Abstract
Acute stress and emotional arousal can enhance the consolidation of long-term memories in a manner that is dependent on β-adrenoceptor activation in the basolateral complex of the amygdala (BLA). The BLA interacts with multiple memory systems in the brain to modulate a variety of classes of memory. However, the synaptic mechanisms of this interaction remain unresolved. This review describes the evidence of modulation of memory and synaptic plasticity produced by emotional arousal, stress hormones, and pharmacological or electrophysiological stimulation of the amygdala. The amygdala modulation of local translation and/or degradation of the synaptic plasticity-related proteins, activity-regulated cytoskeletal-associated protein and calcium/calmodulin-dependent protein kinase II α, is offered as a potential mechanism for the rapid memory consolidation that is associated with emotionally arousing events. This model shares features with synaptic tagging and the emotional tagging hypotheses.
Keywords: amygdala, Arc, CaMKIIα, local translation, memory, synaptic plasticity
Introduction
Emotional arousal modulates the consolidation of long-term memories (McGaugh, 2004). Experiences that produce acute stress, fear, or excitement are typically remembered better than emotionally neutral experiences. This effect is observed in both humans (Cahill et al., 1994, 1996; Cahill and Alkire, 2003; Kuhlmann and Wolf, 2006) and nonhuman animals (Gold et al., 1975; Cahill and McGaugh, 1996; Quirarte et al., 1998; Ferry et al., 1999b; McIntyre et al., 2002; Okuda et al., 2004). Both the invasive studies in animals and the imaging studies in humans corroborate a role for the amygdala in this memory enhancement (Cahill et al., 1994; Ferry et al., 1999b; Roozendaal, 2000; McGaugh and Roozendaal, 2002; Cahill and Alkire, 2003; Roozendaal et al., 2006a, 2008a). More generally, the stress hormones epinephrine (adrenalin) and cortisol (corticosterone in rats) are released by the adrenal glands into the bloodstream to enhance the fight-or-flight response that may be important for survival. These stress hormones also modulate the storage of memories of events that have a bearing on survival through mechanisms involving the activation of β-adrenoceptors in the basolateral complex of the amygdala (Quirarte et al., 1997; Ferry and McGaugh, 1999; Ferry et al., 1999a; Miranda et al., 2003; Roozendaal et al., 2006c). The evidence implicating this pathway stems from an original finding by Liang et al. (1986) indicating that the infusions of the β-adrenoceptor antagonist propranolol into the basolateral complex of the amygdala (BLA) blocked the memory-enhancing effect of posttraining, systemic administration of epinephrine in rats. Many subsequent studies have supported this finding and have demonstrated that the glucocorticoid effects on memory also require β-adrenoceptor activation in the amygdala (Quirarte et al., 1997; Roozendaal et al., 2002, 2004, 2006a, 2006b, 2006c, 2007, 2008b).
A relatively unchallenged assumption in the field is that memory is supported by changes that occur in the brain at the level of the synapse. Molecular and cellular processes contribute to synaptic changes, which likely influence larger brain systems and the local circuitry. Whereas emotional memory-related synaptic changes are seen within the lateral nucleus of the amygdala (Rodrigues et al., 2004), there is also evidence indicating that the cellular processes in the amygdala influence cellular processes in efferent brain regions and, in so doing, may modulate synaptic plasticity in a distributed manner (McGaugh, 2004). Accordingly, amygdala modulation of synaptic plasticity in the hippocampus and cortex contributes to the contextual and sensory features of an emotionally arousing memory.
Packard et al. (1994) were among the first to identify the amygdala as a modulator of multiple memory systems. They observed that posttraining administration of amphetamine into the dorsal hippocampus or caudate nucleus enhanced the consolidation of a spatial or cued version of the water maze task, respectively. Posttraining infusions of amphetamine into the amygdala enhanced consolidation of both versions of the water maze task. Importantly, inactivation of the amygdala prior to testing 24 h after training did not affect the memory enhancement produced by posttraining intra-amygdala amphetamine treatment, indicating that the amygdala was not the locus of the memory trace but instead a modulator of memory consolidation that likely interacted with the hippocampus and caudate nucleus to exert its effects. Since this influential discovery, many have observed interactions of the amygdala with other brain regions that play a role in memory consolidation. These regions include, but are not limited to, the nucleus accumbens (Setlow et al., 2000), the entorhinal cortex (Roesler et al., 2002), the insular cortex (Miranda and McGaugh, 2004), the rostral anterior cingulate cortex (Malin et al., 2007), and the medial prefrontal cortex (Roozendaal et al., 2009). Given the evidence that the amygdala is not the locus of hippocampus- or caudate nucleus-dependent memory but can modulate its consolidation, most of this research considers the influence of the amygdala on efferent structures. However, a role for the amygdala in the modulation of processing in efferent brain regions does not exclude the possibility of a critical feedback or feed-forward modulation of the amygdala (Rodrigues et al., 2009; Roozendaal et al., 2009). The amygdala sends extensive projections to cortical and subcortical regions (McDonald and Jackson, 1987; Price, 2003); however, a monosynaptic connection is not a requirement of this model of amygdala modulation of synaptic plasticity.
In this review, research examining the influence of emotional arousal, stress hormones, and electrical or pharmacological manipulations of the BLA on synaptic plasticity in downstream brain regions is described. The preponderance of evidence for amygdala modulation of efferent plasticity comes from studies of the hippocampus. Research examining the influence of electrical or pharmacological stimulation of the amygdala on hippocampal long-term potentiation (LTP) and expression of synaptic plasticity-related proteins is discussed, with particular emphasis on the synaptic plasticity-related proteins activity-regulated cytoskeletal-associated protein (Arc) and calcium/calmodulin-dependent kinase II α (CaMKII α ). Both synaptic tagging and emotional tagging hypotheses are considered in light of evidence of amygdala modulation of synaptic plasticity-related proteins in the hippocampus. The aim of the proposed model is to expand the current thinking about the effects of emotional arousal on synaptic plasticity.
BLA influence on synaptic plasticity
Many of the mechanisms of LTP are shared with mechanisms required for memory. Just as memory formation can be separated into encoding and consolidation phases, LTP can be separated into induction and maintenance phases. The maintenance of LTP is comparable to the consolidation phase of memory in that both are protein synthesis dependent and both exhibit similar biochemical changes, such as changes in the phosphorylation state of the GluR1 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor (Davis and Squire, 1984; Kelleher et al., 2004; Whitlock et al., 2006). The study of LTP has facilitated our understanding of the influence of BLA activation on synaptic strength in the efferent regions of the brain, particularly the hippocampus. The nuclei of the BLA (lateral and basolateral) project directly to the hippocampus and also to the entorhinal cortex (Price, 2003). Stimulation of the BLA enhances, and lesions of the BLA impair, perforant path stimulation-induced LTP in the dentate gyrus (Ikegaya et al., 1994, 1996). The effect of BLA stimulation on dentate gyrus synapses can be replicated by the stimulation of the lateral perforant path, implicating the BLA-entorhinal cortex pathway in dentate gyrus plasticity (Nakao et al., 2004). The early phase of LTP (lasting ~4 h) can be converted to longer-lasting late-phase LTP with stimulation of the BLA (Frey et al., 2001). This late phase of LTP is dependent on protein kinase A activation (Huang et al., 1995; Abel et al., 1997), indicating a role for catecholamines. More specifically, bath application of norepinephrine (NE) facilitates CA1 LTP in a time-dependent manner (Hu et al., 2007). Furthermore, administration of a β-adrenoceptor agonist to dendrites of the stratum radiatum, paired with subthreshold stimulation of the Schaffer collaterals, promotes late-phase LTP. This effect remains when the dendrites are mechanically separated from the cell body layer, indicating that NE can modulate LTP through protein synthesis-dependent mechanisms that are independent of the soma (Gelinas and Nguyen, 2005). Noradrenergic activation in the BLA also plays a role in hippocampal LTP. Blockade of BLA β-adrenoceptors impairs perforant path stimulation-induced LTP in the dentate gyrus of the hippocampus (Ikegaya et al., 1997). In the intact animal, the stress response would promote locus coeruleus activation (Abercrombie and Jacobs, 1987) and increase release of NE in both the amygdala (McIntyre et al., 2002) and the hippocampus (Rosario and Abercrombie, 1999). Indeed, emotional arousal influences the maintenance of LTP. After induction in freely moving rats, LTP in the hippocampus can be reinforced with aversive or appetitive stimuli, and this reinforcement is dependent on β-adrenoceptors in the BLA as it is blocked with the β-adrenoceptor antagonist propranolol (Seidenbecher et al., 1997). Although NE application to the bath is sufficient to influence LTP in hippocampal slices, NE activation of β-adrenoceptors in the BLA appears to play a necessary role in the enhanced hippocampal plasticity that results from emotional arousal in the intact animal. Furthermore, lesions of the locus coeruleus or blockade of β-adrenoceptors in the medial septum block the BLA-influenced transformation of early to late-phase LTP in the dentate gyrus (Bergado et al., 2007), suggesting that systems, including the medial septum-hippocampus and the BLA, work in concert to enhance the storage of emotionally arousing events.
Synaptic tagging and emotional tagging
Over a decade ago, Frey and Morris (1997) identified ‘synaptic tagging’ as an explanation for the synapse specificity of LTP. The more recently proposed ‘ behavioral tagging’ and ‘emotional tagging’ hypotheses (Richter-Levin and Akirav, 2003; Ballarini et al., 2009) incorporated an element of memory specificity to the tagging concept. Synaptic tagging is based on the observation that the induction of LTP creates the potential for, but not commitment to, lasting changes in synaptic efficacy. This is similar to memory formation in that experiencing an event creates the potential for, but not commitment to, a long-lasting memory for the experience. The core components of synaptic tagging are (1) the setting of a local tag at a synaptic location where long-lasting changes may occur and (2) the synthesis of plasticity-related proteins that are captured by tagged synapses to produce lasting changes in synaptic strength (Redondo and Morris, 2011). The emotional tagging hypothesis is based on the observation that irrelevant information, which would normally be rapidly forgotten, is stored as long-term memory when associated with emotional arousal (Bergado et al., 2011). According to the emotional tagging hypothesis, the activation of the hippocampus by experience sets the local synaptic tag that is reinforced by emotional arousal, resulting in lasting synaptic modifications that support long-term memories. Given that emotional arousal increases NE in the amygdala, and the β-adrenoceptor activation in the BLA is critically involved in the memory-enhancing effects of stress and stress hormones on memory consolidation, the BLA is a likely participant in the emotional tagging process. Here, we discuss evidence indicating that the BLA influences the expression of plasticity-related proteins, promoting the maintenance of long-lasting synaptic modifications in the hippocampus.
BLA influence on expression of plasticity-related proteins in the hippocampus
Although hundreds of known plasticity-related proteins are expressed in the hippocampus, this review will focus on two proteins: CaMKIIα and Arc. These dendritically localized plasticity-related proteins share some similar characteristics. Cap-dependent initiation is a common mechanism triggering translation, requiring the formation of a eukaryotic initiation factor complex to bind ribosomal subunits to the 7-methyl-GTP cap structure at the 5′ end of the messenger RNA (mRNA; Klann and Dever, 2004). However, Arc and CaMKIIα also have internal ribosomal entry sites (IRES) that undergo translation initiation in a cap-independent manner, which may confer a translational advantage (Pinkstaff et al., 2001). The decreased availability and dephosphorylation of the cap-dependent initiation factor eIF4E can result in a switch from predominantly cap-dependent initiation to a cap-independent initiation of translation of mRNAs that have IRES sequences (Dyer et al., 2003; Svitkin et al., 2005). CaMKIIα and Arc protein appear specifically in stimulated regions of dendrites and in spines of hippocampal neurons (Steward et al., 1998; Mori et al., 2000; Moga et al., 2004; Lee et al., 2009). Knockout models have demonstrated that CaMKIIα and Arc are important for long-term memory formation and long-term plasticity in the hippocampus (Silva et al., 1992a,b; Plath et al., 2006). In fact, dendritic targeting of CaMKIIα mRNA is essential for synaptic plasticity and memory. A mutation of the CaMKIIα gene preventing dendritic localization and restricting CaMKIIα mRNA to the soma results in reduced late-phase LTP as well as impairments in spatial memory, associative fear conditioning, and object recognition memory (Miller et al., 2002). This result demonstrates the importance of local translation for plasticity and memory. Arc can also be translated in synapses. Both LTP-inducing stimuli and long-term depression (LTD)-inducing stimuli increase the expression of Arc protein in isolated synapses in vitro (Yin et al., 2002; Waung et al., 2008). These data suggest that there might be a group of dendritically localized plasticity-related genes whose local translation contributes to long-term plasticity and memory consolidation (Sutton and Schuman, 2006).
The translation of Arc mRNA appears to be an important event for hippocampal plasticity and memory. Arc protein expression is necessary in the dorsal hippocampus, the rostral anterior cingulate cortex, and the lateral amygdala for the optimal consolidation of long-term memory (McIntyre et al., 2005; Ploski et al., 2008; Holloway and McIntyre, 2011). Intrahippocampal infusions of anti-sense oligodeoxynucleotides block the expression of Arc and impair the maintenance of hippocampal late-phase LTP without affecting induction. This blockade of hippocampal Arc expression also impairs consolidation but not acquisition of spatial memory (Guzowski et al., 2000). These data, combined with the fact that the knockout of the Arc gene resulted in intact short-term memory but impaired long-term memory, suggest a role for Arc in the consolidation of synaptic plasticity and memory (Plath et al., 2006).
The role of Arc protein in modifying synaptic strength has been well reviewed by others (Bramham et al., 2008, 2010; Korb and Finkbeiner, 2011; Shepherd and Bear, 2011). Briefly, Arc is implicated in actin polymerization (Messaoudi et al., 2007) and AMPA receptor endoytosis (Chowdhury et al., 2006; Rial Verde et al., 2006). It is necessary for LTD (Waung et al., 2008) as well as for potentiation and appears to influence spine shape and density (Peebles et al., 2010). According to one view, Arc expression maintains synaptic homeostasis in a dynamic environment (Shepherd et al., 2006).
The BLA modulates the expression of Arc in the hippocampus. We found that memory-enhancing infusions of a β adrenoceptor agonist into the BLA after training on an aversive inhibitory avoidance task increased Arc protein expression in the hippocampus without influencing mRNA levels (McIntyre et al., 2005). These data suggest that the BLA modulates the synaptic plasticity-related protein Arc in the hippocampus through a post-transcriptional mechanism. Huff et al. (2006) reported that infusions of musicmol into the BLA following contextual fear conditioning attenuated training-induced increases in Arc protein expression in the hippocampus; however, this group observed a reduction in Arc mRNA as well. The apparent discrepancy in hippocampal Arc mRNA measurements across studies may indicate that the BLA can exert a transcriptional influence and a posttranscriptional influence on Arc expression in the hippocampus. Alternatively, our method for mRNA analysis (fluorescence in situ hybridization with densitometry) may not have been sensitive enough to detect a difference in mRNA levels. However, we did detect an increase in Arc mRNA as a result of training. In support of the evidence for a posttranscriptional influence of the BLA on hippocampal Arc expression, Ren et al. (2008) reported that the memory-impairing anesthetic proprofol decreased hippocampal Arc expression, but intra-BLA infusions of musicmol reversed the effect on Arc protein, but not mRNA, in the hippocampus. Using real-time PCR to detect Arc mRNA, this group found evidence in support of BLA modulation of hippocampal Arc expression through a posttranscriptional mechanism. Taken together with evidence of local translation of Arc in vitro, these findings suggest that the BLA may modulate long-term memory for the inhibitory avoidance task by a mechanism that involves posttranscriptional modulation of Arc in the hippocampus through an influence on local synthesis of Arc at the synapse. In support of this theory, the posttraining administration of a memory-enhancing dose of the stress hormone corticosterone increases Arc protein expression in hippocampal synaptoneurosomes and this effect is attenuated by the blockade of BLA β-adrenoceptors (McReynolds et al., 2010).
In recent years, our research has focused on understanding the limitations of this BLA-modulated effect. We have examined the influence of BLA β-adrenoceptors on Arc expression in areas beyond the hippocampus, including the medial prefrontal and rostral anterior cingulate cortex. We have also addressed the issue of whether the effect is limited to Arc expression that is associated with emotional arousal. Finally, we have begun to investigate other plasticity-related proteins, such as CaMKIIα. The results indicate that the posttraining activation of the β-adrenoceptors in the BLA increases both Arc and CaMKII α protein expression in the rostral anterior cingulate cortex (Holloway-Erickson et al., 2012). The influence of the BLA on Arc protein expression at the synapse is not limited to aversive training, as we have observed a similar increase in Arc protein expression in hippocampal synapses following an appetitive conditioned cue preference task (unpublished observations). Therefore, it appears that the BLA may contribute to the long-lasting synaptic changes that support memory by modulating plasticity-related proteins, such as Arc and CaMKIIα, at the synapses that are activated by that particular event.
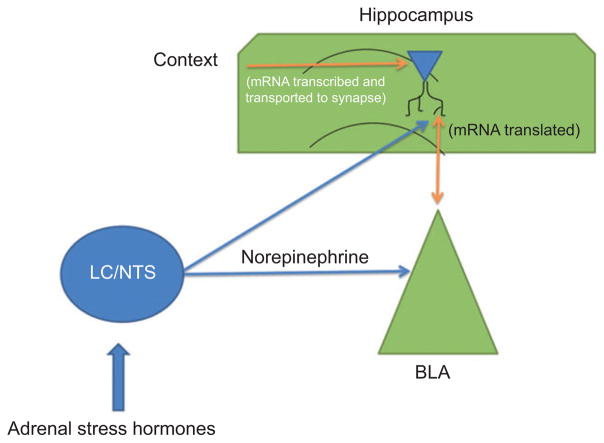
According to our working model, a novel context activates specific synapses in the hippocampus resulting in mRNA transport to those synapses. If this context exposure is coupled with emotional arousal, the noradrenergic activation of the BLA will influence the expression of plasticity-related proteins, such as Arc and CaMKIIα, at the synapse. This may occur through an influence on transport of the mRNA, local translation, or degradation of the protein. Figure 1 illustrates the proposed interaction of the stress hormones and the BLA on synaptic expression of Arc in the hippocampus.
Figure 1.
A novel context produces the transcription of Arc mRNA and the transport to stimulated synapses in the hippocampus. Stress enhances memory through noradrenergic actions on the BLA, which, in turn, influences the translation of the transcript that is already present in the appropriate synapses. Arrows indicate the connections between brain regions, but they are not necessarily monosynaptic.
Potential mechanisms for regulation of plasticity-related proteins
The BLA appears to influence Arc expression in the hippocampus in a posttranscriptional manner, although the mechanism of this effect is not known. Arc expression is tightly regulated and there are a multitude of different stages at which synaptic Arc expression in efferent brain regions may be influenced by the BLA. Here, some of the stages of the expression of Arc and other plasticity-related proteins that may be regulated by the BLA are addressed. The mechanisms of regulation of the transcript and the protein product of Arc have been reviewed extensively elsewhere (Korb and Finkbeiner, 2011; Shepherd and Bear, 2011). We will focus on the transport of dendritically localized mRNA to the synapse, the translation of the mRNA, and the degradation of the mRNA and protein.
Transport
Although it appears that the BLA does not influence Arc mRNA levels in the hippocampus, the influence of the BLA may result in increased availability of dendritically localized mRNAs at the synapse through an influence on their packaging and transport to the dendrites. The exact machinery involved in this transport has not been fully elucidated. However, many of the individual components involved in the packaging of the dendritically localized mRNAs into messenger ribonucleoprotein complexes (mRNPs) and the transport of those mRNPs out to the dendrites have been investigated. Arc and CaMKIIα mRNAs have a conserved dendritic targeting sequence called the A2 response element (A2RE). This cis-acting sequence is the target of a trans-acting RNA-binding protein, hnRNP-A2, which appears to be involved in packaging the mRNA into granules for transport to the dendrites (Gao et al., 2008). The A2RE sequence is sufficient for dendritic targeting, and the deletion of this sequence prevents the dendritic targeting of Arc, CaMKIIα, neurogranin, and MAP2 in vitro (Gao et al., 2008). In addition, there appears to be a competition for space within the mRNP, as the overexpression of one mRNA reduces the amount of the other mRNAs in the mRNP (Gao et al., 2008). Another trans-acting factor that seems to target the cis -acting A2RE sequence is CBF-A, which facilitates the dendritic transport and the localization of Arc, brain-derived neurotrophic factor (BDNF), and CaMKIIα (Raju et al., 2011). This suggests that there may be multiple components that target the same sequence to facilitate the packaging into mRNPs and the transport out to the dendrites.
Staufen-2 is an RNA-binding protein that is necessary for mRNPs to be trafficked out to the dendrites (Tang et al., 2001; Barbee et al., 2006; Johnson et al., 2006; Kim and Kim, 2006). In addition, Staufen-2 is regulated by extracellular signal-regulated kinase 1/2 (ERK 1/2), a component of the mitogen-activated protein kinase (MAPK) signaling pathway. Staufen-2 has a docking site for ERK 1/2 and the deletion of that site both reduces the amount of Staufen-2-containing mRNPs in the dendrites and completely abolishes the depolarization-induced increase in the amount of Staufen-2-containing mRNPs (Nam et al., 2008). Indeed, both actin polymerization and ERK phos-phorylation are required for Arc mRNA to be targeted to activated synaptic sites (Huang et al., 2007). This suggests that ERK phosphorylation may be one potential target of the BLA for the regulation of plasticity-related protein expression at the synapse. In fact, manipulations of the BLA influence the phosphorylated levels of ERK in memory-related brain regions such as the medial prefrontal cortex (Roozendaal et al., 2009). There are a number of different components in the mRNPs that mediate the transport and localization of the dendritically localized mRNAs and it remains to be seen whether these components can be influenced by the BLA.
Translation
It is well established that both LTP and long-term memory require de novo protein synthesis (Davis and Squire, 1984; Kelleher et al., 2004). The translation of proteins is composed generally of three main stages: initiation, elongation, and termination.
The initiation stage of protein translation is a complex process and a likely target for regulation (Klann and Dever, 2004). As discussed previously, BLA stimulation produces a protein synthesis- and NE-dependent effect on hippocampal LTP, converting it from early-phase to late-phase LTP. Arc and CaMKIIα mRNAs are transported to the stimulated regions of dendrites and can be translated in isolated synapses in vitro. Taken together with the evidence that activation of β-adrenoceptors in the BLA enhances memory and produces a posttranscriptional effect on Arc expression, and increases both Arc and CaMKIIα protein in synaptoneurosomes, these findings suggest that an influence on the initiation of local translation is a plausible mechanism for BLA modulation of memory consolidation. In support of this hypothesis, the same posttraining activation of β-adrenoceptors in the BLA that increases the expression of Arc and CaMKIIα does not appear to modulate the expression of the somatic activity-dependent protein c-Fos (McIntyre et al., 2005; Holloway-Erickson et al., 2012).
Here, we discuss two of a number of translation initiation factors that are involved in long-term plasticity and memory. The fragile X mental retardation protein (FMRP)-mediated repression of translation has been identified as a translational repressor for both Arc and CaMKIIα, and it plays a critical role in the metabotropic glutamate-mediated plasticity in the hippocampus (Huber et al., 2002; Zalfa et al., 2003). Another potential target for modulation is the eukaryotic initiation factors eIF2 α and eIF4E. The mechanisms for translational regulation in synaptic plasticity and memory have been reviewed extensively elsewhere (Klann and Dever, 2004; Costa-Mattioli et al., 2009; Richter and Klann, 2009). Finally, the role of the elongation factor, eukaryotic elongation factor 2 (eEF2), in the translation of plasticity-related proteins such as Arc is described.
Initiation
A potential influence on local translation is the removal of a translational repressor. There are a number of different RNA-binding proteins that are studied for their role in translational repression, but here we focus on FMRP. In purified synaptoneurosomes taken from mice lacking the Fmr1 gene, Arc and CaMKIIα protein expression is elevated and association of the mRNA with polyribosomes is increased, indicating active local translation (Zalfa et al., 2003). FMRP may repress translation by interacting with the noncoding neuronal RNA BC1. The noncoding neuronal RNA BC1 interacts with the initiation factor eIF4A and the poly(A)-binding protein to inhibit the formation of a complex important for translation initiation. Furthermore, BC1 may increase FMRP interaction with CYFIP1/ Sra-1, a recently identified neuronal eIF4E-binding protein (4E-BP), which would also prevent the formation of a complex important for initiation (Zalfa et al., 2003; Napoli et al., 2008). It remains to be seen whether manipulations of the BLA influence FMRP expression or its association with CaMKIIα, Arc, and other target mRNAs in memory-related efferent brain regions.
The initiation of translation is typically composed of three main stages: the formation of the 43S preinitiation complex, the binding of mRNA to the 43S preinitiation complex, and the formation of the 80S ribosomal complex (Klann and Dever, 2004). There are a number of different initiation factors that are involved in these processes, although two of the most commonly studied in plasticity and learning and memory are the eukaryotic initiation factors eIF2 α and eIF4E. The initiation factor eIF2 is involved in the formation of the 43S preinitiation complex and the phosphorylation at the serine51 site in the α-subunit prevents normal recycling of eIF2 and results in a decrease in general translation (Klann and Dever, 2004). The phosphorylation/dephosphorylation of this initiation factor is important for plasticity and memory. If the serine51 site is mutated and the phosphorylation is reduced, the threshold for eliciting LTP is reduced and the memory is enhanced. Conversely, if dephosphorylation of eIF2α is prevented by a small-molecule inhibitor, which would correspond to a decrease in general translation, LTP can be induced but not maintained and long-term memory is impaired (Costa-Mattioli et al., 2007). It is suggested that the phosphorylation/dephosphorylation of eIF2 α might serve as a bidirectional biochemical switch between short-term and long-term memory (Costa-Mattioli et al., 2007). Therefore, the phosphorylation and dephosphorylation of eIF2 α provide a possible target for a BLA influence on translational regulation in a manner that would influence synaptic plasticity and memory, although this effect may not be specific to the plasticity-related proteins.
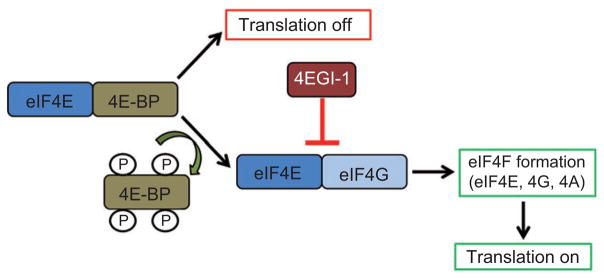
A cap-binding complex is required for cap-dependent translation and is thought to promote the binding of the mRNA to the 43S preinitiation complex to form a 48S complex (Klann and Dever, 2004). This cap-binding complex binds to a 7-methyl-GTP cap structure at the 5′ end of the mRNA and contains initiation factors such as eIF4E, eIF4G, and eIF4A, which together form the eIF4F complex (Klann and Dever, 2004). The eukaryotic initiation factor eIF4E is a target for translational regulation. The interaction between eIF4E and eIF4G is important for cap-dependent translation and learning and memory. A small-molecule inhibitor of cap-dependent translation 4EGI-1, which inhibits the interaction between eIF4E and eIF4G, given into the amygdala blocks the consolidation of fear memory (Hoeffer et al., 2011). This suggests that the cap-dependent initiation of translation is necessary for long-term memory formation. In addition, high-frequency stimulation, which induces LTP and Arc protein expression, increases the levels of phosphorylated eIF4E and eIF2α in an ERK-dependent manner (Panja et al., 2009). However, control of translation is also necessary for proper memory formation. The 4E-BPs are translational regulators that compete with eIF4G for binding of eIF4E and, when bound to eIF4E, prevent eIF4F complex formation. The failure of eIF4F complex formation would result in the inhibition of general protein synthesis. Figure 2 illustrates the influence of phosphorylated 4E-BPs on eIF4E formation and translation. When the 4E-BPs are hyper-phosphorylated, they no longer bind to eIF4E and the initiation factor is free to form the cap-binding complex, thus facilitating eIF4F formation (Klann and Dever, 2004). The controlled regulation of translation is necessary for proper memory formation and the 4E-BPs are important for that regulation. Mice that are lacking 4E-BP2, and should therefore have increased protein translation, show normal acquisition and short-term memory but impaired long-term memory for spatial and conditioned fear associative tasks. These findings suggest that unregulated increases in translation do not provide an advantage for memory consolidation. Rather, the controlled regulation of translation is necessary for long-term memory formation (Banko et al., 2005).
Figure 2. Translational machinery.
When the 4E-BPs are hyperphosphorylated, they no longer bind to eIF4E, leaving the initiation factor to form the eIF4F complex and promote cap-dependent initiation of translation. 4EGI-1 is a small molecule that inhibits cap-dependent translation by interfering with the interaction between eIF4E and eIF4G.
As discussed previously, the packaging and transport of mRNAs to dendrites involve ERK signaling. In addition, ERK signaling plays a role in the initiation of translation. The initiation factor eIF4E is phosphorylated by MAPK-interacting kinase (Mnk), which is a substrate of ERK 1/2 (Klann and Dever, 2004). It is suggested that the phosphorylation of this initiation factor is correlated with enhanced binding of eIF4E to capped mRNA (Minich et al., 1994). The pharmacological inhibition of Mnk1 blocks high-frequency stimulation-induced eIF4F formation and eIF4E phosphorylation (Panja et al., 2009). The pharmacological inhibition of Mnk1 also has a posttranscriptional effect on Arc expression as it blocks Arc translation without affecting Arc transcription (Panja et al., 2009). This is similar to the effect that is seen in the hippocampus following BLA manipulations. Cap-dependent translation can be influenced by neuromodulators as well. The stimuli that would only result in E-LTP, when paired with the bath application of a β-adrenoceptor agonist to hippocampal slices, produce L-LTP as well as the activation of eIF4E and Mnk1 and the inhibition of 4E-BP (Gelinas et al., 2007). These data suggest that the machinery involved in the cap-dependent initiation of translation may be involved in the BLA influence on expression of plasticity-related proteins at the synapse.
Elongation
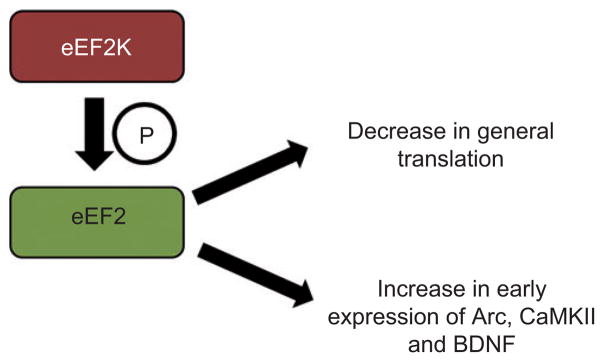
One target for regulation of the elongation stage of translation is eEF2. Figure 3 illustrates the impact of phosphorylation of this elongation factor on translation. When eEF2 is phosphorylated by eEF2 kinase (eEF2K), the elongation stage of translation is inhibited and protein translation is slowed (Ryazanov et al., 1988). The phosphorylation state of eEF2 is influenced by behavioral testing with dephosphorylation seen in the hippocampus and amygdala following a fear conditioning test (Im et al., 2009). Interestingly, although the phosphorylation of eEF2 slows down general translation, it increases the translation of some dendritically localized mRNAs such as Arc, CaMKIIα, and BDNF (Chotiner et al., 2003; Sutton et al., 2007; Park et al., 2008; Verpelli et al., 2010). The slowing down of general translation could increase the availability of initiation factors for mRNAs that might otherwise be poorly translated. In accordance with this supposition, general protein synthesis is decreased 1 h after high-frequency stimulation, but Arc protein expression and eEF2 phosphorylation are increased (Chotiner et al., 2003). Therefore, the BLA may influence a select group of plasticity-related proteins through an influence on eEF2 phosphorylation.
Figure 3. Elongation.
When the eukaryotic elongation factor, eEF2, is phosphorylated by the eEF2K, the elongation stage of translation is slowed and general protein synthesis is decreased. Although the general protein synthesis is decreased, the early expression of Arc, CaMKIIα, and BDNF is increased.
Degradation
Another way that expression of plasticity-related proteins may be increased at the synapse is through regulation of mRNA and protein degradation. After the stop codon, Arc and CaMKIIα have exon-exon junction complexes that allow the mRNA to undergo nonsense-mediated decay (NMD) after a single round of translation. These mRNAs are upregulated when a component of the exon-junction complex, eIF4AIII, or a component of the NMD machinery, Upf1, is blocked by RNA interference (Giorgi et al., 2007). Therefore, if the interaction of the mRNA with the exon-junction complex is impeded, or if the NMD machinery is interfered with, Arc mRNA would likely remain available for additional rounds of translation. This would be one way in which the processes within the BLA may influence the expression of Arc at a posttranscriptional level, although it is not known at this time if training on learning and memory tasks or manipulations of the BLA can influence components of the NMD process.
Protein degradation is just as critical to synaptic plasticity as protein synthesis. There is a balance between synthesis and degradation, as the blockade of either synthesis or degradation results in impaired L-LTP but not if both synthesis and degradation are blocked (Fonseca et al., 2006). There is evidence for local degradation of proteins as components of the ubiquitin-proteasome system, such as ubiquitin and proteasome subunits and associated enzymes, are found at synapses and at the postsynaptic density (Bingol and Schuman, 2005). Furthermore, synaptic stimulation results in an N-methyl-D-aspartate receptor-dependent redistribution of proteasomes from dendritic shafts into synaptic spines (Bingol and Schuman, 2005). Recently, the E3 ligase for Arc was identified. Ube3a is an ubiquitination ligase whose loss is implicated in Angel-man syndrome and whose expression is activity dependent (Greer et al., 2010). Arc contains a Ube3a-binding domain and undergoes activity-dependent ubiquitination by Ube3A. Mice lacking Ube3A show greater Arc protein expression in response to kainic acid or an enriched environment, and this effect was greatest for synaptic Arc protein expression levels. Because some of the changes were only seen with 6 h of treatment, it has been suggested that Ube3A serves a role of returning Arc to baseline levels following activity and, consequently, controlling AMPA receptor endocytosis (Greer et al., 2010; Korb and Finkbeiner, 2011). Whether learning and memory events change the levels of Ube3A or its association with Arc remains an intriguing area of study.
Concluding remarks
Although the role of the BLA in the modulation of emotional memory consolidation and the regulation of local expression of plasticity-related proteins such as Arc have been extensively studied separately, little is known about the exact mechanisms of the BLA influence on synaptic plasticity and the expression of plasticity-related proteins in efferent brain regions. The activation of the BLA does not appear to simply produce a general increase in neuronal activity in these efferent brain regions as β-adrenoceptor activation increases the expression of plasticity-related proteins, Arc and CaMKIIα, but not a common neuronal activity marker, c-Fos, in the anterior cingulate cortex (Holloway-Erickson et al., 2012). These findings suggest that the BLA preferentially influences the dendritically localized, synaptic plasticity-related proteins. An influence of the BLA on plasticity-related protein expression has been observed in efferent brain regions such as the hippocampus, the prefrontal cortex, and the rostral anterior cingulate cortex. How the BLA is able to exert its effects remains to be determined. One possibility is that a coincidence of the stimuli-induced ‘ synaptic tag ’ together with the modulatory effect produced by an emotional event and mediated by the BLA is required for the increase in plasticity-related protein expression at the synapse for the conversion of short-term plasticity and memory into long-term plasticity and memory. These two separate events must converge somewhere inside the cell through intracellular signaling pathways to elicit increases in specific sets of proteins. Future research aimed at determining where this convergence occurs may elucidate the mechanisms underlying the rapid and long-lasting storage of emotionally arousing memories.
Biographies
 Jayme R. McReynolds completed her Bachelor of Science degree at the University of California, Irvine, where she examined stress effects on learning and memory in the laboratory of Dr. James L. McGaugh. She went on to complete her PhD in cognition and neuroscience at the University of Texas at Dallas under the guidance of Dr. Christa McIntyre. Her graduate research was focused on the role of the basolateral complex of the amygdala in modulating emotionally arousing memory and expression of plasticity-related proteins in efferent brain regions. She is now a postdoctoral fellow in Dr. John Mantsch’s laboratory at Marquette University studying the influence of stress on addictive behavior.
Jayme R. McReynolds completed her Bachelor of Science degree at the University of California, Irvine, where she examined stress effects on learning and memory in the laboratory of Dr. James L. McGaugh. She went on to complete her PhD in cognition and neuroscience at the University of Texas at Dallas under the guidance of Dr. Christa McIntyre. Her graduate research was focused on the role of the basolateral complex of the amygdala in modulating emotionally arousing memory and expression of plasticity-related proteins in efferent brain regions. She is now a postdoctoral fellow in Dr. John Mantsch’s laboratory at Marquette University studying the influence of stress on addictive behavior.
 Christa K. McIntyre is an Assistant Professor of Cognition and Neuroscience in the School of Behavioral and Brain Sciences at the University of Texas at Dallas. She earned her PhD at the University of Virginia under the mentorship of Dr. Paul Gold and did her postdoctoral work with Dr. James L. McGaugh at the University of California, Irvine. Her research examines the neurobiology of memory consolidation, with a focus on the influence of emotional arousal on memory and synaptic plasticity. Together with her graduate students, Christa McIntyre studies the effects of the autonomic nervous system on interacting brain systems with the aim of understanding how events of a single emotionally arousing experience are stored as long-term memories, whereas memories of nonarousing events are lost forever.
Christa K. McIntyre is an Assistant Professor of Cognition and Neuroscience in the School of Behavioral and Brain Sciences at the University of Texas at Dallas. She earned her PhD at the University of Virginia under the mentorship of Dr. Paul Gold and did her postdoctoral work with Dr. James L. McGaugh at the University of California, Irvine. Her research examines the neurobiology of memory consolidation, with a focus on the influence of emotional arousal on memory and synaptic plasticity. Together with her graduate students, Christa McIntyre studies the effects of the autonomic nervous system on interacting brain systems with the aim of understanding how events of a single emotionally arousing experience are stored as long-term memories, whereas memories of nonarousing events are lost forever.
Contributor Information
Jayme R. McReynolds, Department of Behavioral and Brain Sciences, The University of Texas at Dallas, 800 West Campbell Road, Richardson, TX 75080, USA
Christa K. McIntyre, Department of Behavioral and Brain Sciences, The University of Texas at Dallas, 800 West Campbell Road, Richardson, TX 75080, USA.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987;7:2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci USA. 2009;106:14599–14604. doi: 10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado JA, Frey S, López J, Almaguer-Melian W, Frey JU. Cholinergic afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. Neurobiol Learn Mem. 2007;88:331–341. doi: 10.1016/j.nlm.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Bergado JA, Lucas M, Richter-Levin G. Emotional tagging – a simple hypothesis in a complex reality. Prog Neurobiol. 2011;94:64–76. doi: 10.1016/j.pneurobio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Bingol B, Schuman EM. Synaptic protein degradation by the ubiquitin proteasome system. Curr Opin Neurobiol. 2005;15:536–541. doi: 10.1016/j.conb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. The neurobiology of memory for emotional events: adrenergic activation and the amygdala. Proc West Pharmacol Soc. 1996;39:81–84. [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiner JK, Khorasani H, Nairn AC, O’Dell TJ, Watson JB. Adenylyl cyclase-dependent form of chemical long-term potentiation triggers translational regulation at the elongation step. Neuroscience. 2003;116:743–752. doi: 10.1016/s0306-4522(02)00797-2. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/ Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, et al. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Dyer JR, Michel S, Lee W, Castellucci VF, Wayne NL, Sossin WS. An activity-dependent switch to cap-independent translation triggered by eIF4E dephospho-rylation. Nat Neurosci. 2003;6:219–220. doi: 10.1038/nn1018. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol Learn Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α1-adrenoceptors. J Neurosci. 1999a;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Role of norepi-nephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biol Psychiatry. 1999b;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Tatavarty V, Korza G, Levin MK, Carson JH. Multiplexed dendritic targeting of α calcium calmodulin-dependent protein kinase II, neurogranin, and activity-regulated cytoskeleton-associated protein RNAs by the A2 pathway. Mol Biol Cell. 2008;19:2311–2327. doi: 10.1091/mbc.E07-09-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. β-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple β-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk RB, McGaugh JL. Effects of hormones on time-dependent memory storage processes. Prog Brain Res. 1975;42:210–211. doi: 10.1016/s0079-6123(08)63665-1. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, et al. The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, et al. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc Natl Acad Sci USA. 2011;108:3383–3388. doi: 10.1073/pnas.1013063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway CM, McIntyre CK. Post-training disruption of Arc protein expression in the anterior cingulate cortex impairs long-term memory for inhibitory avoidance training. Neurobiol Learn Mem. 2011;95:425–432. doi: 10.1016/j.nlm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Holloway-Erickson CM, McReynolds JR, McIntyre CK. Memory-enhancing intra-basolateral amygdala infusions of clenbuterol increase Arc and CaMKIIα protein expression in the rostral anterior cingulate cortex. Front Behav Neurosci. 2012;6:17. doi: 10.3389/fnbeh.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchouladze R. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci. 2007;27:9054–9067. doi: 10.1523/JNEUROSCI.2410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. Attenuated hippocampal long-term potentiation in basolateral amygdala-lesioned rats. Brain Res. 1994;656:157–164. doi: 10.1016/0006-8993(94)91377-3. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. Dentate gyrus field potentials evoked by stimulation of the basolateral amygdaloid nucleus in anesthetized rats. Brain Res. 1996;718:53–60. doi: 10.1016/0006-8993(95)01465-9. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Nakanishi K, Saito H, Abe K. Amygdala β-noradrenergic influence on hippocampal long-term potentiation in vivo. NeuroReport. 1997;8:3143–3146. doi: 10.1097/00001756-199709290-00027. [DOI] [PubMed] [Google Scholar]
- Im HI, Nakajima A, Gong B, Xiong X, Mamiya T, Gershon ES, Zhuo M, Tang YP. Post-training dephosphorylation of eEF-2 promotes protein synthesis for memory consolidation. PLoS ONE. 2009;4:e7424. doi: 10.1371/journal.pone.0007424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EM, Kinoshita Y, Weinreb DB, Wortman MJ, Simon R, Khalili K, Winckler B, Gordon J. Role of Pur α in targeting mRNA to sites of translation in hippocampal neuronal dendrites. J Neurosci Res. 2006;83:929–943. doi: 10.1002/jnr.20806. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kim KC, Kim HK. Role of staufen in dendritic mRNA transport and its modulation. Neurosci Lett. 2006;397:48–52. doi: 10.1016/j.neulet.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011;34:591–598. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci. 2006;120:217–223. doi: 10.1037/0735-7044.120.1.217. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol Learn Mem. 2007;87:295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci USA. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem. 2010;93:312–321. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Minich WB, Balasta ML, Goss DJ, Rhoads RE. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc Natl Acad Sci USA. 1994;91:7668–7672. doi: 10.1073/pnas.91.16.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, McGaugh JL. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: involvement of the basolateral amygdala. Learn Mem. 2004;11:312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, LaLumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. Eur J Neurosci. 2003;18:2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Mori Y, Imaizumi K, Katayama T, Yoneda T, Tohyama M. Two cis-acting elements in the 3′ untranslated region of α-CaMKII regulate its dendritic targeting. Nat Neurosci. 2000;3:1079–1084. doi: 10.1038/80591. [DOI] [PubMed] [Google Scholar]
- Nakao K, Matsuyama K, Matsuki N, Ikegaya Y. Amygdala stimulation modulates hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004;101:14270–14275. doi: 10.1073/pnas.0405709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam YJ, Cheon HS, Choi YK, Kim SY, Shin EY, Kim EG, Kim HK. Role of mitogen-activated protein kinase (MAPK) docking sites on Staufen2 protein in dendritic mRNA transport. Biochem Biophys Res Commun. 2008;372:525–529. doi: 10.1016/j.bbrc.2008.05.047. [DOI] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja D, Dagyte G, Bidinosti M, Wibrand K, Kristiansen AM, Sonenberg N, Bramham CR. Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J Biol Chem. 2009;284:31498–31511. doi: 10.1074/jbc.M109.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/ Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles CL, Yoo J, Thwin MT, Palop JJ, Noebels JL, Finkbeiner S. Arc regulates spine morphology and maintains network stability in vivo. Proc Natl Acad Sci USA. 2010;107:18173–18178. doi: 10.1073/pnas.1006546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkstaff JK, Chappell SA, Mauro VP, Edelman GM, Krushel LA. Internal initiation of translation of five dendritically localized neuronal mRNAs. Proc Natl Acad Sci USA. 2001;98:2770–2775. doi: 10.1073/pnas.051623398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of Pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Ann NY Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Raju CS, Fukuda N, López-Iglesias C, Göritz C, Visa N, Percipalle P. In neurons, activity-dependent association of dendritically transported mRNA transcripts with the transacting factor CBF-A is mediated by A2RE/RTS elements. Mol Biol Cell. 2011;22:1864–1877. doi: 10.1091/mbc.E10-11-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhang FJ, Xue QS, Zhao X, Yu BW. Bilateral inhibition of γ-aminobutyric acid type A receptor function within the basolateral amygdala blocked propofol-induced amnesia and activity-regulated cytoskeletal protein expression inhibition in the hippocampus. Anesthesiology. 2008;109:775–781. doi: 10.1097/ALN.0b013e31818a37c4. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. Emotional tagging of memory formation—in the search for neural mechanisms. Brain Res Brain Res Rev. 2003;43:247–256. doi: 10.1016/j.brainresrev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Roesler R, Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of 8-Br-cAMP infused into the entorhinal cortex of rats after training. Eur J Neurosci. 2002;15:905–910. doi: 10.1046/j.1460-9568.2002.01924.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor-cAMP/cAMP/PKA system in influencing memory consolidation. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered β-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004;81:150–154. doi: 10.1016/j.nlm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem. 2006a;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006b;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 2006c;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Lengvilas R, McGaugh JL, Civelli O, Reinscheid RK. Orphanin FQ/nociceptin interacts with the basolateral amygdala noradrenergic system in memory consolidation. Learn Mem. 2007;14:29–35. doi: 10.1101/lm.403607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008a;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the β-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci. 2008b;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: correlation with stress-induced norepinephrine efflux in the hippocampus of Sprague-Dawley rats. Brain Res Bull. 1999;48:595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D. A post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci USA. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. Eur J Neurosci. 2000;12:367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14:279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science. 1992a;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in a-calcium-calmodulin kinase II mutant mice. Science. 1992b;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Svitkin YV, Herdy B, Costa-Mattioli M, Gingras AC, Raught B, Sonenberg N. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol Cell Biol. 2005;25:10556–10565. doi: 10.1128/MCB.25.23.10556-10565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Meulemans D, Vazquez L, Colaco N, Schuman E. A role for a rat homolog of staufen in the transport of RNA to neuronal dendrites. Neuron. 2001;32:463–475. doi: 10.1016/s0896-6273(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Piccoli G, Zanchi A, Gardoni F, Huang K, Brambilla D, Di Luca M, Battaglioli E, Sala C. Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. J Neurosci. 2010;30:5830–5842. doi: 10.1523/JNEUROSCI.0119-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]