Abstract
IL-1RA has been used intra-cerebrally to ameliorate neuroinflammatory responses. The present study explored the possibility that the bioactivity of IL-1RA administered intra-cerebrally may be prolonged in the CNS. hIL-1RA was detected in hippocampus from 2 h to 14 d post-ICM treatment. hIL-1RA ameliorated both the hippocampal cytokine (TNFα and NFκBIα:) and sickness response to peripheral LPS administered 4 d after hIL-1RA. Four days post treatment, hIL-1RA reduced the basal expression of IL-1R1, Iba-1, MHCII, and TLR4 and blunted the microglial IL-1β and IL-6 response to LPS ex vivo. IL-1RA might be administered prophylactically to prevent the neuroinflammatory effects of trauma.
Keywords: Pro-inflammatory, Microglia, Macrophage, Hippocampus
1. Introduction
Interleukin-1 receptor antagonist (IL-1RA) functions as a competitive inhibitor of IL-1α and IL-1β binding to the IL-1 type 1 receptor with an affinity equal to IL-1β without any agonist activity (Arend et al., 1990; Dripps et al., 1991; Hannum et al., 1990). In doing so, IL-1RA prevents IL-1 activation of pro-inflammatory signal transduction proteins such as NFκB and AP-1 (Dinarello, 1997) and subsequent pro-inflammatory immune responses (Weber et al., 2011).
Intracerebral administration of IL-1RA has been shown to reliably block the induction of sickness responses by IL-1β or LPS (Bluthe et al., 1992; Cartmell et al., 1999; Kent et al., 1992; Luheshi et al., 1996; Opp and Krueger, 1991). In these experiments the proinflammatory stimulus was administered concurrently with, or immediately before, the intracerebral IL-1RA treatment, and proinflammatory effects were measured within hours of treatment. Also, intracerebral administration of IL-1RA attenuates aging-induced sensitization of neuroinflammatory and sickness responses to LPS (Abraham and Johnson, 2009). Interestingly, Abraham and Johnson (2009) found that exogenous IL-1RA exerted anti-inflammatory effects in aged mice 24 h post-IL-1RA administration. This finding is particularly noteworthy because the duration of IL-1RA effects on neuroinflammation (24 h) far exceeded the plasma half-life (<2 h) of IL-1RA. Plasma IL-1RA levels decline rapidly after intra-venous infusion and exhibit an initial half-life <30 min and a terminal half-life <2 h (Granowitz et al., 1992). Notably, systemic administration of IL-1RA results in detectable levels in brain and CSF up to 18 h post-injection and was neuroprotective against cerebral ischemia (Cawthorne et al., 2011; Greenhalgh et al., 2010). We conducted a study in which a single dose of IL-1RA was administered intra-cisterna magna (ICM) in aged rats concurrent with a peripheral administration of Escherichia coli. E. coli given to aging rats typically produces impairments in memory of new learning occurring 4 d later, as well as producing persistent neuroinflammation still present at 4 d. Four days after IL-1RA and E. coli administration, IL-1RA treatment blocked these memory deficits and neuroinflammatory effects (Frank et al., 2010). One possible explanation for the prolonged anti-inflammatory effects of IL-1RA administration in the CNS is that IL-1RA bioactivity may be extended in the CNS relative to the short duration of IL-1RA action after peripheral administration. The present study explored this possibility by examining the duration of IL-1RA effects on subsequent LPS-induced neuroinflammatory processes and sickness responses after a single ICM injection of IL-1RA.
2. Materials and methods
2.1. Rats
Male Sprague–Dawley rats (60–90 d old; Harlan Sprague–Dawley, Inc., Indianapolis, IN, USA) were pair-housed with food and water available ad libitum. The colony was maintained at 22–23 °C on a 12 h light/dark cycle (lights on at 07:00 h). All experimental procedures were conducted in accordance with protocols approved by the University of Colorado Institutional Animal Care and Use Committee.
2.2. Drugs
Recombinant human IL-1RA (hIL-1RA) protein was obtained from Amgen (Thousand Oaks, CA). Lipopolysaccharide (LPS; E. coli serotype 0111:B4) was obtained from Sigma (St. Louis, MO).
2.3. Experimental designs
2.3.1. Experiment 1. Duration of hIL-1RA in the hippocampus after a single intra-cisterna magna (ICM) injection
hIL-1RA protein was assayed in the hippocampus 2 h, and 1, 2, 4 and 14 d post-ICM treatment with hIL-1RA (100 μg). A separate group of rats was injected with vehicle (0.9% saline) ICM and hIL-1RA measured 2 h post-injection. This experimental group was included to assess whether the anti-hIL-1RA antibody utilized in the ELISA assay cross-reacts with rat IL-1RA.
2.3.2. Experiment 2. Effect of hIL-1RA on LPS-induced hippocampal pro-inflammatory cytokines
Vehicle (0.9% saline) or LPS (100 μg/kg) was administered i.p. 4 d post-ICM injection of either vehicle (0.9% saline) or hIL-1RA (100 μg) and the hippocampal neuroinflammatory response measured 2 h post-LPS or vehicle treatment. It should be noted that a vehicle i.p./hIL-1RA ICM group was not included in this design as we have previously found that hIL-1RA injected ICM does not significantly alter hippocampal pro-inflammatory cytokine expression in rats treated i.p. with vehicle (Frank et al., 2010).
2.3.3. Experiment 3. Effect of hIL-1RA on the LPS-induced sickness response
Vehicle (0.9% saline) or LPS (100 μg/kg) was administered i.p. 4 d or 14 d post-ICM injection of either vehicle (0.9% saline) or hIL-1RA (100 μg). Baseline social exploration was measured 24 h prior to ICM treatment (baseline test 1) and 24 h prior to LPS/vehicle treatment (baseline test 2). Experimental groups were matched for baseline social exploration levels. At 2, 6 and 24 h post-LPS/vehicle treatment, social exploration was measured.
2.3.4. Experiment 4. Effect of hIL-1RA on hippocampal macrophage/microglial and astroglial activation markers
Four days post-ICM injection of either vehicle (0.9% saline) or hIL-1RA (100 μg), gene expression of macrophage and glial antigens was measured in the hippocampus.
2.3.5. Experiment 5. Effect of hIL-1RA on the microglial pro-inflammatory response to LPS ex vivo
Four days post-ICM injection of vehicle (0.9% saline) or hIL-1RA (100 μg), hippocampal microglia were isolated, exposed to LPS ex vivo for 2 h and the pro-inflammatory cytokine response measured.
2.4. ICM administration of hIL-1RA protein
Rats were briefly anesthetized (<2 min) with halothane. The dorsal aspect of the skull was shaved and swabbed with 70% ETOH. A 27-gauge needle attached via PE50 tubing to a 25 μl Hamilton syringe was inserted into the cisterna magna. To verify entry into the cisterna magna, ∼2 μl of CSF was drawn. In all cases, CSF was clear of red blood cells indicating entry into the cisterna magna. Vehicle (0.9% saline) or hIL-1RA (100 μg) was administered in 3 μl total volume.
2.5. Euthanasia and tissue collection
Rats were given a lethal dose of sodium pentobarbital and transcardially perfused with ice-cold saline (0.9%) for 3 min. The hippocampus was rapidly dissected, flash frozen in liquid nitrogen, and stored at − 80 °C. For the microglia ex vivo study, hippocampus was rapidly dissected and microglia immediately isolated.
2.6. hIL-1RA ELISA
hIL-1RA protein was measured using a commercially available ELISA kit (R & D Systems, Minneapolis, MN). The hippocampus was sonicated using a tissue extraction reagent (Invitrogen, Carlsbad, CA) supplemented with a protease inhibitor cocktail (Sigma). Ho-mogenate was centrifuged (10 min, 14,000×g, 4 °C) and supernatant collected and stored at − 20 °C until assayed. Total protein levels were quantified using a Bradford assay. hIL-1RA protein was assayed according to the manufacturers' protocol. Of note, the IL-1RA ELISA utilized here specifically detects human IL-1RA and recognizes both the natural and recombinant forms of hIL-1RA. The manufacturer certifies that no significant cross-reactivity for rat IL-1RA was observed with this hIL-1RA assay. hIL-1RA data are presented as pg/mg total protein.
2.7. Real time RT-PCR
Total RNA was isolated from whole hippocampus utilizing a standard method of phenol:chloroform extraction (Chomczynski and Sacchi, 1987). For detailed descriptions of RNA isolation, cDNA synthesis, and PCR amplification protocols refer to prior publication (Frank et al., 2006). cDNA sequences were obtained from GenBank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Genomic sequence accession numbers are listed after each gene symbol. Measurement of gene expression included the pro-inflammatory cytokines Il1b (NC_055102), Il6 (NC_005103) and Tnf (NC_005119); cytokine signaling molecules IL-1 Type 1 receptor (Il1r1, NC_005108) and NFκB inhibitor α (Nfkbia, NC_005105); the macrophage/microglial antigens Cd163 (NC_005103) CD200 receptor 1 (Cd200r1, NC_005110), Ionized Calcium Binding Adapter Protein (Aif1, NC_005119, the alias Iba-1 is used in text), Major Histocompatibility Complex II Antigen RT1-D alpha (RT1Da, NC_005119, alias MHCII is used in text), Toll-Like Receptor 2 (Tlr2, NC_005101) and Tlr4 (NC_005104); the astrocyte antigen Glial Fibrillary Acidic Protein (Gfap, NC_005109); and the housekeeping gene beta-actin (Actb, NC_005111.2). Primer sequences were designed using the Operon Oligo Analysis Tool (http://www.operon.com/technical/toolkit.aspx) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul et al., 1997). Primers were obtained from Invitrogen. Primer specificity was verified by melt curve analyses. All primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA Primer sequences are presented in Table 1. PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA). Formation of PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA). Relative gene expression was determined by taking the expression ratio of the gene of interest to β-Actin.
Table 1.
Primer Sequences.
| Gene | Primer sequence 5′ → 3′ | Function |
|---|---|---|
| β-Actin | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein(housekeeping gene) |
| CD163 | F: GTAGTAGTCATTCAACCCTCAC R: CGGCTTACAGTTTCCTCAAG |
Macrophage specific antigen |
| CD200R | F: TAGAGGGGGTGACCAATTAT R: TACATTTTCTGCAGCCACTG |
Microglia/macrophage antigen |
| GFAP | F: AGATCCGAGAAACCAGCCTG R: CCTTAATGACCTCGCCATCC |
Astrocyte antigen |
| IL-1β | F: CCTTGTGCAAGTGTCTGAAG R: GGGCTTGGAAGCAATCCTTA |
Pro-inflammatory cytokine |
| IL-1R1 | F: TAGATGACAGCAAGAGGGA R: ACTTCCAGTAGACAAGGTC |
Receptor mediating IL-1β pro-inflammatory signaling |
| IL-6 | F: AGAAAAGAGTTGTGCAATGGCA R: GGCAAATTTCCTGGTTATATCC |
Pro-inflammatory cytokine |
| Iba-1 | F: GGCAATGGAGATATCGATAT R: AGAATCATTCTCAAGATGGC |
Microglia/macrophage antigen |
| MHCII | F: AGCACTGGGAGTTTGAAGAG R: AAGCCATCACCTCCTGGTAT |
Microglia/macrophage antigen |
| NFκBIα | F: CACCAACTACAACGGCCACA R: GCTCCTGAGCGTTGACATCA |
Induced by NFκB to inhibit NFκB function |
| TNFα | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
Pro-inflammatory cytokine |
| TLR2 | F: TGGAGGTCTCCAGGTCAAATC R: ACAGAGATGCCTGGGCAGAAT |
Pattern recognition receptor for motifs of gram-positive bacteria |
| TLR4 | F: TCCCTGCATAGAGGTACTTC R: CACACCTGGATAAATCCAGC |
Pattern recognition receptor for motifs of gram-negative bacteria |
2.8. Social exploration test
LPS-induced sickness behavior was assessed by measuring decreases in duration of social exploration of a conspecific juvenile (Bluthe et al., 1999). Each experimental subject was allocated a separate plastic tub cage with shaved wood bedding, food and a wire lid. Subjects were transferred to a dimly lit room (40 lx) and allowed to habituate to the novel environment for 1 h prior to testing. After 1 h, a 28–32-day-old juvenile was introduced to the cage with the test subject for 3 min and the amount of time spent by the experimental rat socially exploring (ano-genital sniffing, body sniffing, pinning, and allo-grooming) the juvenile was measured. Exploratory behaviors were timed by an observer blind to treatment condition.
2.9. Ex vivo immune stimulation of hippocampal microglia with LPS
Hippocampal microglia were isolated using a Percoll density gradient as previously described (Frank et al., 2006). Microglia were suspended in DMEM+10% FBS and microglia concentration determined by trypan blue exclusion. Microglia concentration was adjusted to a density of 5×103 cells/100 μl and 100 μl added to individual wells of a 96-well v-bottom plate. Cells were incubated with LPS (0.1, 1, 10, and 100 ng/ml) or media alone for 2 h at 37 °C, 5% CO2. The plate was centrifuged at 1000×g for 10 min at 4 °C to pellet cells and cells washed 1× in ice cold PBS and centrifuged at 1000×g for 10 min at 4 °C. Cell lysis/homogenization and cDNA synthesis were performed according to the manufacturer's protocol using the SuperScript III CellsDirect cDNA Synthesis System (Invitrogen).
2.10. Statistical analysis and data presentation
All data are presented as mean±SEM. Statistical analyses consisted of ANOVA followed by post-hoc tests (Newman–Keuls) using Prism 5 (Graphpad Software, Inc., La Jolla, CA). Threshold for statistical significance was set at α=.05. Sample sizes are presented in figure legends.
3. Results
3.1. Experiment 1. Duration of hIL-1RA in the hippocampus after a single ICM injection
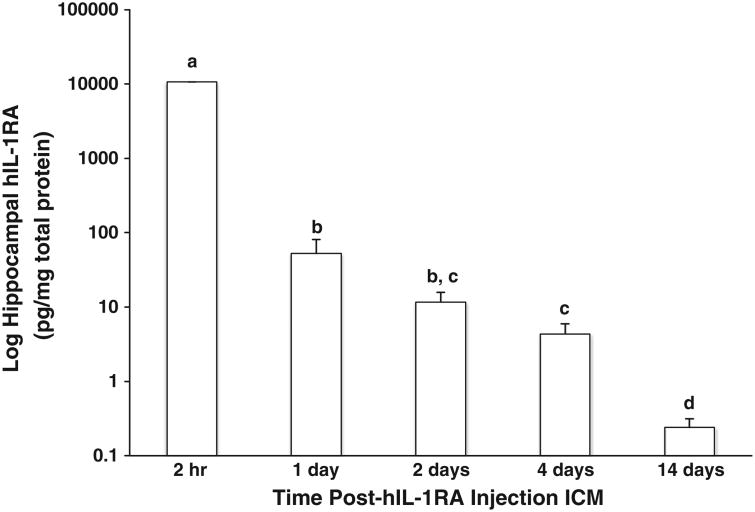
As shown in Fig. 1, hIL-1RA was detected in the hippocampus at all time-points post-ICM injection of hIL-1RA. hIL-1RA levels in the hippocampus significantly declined from 2 h to 14 d post-ICM injection (df=4, 18, F=2.3×10ˆ5, p<.0001). Simple effects analysis is provided in Fig. 1. hIL-1RA was not detected in the hippocampus of vehicle treated rats 2 h post-treatment (data not shown). The lack of hIL-1RA detection in vehicle treated rats validated the lack of cross-reactivity with rat IL-1RA, a finding consistent with the ELISA manufacturer's certification. To determine whether hIL-1RA injected ICM enters peripheral compartments, hIL-1RA was measured in serum and liver 2 h and 24 h post-ICM injection of hIL-1RA. At 2 h post-injection, hIL-1RA was detected in serum (5.01 ×10ˆ4 pg/ml) and liver (4.35×10ˆ3 pg/ml). However, at 24 h post-injection, hIL-1RA was not detectable in serum or liver (data not shown).
Fig. 1.
Duration of hIL-1RA in the hippocampus after a single ICM injection. hIL-1RA protein was assayed in the hippocampus 2 h, 1 d, 2 d, 4 d and 14 d post-ICM treatment with hIL-1RA (100 μg). Means with different letter designations (a, b, c, or d) are significantly different (p<.05) from each other. Data are presented as the mean±SEM.
3.2. Experiment 2. Effect of hIL-1RA on LPS-induced hippocampal pro-inflammatory cytokines
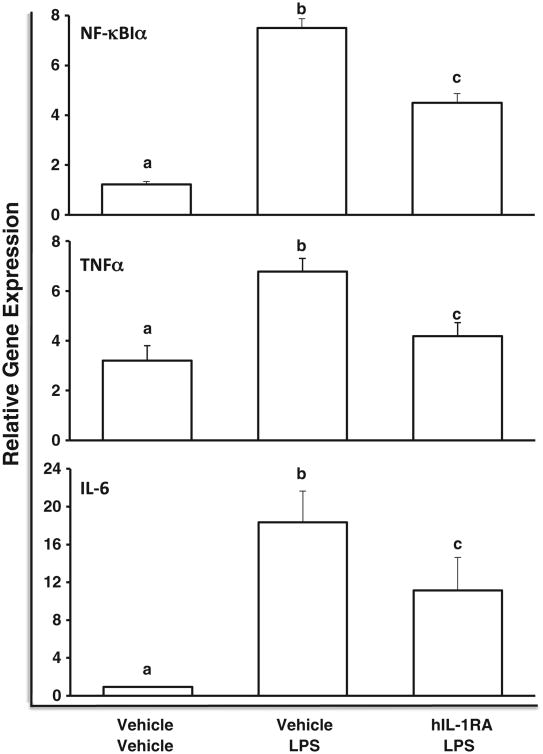
In rats treated ICM with hIL-1RA, hIL-1RA was detectable in the hippocampus at levels (mean = 2.58 pg/mg total protein) comparable to those observed in the time-course experiment and hIL-1RA was not detected in the hippocampus of any rats treated with vehicle ICM (data not shown). hIL-1RA blunted to varying degrees the LPS induced increase in several hippocampal pro-inflammatory mediators (Fig. 2). hIL-1RA significantly blunted the LPS-induced increase in hippocampal TNFα (df=2, 9, F=7.5, p<.01). In vehicle ICM treated rats, LPS induced a significant increase in TNFα compared to vehicle i.p./vehicle ICM rats (p<.05). hIL-1RA treatment significantly decreased LPS-induced TNFα expression compared to LPS i.p./vehicle ICM rats (p<.05).TNFα levels did not differ between vehicle i.p./vehicle ICM rats and LPS i.p./IL-1RA ICM rats. Similarly, hIL-1RA significantly blunted the LPS-induced increase in hippocampal NF-κBIα (df=2, 9, F = 51.33, p<.0001). In vehicle ICM treated rats, LPS induced a significant increase in NF-κBIα compared to vehicle i.p./vehicle ICM rats (p<.05), while hIL-1RA treatment significantly reduced LPS-induced NF-κBIα expression compared to LPS i.p./vehicle ICM rats (p<.05). NF-κBIα levels were significantly elevated in LPS i.p./hIL-1RA ICM rats compared to vehicle i.p./vehicle ICM rats. LPS induced a significant increase in IL-6 independent of hIL-1RA treatment (df=2, 9, F = 4.77, p<.05). hIL-1RA failed to blunt the LPS-induced increase in IL-1β (data not shown).
Fig. 2.
Effect of hIL-1RA on LPS-induced hippocampal pro-inflammatory cytokines. Vehicle (0.9%) or LPS (100 μg/kg) was administered i.p. 4 d post-ICM injection of either vehicle (0.9%) or hIL-1RA (100 μg) and the hippocampal neuroinflammatory response measured 2 h post-LPS or vehicle treatment. Means with different letter designations (a, b, or c) are significantly different (p<.05) from each other. Data are presented as the mean±SEM.
3.3. Experiment 3. Effect of hIL-1RA on the LPS-induced sickness response
In light of the above observation that hIL-1RA treatment blunts neuroinflammatory responses to LPS administered 4 d after hlL-1RA treatment, the functional relevance of these anti-inflammatory effects was assessed using the social exploration test, which serves as a behavioral measure of the LPS-induced sickness response. The 3-way interaction between time, hIL-1RA treatment, and LPS treatment on social exploration was significant (Fig. 3A; df=3, 60, F=3.674, p<.02). Simple effects analysis (Fig. 3B) shows that in subjects treated with vehicle-ICM, LPS treatment significantly reduced social exploration behavior at 2, 6, and 24 h post-LPS compared to baseline levels (0 h). In rats treated with hIL-1RA-ICM, LPS significantly reduced social exploration at 2 and 6 h post-LPS treatment compared to baseline. However, between subjects analysis at each time-point shows that in rats treated with hIL-1RA-ICM and LPS-i.p., social exploration levels were significantly greater than levels observed in rats treated with vehicle-ICM and LPS-i.p. at 2, 6, and 24 h post-LPS treatment. At 2 and 6 h post-treatment, the duration of social exploration was significantly blunted in both LPS treatment groups compared to both vehicle-i.p. treatment groups. However, at 24 h post-treatment i.p., social exploration was no longer significantly decreased in rats treated with hIL-1RA-ICM and LPS-i.p. as compared to vehicle-i.p. treatment groups, while exploratory activity was still significantly decreased in rats treated with vehicle-ICM and LPS-i.p. compared to all other treatment groups. To further characterize the effect of hIL-1RA treatment on the LPS-induced sickness response, the effect of hIL-1RA on LPS-induced weight change was immediately measured after the 24 h social exploration test (Fig. 3C). The interaction between LPS and hIL-1RA treatment was significant (df=1, 20, F=21.35, p<.001). hIL-1RA treatment significantly attenuated the effect of LPS on body weight to levels comparable to levels observed in vehicle/hIL-1RA treated rats, but significantly lower than vehicle/vehicle treated rats. At 14 d post-treatment, the effect of hIL-1RA on LPS-induced sickness behavior was assessed at this time point post-ICM treatment. hIL-1RA failed to significantly attenuate LPS-induced decreases in social exploration and body weight (data not shown).
Fig. 3.
Effect of hIL-1RA on the LPS-induced sickness response. Vehicle (0.9%) or LPS (100 μg/kg) was administered i.p. 4 d post-ICM injection of either vehicle (0.9%) or hIL-1RA (100 μg). At 2 h, 6 h and 24 h post-LPS/vehicle treatment, social exploration was measured. A: Social exploration data is presented as a function of time post-vehicle/LPS treatment. B: Simple effects analysis of data in A presented as a function of treatment group. Means with different letter designations (a, b, c, d, or e) are significantly different (p<.05) from each other. C: LPS-induced weight change. Body weight was measured 1 d prior to vehicle/LPS treatment and immediately after the 24 h social exploration test. Means with different letter designations (a, b, or c) are significantly different (p<.05) from each other. Data are presented as the mean±SEM.
3.4. Experiment 4. Effect of hIL-1RA on hippocampal macrophage/microglial and astroglial activation markers
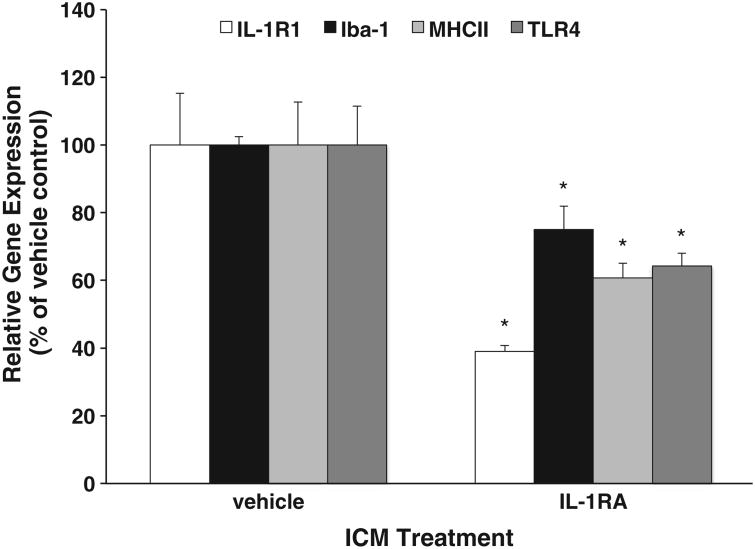
The findings presented thus far are obscure as to the mechanism of hIL-1RA anti-inflammatory effects because it is unclear whether hIL-1RA functioned as an antagonist at the time of LPS challenge and/or modulated the immunophenotype or activational state of CNS immune cells (i.e. microglia and macrophages) prior to LPS challenge, thereby desensitizing these cells to LPS-induced pro-inflammatory signals in the CNS. Compared to vehicle treated rats, hIL-1RA significantly reduced the gene expression levels of several CNS macrophage/microglial antigens (Fig. 4) including Iba-1 (df=11, t = 2.93, p<.02), IL-1R1 (df=10, t = 3.98, p<.01), MHCII (df=15, t = 2.79, p<.02), and TLR4 (df=10, t = 2.96, p<.02). The astrocyte antigen GFAP was unaltered by hIL-1RA treatment (data not shown). Several macrophage/microglial antigens were also not changed by hIL-1RA treatment including CD163, CD200R, and TLR2 (data not shown). At 14 d post-ICM treatment, hIL-1RA treatment significantly decreased (30%) the expression level of IL-1R1 compared to vehicle controls (df= 10,t = 3.97, p<.01), however expression of Iba-1, MHCII, and TLR4 was no longer significantly reduced by hIL-1RA treatment compared to vehicle controls (data not shown).
Fig. 4.
Effect of hIL-1RA on hippocampal macrophage/microglial activation markers. Four days post-ICM injection of either vehicle (0.9%) or hIL-1RA (100 μg), gene expression of macrophage and glial antigens was measured in the hippocampus. For each marker, hIL-1RA treatment means were significantly different (*p<.05) than their respective vehicle control. Data are presented as the mean±SEM.
3.5. Experiment 5. Effect of hIL-1RA on the microglial pro-inflammatory response to LPS ex vivo
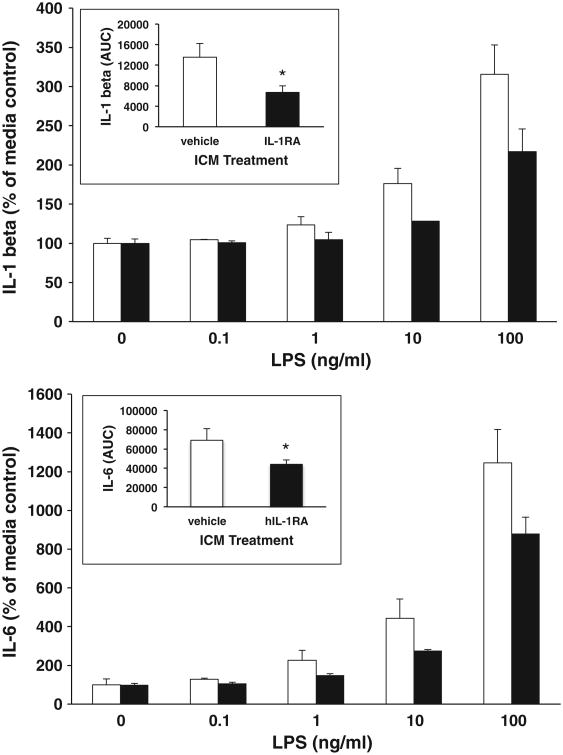
The effect of hIL-1 RA on macrophage/microglial activation markers suggests the possibility that hIL-1RA treatment may alter the functional responsiveness of microglia to pro-inflammatory stimuli. The immunophenotype of microglia (Iba-1 +/CD11b+/CD163−/GFAP−) as measured by PCR indicated highly pure microglia (data not shown). hIL-1RA treatment in vivo blunted the LPS-induced increase in both IL-1β and IL-6 across all concentrations of LPS (Fig. 5). Area under the LPS concentration curve was significantly decreased in hIL-1RA treated rats compared to vehicle treated rats for both IL-1β (df=6,t = 2.35, p<.03) and IL-6 (df = 6, t = 2.25, p<.04) (Fig. 5 insets). Compared to vehicle treated rats, hIL-1RA failed to alter the LPS-induced increase in both TNFα and NFκBIα (data not shown).
Fig. 5.
Effect of hIL-1RA on the microglial pro-inflammatory response to LPS ex vivo. Four days post-ICM injection of vehicle (0.9%) or hIL-1RA (100 μg), hippocampal microglia were isolated, exposed to LPS ex vivo for 2 h and the pro-inflammatory cytokine response measured. Open bars indicate vehicle treatment and closed bars indicate hIL-1RA treatment in vivo. Insets: For IL-1 and IL-6, area under the LPS concentration curve (AUC) was computed for vehicle and hIL-1RA treatment groups and means compared (*p<.05). Data are presented as the mean±SEM.
4. Discussion
The present set of experiments shows that a single administration of hIL-1RA into the cisterna magna resulted in detectable levels of hIL-1RA in the hippocampus, which persisted for at least 14 d post-administration. ICM injection is an effective, minimally invasive method to deliver compounds throughout the CNS (Proescholdt et al., 2000). In the present study, hIL-1RA levels were maximal at 2 h post-ICM injection and then declined substantially at 24 h post-ICM injection. The rate of decline of hIL-1RA levels in the hippocampus progressively slowed from 2 h to 14 d post-ICM treatment. Detection of hIL-1RA in the hippocampus 14 d after ICM injection suggests that the half-life of hIL-1RA in the CNS may be considerably longer than observed in the periphery (<2 h) (Granowitz et al., 1992). However, pharmacokinetic studies are needed to characterize the half-life of hIL-1RA in CNS after ICM injection. Nonetheless, the present results suggest that the CNS microenvironment may promote an extended half-life of hIL-1RA injected ICM.
We have previously demonstrated that a single dose of hIL-1RA (identical to the dose used here) injected ICM concurrent with live replicating E. coli, completely blocked the hippocampal pro-inflammatory cytokine response measured 4 d after the E. coli (Frank et al., 2010). These findings suggested the possibility that hIL-1RA may retain bioactivity in the CNS 4 d post-administration. Indeed, we found here that a single administration of hIL-1RA in to the CNS exhibited anti-inflammatory activity 4 d post-treatment. hIL-1RA completely blocked the hippocampal TNFα response and significantly attenuated the NF-κBIα response to LPS injected i.p. 4 d after hIL-1RA treatment. To determine whether the anti-inflammatory effects of hIL-1 RA were behaviorally relevant, the effect of hIL-1RA treatment on the LPS-induced sickness response was assessed. A single administration of hIL-1RA ICM 4 d prior to a peripheral challenge with LPS significantly blunted the LPS-induced decreases in social exploration. However, IL-1RA administered 14 d prior to LPS challenge failed to modulate the LPS-induced sickness response. Consistent with prior reports (Bluthe et al., 1992), the dose of LPS (100μg/kg) used here resulted in profound decreases in the time spent by the LPS-treated rats investigating and exploring a conspecific juvenile. We routinely observed the LPS-treated animal not engaging the juvenile even though the juvenile was initiating contact with the adult. Prior treatment with hIL-1RA significantly ameliorated these LPS-induced decrements in social investigation at all time-points post-LPS treatment. However, Bluthe et al. (1992) failed to show an effect of hIL-1RA given ICV on LPS-induced decreases in social exploration. Several differences between studies may account for these discrepant findings, including the dose of LPS (250 μg/kg vs 100 μg/kg), the dose of hIL-1RA (60 μg vs 100 μg) and the route of hIL-1RA administration (ICV vs ICM). However, several other studies have shown that hIL-1RA administered ICV at a dose comparable to the dose used here results in a significant blunting of fever, another LPS-induced sickness response (Cartmell et al., 1999; Luheshi et al., 1996). hIL-1RA administered ICV also is capable of blocking IL-1β-induced fever and decrements in social exploration (Kent et al., 1992; Opp and Krueger, 1991). It is important to note that in each of these prior studies of hIL-1RA central anti-inflammatory effects, hIL-1RA was administered concurrent or within 1–2 h of treatment with the pro-inflammatory stimulus. The present results show that hIL-1RA administered several days prior to a pro-inflammatory insult significantly ameliorated the neuroinflammatory and sickness response, suggesting that hIL-1RA can be administered prophylactically.
One explanation for these results is that the levels of hIL-1RA detected in the hippocampus at 4 d post-treatment retained adequate anti-inflammatory bioactivity for active antagonism at the IL-1 type 1 receptor, thereby blocking LPS-induced IL-1 signaling in the CNS and downstream neuroinflammatory effects. However, basal levels of pro-inflammatory cytokines such IL-1β and IL-6 also play pivotal roles in physiological functions in the CNS such as sleep and thermal regulation as well as learning and memory (Besedovsky and del Rey, 2011). Therefore, an alternate explanation is that hIL-1RA antagonized basal IL-1 signaling in the hippocampus during some part of the 4 d period between hIL-1RA treatment and LPS administration, resulting in a shift in the CNS microenvironment toward an anti-inflammatory immunophenotype. In other words, hIL-1RA treatment may have desensitized the CNS microenvironment or innate immune effector cells such as perivascular macrophages and microglia to pro-inflammatory insults. To assess this possibility, several proteins involved in glial regulation, activation, and pro-inflammatory signaling were measured in the hippocampus 4 d after ICM treatment with vehicle or hIL-1RA. Four days after administration, hIL-1RA treatment resulted in a significant downregulation of several macrophage/microglial antigens including IL-1R1, Iba-1, MHCII, and TLR4. By 14 d post-ICM treatment, IL-1R1 expression was still significantly downregulated, however the expression of Iba-1, MHCII, and TLR4 was no longer downregulated at this time-point. These results suggest that a single administration of hIL-1RA into the CNS may induce a transient shift in the CNS microenvironment toward an anti-inflammatory state by attenuating pro-inflammatory drive (i.e. blocking ongoing basal IL-1 signaling). These results suggest the possibility that hIL-1RA, when administered prophylactically, may function, in part, to desensitize CNS macrophages/microglia to pro-inflammatory insults. To further explore this possibility, an ex vivo experimental approach was taken to examine the effects of in vivo hIL-1RA treatment on the microglial response to LPS ex vivo. Here, LPS was used to probe the sensitization status of microglia after in vivo treatment. Hippocampal microglia isolated 4 d after hIL-1RA treatment exhibited, in part, a blunted pro-inflammatory response (IL-1β and IL-6) to LPS. These results are consistent with the finding of hIL-1RA downregulation of microglial antigens in vivo, which provides additional support for the notion that hIL-1RA, when given pro-phylactically, alters the immunophenotype of microglia and blunts the pro-inflammatory response of microglia to inflammatory insults. However, it is unclear from these data to what degree, if any, hIL-1RA may also be functioning as a receptor antagonist at the time of the pro-inflammatory challenge, which is given several days after hIL-1RA treatment.
The present results suggest a new caution with regard to how the effects of intracerebral IL-1RA administration can be interpreted. The common assumption has been that, if IL-1RA blocks the neuroinflammatory effects of some manipulation such as peripheral LPS or E. coli administration, then these neuroinflammatory effects of the pro-inflammatory stimulus must have been mediated by their effects on brain IL-1 signaling. Indeed, this is how Frank et al. (2010) interpreted the IL-1RA blockade of E. coli induced memory deficits to new learning occurring 4 d later. However, now this conclusion must be viewed with some reservation. In the present studies microglia/macrophage activation markers in vivo, and microglial sensitivity ex vivo, were examined 4 d after ICM hIL-1RA treatment. Here, hIL-1RA shifted the cells to a less inflammatory phenotype presumably by antagonizing basal IL-1 signaling prior to pro-inflammatory challenge. In light of these findings, an alternate interpretation of the Frank et al. (2010) data is that hIL-1RA treatment desensitized hippocampal microglia (e.g. downregulated IL-1R1) to the neuroinflammatory effects (IL-1) of peripheral E. coli., thus abrogating the E. coli-induced memory deficits and pro-inflammatory cytokine effects observed 4 d after hIL-1RA and E. coli treatment. It is to be noted that if this immunophenotypic shift were to occur more quickly (not measured here), then many reported effects of intracerebral IL-1RA may have a different interpretation.
Finally, the present results suggest that IL-1RA might be effectively administered prophylactically, thereby providing a therapeutic window of opportunity, wherein neuroinflammatory effects of trauma may be ameliorated. The prophylactic use of IL-1RA may be particularly beneficial in clinical settings such as where surgical trauma results in cognitive dysfunction (post-operative cognitive dysfunction), in which neuroinflammation has been implicated as an etiologic factor (Monk and Price, 2011).
Acknowledgments
Julia Sobesky provided assistance with ICM injections, tissue collection, and ELISAs.
Johanna Flyer provided assistance with behavioral measurements of social exploration.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP, Welgus HG, Thompson RC, Eisenberg SP. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990;85:1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. Central and peripheral cytokines mediate immune-brain connectivity. Neurochem Res. 2011;36:1–6. doi: 10.1007/s11064-010-0252-x. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Rothwell NJ. Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J Physiol. 1999;518(Pt 2):585–594. doi: 10.1111/j.1469-7793.1999.0585p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorne C, Prenant C, Smigova A, Julyan P, Maroy R, Herholz K, Rothwell N, Boutin H. Biodistribution, pharmacokinetics and metabolism of interleukin-1 receptor antagonist (IL-1RA) using [(1)(8)F]-IL1RA and PET imaging in rats. Br J Pharmacol. 2011;162:659–672. doi: 10.1111/j.1476-5381.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Dripps DJ, Brandhuber BJ, Thompson RC, Eisenberg SP. Interleukin-1 (IL-1) receptor antagonist binds to the 80-kDa IL-1 receptor but does not initiate IL-1 signal transduction. J Biol Chem. 1991;266:10331–10336. [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, Catalano MA, Wolff SM, Dinarello CA. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- Greenhalgh AD, Galea J, Denes A, Tyrrell PJ, Rothwell NJ. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol. 2010;160:153–159. doi: 10.1111/j.1476-5381.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am J Physiol. 1996;270:E91–E95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17:376–381. doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol. 1991;260:R453–R457. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- Proescholdt MG, Hutto B, Brady LS, Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience. 2000;95:577–592. doi: 10.1016/s0306-4522(99)00417-0. [DOI] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M. Inteleukin-1 (IL-1) pathway. Sci Signal. 2011 doi: 10.1126/scisignal.3105cm1. 3 cm1. [DOI] [PubMed] [Google Scholar]