-4x: a phase 2 randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2014;14:291–300. doi: 10.1016/S1473-3099(13)70343-8. [DOI] [PubMed] [Google Scholar]
- R Core Team . Foundation for Statistical Computing; 265 Vienna, Austria: 2015. R: A Language and Environment for Statistical Computing.http://www.R-project.org (accessed 06.09.2017) [Google Scholar]
- Rockstroh J.K., Asmuth D., Pantaleo G., Clotet B., Podzamczer D., Van Lunzen J., Arastéh K., Mitsuyasu R., Peters B., Lazzarin A., Jolliffe D., Ökvist M., Krogsgaard K., Mørk A., Sommerfelt M.A. Vacc-4x, during ART. IAS 2015 Vancouver Canada 19th–22nd July. Abstract # TUPEB296. 2015. Reduction in total HIV-1 proviral DNA following re-boost immunizations using the peptide-based therapeutic vaccine candidate. [Google Scholar]
- Saing T., Valdiva A., Hussain P., Ly J., Gonzalez L., Guilford F.T., Pearce D., Venketaraman V. Data on pro-inflammatory cytokines IL-1β, IL-17 and IL-6 in the peripheral blood of HIV-infected individuals. Data Brief. 2016;19:1044–1047. doi: 10.1016/j.dib.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen B., Sommerfelt M.A., Stjernholm G., Smith P.L., Ökvist M., Hovden A.O., Hoddevik G., Redfield R., Ustina V., Jelmert Ø., Zeldis J., Dalgleish A. Correlation of antibody responses to a peptide antigen gp120-C5501-512/gp41732-744 with HIV disease progression. AIDS Res. Hum. Retrovir. 2017;33(6):558–566. doi: 10.1089/AID.2016.0184. [DOI] [PubMed] [Google Scholar]
- Thakur A., Pedersen L.A., Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine. 2012;30:4907–4920. doi: 10.1016/j.vaccine.2012.05.049. [DOI] [PubMed] [Google Scholar]
- Thompson M., Heath S.L., Sweeton B., Williams K., Cunningham P., Keele B.F., Sen S., Palmer B.E., Chomont N., Xu Y., Basu R., Hellerstein M.S., Kwa S., Robinson H.L. DNA/MVA Vaccination of HIV-1infected participants with viral suppression on antiretroviral therapy, followed by treatment interruption: elicitation of immune responses without control of re-emergent virus. PLoS One. 2016;11(10):e0163164. doi: 10.1371/journal.pone.0163164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardas E., Stanescu I., Leinonen M., Ellefsen K., Pantaleo G., Valtavaara M., Ustav M., Reijonen K. Indicators of therapeutic effect in FIT-06, a phase II trial of a DNA vaccine, GTU®-Multi-HIVB, in untreated HIV-1 infected subjects. Vaccine. 2012;30:4046–4054. doi: 10.1016/j.vaccine.2012.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables