Abstract
Introduction
When administered during pregnancy, antibodies and other biologic drugs that contain the Fc part of the IgG molecule can traverse the placenta. Although it is generally accepted that the FcRn receptor mediates this process, gaps remain in our understanding of underlying details in humans and in common laboratory animal species.
Methods
We expanded our previous studies in timed-pregnant guinea pigs to both measure the transport of human (h) IgG at earlier gestation ages in vivo and evaluate FcRn function in vitro using Surface Plasmon Resonance (SPR) and Madin–Darby canine kidney cells (MDCK) that express guinea pig (gp) FcRn.
Results
In timed-pregnant guinea pigs both the average concentration of hIgG in the fetus and its ratio to maternal hIgG concentration increase exponentially with gestation age. Thus, hIgG fetal:maternal concentration ratios increase from an average of 1% to 3%, 17%, and 76% on GD ~26, 35, 46, and 54, respectively. In vitro, gpFcRn immobilized on a solid surface can bind hIgG and gpIgG preparations in a similar manner. All engineered human Fc isotype-specific constructs were internalized by MDCK-gpFcRn cells at significant levels. While not significant, their recycling and hIgG transcytosis by this cell line also trend higher than background controls.
Discussion
Pregnant guinea pigs exhibit similarities with humans in the degree and timing of trans-placental transfer as well as the ability of their FcRn to bind and internalize hIgG in vitro. Further studies are needed to guide building appropriate systems for the evaluation of FcRn mediated function of human immunoglobulin therapies.
Keywords: Transplacental immunoglobulin transfer
1. Introduction
Finding appropriate in vivo and ideally in vitro models for assessing safety and efficacy of protein therapeutics during pregnancy is not trivial. For many such biologics, the placenta provides an effective barrier that prevents fetal exposure to a biologic drug. This is not the case for antibody therapeutics consisting of intact antibodies and engineered biologics that contain the Fc part of IgG. An active, receptor mediated process ensures the transfer of these products from maternal to fetal blood [1]. This process is believed to start in the second trimester of human pregnancy [2], with 17–22 weeks fetuses having IgG concentrations that are at least ten times lower than maternal levels [3]. As human pregnancy progresses, fetal IgG levels continuously increase to reach and even surpass maternal levels at the end of the third trimester [4–7], resulting in fetal:maternal ratios higher than one. The same receptor, neonatal Fc receptor (FcRn) also plays a major role in rescuing IgG from catabolism, thus prolonging the half-life of IgG in the circulation [8–11].
Information on trans-placental transfer of human antibody preparations in common laboratory animal species is incomplete. At the end of gestation many species, including rabbits and monkeys exhibit trans-placental transfer of human antibodies [12,13]. Others, such as newborn rats and mice obtain most of their antibodies from their dam through lactation [13–15], although it has been shown that maternally administered human IgG can also be transferred to the fetus to achieve fetal:maternal ratios higher than one [16].
We and others have shown that the pregnant guinea pig is a good model for studying antibody transfer during pregnancy [17–21] and can transfer all human IgG subclasses at the end of gestation [18]. Based on these findings, we set out to test the hypothesis that, like in humans, fetal concentration of the human IgG in the pregnant guinea pig increases with gestation. By providing data on fetal exposure to hIgG at different stages of the development and on species specific FcRn activity, our studies may help guide choosing appropriate models to assess safety and efficacy of antibody therapies during pregnancy.
2. Materials and methods
2.1. Animal studies
All animal procedures were performed in accordance with protocols approved by the CBER Animal Care and Use Committee. Hartley Albino (Crl:HA) guinea pigs were purchased from commercial sources and mated to produce timed pregnancies as previously described [20]. Four groups of pregnant sows, one for each gestational age, n = 3–4/group, were used. On gestation day (GD) ~22, ~30, ~41 and ~49, approximately corresponding to the end of first trimester, middle and end of second trimester and middle of the third trimester, the animals were weighed and a polyclonal commercial human IgG purified from pooled plasma of healthy donors with high titers of antibodies against Hepatitis B, HepaGam®(Emergent Biosolutions, 549 IU/mL and 41 mg/mL) was administered intravenously at a dose 100 IU/kg (0.182 ml/kg or ~7 mg/kg). Dose was chosen to correspond with the approved dose for infants born to mothers testing positive for Hepatitis B [22]. Five days after injection, i.e. GD ~35, ~46, and ~54, blood samples were collected from all dams, two of the litters on GD ~46, and all the litters of GD ~54 via cardio- or cordo-centesis; whole fetuses were collected from all the remaining animals (Table 1, italics). Due to a technical error, terminal samples in the group that received injections on GD ~22 were collected on GD ~26, four days after injection. Five days post-injection was used as the sampling point because previously we observed a plateau in the fetal concentration of maternally administered hIgG at 4–5 days post-administration [18], but also because it is short enough to allow for the assessment of administered product free from the interference of anti-drug antibodies to the foreign biologic. The GD designations were approximate because the breeding procedure allowed for a maximum of three days of co-habitation for the mating pair. Thus, the litter designated GD ~22 was obtained from sows that could be 22 ± 1 days pregnant.
Table 1.
Gestation age dependent transfer of human IgG in the pregnant guinea pig. Maternal and fetal hIgG concentrations were determined by fitting absorbance data measured in ELISA to a standard curve. Italics signify data from measurements in total body homogenates. Concentrations from all litter-mates were averaged to obtain the mean litter concentration and the fetal:maternal ratio was calculated by dividing this value by the hIgG concentration from the respective dam. The assumption was made that in the fetus IgG was distributed equally in tissue and serum, and no adjustment was made for the concentration measured in total body homogenates versus serum. One way ANOVA with Bonferroni post hoc analysis was used to compare gestation dependent hIgG fetal concentration or concentration ratios.
| Gestation day | GD26 | GD35 | GD46 | GD54 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| hIgG Concentration | Maternal Concentration, μg/mL |
Mean Litter Concentration (n), μg/mL |
Fetal:Maternal Concentration Ratio (%) |
Maternal Concentration, μg/mL |
Mean Litter Concentration (n), μg/mL |
Fetal:Maternal Concentration Ratio (%) |
Maternal Concentration, μg/mL |
Mean Litter Concentration (n), μg/mL |
Fetal:Maternal Concentration Ratio (%) |
Maternal Concentration, μg/mL |
Mean Litter Concentration (n), μg/mL |
Fetal:Maternal Concentration Ratio (%) |
| Individual Dams/Litters | 77.6 | 1.6 (2) | 2.1 | 12.2 | 0.5 (2) | 4.1 | 74.3 | 15.1 (3) | 20.3 | 59.1 | 26.6 (4) | 45.0 |
| 107.3 | 1.1 (3) | 1.0 | 73.4 | 2.6 (3) | 3.5 | 97.9 | 11.6 (4) | 11.8 | 76.8 | 72.0 (3) | 93.8 | |
| 136.4 | 1.4 (4) | 1.0 | 110.3 | 4.1 (4) | 3.7 | 93.1 | 3.8 (2) | 4.1 | 99.1 | 88.4 (3) | 89.2 | |
| 91.6 | 1.2 (3) | 1.3 | 103 | 34.4 (3) | 33.4 | |||||||
| Group Mean | 107.1 | 1.4 | 1.4 | 71.9 | 2.1 | 3.2 | 92.1 | 16.2 | 17.4 | 78.3 | 62.3* | 76.0# |
| Std. Deviation | 29.4 | 0.2 | 0.6 | 42.6 | 1.6 | 1.3 | 12.5 | 13.0 | 12.6 | 20.0 | 32.0 | 26.9 |
| N (n) | 3 | 3 (9) | 3 | 4 | 4 (12) | 4 | 4 | 4 (12) | 4 | 3 | 3 (10) | 3 |
hIgG concentration or
its fetal:maternal ratio is statistically different from all the other gestation ages sampled.
Fetuses were carefully separated from the placenta, cleaned with cold PBS, weighed, flash frozen individually, and then homogenized by placing 50% tissue:PBS w:v mixture on ice with an OMNI TH apparatus (Omni International, Kennesaw, GA). The mixture was centrifuged at 10,000× g for 10 min at 4 °C and the supernate frozen at −80 °C for storage until use. hIgG in the serum and tissue homogenates was determined with a human IgG ELISA kit (Assaypro, St. Charles, MO). All samples were measured in duplicates and data points out of data fitting range or with CV > 15% were excluded from analysis and repeated measurements taken, if possible. The kit did not cross-react with guinea pig serum or homogenates from controls that did not receive hIgG.
2.2. Plasmids and cell culture
2.2.1. Gaussia Luciferase-hFc1, 2, 3, and 4 chimeric proteins
Gaussia luciferase gene (GLuc, New England Biolabs, Ipswich, MA) was inserted by restriction digest onto each of four plasmids for mammalian expression, pFUSE-hIgG(n)-Fc1 (n = 1,2, 3, or 4, InvivoGen, San Diego, CA) containing the Fc part of the hIgG1, 2, 3, and 4 respectively which were then transfected into Huh7 cells using published protocols [20]. Secretion of soluble GLuc-Fc was monitored by western blot or by measuring the activity of luciferase in the cell growth media with the BioLUX®Gaussia Luciferase Flex Assay kit (New England BioLabs) recorded with an Infinite F500 microplate reader (TECAN, Männedorf, Switzerland).
2.2.2. Epithelial cells expressing FcRn
Sequences for guinea pig beta 2 microglobulin (B2M) and the alpha chain of the FcRn receptor (FCGRT) were synthesized de novo (Origene USA, Rockville, MD) from cDNA sequences obtained from Cavia porcellus genome in the ensembl server database as described [20]. Each DNA sequence was amplified via PCR with custom primers and inserted into the bicistronic plasmid pBudCE4.1 (Life Technologies, USA) under the control of pCMV and pEF-1α promoters, respectively. Either plasmid backbone or plasmid with the two inserts was transfected into MDCK cells (NBL-2 CCL-34, ATCC); stable cell lines were obtained in about two-three weeks. Stable MDCK II cells expressing hFcRn and controls were a gift from Dr. Bloomberg [23].
The expression of gpFcRn was confirmed with western blot, pull-down assay and fluorescent imaging. For western blots, ~105–106 cells in sample loading buffer (Invitrogen) underwent electrophoresis, then transferred to a nitrocellulose membrane, probed with polyclonal rabbit antibodies specific for the FcRn alpha chain (anti-human FCGRT, Proteintech, 1:200) and anti-β–actin (PA1-46296, Thermo Scientific, 1:2000), then goat anti-rabbit antibodies (1:2000) and imaged as before [20].
For pull-down assay, ~105–106 MDCK cells with or without FcRn in 100 μL NP-40 PBS buffer with protease inhibitor were incubated for 30 min on ice, span down to remove cell debris and the supernatant adjusted to 1000 μL with Hanks Balanced Salts Solution (HBSS, Sigma Aldrich) pH 6.0. Subsequently, 1 μg polyclonal gpIgG (Lampire Biological Laboratories) or hIgG (Hizentra®, CSL Behring) and protein G beads were added and the mixture incubated for 1 h at RT or overnight at 4 °C with mixing. Beads were extensively washed with HBSS pH 6.0, the proteins were eluted with sample loading buffer (Invitrogen), and then underwent all the subsequent steps described for the western blots.
For fluorescent imaging, 0.5 × 105 MDCK cells with or without gpFcRn receptor were seeded onto 18 mm round coverslip inside a 12 well plate. The following day, coverslips were fixed in cold 1:1 volume:volume methanol-acetone mixture for 10 min, blocked for 30 min in blocking buffer (PBS pH 7.4, 10% FBS) and incubated 1 h with primary antibodies (mouse monoclonal antibody for E-Cad-herin, 4A2C7, Life Technologies, 10 μg/mL and rabbit polyclonal anti-hFCGRT, Proteintech, 20 μg/mL). Goat antibodies conjugated to fluorescent dyes (anti mouse AlexaFlour 488, Life Technologies, 10 μg/mL and anti rabbit-Dylight550, Pierce, 10 μg/mL) were used as secondary antibodies. Images were recorded in grayscale with a digital camera (DP71, Olympus) coupled to Olympus IX51 microscope equipped with bandpass emission fluorescence filter optical blocks. During the processing stage, individual image channels were pseudocolored with RGB values corresponding to each of the fluorophore emission spectral profiles.
2.2.3. Soluble FcRn
The soluble gpFcRn receptor was engineered by removing the transmembrane domain of gpFCGRT and adding both a flexible linker between the alpha and beta chains and a C-terminal Fc tag for ease of purification as described [20]. DNA for this construct was transfected into CHO cells and the secreted soluble receptor was purified by protein A affinity chromatography (Syd Labs, Natick, MA).
2.2.4. Internalizing, recycling and transcytosis
All the internalizing, recycling and transcytosis experiments were performed at 37 °C. For transcytosis experiments, MDCK cells containing either human, guinea pig FcRn or empty respective cloning vectors, were grown in replicates of three or more onto 0.33 cm2 or 1.12 cm2 transwell semi-permeable membranes (0.4 μm pore size) placed into 24 or 12 well plates (Corning, Sigma–Aldrich, USA) using published protocols [24]. Cell growth was monitored by measuring trans epithelial electrical resistance (TEER) via EVOM2 voltohmmeter (World Precision Instruments, Sarasota, Fl). When TEER reached 170–200 Ω*cm2 or more, usually on day 4 of experiment, transcytosis experiment was performed for 90–120 min by adding hIgG (HepaGam®, Emergent Biosolutions, 10 μg/mL) in 0.2 mL pre-warmed HBSS pH 6.0 inside and 0.2 mL HBSS pH 7.4 outside the transwell.
To measure internalizing and recycling of GLuc-hFc1, 2, 3, or 4 chimeric proteins, we adapted a published protocol [25]. Briefly, 1 × 105 MDCK cells with or without gpFcRn were seeded onto a 96 well white plates (Nunc-Immuno™ MicroWell™, Sigma–Aldrich). The following day, cells were washed with 0.2 mL pre-warmed HBSS pH 6.0 for 5 min, incubated for 1 h with 0.1 mL HBSS pH 6.0 containing GLuc or GLuc-hFc1, 2, 3, or 4 chimeric proteins (0.2–1 × 106 RU/well, depending on the construct, three replicates per each chimera); residual luciferase activity in the media was then quantified. Preferential internalizing of the Fc-containing chimeras would result in decreased GLuc activity in GLuc-Fc wells versus GLuc alone wells. Subsequently, the same plate was washed, incubated with HBSS pH 7.4 for 1.5 h to allow for the chimeric IgG to be exocytosed, then re-assayed for luciferase activity. Recycling of GLuc-Fc constructs would result in increased Gluc signal in GLuc-Fc wells compared to those containing GLuc alone.
2.3. Surface plasmon resonance (SPR)
Binding assays were performed at 25 °C using the Biacore 3000 instrument (GE Healthcare, Piscataway, NJ, USA) as previously described [18]. Briefly, the soluble gpFcRn was purified using affinity chromatography, diluted in 10 mM sodium acetate, pH 5.5 and immobilized on a CM5 chip using an amine coupling kit (GE Healthcare, Piscataway, NJ, USA) to a density of 2000 resonance units (RU). A commercial polyclonal hIgG product purified from pooled human plasma (Hizentra®, CSL Behring AG [26]) and a polyclonal gpIgG purified from guinea pig serum (Lampire Biological Laboratories) were serially diluted threefold in binding buffer (50 mM Na phosphate pH 6.0, 150 mM NaCl, and 0.005% Tween 20) to obtain concentrations of 5000–60 nM. The diluted IgG preparations were injected in duplicate across the reference and gpFcRn surface at 30 μL/min for 2 min. After dissociating for 2.5 min with the either binding buffer or a similar buffer at pH 7.4, the chip surface was regenerated using elution buffer.
2.4. Data transformation and analysis
Absorbance values from ELISA were transformed into human IgG concentration by fitting them to an equation derived from a five parameter fit of the standard curve (SoftMax Pro, Molecular Devices). IgG concentrations (μg/mL) from all the siblings in each litter were averaged to obtain litter average. The litter was used as unit for statistical analysis.
Transcytosis of IgG from epithelial cells was expressed in units of ng/cm2 using the surface area of 0.33 or 1.12 cm2 for 24 or 12 well plate inserts, respectively. The luciferase activity recording for each construct in gpFcRn-MDCK cells was normalized by dividing it with the average recording from the respective wells with empty vector/MDCK.
The stability of the human or gpIgG complex with gpFcRn was evaluated during the first 2 min of dissociation by calculating percent stability for all IgG dilutions as described [27]. Briefly, for each concentration, the percent differences in SPR signal at the beginning of dissociation (R0) and again 2 min later (R1) were computed and an average for each analyte at each pH was calculated.
The normalized luciferase activity values for each construct, the transcytosis values for each experiment, and the average stabilities from SPR measurements were analyzed using ANOVA with Bonferroni post hoc comparison; p values < 0.05 were considered significant.
3. Results
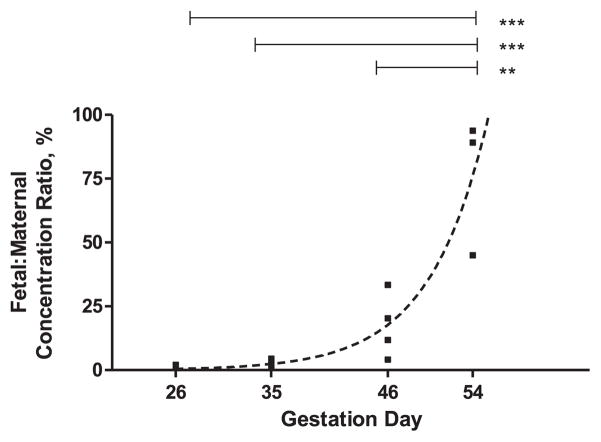
In this study we set out to measure the fetal concentration and fetal:maternal ratio of human IgG administered at different gestation ages to timed-pregnant guinea pigs. We found that the transfer of human IgG from the pregnant dam to its litter increases with gestation age. Both the average fetal concentration of the human IgG and fetal:maternal concentration ratios increase with gestation to reach significantly higher levels on GD54 compared to earlier time points (Table 1 and Fig. 1). Thus, fetal:maternal ratios increase from an average of 1% to 3%, 17% and 76% on gestation days 26, 35, 46 and 54, respectively. The increase in fetal:maternal ratio fits an exponential growth curve (r2 = 0.86), with y0 = 0.004%, k = 0.188 days−1, and doubling time 3.817 days (Fig. 1, broken line). A similar fit, albeit of a lower quality (r2 = 0.75) can also be obtained for the average fetal concentration values.
Fig. 1.
Transplacental transfer of human (h) IgG in timed pregnant guinea pigs at different gestation ages. Fetal:maternal concentration ratio was calculated by dividing the mean litter hIgG concentration by the hIgG concentration from the respective dam (Table 1). One way ANOVA with Bonferroni post hoc analysis was used to compare fetal:maternal ratios in each gestation age; **p < 0.01, ***p < 0.005. Data was fitted using GraphPad Prism (GraphPad Software, San Diego, CA) and the exponential fit shown by the broken line (R2 = 0.86).
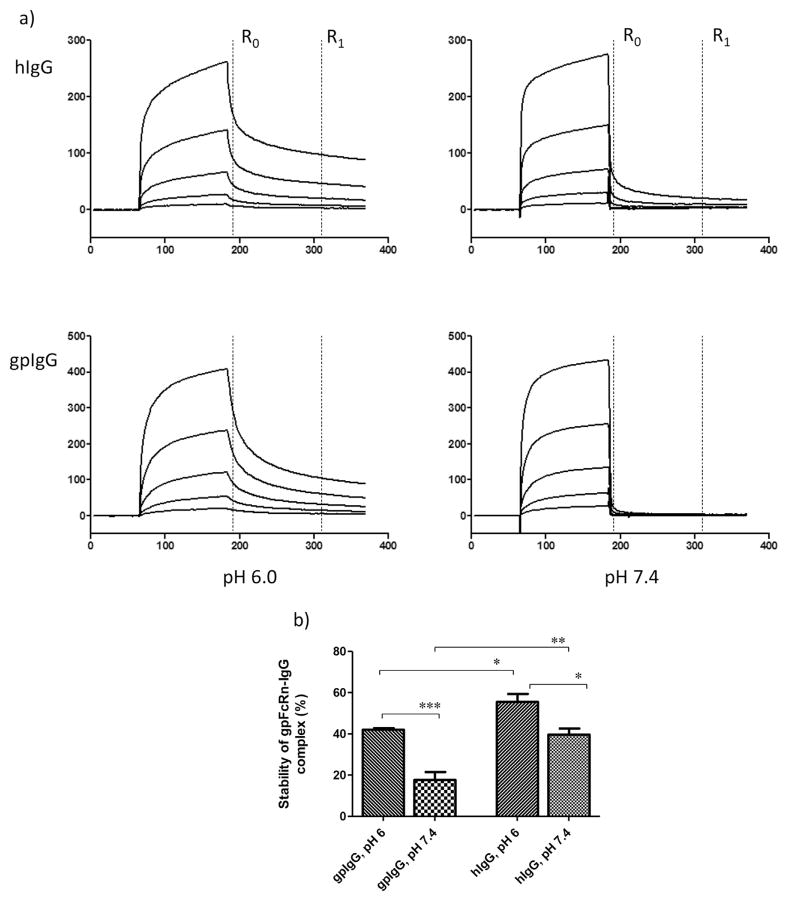
We also measured guinea pig FcRn binding to IgG using SPR. The sensograms (Fig. 2) show the binding and dissociation of the soluble gpFcRn to the polyclonal hIgG (Fig. 2a, top panels) and a comparator, gpIgG (Fig. 2a, bottom panels). They have a very similar shape indicative of complex binding common for polyclonal IgG preparations. Thus, we could not adequately fit these sensograms to any mathematical model of binding. We used the “percent stability” approach [27] to compare respective binding of the h-and gpIgG to gpFcRn. Both IgG preparations displayed the characteristic pH dependent binding to FcRn reflected by the significantly higher stability of IgG-gpFcRn complexes at pH 6.0 compared to pH 7.4 (Fig. 2b). There were differences in the calculated average stabilities between gpFcRn and IgG from either species (Fig. 2b), with the hIgG-gpFcRn complex having significantly higher apparent stability than the gpIgG-gpFcRn complex.
Fig. 2.
a) Sensograms from surface plasmon resonance (SPR) of guinea pig (gp) FcRn binding to human (h) IgG and its comparator, gpIgG. Purified soluble gpFcRn was immobilized on a CM5 chip to a density of 2000 resonance units (RU). Serially diluted polyclonal hIgG and gpIgG in binding buffer were injected in duplicate, and then dissociated with either binding (pH 6) or neutral pH buffer. For simplicity, only a single replicate is shown. b) The average percent stability of the hIgG and gpIgG complex with gpFcRn. Percent stability was calculated from SPR signal at the beginning of dissociation (R0) and again 2 min later (R1) for each dilution. ANOVA with Bonferroni post hoc analysis was used to compare average values for each analyte at each pH; *p < 0.05, **p < 0.01, ***p < 0.005.
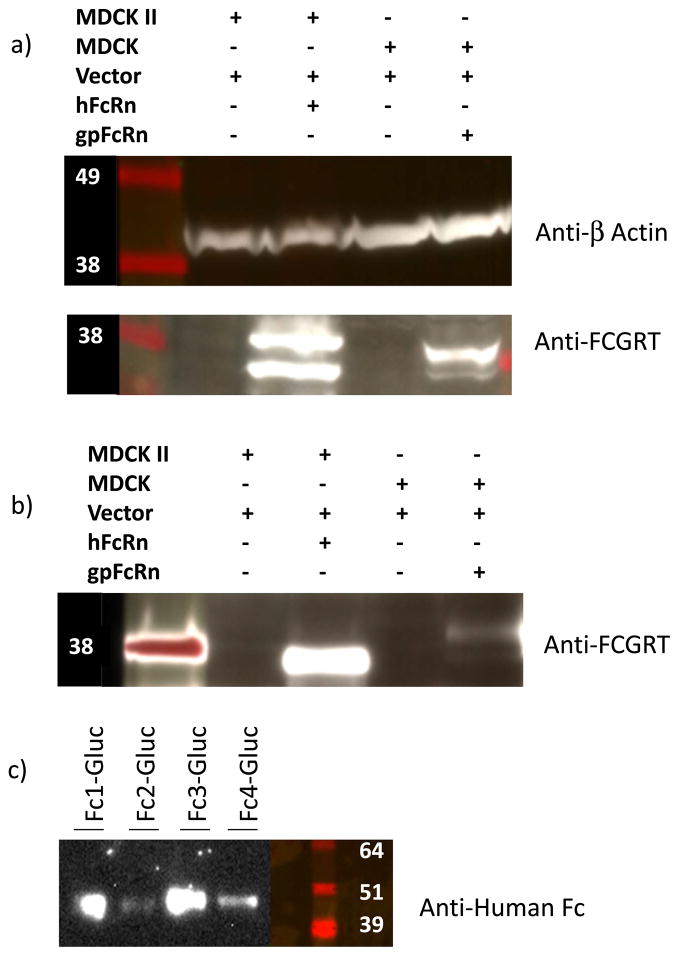
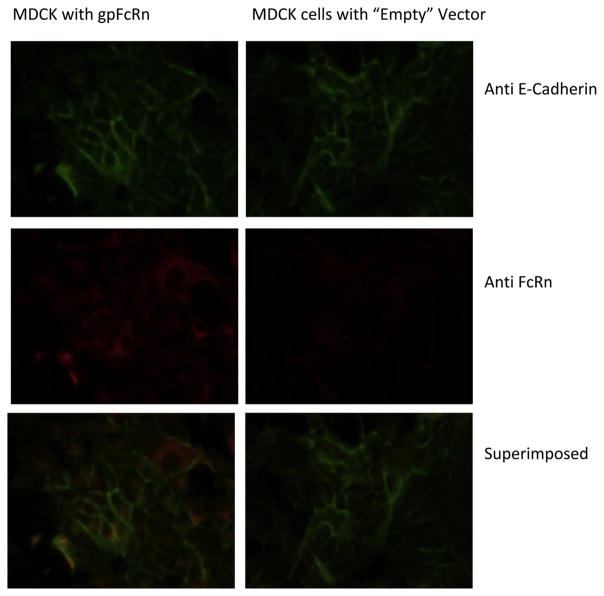
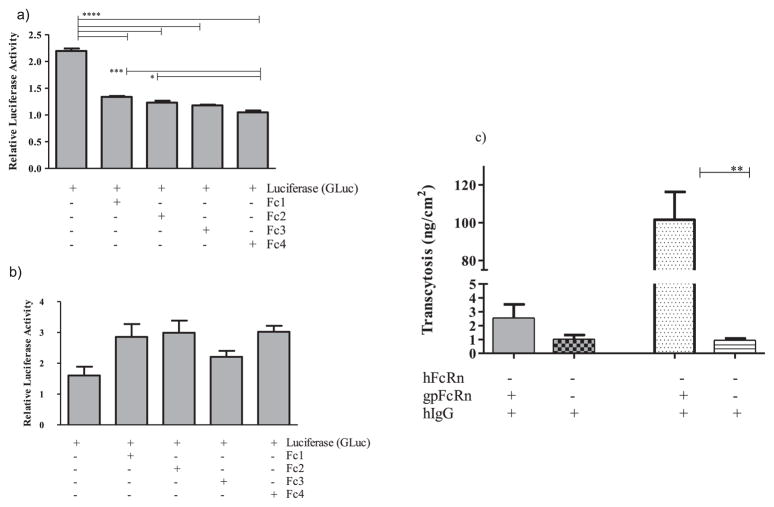
As demonstrated by Western blot (Fig. 3a) and fluorescent imaging (Fig. 4), we created an MDCK cell line that expressed gpFcRn at high levels compared to negative, plasmid only, controls. Pull-down assays with gpIgG (Fig. 3b) and hIgG (data not shown) indicated that the receptor expressed by these cells is functional, although it appears to be expressed at much lower levels compared to hFcRn expression by an MDCK II cell line. We grew these cells to confluent layers in 96 well plates and used human Fc-isotype specific constructs consisting of human Fc subclasses conjugated to gaussia luciferase (GLuc-hFc1, 2, 3, and 4, Fig. 3c) to assess internalization and recycling of human IgG subclasses by the gpFcRn receptor. Our data showed that all four human Fc isotype-specific constructs entered gpFcRn-MDCK cells at pH 6.0 as reflected by decreased luciferase activity in cell media compared GLuc alone (Fig. 5a). After washing, we incubated these cells at pH 7.4 to allow for exocytosis and subsequently repeated luciferase activity measurement. Although not statistically significant, we saw a trend toward a higher average activity in cells with GLuc-hFc chimeras compared to GLuc alone negative control (Fig. 5b).
Fig. 3.
a) Expression of guinea pig (gp) FcRn by MDCK cells assessed by probing with anti human (h) FcRn. hFcRn-MDCK II and cells with respective empty vectors were used as comparators; β-actin served as loading control. Theoretical molecular weight for the alpha chain of gpFcRn is ~36 KDa. High levels of expression can be seen in cells transfected with vector/receptor DNA, but not in cells transfected with vector alone. gpFcRn expression appears lower than hFcRn. b) Pull down by gpIgG of gpFcRn expressed by FcRn-MDCK cells compared to hFcRn-MDCK II and cells with respective empty vectors. Recovery of gpFcRn from cells transfected with gpFcRn is higher than negative controls but much less compared to hFcRn. Similar results were obtained with hIgG (data not shown). c) Secretion of chemoluminescent human Fc isotype specific constructs (GLuc-hFc1, 2, 3, and 4 chimeras) from stably transfected cells. Theoretical molecular weight of the chimeric proteins without glycosylation is 44–46 kDa. Secretion was also confirmed by measuring luciferase activity (data not shown).
Fig. 4.
Expression of guinea pig (gp) FcRn by MDCK cells stably transfected with FcRn (left) or empty vector (right), detected using immunofluorescence. FcRn (in red) was only expressed in cells transfected with the receptor containing plasmid (left), but not in those with the “empty” vector (right). It can be seen co-localizing with E-Cadherin in the cell membrane (in yellow, bottom row); considerable intracellular expression is also noted.
Fig. 5.
a) Internalizing and b) recycling of human (h) Fc subclasses 1 through 4 conjugated to Gaussia luciferase (GLuc-hFc1, 2, 3 and 4) by guinea pig (gp) FcRn-MDCK cells. a) GLuc-hFc1, 2, 3 and 4 chimeras or Gaussia luciferase (GLuc) alone in HBSS pH 6.0 were added apically onto MDCK cells with or without gpFcRn. After 1 h incubation, significantly lower GLuc activities in culture media for GLuc-hFc1, 2, 3 and 4 constructs were measured compared to GLuc alone, consistent with gpFcRn-MDCK cells preferentially internalizing all hFc subclass-GLuc chimeras. b) After being washed, cells were incubated with HBSS pH 7.4 for 1.5 h; GLuc activity in culture media was subsequently measured again. Wells containing GLuc-hFc constructs show a non-significant trend toward higher activity compared to those with GLuc alone, consistent with some degree of preferential release of GLuc-hFc chimeras from gpFcRn-MDCK cells. c) Transcytosis of polyclonal hIgG by gp FcRn-MDCK cells; hFcRn-MDCK II cells and respective vector-only controls were used as comparators. Transcytosis of hIgG by gpFcRn-MDCK epithelial layer is more than two times higher than negative controls, but significantly lower compared to hFcRn-MDCK II stable cell line. Differences in receptor expression and MDCK cell lineages likely underlie this observation. *p < 0.05, **p < 0.01, ***p < 0.005, and ****p < 0.001.
Finally, we measured transcytosis of hIgG across semi-permeable layers of MDCK cells that expressed gpFcRn. Consistent with recycling experiments, although not statistically significant, the amount of hIgG transcytosed by gpFcRn cells trended higher than no-receptor negative controls (Fig. 5c). By comparison, hFcRn-MDCK II controls displayed significantly higher transcytosis of the same polyclonal hIgG.
4. Discussion
Aiming to better understand transplacental transfer of human IgG and Fc-containing biologics, we used both the timed-pregnant guinea pig model and FcRn mediated in vitro assays with intriguing results. In vivo, we demonstrated that robust gestation age dependent (Fig. 1) transfer of human IgG does occur in the pregnant guinea pig. This results in both progressively higher fetal concentrations and fetal:maternal concentration ratios of human IgG with increased development, and significantly higher average fetal concentration on GD~54 when compared to earlier sampled time-points (Table 1). Although an experimental time point at the very end of gestation was not included in this study, our previous investigations have shown that newborn piglets delivered 4–5 days after maternal administration of human IgG averaged fetal:maternal concentration ratios of 82–129% [18], thus higher than the 76% ratio we observed on GD54. We believe this data is consistent with the FcRn mediated transplacental transfer of hIgG in the pregnant guinea pig during the third trimester. As such, this process resembles what is seen in primates including humans, but is distinct from the transfer of antibodies through the yolk sac (characterized by much lower levels of transfer), also present in guinea pigs earlier in gestation [17,19].
Using SPR, we also showed that gpFcRn binds to both autologous (gp) and hIgG in a similar pH dependent manner (Fig. 2). The hIgG-gpFcRn complex appears more stable than the gpIgG-gpFcRn complex at both neutral and acidic pH. Further studies are needed to ascertain to what extent the differences in the IgG preparations (one approved human biologic and the other a research grade reagent) contribute to these results, and if higher complex stability is an intrinsic characteristic of hIgG binding to gpFcRn. Despite this binding, under the conditions of our in vitro experiments, MDCK cells expressing gpFcRn mediated only limited trans-epithelial transport of hIgG which was neither statistically significant nor as robust as we saw in a similar cell line (MDCK II) expressing hFcRn (courtesy of Dr. Bloomberg) (Fig. 5c). Differences in receptor expression levels (Fig. 3a, b) in addition to differences in MDCK cell lineages [28] may underlie these results. This variability underscores the complexities and limitations in our ability to consistently recapitulate aspects of transplacental transport in vitro using common laboratory cell lines. Further studies are needed to explore inter-species differences in FcRn expression and binding, and to guide in building appropriate systems for the evaluation of FcRn mediated function of human immunoglobulin therapies.
Acknowledgments
We acknowledge the US FDA Offfce of Women’s Health for funding this work. We thank Drs. R. Bloomberg and T. Kuo from Brigham and Women’s Hospital for providing hFcRn-MDCK II cell line and control, as well as advice on performing transcytosis experiments.
Footnotes
Disclaimer
The findings and conclusions in this presentation have not been formally disseminated by the Food and Drug Administration and should not be construed to represent Agency determination or policy.
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 2.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 3.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 4.Hashira S, Okitsu-Negishi S, Yoshino K. Placental transfer of IgG subclasses in a Japanese population. Pediatr Int. 2000;42:337–342. doi: 10.1046/j.1442-200x.2000.01245.x. [DOI] [PubMed] [Google Scholar]
- 5.Malek A, Sager R, Schneider H. Maternal-fetal transport of immunoglobulin G and its subclasses during the third trimester of human pregnancy. Am J Reprod Immunol. 1994;32:8–14. doi: 10.1111/j.1600-0897.1994.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 6.Pitcher-Wilmott RW, Hindocha P, Wood CB. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 1980;41:303–308. [PMC free article] [PubMed] [Google Scholar]
- 7.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 11.Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, Waldmann TA, Robinson JM, Anderson CL. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci U S A. 2006;103:5084–5089. doi: 10.1073/pnas.0600548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pentsuk N, van der Laan JW. An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res B Dev Reprod Toxicol. 2009;86:328–344. doi: 10.1002/bdrb.20201. [DOI] [PubMed] [Google Scholar]
- 13.Barrow P. Developmental and reproductive toxicity testing of vaccines. J Pharmacol Toxicol Methods. 2012;65:58–63. doi: 10.1016/j.vascn.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 14.DeSesso JM, Williams AL, Ahuja A, Bowman CJ, Hurtt ME. The placenta, transfer of immunoglobulins and safety assessment of biopharmaceuticals in pregnancy. Crit Rev Toxicol. 2012;42:185–210. doi: 10.3109/10408444.2011.653487. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Mohanty S, Ganesan LP, Hua K, Jarjoura D, Hayton WL, Robinson JM, Anderson CL. FcRn in the Yolk Sac endoderm of mouse is required for IgG transport to fetus. J Immunol. 2009;182:2583–2589. doi: 10.4049/jimmunol.0803247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowman CJ, King LE, Stedman DB. Embryo-fetal distribution of a bio-pharmaceutical IgG2 during rat organogenesis. Reprod Toxicol. 2012;34:66–72. doi: 10.1016/j.reprotox.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Leissring JC, Anderson JW. The transfer of serum proteins from mother to young in the guinea pig. I. Prenatal rates and routes. Am J Anat. 1961;109:149–155. doi: 10.1002/aja.1001090205. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Norton MG, Mahmood I, Zhao Z, Zhong L, Zhang P, Struble EB. Transplacental transfer of hepatitis B neutralizing antibody during pregnancy in an animal model: implications for newborn and maternal health. Hepat Res Treat. 2014;2014:159206. doi: 10.1155/2014/159206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes JM. Antitoxin transfer from mother to foetus in the guinea-pig. J Pathol Bacteriol. 1959;77:371–380. doi: 10.1002/path.1700770206. [DOI] [PubMed] [Google Scholar]
- 20.Struble EB, Ma L, Zhong L, Lesher A, Beren J, Zhang P. Human antibodies can cross guinea pig placenta and bind its neonatal Fc receptor: implications for studying immune prophylaxis and therapy during pregnancy. Clin Dev Immunol. 2012;2012:538701. doi: 10.1155/2012/538701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulangara AC, Schechtman AM. Do heterologous proteins pass from mother to fetus in cow, cat and guinea pig? Proc Soc Exp Biol Med. 1963;112:220–222. [Google Scholar]
- 22.HepaGam B. Prescribing Information. 2012 http://www.hepagamb.com/pdfs/HepaGamBPI.pdf.
- 23.Claypool SM, Dickinson BL, Wagner JS, Johansen FE, Venu N, Borawski JA, Lencer WI, Blumberg RS. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol Biol Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo TT, de Muinck EJ, Claypool SM, Yoshida M, Nagaishi T, Aveson VG, Lencer WI, Blumberg RS. N-glycan moieties in neonatal Fc receptor determine steady-state membrane distribution and directional transport of IgG. J Biol Chem. 2009;284:8292–8300. doi: 10.1074/jbc.M805877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS, Lencer WI. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hizentra Prescribing Information. 2012 http://labeling.cslbehring.com/PI/US/Hizentra/EN/Hizentra-Prescribing-Information.pdf.
- 27.Leonard P, Safsten P, Hearty S, McDonnell B, Finlay W, O’Kennedy R. High throughput ranking of recombinant avian ScFv antibody fragments from crude lysates using the biacore A100. J Immunol Methods. 2007;323:172–179. doi: 10.1016/j.jim.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Dukes JD, Whitley P, Chalmers AD. The MDCK variety pack: choosing the right strain. BMC Cell Biol. 2011;12:43. doi: 10.1186/1471-2121-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]