Abstract
There is evidence that pain can impact cognitive function in people. The present study evaluated whether Pavlovian fear conditioning in rats would be reduced if conditioning were followed by persistent inflammatory pain induced by a subcutaneous injection of dilute formalin or complete Freund's adjuvant (CFA) on the dorsal lumbar surface of the back. Formalin-induced pain specifically impaired contextual fear conditioning but not auditory cue conditioning (Experiment 1A). Moreover, formalin pain only impaired contextual fear conditioning if it was initiated within 1 h of conditioning and did not have a significant effect if initiated 2, 8 or 32 h after (Experiments 1A and 1B). Experiment 2 showed that formalin pain initiated after a session of context pre-exposure reduced the ability of that pre-exposure to facilitate contextual fear when the rat was limited to a brief exposure to the context during conditioning. Similar impairments in context- but not CS-fear conditioning were also observed if the rats received an immediate post-conditioning injection with CFA (Experiment 3). Finally, we confirmed that formalin and CFA injected s.c. on the back induced pain-indicative behaviours, hyperalgesia and allodynia with a similar timecourse to intraplantar injections (Experiment 4). These results suggest that persistent pain impairs learning in a hippocampus-dependent task, and may disrupt processes that encode experiences into long-term memory.
Keywords: Pain, Formalin, Fear conditioning, Rat, Memory
1. Introduction
Pain interferes with cognitive function in general, and memory in particular. Evidence for this comes from several sources. First, patients with chronic pain complain of significant memory disturbances [1–5]. These complaints have been verified in standardised neurocognitive assessments of teenage, adult and elderly chronic pain patients across a range of chronic pain-causing conditions, and the severity of impairment is proportional to the degree of pain experienced at assessment [6–18]. Second, experimentally inducing pain in otherwise healthy volunteers interferes with memory function if the painful stimulus is applied during encoding and/or retrieval in long-term memory tasks [18–21]. These memory deficits would have a limiting effect on a person suffering from pain, contribute to pain disability, and are a barrier to successful completion of treatment programmes [22].
However, the causes of these pain-induced memory deficits remain unclear. Studies of the effect of pain on memory with clinical populations are often hampered by small samples, variation in the source and extent of pain, less than adequate controls, and the presence of other disease-related complications or psychosocial distress that might confound conclusions. Research with healthy volunteers can be constrained by obvious ethical concerns and methodological difficulties that limit the ability to test hypotheses about mechanisms, and to test potential treatments.
Understanding the mechanisms by which pain causes memory dysfunctions requires the use of animal models. Therefore the present study aimed to demonstrate that persistent pain would affect long-term memory in the laboratory rat using Pavlovian fear conditioning, a standard procedure for studying the neurobiology of memory. In this paradigm, a rat is placed in a conditioning chamber where, after a couple of minutes of exploration, it is presented with a discrete tone conditioned stimulus (CS). At the end of the tone CS, the rat receives a mild but startling electric stimulus to its feet (unconditioned stimulus) that provokes a fearful response (unconditioned response). The next time the rat experiences the tone CS it displays its natural freezing defensive response and becomes immobile (conditioned response). However, separately from freezing to the tone CS, the rat will also freeze in the presence of the contextual cues of the conditioning chamber (context). Fear conditioning to the tone CS is a simple and rapid process whereas fear conditioning to the context requires the integration of spatially diffuse cues, and this extra information processing demand requires longer periods of time and uses neurobiological mechanisms that are more susceptible to post-conditioning interference than for a simple CS [23,24].
The present experiments aimed to examine the effect of pain on contextual and tone CS fear conditioning. After conditioning, rats were given a subcutaneous injection of either dilute formalin or complete Freunds adjuvant (CFA). Formalin produces an immediate behavioural response indicative of pain [25], and both formalin and CFA induce hyperalgesia and allodynia that can persist for weeks [26,27]. The rats were then tested 4 or 7 days later to see if pain experienced after conditioning had affected memory of conditioning.
2. Materials and methods
2.1. Subjects
Adult male Sprague-Dawley rats weighing between 350 and 450 g were used in all experiments. Experiments 1 and 2 were conducted at the University of Colorado and used rats from Harlan Labs, Madison, WI. Experiments 3 and 4 were conducted at the University of Sydney and used rats from the Animal Resource Centre, Perth, WA, Australia. On arrival to the colony, the rats were housed in temperature-controlled (23 ± 3 °C) and light-controlled (12/12 h light/dark cycle; lights on at 07:00 h) rooms with standard rodent chow and water available ad libitum. Behavioural testing was performed between 07:00 and 16:00 h. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder, and by the Animal Ethics Committee at the University of Sydney.
2.2. Apparatus
Experiments 1 and 2 used two identical conditioning chambers (26 cm × 21 cm × 24 cm, l × w × h) made of clear plastic and with window mesh roofs, each held within different igloo ice chests (54 cm × 30 cm × 27 cm; l × w × h) with white interiors. A speaker and activated 24-V DC light bulb were mounted on the ceiling inside each chest. The unconditioned stimulus was a 2 s, 0.55-mA shock (as measured at the rod floor) delivered through a removable floor of stainless steel rods 1.5 cm in diameter spaced 1.2 cm center to center. Each rod was wired to a shock generator and scrambler (Lafayette Instruments Model 8240415-SS, Lafayette, IN). The conditioning chambers rested on the steel rods and the floor of each chamber was cleaned with water between each training or testing session. The 2976 Hz tone CS was presented at 74 dB as measured in the test chamber by a Triplet sound level meter (Model 370 set on the C scale). The test chambers were changed when the tone was tested to prevent the generalization of conditioned context fear to the CS test context. The shape of the conditioning chambers was changed from rectangular to triangular by placing a 34 cm × 10 cm plastic panel diagonally inside the conditioning chambers. Moreover, the stainless steel rod floor was removed so the chamber sat on a flat plastic surface and the background lights were replaced with red globes to darken the chambers. Background noise in the chambers was measured at 68 dB.
Experiment 3 occurred in 4 identical chambers (30 cm long, 25 cm wide and 31 cm high) made of clear plastic and aluminum, each in held in wooden sound attenuation boxes with a 40 W light globe inside each. A 1500 Hz tone generated programmatically by a computer and played through speakers behind the conditioning chambers was presented at 74 dB (against 68 dB background noise) served as the CS. The unconditioned stimulus was a 1 s, 0.75 mA shock (as measured at the rod floor) delivered through a removable floor of stainless steel rods of the same dimensions described above. Each rod was wired to a shock generator and scrambler (Med Associates, VT). The floor of each chamber was cleaned with water between each session. The tone CS test was conducted in modified shuttle boxes (34 cm high, 29 cm long, and 26 cm wide). The doors between each chamber of the shuttle box were closed to create separate testing chambers, and a plastic mesh was laid on the floor to change tactile cues. The room lights were turned off and testing was conducted under red lighting.
2.3. Procedures
2.3.1. Formalin or complete Freund's adjuvant injections
Subcutaneous injections of dilute formalin or CFA are commonly used methods to induce persistent or chronic inflammatory nociceptive stimulation in animal studies of pain [25,26]. In the standard procedure, formalin or CFA is administered into the plantar surface of the foot. However, pilot studies indicated that injecting into the paw with vehicle after conditioning increased fear conditioning compared to non-injected controls. Given this limitation our injections were instead made into the loose subcutaneous tissue on the dorsal lumbar surface of the back at the region identified by Takahashi et al. [28,29] to be within the same L5 dermatome that innervates the plantar surface of the paw. Formalin (4% in 0.9%, w/v saline at 0.1 mL), complete Freund's adjuvant (0.2 mL containing 200 μg Mycobacterium tuberculosis; Sigma, St. Louis, MO) or vehicle injections occurred in a room adjacent to their colony room. The rats were lightly held in toweling and rapidly injected subcutaneously (s.c.). To ensure that the person scoring the behaviour of the rat was blind to the treatment given the rat, one person injected the rats and a different person scored fear conditioning or pain behaviour.
2.3.2. Fear conditioning
The rats were handled on the day prior to conditioning. Conditioning and testing of contextual fear occurred between 9 a.m. and 12 p.m. whereas testing of conditioning to the tone CS occurred between 3 and 5 p.m. Unless specified elsewhere, on the day of conditioning, the rats were transported to the conditioning room in their home cages covered by a white lab coat and immediately placed in the conditioning chambers. 120 s after the rats were placed in the chamber, the rats received a 15-s presentation of the tone CS that terminated with the onset of the shock unconditioned stimulus, and the rats were removed 30 s after the shock.
Conditioned fear was assessed by transporting the animal cages to the conditioning chambers under a lab coat and placing each rat in the conditioning chamber for 10 min. Freezing was assessed using a time sampling technique when the rats were judged as being active or freezing once every 10 s. Freezing was defined as the complete absence of movement except for respiration.
Assessment of fear to the tone CS occurred the next day. The animals were transported to the conditioning room without a lab coat covering their cages and were assessed in the modified CS-testing chambers under red light. Different testing chambers were used to reduce the potential effects of generalized contextual fear on the amount of freezing to the CS. Moreover, CS-fear testing occurred in the afternoon to provide a different temporal context. The tone cue test lasted for 12 min and consisted of a 2-min pre-CS period in the absence of the auditory cue followed by a 10-min presentation of the tone CS.
2.3.3. Pain assessment
2.3.3.1. Spontaneous nociceptive behaviours
Following injection with formalin, CFA or vehicle, the rats were observed for pain evoked behaviour. A time-sampling procedure was used in which the rats' behaviour was observed and assessed once every 30 s. The rats' behaviour was scored used a weighted scoring system similar to that developed by Dubuisson and Dennis [30] and modified for behaviours relevant to the site of injection. In each observation, the rats were scored as 0 if they displayed no nociceptive behaviours, 1 if they were writhing or lying with an arched or curved back, or 2 if the animals were attempting to lick or scratch the site of injection. Animals were observed for 5-min periods and the pain scores across 10 observations were averaged.
2.3.3.2. Tail immersion test for thermal hyperalgesia
The tail immersion test, which measures the latency for the rat to withdraw its tail from a heated water bath, was used to measure thermal nociceptive sensitivity. Rats were habituated to the testing apparatus and procedures for 5 days prior to testing. During testing, rats were transported to the laboratory and placed in clear Plexiglas containers (35 cm × 21 cm × 19 cm) with metal mesh ceiling and wood chip bedding for 20 min. Each rat was removed from its container and the distal 4-cm portion of the tail was immersed in water maintained at 49 °C by a Thermoline water thermoregulator. The latency to entirely remove the tail from the water was recorded, the tail was cleaned dry with a towel, and the rat immediately returned to its container. Four measurements were recorded over 20 min to determine the baseline latency for each rat. Following injection with formalin, CFA or vehicle, tail flick latencies were recorded once only at each time point.
2.3.3.3. Von Frey test for mechanical allodynia
The von Frey test measures response thresholds to calibrated light pressure stimuli applied to the ventral surface of the paws. The test was performed using 0.406–15.136 g stimuli as described in detail previously [31]. The rats were habituated to the testing cages for 5 days prior to testing. On the day of testing, the rats were first assessed for baseline response thresholds prior to tail immersion testing and injection, and the average response threshold from both feet was calculated. The monofilament was applied perpendicularly to the mid-plantar or heel of a weight-bearing hindpaw for 8 s. Paw withdrawal responses included flinching during the maintained application of the monofilament, but not at initial contact or removal of the monofilament. Monofilaments were tested in ascending order of stiffness until two consecutive withdrawal responses were recorded with the same monofilament. There was at least 2 min between applications of monofilaments to a paw. The behavioural responses were used to calculate the log pressure that would have resulted in the 50% paw withdrawal threshold, by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method [32,33] as used previously [31].
2.3.4. Statistics
The dependent variable in experiments 1–3 was the percentage of samples that the rats were observed to be freezing during the test period. In Experiment 4, data from the tail immersion tests were analysed as the withdrawal latency in seconds, the data from the von Frey tests were analysed as the interpolated 50% threshold in log 10 of stimulus intensity (monofilament stiffness in milligrams × 10), and data from the nociceptive behaviour observations were analysed as average pain score for each 5-min period. The data were analysed by repeated measures ANOVAs or by planned orthogonal contrasts, and statistical significance was set with alpha at p < 0.05. For post-hoc analyses, alpha was maintained at 0.05 with Scheffé's procedure.
2.4. Experiments 1A and 1B: effect of post-conditioning formalin pain on contextual- and CS-fear conditioning
The Pavlovian fear conditioning model has several advantages for studying the effect of pain on memory. First, unlike other animal models of memory that require extensive training, fear conditioning can occur in a single session so that the effect of pain on the rat's memory for a discrete experience can be tested. Second, fear conditioning to a tone CS is a rapid process whereas fear conditioning to the context is followed by a critical time period during which the processes involved in context memory consolidation are susceptible to interference. This is revealed by studies showing that contextual, but not tone CS fear conditioning, can be impaired by hippocampal damage, immune challenge, or social isolation if done within a critical time period after the conditioning trial [34–38]. However, this period is transient and manipulations that can reduce contextual fear if experienced soon after conditioning have little or no effect if performed some time after that episode.
Therefore, this study was designed to test whether experience with pain immediately following fear conditioning will disrupt contextual but not tone CS fear conditioning. Experiment 1A examined whether formalin pain would reduce contextual or tone CS fear conditioning if s.c. formalin-induced pain begins 1 h after conditioning compared to pain-free controls. Experiment 1B examined if formalin pain had to be experienced within a critical time period to reduce fear conditioning. Here, all rats experienced formalin pain. However, formalin was administered 0.5, 2, 8, or 32 h after conditioning.
On the first day of the experiment, the rats were transported to the laboratory, conditioned, and then returned to their colony room. In Experiment 1A, the rats received a s.c. injection of either formalin [n = 8] or equivolume saline [n = 8] on the back 1 h after conditioning. In Experiment 1B, the rats received a s.c. injection of formalin 0.5, 2, 8 or 32 h after conditioning. Group sizes were 0.5 h [n = 8], 2 h [n = 8], 8 h [n = 8] and 32 h [n = 8]. In both experiments, the rats were given a 4-day recovery period between conditioning and testing for contextual fear, followed by a test for auditory cue fear approximately 30 h later.
2.5. Experiment 2: effect of formalin pain on context pre-exposure in an immediate shock conditioning procedure
Rats need longer periods of time to learn to associate stimuli with contextual cues than simple tone CSs. Robust tone CS fear conditioning can occur in one pairing, even when the CS and shock are simultaneously presented [39]. However, studies that manipulate the time a rat spends in the context prior to a shock show that contextual fear conditioning is weak if the preshock exploration period in the context is very short (e.g., less than 27 s; [40,41]). That is, there is very little fear conditioned to the context if a shock is administered very quickly after placement into the novel context. This immediate shock deficit can be overcome by increasing the time the rat is exposed to the context, either by extending the interval between placement in the context and footshock during the conditioning trial or by allowing the rat to explore the context in a separate session prior to the conditioning trial [40,41]. These results suggest that the processes underlying contextual fear conditioning can be dissociated into two processes: A relatively slow integration of the available contextual stimuli into memory, and the association of this contextual memory trace with the aversive US. This experiment aims to test if pain interferes with the consolidation of contextual cues into memory independently of the fear conditioning process. That is, this experiment will examine whether formalin pain experienced after a session of pre-exposures to the to-be-conditioned context will attenuate the effect of context pre-exposure on the immediate shock deficit when conditioning occurs the following day.
On the first day of the experiment half the rats were transported to the laboratory, and were given five 2-min pre-exposure (PreX) sessions in the conditioning chambers separated by 5-min intervals in their home cages. The remaining rats were given equivalent handling in their home cages (HC). 30-min after the context pre-exposure or after handling, the rats received a s.c. injection of either formalin (4% in saline at 0.1 ml) or equivolume saline above the tail on the lumbar portion of the back. Group sizes were HC-Saline [n = 8], HC-Formalin [n = 8], PreX-Saline [n = 9], and PreX-Formalin [n = 9]. At the same time the next day, all rats were transported to the laboratory, placed in the conditioning context for 10 s when they received a single unsignalled footshock US, were removed immediately from the conditioning context and returned to their colony room. 4 days later the rats were again transported to the conditioning chambers and assessed for contextual fear.
2.6. Experiment 3: effect of post-conditioning complete Freund's adjuvant on contextual- and CS-fear conditioning
Experiment 3 attempted to extend the findings of Experiment 1 by examining whether contextual fear conditioning would also be reduced in rats given post-conditioning s.c. injections of a different inflammatory stimulus. CFA is widely used to model chronic inflammatory and polyathritic pain in rodents, and induces chronic and progressive hyperalgesia and allodynia [26]. On the first day of the experiment, the rats were transported to the laboratory, and conditioned as described above. Immediately after conditioning, they received a s.c. injection of CFA [n = 8] or vehicle [n = 8] in the same location as above, and were then returned to their colony room. 7 days after conditioning, the rats were again transported to the conditioning chambers and assessed for contextual fear, followed by a test for auditory cue fear test approximately 30 h later.
2.7. Experiment 4: the behavioural effect of formalin or complete Freund's adjuvant injected subcutaneously onto the dorsal lumbar surface of the back of the rat
The previous experiments investigated the effect of formalin or CFA injected s.c. into the loose subcutaneous tissue on the dorsal lumbar surface of the back. As the standard procedure is to administer formalin or CFA into the plantar surface of the foot, the aims of this experiment were to verify that s.c. lumbar formalin and/or CFA at the doses used here were suitably noxious stimuli, and to determine if the timecourse of changes in behaviour and pain sensitivity are similar to those reported with intraplantar formalin or CFA. Prior to injection with formalin [n = 8], CFA [n = 8] or saline [n = 8], the rats were assessed for baseline tail flick latencies in the immersion test, and withdrawal thresholds to the von Frey hairs. Following injection, tail flick latencies were recorded 5-min, 15-min, 30-min, 60-min, 120-min, 180-min, 360-min, 1-day, 4-day and 7-day post-injection. The rats were also assessed in the von Frey test at 80-min, 200-min and 380-min, and 1-day, 4-day and 7-day post-injection. The rats's pontaneous behaviour was observed for the first 60-min post-injection, and then for 5-min periods prior to tail immersion testing at 120-min, 180-min and 360-min and 1-day, 4-day, and 7-day post-injection.
3. Results
3.1. Experiments 1A and 1B: effect of post-conditioning formalin pain on contextual-and CS-fear conditioning
The results of Experiment 1A are shown in Fig. 1A. It shows that the expression of contextual- but not auditory-cue fear was reduced in rats receiving a s.c. injection of formalin 1 h after conditioning compared to controls. These observations were confirmed in statistical analyses. There was no main effect of type of injection (formalin vs. saline), F(1,14) = 3.5, p > 0.05, but there was a main effect of test-type (context vs. CS), F(1,14) = 6.0, p < 0.03, and there was a significant interaction between injection and test-type, F(1,14) = 6.3, p <0.03.
Fig. 1.
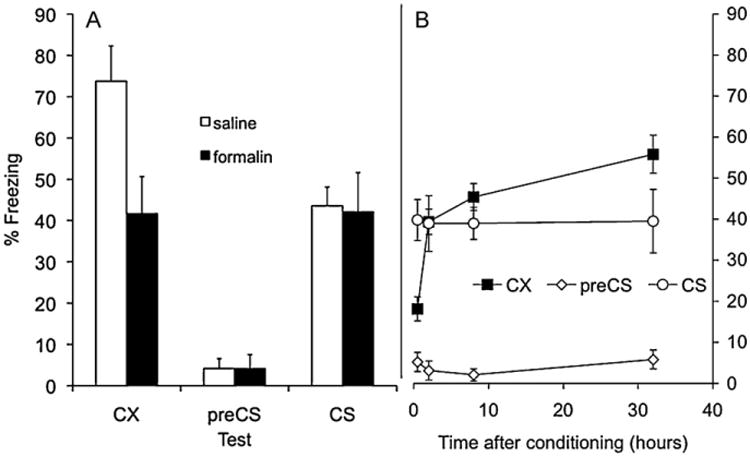
(A.) Effect of s.c. formalin or saline (administered 1 h after conditioning) on freezing in a test of conditioned fear to the conditioning contextual cues (CX) or to the tone conditioned stimulus (CS) when tested in a different context. Pre-CS refers to freezing prior to the CS being switched on in the CS test. (B) Effect of s.c. formalin when administered 0.5, 2, 8, or 32 h after conditioning on freezing in a test of conditioned fear to the conditioning contextual cues (CX) or to the tone conditioned stimulus (CS) when tested in a different context. Pre-CS refers to freezing prior to the CS being switched on in the CS test.
The results of Experiment 1B are shown in Fig. 1B. It shows that the reduction in freezing to the contextual-cues by formalin was time-dependent. Maximum freezing to the context was observed when formalin was administered 32 h after conditioning but the levels of freezing diminished the shorter the period of time between conditioning and injection. Consistent with the previous experiment however, there was no effect of formalin on freezing to the CS at any time-point. Statistical analysis confirmed these observations. There was no main effect of test-type (context vs. CS) on freezing (F < 1) but there was a reliable difference in freezing when comparing rats injected 0.5 h after conditioning to the other groups (2, 8 and 32 h), F(1,30) = 11.0, p < 0.002, and there was a significant interaction between test-type and this time-point (0.5 h vs. 2, 8 and 32 h). There were no reliable differences in comparisons made between rats injected 2 h after conditioning or those injected later (8 and 32 h), in comparisons between rats injected 4 or 8 h after conditioning, nor were there significant interactions between these time-points and test-type (all Fs < 2).
3.2. Experiment 2: effect of formalin pain on context pre-exposure in the immediate shock conditioning procedure
The results of Experiment 2 are shown in Fig. 2. It shows that the level of freezing to the context was less in rats who received a single immediate shock compared to rats who had received five 2-min pre-exposure sessions the day before. Fig. 2 also shows that the increase in freezing in Pre-X rats was reduced in rats that received an injection of formalin 30 min after the pre-exposure sessions. These observations were confirmed in statistical analyses. There was a main effect of type of pre-exposure, F(1,30) = 120.6, p < 0.001, but no main effect of formalin, F(1,30) = 1.3, p > 0.05. Importantly, there was a significant interaction between pre-exposure and formalin, F(1,30) = 8.2, p < 0.01.
Fig. 2.
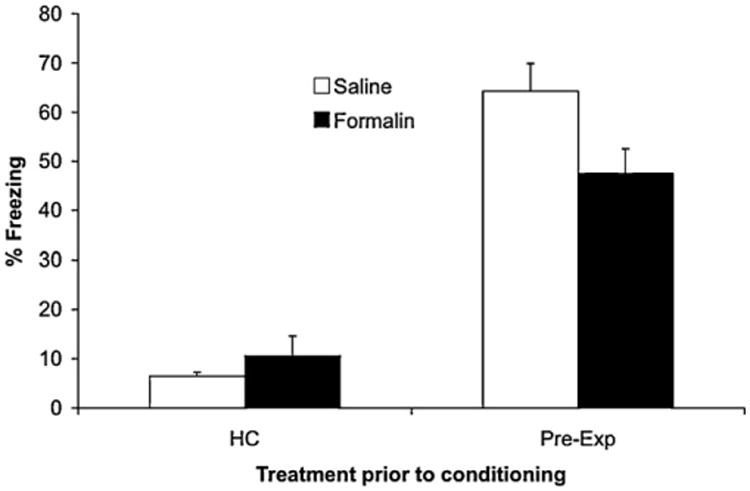
The effect of s.c. formalin or saline 1 h after pre-exposures to the to-be-conditioned context on the ability of context pre-exposure to reduce the immediate shock deficit when conditioning occurs the following day. Pre-exp refers to rats that had a session of five 2-min exposures to the context on the day prior to conditioning. HC refers to rats that received equivalent handling in their homecages on the day prior to conditioning.
3.3. Experiment 3: effect of post-conditioning complete Freund's adjuvant on contextual- and CS-fear conditioning
The results of Experiment 3 are shown in Fig. 3. It shows that the expression of contextual- but not auditory-cue fear was reduced in rats receiving a s.c. injection of CFA immediately after conditioning compared to controls. These observations were confirmed in statistical analyses. There was a main effect of type of injection (CFA vs. saline), F(1,14) = 6.0, p <0.03, a main effect of test-type (context vs. CS), F(1,14) = 5.4, p < 0.05, and there was a significant interaction between injection and test-type, F(1,14) = 5.8, p < 0.05.
Fig. 3.
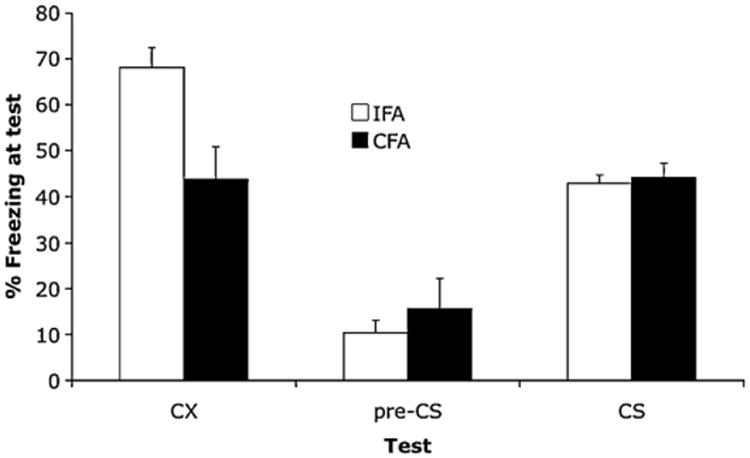
The effect of s.c. complete Freund's adjuvant (CFA) or incomplete Freund's adjuvant (IFA) immediately after conditioning on the levels of freezing in a test of conditioned fear to the conditioning contextual cues (CX) or to the tone conditioned stimulus (CS) when tested in a different context. Pre-CS refers to freezing prior to the CS being switched on in the CS test.
3.4. Experiment 4: the behavioural effect of formalin or complete Freund's adjuvant injected subcutaneously onto the dorsal lumbar surface of the back of the rat
Fig. 4A shows the change in the expression of pain behaviours after injection with formalin, CFA or vehicle. Rats injected with formalin showed a range of behavioural responses indicating pain from the injection, such as turning to scratch or groom the injection site, writhing, vocalizations, increased locomotor activity, leg kicking and hopping, and lying on the stomach while stretching the back. These behaviours were observed for the first 60 min after injection but were absent by 120 min. CFA or saline treated rats did not display these behaviours at any timepoint observed during the study. Statistical analysis of the scoring of the pain behaviours revealed significant overall between-group differences between the formalin and saline treated rats, F(1,21) = 763.496, p < 0.001, between formalin and CFA treated rats, F(1,21) = 692, p < 0.001, but not between CFA and saline treated rats, F(1,21) = 1.8. Post-hoc analysis of each time point revealed significant differences between vehicle- and formalin-treated rats from 5-to 55-min post-injection, all F(1,21)>34.6, critical F=8.65, but not at 60min or later time points.
Fig. 4.
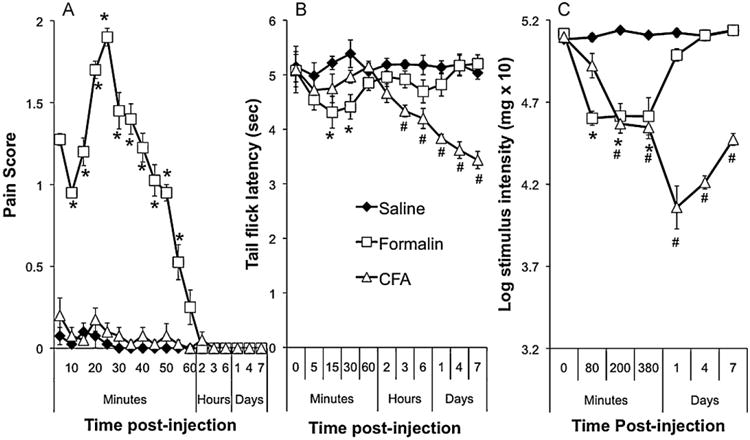
(A) Intensity of observed pain behaviours following s.c. injection of formalin, CFA or saline on the lumbar region of the back. (B) Change in tail flick latencies from baseline after a s.c. injection of formalin, CFA or saline on the lumbar region of the back. (C) Change in paw withdrawal thresholds from baseline in the von Frey test of mechanical allodynia after a s.c. injection of formalin, CFA or saline on the lumbar region of the back. *Significant difference between formalin- and vehicle-treated rats, #Significant difference between CFA- and vehicle treated rats using Scheffé's test for post-hoc contrasts.
Fig. 4B shows the change in tail flick latencies following injection with formalin, CFA or saline. Formalin produced a decrease in tail flick latencies immediately after conditioning that had resolved by the first hour of testing. CFA produced a mild hyperalgesia that became apparent within hours of injection and that became progressively stronger over the course of testing. Statistical analysis revealed significant overall between-group differences between formalin and vehicle, F(1,21) = 9.4, p < 0.01, between CFA and vehicle, F(1,21) = 44.0, p < 0.001, and between CFA and formalin treated rats, F(1,21) = 12.6, p < 0.002. Post-hoc analysis revealed significant differences between vehicle and formalin treated rats at 15- and 30-min post-injection, F(1,21) = 29.5 and 12.9 respectively, critical F = 8.65. Post-hoc analysis revealed a significant difference between vehicle and CFA treated rats at 3-h post-treatment and for all subsequent time points, F(1,21)>16.6, critical F =8.65. There were no differences in tail flick latencies at baseline (Fs< 1).
Fig. 4C shows the change in withdrawal thresholds to the calibrated von Frey hairs. Injection with formalin induced a rapid decrease in response thresholds that persisted for at least 6 h. CFA also induced a decrease in withdrawal response thresholds apparent within 2 h and that persisted for the 7 days of testing. Statistical analysis revealed significant overall between-group differences between formalin and vehicle, F(1,21) = 37.4, p < 0.001, between CFA and vehicle, F(1,21) = 72.4, p < 0.001, and between CFA and formalin treated rats, F(1,21) 213.9, p<0.001. Post-hoc analyses revealed significant differences between formalin- and vehicle-treated rats at 80-, 200-, and 380-min post-treatment, F(1,21) = 45.9, 43.4, and 20.3 respectively, critical F = 8.65, and between CFA- and vehicle-treated rats from 200 min onwards, all F(1,21) > 26.5, critical F = 8.65. There were no differences in withdrawal response thresholds at baseline (all Fs < 2).
4. Discussion
Subcutaneous injection of formalin is widely used to model moderate continuous pain generated by injured tissue, and induces a characteristic biphasic behavioural response (e.g., grooming, licking, flinching and protecting the injection site) lasting for approximately an hour [30], and allodynia and hyperalgesia that may persist for weeks [27,42]. Subcutaneous injection of CFA is a widely used model of chronic and progressive inflammatory or polyarthritic pain with hyperalgesia and mechanical allodynia that develops within a few hours or days and persists for many weeks [26,43,44]. Animals may begin to show protective behaviours within a few days of injection with CFA [45]. Here we observed that s.c. injection of formalin on the back produced a time-course of pain-indicative behaviours similar to those expressed when formalin is injected into the foot. These behaviours persisted for an hour and were no longer observable by 2 h. CFA did not produce any behavioural change in the rats during this period. In addition to the overt nocifensive behaviours, formalin and CFA injected onto the back also induced thermal hyperalgesia in the tail and allodynia on the feet. This effect began immediately and persisted for up to 6 h after formalin, and began within hours and persisted for at least 7 days after CFA This is consistent with previous observations that injection of formalin [27,42,46,47] or CFA [45,48–50] into the foot, back or tail not only affects tactile and nociceptive sensitivity at the site of injection, but also at non-injected sites. Importantly, this strongly suggests that injecting formalin or CFA onto the lumbar region of the back recruits similar neuroplastic events in the CNS that have been identified in previous studies as underlying the prolonged hyperalgesia and allodynia induced by formalin or CFA injected in other locations.
The major novel finding of these studies is that post-conditioning experience with pain disrupted memory in the Pavlovian fear-conditioning paradigm. Moreover, this effect occurred with two different noxious stimuli: Formalin and CFA. It could be argued that formalin or CFA-induced pain, hyperalgesia or allodynia produced some non-specific change in the rats that interfered with their ability to demonstrate freezing at test. For instance, persistent motivational or hyperalgesic effects of formalin or CFA may have affected the freezing response at test. Or perhaps the results reflect a loss of state-dependent learning as the animals were pain free during conditioning and may have experienced persistent pain or hyperalgesia at test. However, these interpretations are unlikely. First, formalin and CFA had a domain-specific effect on freezing: Freezing was only reduced in the presence of the contextual cues but not to the CS. Second, all rats received a formalin injection in Experiment 1B, yet only rats injected within 2 h after conditioning showed a reduction in freezing to the context at test. It seems unlikely that any persistent motivational or state-dependent effects of formalin or CFA would be both stimulus specific and temporally graded with respect to time between conditioning and injection.
Rather, the present results suggest that pain interferes with the post-conditioning processes that consolidate context conditioning into long-term memory. It has been suggested that this requires two separate learning processes [23,24]. The first involves a relatively slow process of integrating the diverse contextual cues into a unique mnemonic representation that can be easily retrieved later. The second is the association of the context representation in active memory with the fear motivational system. There are multiple lines of evidence for the dissociation of these two processes in context fear conditioning studies, however the most relevant for present purposes comes from studies investigating the effects of context pre-exposure on fear conditioning. Rats that have had an adrenalectomy, or hippocampal lesions, or that experience post-conditioning social isolation stress, morphine injections or bacterial lipopolysaccharide-induced inflammation show reduced CX- but not CS-fear. However, pre-exposing the rats to the context prior to conditioning can prevent these effects [35–37,51,52]. Similarly, rats that have had long periods of time to explore the context prior to the shock (say, 120 s) show greater CX-fear conditioning than rats that are shocked shortly after being placed in the context (<27 s). However, this immediate shock deficit can be reduced if the rats have been pre-exposed to the conditioning context [40,41]. Taken together, these observations suggest that pre-exposure sessions allow the rats time to encode a durable representation of the context into long-term memory, and this representation is then able to be rapidly retrieved and associated with the foot shock during conditioning.
The fact that formalin pain did not affect CS-fear conditioning suggests that it did not impair mechanisms that associate stimuli with the fear motivational system. Rather, Experiment 4 demonstrated that the facilitatory effect of pre-exposure on the immediate shock deficit was reduced when context pre-exposure was followed by formalin pain. This suggests that pain selectively impairs the processes that support the formation and storage of the contextual representation.
Context conditioning depends on an intact hippocampus. Lesions of the hippocampus prior to, or within 21 days of conditioning or post-conditioning hippocampal infusions of the proinflammatory cytokine interleukin-1 beta (IL-1) impairs the expression of CX- but not CS-fear at test [38,53,54]. Within the hippocampus, NMDA receptor-dependent plasticity is critical for CX-fear conditioning: Intra-hippocampal infusions of antagonists against NMDA receptors or genetic modifications of hippocampal NMDA receptors reduce CX-fear conditioning [52,55]. More recently, it has been shown that the facilitation of immediate shock by context pre-exposure can be reduced by post-exposure infusions of IL-1, anisomycin or muscimol and is associated with changes in the expression of hippocampal brain derived neurotrophic hormone (BDNF) [56–58].
Given this background, it is interesting to note that formalin and CFA increases activity in the hippocampus as measured by fMRI or PET [59–61], c-fos expression [62,63], or electrophysiological recordings of neurons [64,65]. Moreover, there is evidence that formalin pain relies on similar hippocampal NMDA receptor-dependent neuroplastic processes important for memory, because injecting lidocaine or NMDA receptor antagonists in the hippocampus reduces formalin pain [66–68]. Indeed, formalin and CFA affect a range of hippocampal processes thought to be important for learning and memory, such as BDNF [69,70] and calcium/calmodulin-dependent protein kinase II activity (pCaMK-II; [71]), and neurogenesis [72]. Importantly, formalin injected into the same region of the back used here has been shown to cause widespread changes in c-fos activity in multiple regions of the brain important for fear conditioning, such as the amygdala, medial prefrontal cortices, and hippocampus [73].
It is noteworthy that although CFA and formalin both caused memory impairments, there was a large difference in the pain-indicative behavioural responses to these stimuli. This suggests that acute pain per se may not be necessary for the memory impairments observed here. Indeed, similar context-specific temporally graded anterograde amnesia can also be induced by post-conditioning experience with a diverse range of stressors or distractors that do not cause pain. Immediate post-conditioning injection of morphine [34], bacterial lipopolysaccharides [37] or live Escherischia coli [74], intracerebral infusions with proinflammatory cytokines [56] all specifically reduce context fear conditioning. In addition, context conditioning can also be reduced by experience with psychological stressors, such as social isolation, after conditioning [36] or by the chronic administration of opioids beginning days after conditioning [35].
Rather, the pain-induced memory impairments may be due to the stimulation of CNS mechanisms common to the responses to other stressors that are known to have negative effects on cognition. There is a significant co-morbidity between chronic pain and various anxiety and depressive disorders in people [75], and animal studies have shown that noxious stimuli, including formalin and CFA, stimulate behavioural, limbic and neuroendocrine activity characteristic of stress and depression [76–79]. Indeed, the effects of CFA on hippocampal neurogenesis described above can be abolished by anti-depressants [80]. Moreover, formalin and CFA both stimulate the release of proinflammatory cytokines in the brain [50,76,81] that have been shown to negatively affect pain sensitivity, mood and cognition in other disorders [82]. Thus, pain-induced cognitive impairments may be the result of the activation of a recuperative motivational state similar to that in the sickness response. Therefore, it is not clear from these results whether it is the induction of pain per se that caused the memory impairments or whether the impairments are secondary to the motivational and emotional changes that accompany pain.
Moreover, the time course of effects of formalin and CFA differed greatly. The amnestic affects of formalin were only apparent if formalin injection occurred within 2 h of context conditioning. However, immediate post-conditioning injection with CFA also reduced context fear conditioning even though the behavioural effects of CFA injection took far longer to emerge. This leaves the possibility that the effects of formalin or CFA injection on memory occurred by different mechanisms. Some models of memory suggest that different neurobiological processes contribute to the consolidation of memory at different stages after an experience. For instance, memories may be initially stored as patterns of synaptic activity in the hippocampus which, through processes involving long-term potentiation, protein synthesis and synaptic plasticity, transition into different stores in various cortical structures [83,84]. Thus, the possibility remains that the rapid and acute pain induced by formalin affects different stages of the memory consolidation process than the more slowly developing and chronic pain induced by CFA.
The results reported here are consistent with experimental reports with human subjects that pain can interfere with memory. For instance, chronic pain patients (who reported pain during testing) showed an overall reduction in learning a list of words compared to healthy controls [18], and performance in healthy volunteers was also affected if they experienced pain via the cold-pressor test during word list learning and/or during retrieval [18,20,21]. In addition, Grisart and Van der Linden [17] showed that chronic pain patients had deficits in memory tasks that required explicit control of memory rather than ones that relied on automatic retrieval of information from memory. This is interesting given the central role of the hippocampus in human declarative memory [85]. In fact, it should be noted here that the amnesic patient H.M., who famously received a bilateral resection of his medial temporal lobes to treat epilepsy and developed impairments in consolidating new information into declarative long-term memory, was also largely indifferent to pain [86]. Given that the present results suggest that pain has a specific effect on a hippocampal-dependent memory system with a high information-processing load, it would seem that studying how pain affects contextual fear conditioning in rats would provide essential insights into how pain impairs memory in humans.
Acknowledgments
This research was supported by Australian Research Council grant DP0667314, and NIH grant DA024044.
References
- 1.Munoz M, Esteve R. Reports of memory functioning by patients with chronic pain. Clin J Pain. 2005;21(4):287–91. doi: 10.1097/01.ajp.0000173993.53733.2e. [DOI] [PubMed] [Google Scholar]
- 2.Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch Phys Med Rehabil. 2005;86(6):1147–54. doi: 10.1016/j.apmr.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 3.McCracken LM, Iverson GL. Predicting complaints of impaired cognitive functioning in patients with chronic pain. J Pain Symptom Manage. 2001;21(5):392–6. doi: 10.1016/s0885-3924(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 4.Jamison RN, Rock DL, Parris WC. Empirically derived Symptom Checklist 90 subgroups of chronic pain patients: a cluster analysis. J Behav Med. 1988;11(2):147–58. doi: 10.1007/BF00848262. [DOI] [PubMed] [Google Scholar]
- 5.Schnurr RF, MacDonald MR. Memory complaints in chronic pain. Clin J Pain. 1995;11(2):103–11. doi: 10.1097/00002508-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Iezzi T, Archibald Y, Barnett P, Klinck A, Duckworth M. Neurocognitive performance and emotional status in chronic pain patients. J Behav Med. 1999;22(3):205–16. doi: 10.1023/a:1018791622441. [DOI] [PubMed] [Google Scholar]
- 7.Iezzi T, Duckworth MP, Vuong LN, Archibald YM, Klinck A. Predictors of neurocognitive performance in chronic pain patients. Int J Behav Med. 2004;11(1):56–61. doi: 10.1207/s15327558ijbm1101_7. [DOI] [PubMed] [Google Scholar]
- 8.Povedano M, Gascon J, Galvez R, Ruiz M, Rejas J. Cognitive function impairment in patients with neuropathic pain under standard conditions of care. J Pain Symptom Manage. 2007;33(1):78–89. doi: 10.1016/j.jpainsymman.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Kewman DG, Vaishampayan N, Zald D, Han B. Cognitive impairment in musculoskeletal pain patients. Int J Psychiatry Med. 1991;21(3):253–62. doi: 10.2190/FRYK-TMGA-AULW-BM5G. [DOI] [PubMed] [Google Scholar]
- 10.Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain. 2002;96(3):279–84. doi: 10.1016/S0304-3959(01)00457-2. [DOI] [PubMed] [Google Scholar]
- 11.Tassain V, Attal N, Fletcher D, Brasseur L, Degieux P, Chauvin M, et al. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non-cancer pain. Pain. 2003;104(1–2):389–400. doi: 10.1016/s0304-3959(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 12.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7(1):60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 13.Hart RP, Martelli MF, Zasler ND. Chronic pain and neuropsychological functioning. Neuropsychol Rev. 2000;10(3):131–49. doi: 10.1023/a:1009020914358. [DOI] [PubMed] [Google Scholar]
- 14.Oosterman JM, de Vries K, Dijkerman HC, de Haan EH, Scherder EJ. Exploring the relationship between cognition and self-reported pain in residents of homes for the elderly. Int Psychogeriatr. 2009;21(1):157–63. doi: 10.1017/S1041610208007941. [DOI] [PubMed] [Google Scholar]
- 15.Grace GM, Nielson WR, Hopkins M, Berg MA. Concentration and memory deficits in patients with fibromyalgia syndrome. J Clin Exp Neuropsychol. 1999;21(4):477–87. doi: 10.1076/jcen.21.4.477.876. [DOI] [PubMed] [Google Scholar]
- 16.Radanov BP, Di Stefano G, Schnidrig A, Sturzenegger M. Psychosocial stress, cognitive performance and disability after common whiplash. J Psychosom Res. 1993;37(1):1–10. doi: 10.1016/0022-3999(93)90118-y. [DOI] [PubMed] [Google Scholar]
- 17.Grisart JM, Van der Linden M. Conscious and automatic uses of memory in chronic pain patients. Pain. 2001;94(3):305–13. doi: 10.1016/S0304-3959(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 18.Pearce SA, Isherwood S, Hrouda D, Richardson PH, Erskine A, Skinner J. Memory and pain: tests of mood congruity and state dependent learning in experimentally induced and clinical pain. Pain. 1990;43(2):187–93. doi: 10.1016/0304-3959(90)91072-Q. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz J, Bromm B. Event-related potential correlates of interference between cognitive performance and tonic experimental pain. Psychophysiology. 1997;34(4):436–45. doi: 10.1111/j.1469-8986.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuhajda MC, Thorn BE, Klinger MR. The effect of pain on memory for affective words. Ann Behav Med. 1998;20(1):31–5. doi: 10.1007/BF02893806. [DOI] [PubMed] [Google Scholar]
- 21.Seltzer SF, Yarczower M. Selective encoding and retrieval of affective words during exposure to aversive stimulation. Pain. 1991;47(1):47–51. doi: 10.1016/0304-3959(91)90010-U. [DOI] [PubMed] [Google Scholar]
- 22.Livengood JM. Psychologic issues in chronic pain: Possible imperdiments to successful treatment outcomes. Curr Pain Headache Rep. 1997;1:320–3. [Google Scholar]
- 23.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 24.Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16(10):573–85. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 26.Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988;31(2):455–551. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- 27.Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J Pain. 2001;2(1):2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi Y, Chiba T, Kurokawa M, Aoki Y. Dermatomes and the central organization of dermatomes and body surface regions in the spinal cord dorsal horn in rats. J Comp Neurol. 2003;462(1):29–41. doi: 10.1002/cne.10669. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Y, Chiba T, Kurokawa M, Aoki Y, Takahashi K, Yamagata M. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine. 2003;28(9):871–80. doi: 10.1097/01.BRS.0000058717.43888.B9. [DOI] [PubMed] [Google Scholar]
- 30.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4(2):161–74. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 31.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861(1):105–16. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 32.Harvey LO. Efficient estimation of sensory thresholds. Behav Res Methods Instr Comp. 1986;18:623–32. [Google Scholar]
- 33.Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- 34.Rudy JW, Kuwagama K, Pugh CR. Isolation reduces contextual but not auditory-cue fear conditioning: a role for endogenous opioids. Behav Neurosci. 1999;113(2):316–23. doi: 10.1037//0735-7044.113.2.316. [DOI] [PubMed] [Google Scholar]
- 35.McNally GP, Westbrook RF. Temporally graded, context-specific retrograde amnesia and its alleviation by context preexposure: effects of postconditioning exposures to morphine in the rat. J Exp Psychol Anim Behav Process. 2003;29(2):130–42. doi: 10.1037/0097-7403.29.2.130. [DOI] [PubMed] [Google Scholar]
- 36.Rudy JW. Postconditioning isolation disrupts contextual conditioning: an experimental analysis. Behav Neurosci. 1996;110(2):238–46. doi: 10.1037//0735-7044.110.2.238. [DOI] [PubMed] [Google Scholar]
- 37.Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12(3):212–29. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- 38.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 39.Albert M, Ayres JJB. One-trial simultaneous and backward excitatory fear conditioning in rats: lick suppression, freezing, and rearing to the CS compounds and their elements. Anim Learn Behav. 1997;25:210–20. [Google Scholar]
- 40.Kiernan MJ, Westbrook RF. Effects of exposure to a to-be-shocked environment upon the rat's freezing response: evidence for facilitation, latent inhibition, and perceptual learning. Q J Exp Psychol B. 1993;46(3):271–88. doi: 10.1080/14640749308401089. [DOI] [PubMed] [Google Scholar]
- 41.Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–70. [Google Scholar]
- 42.Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101(4):1127–35. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 43.Pearson C, Wood FD. Studies of polyarthritis and other lesions in rats by injection of mycobacterial adjuvant. I. General clinical and pathological characterstics and some modifying factors. Arthritis Rheum. 1959;2:440–59. [Google Scholar]
- 44.Rainsford KD. Adjuvant polyarthritis in rats: is this a satisfactory model for screening anti-arthritic drugs? Agents Actions. 1982;12(4):452–8. doi: 10.1007/BF01965926. [DOI] [PubMed] [Google Scholar]
- 45.Calza L, Pozza M, Arletti R, Manzini E, Hokfelt T. Long-lasting regulation of galanin, opioid, and other peptides in dorsal root ganglia and spinal cord during experimental polyarthritis. Exp Neurol. 2000;164(2):333–43. doi: 10.1006/exnr.2000.7442. [DOI] [PubMed] [Google Scholar]
- 46.Wiertelak EP, Furness LE, Horan R, Martinez J, Maier SF, Watkins LR. Subcutaneous formalin produces centrifugal hyperalgesia at a non-injected site via the NMDA-nitric oxide cascade. Brain Res. 1994;649(1–2):19–26. doi: 10.1016/0006-8993(94)91044-8. [DOI] [PubMed] [Google Scholar]
- 47.Bianchi M, Panerai AE. Formalin injection in the tail facilitates hindpaw withdrawal reflexes induced by thermal stimulation in the rat: effect of paracetamol. Neurosci Lett. 1997;237(2–3):89–92. doi: 10.1016/s0304-3940(97)00819-7. [DOI] [PubMed] [Google Scholar]
- 48.Larson AA, Brown DR, el-Atrash S, Walser MM. Pain threshold changes in adjuvant-induced inflammation: a possible model of chronic pain in the mouse. Pharmacol Biochem Behav. 1986;24(1):49–53. doi: 10.1016/0091-3057(86)90043-2. [DOI] [PubMed] [Google Scholar]
- 49.Gutstein HB, Trujillo KA, Akil H. Does chronic nociceptive stimulation alter the development of morphine tolerance? Brain Res. 1995;680(1–2):173–9. doi: 10.1016/0006-8993(95)00259-s. [DOI] [PubMed] [Google Scholar]
- 50.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20(2):467–73. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 51.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111(3):503–11. [PubMed] [Google Scholar]
- 52.Young SL, Bohenek DL, Fanselow MS. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci. 1994;108(1):19–29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- 53.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88(2):261–74. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 54.Hein AM, Stutzman DL, Bland ST, Barrientos RM, Watkins LR, Rudy JW, et al. Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience. 2007;150(4):754–63. doi: 10.1016/j.neuroscience.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290(5494):1170–4. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 56.Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134(1–2):291–8. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 57.Barrientos RM, O'Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav brain Res. 2002;134(1–2):299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 58.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role ofthe dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shih YY, Chang C, Chen JC, Jaw FS. BOLD fMRI mapping of brain responses to nociceptive stimuli in rats under ketamine anesthesia. Med Eng Phys. 2008;30(8):953–8. doi: 10.1016/j.medengphy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Shih YY, Chen YY, Chen CC, Chen JC, Chang C, Jaw FS. Whole-brain functional magnetic resonance imaging mapping of acute nociceptive responses induced by formalin in rats using atlas registration-based event-related analysis. J Neurosci Res. 2008;86(8):1801–11. doi: 10.1002/jnr.21638. [DOI] [PubMed] [Google Scholar]
- 61.Shih YY, Chiang YC, Chen JC, Huang CH, Chen YY, Liu RS, et al. Brain nociceptive imaging in rats using (18)f-fluorodeoxyglucose small-animal positron emission tomography. Neuroscience. 2008;155(4):1221–6. doi: 10.1016/j.neuroscience.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Aloisi AM, Zimmermann M, Herdegen T. Sex-dependent effects of formalin and restraint on c-Fos expression in the septum and hippocampus of the rat. Neuroscience. 1997;81(4):951–8. doi: 10.1016/s0306-4522(97)00270-4. [DOI] [PubMed] [Google Scholar]
- 63.Khanna S, Chang LS, Jiang F, Koh HC. Nociception-driven decreased induction of Fos protein in ventral hippocampus field CA1 of the rat. Brain Res. 2004;1004(1-2):167–76. doi: 10.1016/j.brainres.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 64.Tai SK, Huang FD, Moochhala S, Khanna S. Hippocampal theta state in relation to formalin nociception. Pain. 2006;121(1–2):29–42. doi: 10.1016/j.pain.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 65.Khanna S. Dorsal hippocampus field CA1 pyramidal cell responses to a persistent versus an acute nociceptive stimulus and their septal modulation. Neuroscience. 1997;77(3):713–21. doi: 10.1016/s0306-4522(96)00456-3. [DOI] [PubMed] [Google Scholar]
- 66.McKenna JE, Melzack R. Analgesia produced by lidocaine microinjection into the dentate gyrus. Pain. 1992;49(1):105–12. doi: 10.1016/0304-3959(92)90195-H. [DOI] [PubMed] [Google Scholar]
- 67.McKenna JE, Melzack R. Blocking NMDA receptors in the hippocampal dentate gyrus with AP5 produces analgesia in the formalin pain test. Exp Neurol. 2001;172(1):92–9. doi: 10.1006/exnr.2001.7777. [DOI] [PubMed] [Google Scholar]
- 68.Soleimannejad E, Naghdi N, Semnanian S, Fathollahi Y, Kazemnejad A. Antinociceptive effect of intra-hippocampal CA1 and dentate gyrus injection of MK801 and AP5 in the formalin test in adult male rats. Eur J Pharmacol. 2007;562(1–2):39–46. doi: 10.1016/j.ejphar.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 69.Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81(3):193–9. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- 70.Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005;133(4):999–1006. doi: 10.1016/j.neuroscience.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Choi SS, Seo YJ, Shim EJ, Kwon MS, Lee JY, Ham YO, et al. Involvement of phos-phorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Res. 2006;1108(1):28–38. doi: 10.1016/j.brainres.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 72.Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7(8):544–55. doi: 10.1016/j.jpain.2006.01.458. [DOI] [PubMed] [Google Scholar]
- 73.Ohtori S, Takahashi K, Chiba T, Takahashi Y, Yamagata M, Sameda H, et al. Fos expression in the rat brain and spinal cord evoked by noxious stimulation to low back muscle and skin. Spine. 2000;25(19):2425–30. doi: 10.1097/00007632-200010010-00002. [DOI] [PubMed] [Google Scholar]
- 74.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–32. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety. 2009;26(10):888–901. doi: 10.1002/da.20600. [DOI] [PubMed] [Google Scholar]
- 76.Jia D, Gao GD, Liu Y, He SM, Zhang XN, Zhang YF, et al. TNF-alpha involves in altered prefrontal synaptic transmission in mice with persistent inflammatory pain. Neurosci Lett. 2007;415(1):1–5. doi: 10.1016/j.neulet.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 77.Boyce-Rustay JM, Zhong C, Kohnken R, Baker SJ, Simler GH, Wensink EJ, et al. Comparison of mechanical allodynia and the affective component of inflammatory pain in rats. Neuropharmacology. 2009;58(2):537–43. doi: 10.1016/j.neuropharm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 78.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31(4):739–50. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 79.Wang HC, Wang YC, Huang AC, Chai SC, Wu YS, Wang CC. Roles of corticosterone in formalin-induced conditioned place aversion in rats. Neurosci Lett. 2009;464(2):122–6. doi: 10.1016/j.neulet.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 80.Duric V, McCarson KE. Effects of analgesic or antidepressant drugs on pain-or stress-evoked hippocampal and spinal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression in the rat. J Pharmacol Exp Ther. 2006;319(3):1235–43. doi: 10.1124/jpet.106.109470. [DOI] [PubMed] [Google Scholar]
- 81.Yabuuchi K, Maruta E, Minami M, Satoh M. Induction of interleukin-1 beta mRNA in the hypothalamus following subcutaneous injections of formalin into the rat hind paws. Neurosci Lett. 1996 Mar;207(2):109–12. doi: 10.1016/0304-3940(96)12505-2. [DOI] [PubMed] [Google Scholar]
- 82.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005 Feb;257(2):139–55. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 83.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997 Nov;68(3):285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 84.McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995 Jul;102(3):419–57. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 85.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 86.Corkin S. What's new with the amnesic patient H.M.? Nat Rev Neurosci. 2002 Feb;3(2):153–60. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]


