Abstract
Migraine headaches are debilitatingly painful and poorly managed. Facial allodynia is often associated with migraine, and clinical evidence indicates that it is a critical point in migraine progression. That is, if the migraine can be treated prior to the onset of facial allodynia, the migraine can be halted using triptans, whereas if treatment is administered after facial allodynia has begun, the treatment is ineffective. The meninges and the immune cells therein have been implicated in migraine facial pain. Indeed, application of inflammatory mediators over the meninges has been used to study changes in pain responsive neurons in trigeminal complex, and changes in their receptive fields. Much of this research has been carried out in anesthetized rats, which limits the clinical application. Our indwelling supradural catheter model, in which inflammatory mediators can be administered to the meninges in awake and freely moving rats, allows for the assessment of behavioral changes shortly after injection. Following administration of inflammatory soup (histamine, serotonin, bradykinin, and prostaglandin E2) or the immunogenic HIV-1 coat protein gp120 results in reliable periorbital mechanical allodynia. This model provides an additional means to study the neurocircuitry and neuropharmacology of facial allodynia. Here, we describe detailed methods for the placement of the catheter, injection procedures, and assessment of facial allodynia.
Keywords: Facial allodynia, Meninges, Migraine, Supradural catheter, Animal model
1. Introduction
Within the migraine population, facial allodynia (1, 2) and corpalgia (extracephalic allodynia) (3) are commonly experienced. Electrophysiological studies have shown that application of immune mediators over the meninges leads to enhanced responsivity to facial stimulation in the brain stem (4, 5). Much of what has been learned to date has been derived from studies performed in anesthetized rats. These studies are limiting because (1) the anesthetics used are known to alter glial and neuronal activity as well as immune activity (6–12) and (2) the nature of the studies are such that the behavioral manifestations of immunogenic manipulation of the meninges cannot be measured, nor can they be manipulated.
The supradural catheter model was developed based on the findings from electrophysiological studies that application of inflammatory mediators over the transverse sagittal sinus activates neurons in the trigeminal nucleus caudalis (5). Thus, our model places the catheter tip such that it terminates at the superior sagittal sinus. Injection of proinflammatory mediators (histamine, serotonin, bradykinin, and prostaglandin E2) via the supradural catheters induces periorbital mechanical allodynia, validating this method as a model of facial allodynia (13). We further showed that application of the immunogenic HIV-1 coat protein gp120 via the supra-dural catheter also induces periorbital mechanical allodynia, supporting the hypothesis that the resident immune cells of the meninges can modulate facial allodynia.
Here, the procedure for supradural catheterization is thoroughly described, as is the procedure for supradural injections. The method for measuring facial allodynia is modified from Ren (14), and is also presented in detail. Determining the 50% threshold from the observed data will be described.
2. Materials
2.1. Indwelling Supradural Catheter Construction and Preparation
Sterile polyethylene tubing (PE-10, Becton Dickinson, Franklin Lakes, NJ).
Scalpel handle with no. 11 scalpel blade.
Sterile 15-mL conical tube (Fisher Scientific, Houston, TX).
Sterile saline.
75% Ethanol.
Black permanent marker.
2.2. Indwelling Supradural Catheter Surgery
2.2.1. Equipment
Stereotaxic apparatus (Kopf Instruments, Tujunga, CA).
Ultra hot glass-bead sterilizer (World Precision Instruments, Sarasota, FL) to sterilize tools before and between uses.
Shaver.
Scalpel handle with no. 11 or no. 10 scalpel blades.
Sterile gauze (4 × 4 in.) to create a drape around the surgical site.
Sterile Q-tips to absorb blood and apply hydrogen peroxide.
Handheld drill used with a Dremel #107 engraving cutter bit.
#5 Fine-tipped forceps (Fine Science Tools, Foster City, CA; # 11254-20).
#7 Fine-tipped curved forceps (Fine Science Tools, #11274-20).
Two serrefine clamps (Fine Science Tools, #18050-35).
50-μL Hamilton syringe.
9-mm Stainless steel wound clips (Stoelting, Wood Dale, IL).
Wound clip applicator (Fine Science Tools, #12031-09).
Supradural injectors.
2.2.2. Reagents
Isoflurane (Halocarbon Laboratories, River Edge, NJ).
Oxygen.
Betadine.
3% Hydrogen peroxide.
Superglue (cyanoacrylate).
Polysporin antibiotic (Pfizer, New York, NY).
Sterile saline.
2.3. Supradural Catheters Injectors and Injection
30-G, ½-in. hypodermic needles (Becton Dickinson, Franklin Lakes, NJ) with plastic hub removed.
PE-10 tubing (PE-10, Becton Dickinson, Franklin Lakes, NJ).
Autoclaved 100-μL Hamilton glass syringes (Fisher Scientific).
Infusion pump (Razel Scientific Instruments, INC, Standford, CT; Model A-99, pump set at 36.9).
2.4. Low-Threshold Mechanical Test
Leather glove.
Calibrated Semmes–Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL). The hair stiffness ranges from 4.56 (4.00 g) through 5.88 (60.00 g) in a logarithmic series of seven monofilaments. Log stiffness of the hairs is defined as Log10 (mg × 10) ∼ nomenclature recognized by the intended reader. The seven stimuli have the following log stiffness values: 4.56 (4 g), 4.74 (6 g), 4.93 (8 g), 5.07 (10 g), 5.18 (15 g), 5.46 (26 g), and 5.88 (60 g).
PsychoFit is the computer program used to estimate 50% threshold values, and is available for both Macintosh and PC computers. The software can be downloaded from L.O. Harvey's website (http://psych.colorado.edu/∼lharvey).
3. Methods
The methods and procedures delineated here are for: (1) construction and preparation of supradural catheters; (2) bilateral supradural catheter placement (see Note 1); (3) construction and preparation of supradural catheter injectors; (4) administration of immunogenic stimuli via supradural catheters; and (5) assessing facial allodynia using von Frey filaments and submitting data to PsychoFit. The goal in the development of this model was to test immunogenic stimuli, and as such, all procedures are performed under sterile conditions. Procedures described for constructing catheters and injectors are carried out on sterile autoclave paper, and all instruments are sterilized using the hot-bead sterilizer.
3.1. Preparation of Supradural Catheter
PE-10 tubing is cut in 6–8 cm lengths (see Note 2).
Prior to the day of surgery, catheters are filled with sterile saline and heat sealed at both ends such that no air bubbles are in the catheter.
Catheters are stored in 75% ethanol for 24 h prior to surgery.
Before implantation, catheters are removed from ethanol, dried, and marked with permanent marker at 4 mm from the end of the catheter that is to be threaded under the skull during surgery, and transferred to sterile saline.
3.2. Supradural Catheter Placement Surgery
All surgical tools are sterilized in a glass-bead sterilizer before surgical procedures, and care is taken to keep the surgery environment as clean as possible.
Animal is placed in stereotaxic apparatus, and secured with ear bars maintaining the head in a level position.
Surgery is carried out under isoflurane anesthesia, 2.5% in oxygen, which is chosen because it has minimal effects on immune cell function compared to other commonly used anesthetics (6– 12).
The surgical site is shaved and topical Betadine is liberally applied to the scalp.
A 3-cm incision is then made to expose bregma.
3% Hydrogen peroxide is applied to the skull surface to minimize bleeding.
The surgical site remained exposed through the use of bilateral serrefine clamps directly attached to the scalp.
The handheld drill is used with Dremel #107 engraving cutter bit to bore an 8- 10-mm-long bilateral trough in the skull. The trough is approximately 2 mm wide and is made 3–4 mm lateral to bregma on either side (see Note 3). Caution is taken not to penetrate the dura. Troughs are drilled such that the thinnest points are 1 mm caudal to bregma (Fig. 1).
Catheters are inserted horizontally along the troughs at the 1 mm caudal point to the 4-mm mark on the catheter.
Once placed, catheters are secured with superglue, and allowed to dry for approximately 15 min.
Catheters are flushed with 5 μL of sterile saline through using a 50-μL Hamilton syringe, making sure to avoid any air bubbles thereby to preventing clogging (see Note 4). The catheter is then resealed with heated forceps.
The wound is then sealed with 9-mm wound clips.
Powdered Polysporin antibiotic is liberally applied to the surgical site.
Rats are removed from stereotaxic apparatus and transferred to a heated recovery box to recover. Animals are returned to their home cage once ambulatory (see Note 5).
Fig. 1.
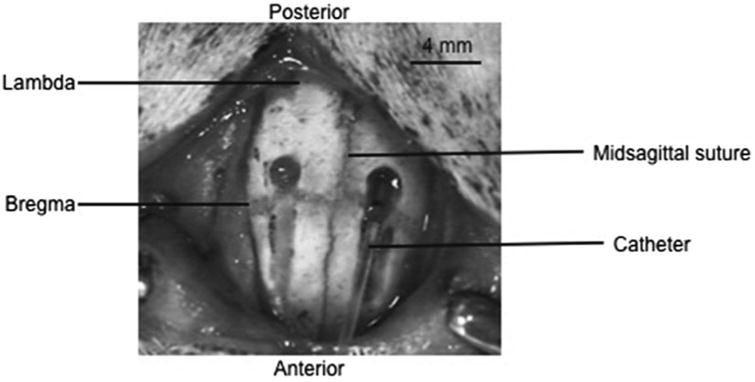
This picture shows the drilled troughs, with a catheter, made from PE-10 tubing, guided into place via the trough. The troughs are drilled 2–3 mm lateral of midline, and stop 1 mm posterior of bregma. The troughs are approximately 4 mm in length, gradually becoming deeper at the most posterior point. The skull is pierced and caution is taken not to penetrate the dura. Modified with permission from ref. 13.
3.3. Supradural Catheter Injectors and Injection Procedures
3.3.1. Supradural Catheter Injectors
Remove the plastic hub from a sterile 30-G, ½-in. hypodermic needle, and insert blunt end into a 30 cm length of PE-10 tubing.
Insert an intact 30-G, ½-in. hypodermic needle into the open end of the PE-10 tubing.
Store prepared injectors in a sterile and dry container until needed.
3.3.2. Supradural Catheter Injections
Tightly connect a PE-10 catheter injector to a 50-μL autoclaved Hamilton glass syringe, both flushed with sterile, endotoxin-free water, and creating an airtight seal.
Create an air bubble by drawing up 1–2 μL prior and then draw up immunogenic stimulus. We have found that the optimal volume of injection is 6 μL. Sufficient volume is drawn up to accommodate injection into the left and right catheter. The air bubble serves as a marker that can be followed during drug administration. Stable, consistent movement of the bubble indicates a good injection, whereas slow movement or compression of the air bubble and lack of movement indicate problems with drug delivery that may confound behavioral testing.
The Hamilton syringe is situated in the infusion pump, and integrity and flow of the injector are confirmed.
Animals are gently wrapped in a towel such that the hands of individual holding the animal is protected from the rat's paws.
The sealed catheter tips are clipped, and the injector connected to the catheter. The injection is slowly passed through the catheter via the infusion pump such that 6 μL is infused over 2 min and 16 s. Following the infusion, the injector remains connected to the catheter for 1 min.
The injector is removed from the catheter, and the tip resealed using heated forceps, and the other catheter injected.
A second injection is then given 2 h after the first. We have shown that this paradigm testing inflammatory soup or gp120 is required to successfully induce facial allodynia (see Note 6) (13).
3.4. Low-Threshold Periorbital Mechanical Allodynia
All animals are habituated to the testing environment as previously described (see Note 7). Briefly, rats are placed, and encouraged to stay, in a leather testing glove in a quiet room for 5 days, 5 min/day prior to surgery (Fig. 2).
Baseline measures are collected the day before surgery, and again just prior to administration of immunogenic stimuli (usually 4–5 days postsurgery).
On the day of testing, the rat is placed in the leather testing glove for 5 min. At the end of the 5 min period, baseline responses to monofilaments is assessed. The periorbital region is stimulated five times with each filament for 2 s on each side of the face, alternating sides, and separating stimulations by approximately 3 s.
A response is counted when the animals moves away from the von Frey stimulus, or moves the stimulus away by paw swipe.
Our testing paradigm begins with testing the behavior response to the lowest monofilament, 4.56 (4 g), and recording responses, until the animal responds to all five stimulations with a single monofilament.
All responses are recorded and submitted to “PsychoFit (fat)” to assess 50% threshold values (see Note 8).
When the experimental paradigm is such that both left and right sides receive the same manipulation, the data from both sides can be averaged prior to analysis.
All testing is performed blinded with respect to group assignment.
Fig. 2.
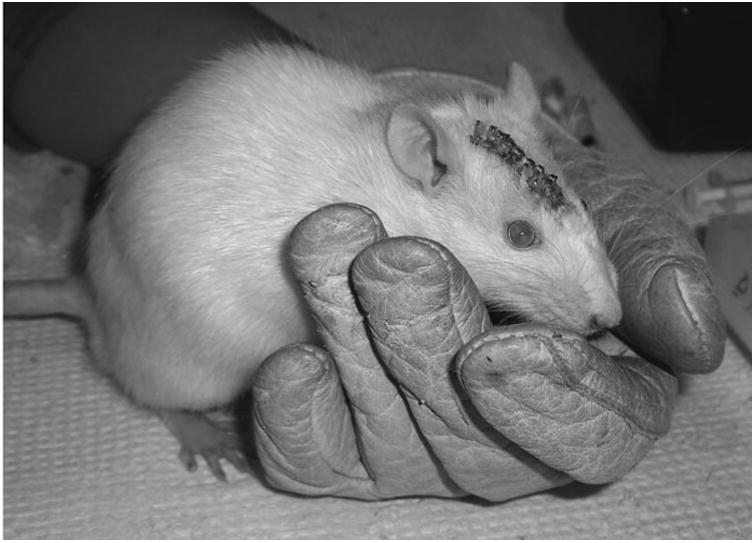
The rat is held in a leather glove for facial allodynia testing. Prior to experimental manipulation, rats are habituated to the glove, and encouraged to stay in the glove during daily habituations over a 5-day period.
4. Notes
We have had the most success performing surgeries on rats 250–350 g, and optimal results in rats 300 g or less. Surgical difficulties have been encountered when larger animals are used. The skulls of the larger animals are noticeably different from the smaller rats, and because of this, the method used for drilling has to be modified dependent on the animal.
Care should be taken in cutting the catheter to size. If the catheter is longer than described, the rats tend to pull them out. A shorter catheter limits the number of possible injections.
A significant challenge of these surgeries is placing the catheter without puncturing the dura. An additional challenge is to place the catheter 2–3 mm lateral to the midsagittal sinus, and 1–2 mm anterior to the transverse sagittal sinus. If the catheter falls beyond these boundaries, behavioral changes in response to immune stimuli are not detectable. The gradually descending troughs were developed to deal with these two issues. When done correctly, the troughs guide between the skull and dura, aiding in not puncturing the dura, and guiding a straight catheter to 2–3 mm from midline.
Clogged catheters can be an issue in this model. Keeping the catheters free of air bubbles eliminates clogging, as does maintaining an extremely clean surgical environment. Here, all tools are sterilized between animals, and gloves are worn for all surgical procedures, during construction of catheters and injectors, and during injections. Using these precautions, it has been our experience that these catheters remain viable for up to 20 days postsurgery.
With practice, an expert surgeon can expect to complete the surgery placing bilateral supradural catheters within 25 min, with a success rate routinely of 100%. A novice to these surgical procedures can expect to complete the surgery within 45–50 min. With experience, the success rate is approximately 84%, with rats being excluded from analysis due to catheters that are misplaced or dislodged/missing (<1%) or to have pierced the dura (∼16%).
Experimental verification of the methods can be tested by two injections of inflammatory soup (5, 13) consisting of 1 mM histamine, serotonin, and bradykinin, and 0.1 mM prostaglandin E2, separated by 2 h. Reliable periorbital mechanical allodynia is observable 2 h after the second injection.
We have found that accurate behavioral measurement is dependent on the amount of handling the rats experience. Rats are given 1 week to acclimate to the colony room, with handling beginning 4 days after the arrive, and continuing to the day of surgery, 10 days after they arrive.
Analysis of behavioral data is as previously described by Milligan et al. (15) with two exceptions. In the downloaded template from L.O. Harvey's website (1) the Alpha (starting value) is set to 4.56 instead of 3.61 and (2) the log force of the stimuli need to changed to the values listed above (See Subheading 2.4, item 2).
Acknowledgments
We would like to thank John Mahoney and Niloofar Rezvani for technical assistance. This work was supported by GlaxoSmithKline, and NIH grants DA015642, DA022042, and DE017782, and well as the University of Colorado Undergraduate Research Opportunities Program.
References
- 1.Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol (Paris) 2005;161:658–660. doi: 10.1016/s0035-3787(05)85109-4. [DOI] [PubMed] [Google Scholar]
- 2.Vickers ER, Cousins MJ. Neuropathic orofacial pain part 1 – prevalence and pathophysiology. Aust Endod J. 2000;26:19–26. doi: 10.1111/j.1747-4477.2000.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 3.Cuadrado ML, Young WB, Fernandezde-las-Penas C, Arias JA, Pareja JA. Migrainous corpalgia: body pain and allodynia associated with migraine attacks. Cephalalgia. 2008;28:87–91. doi: 10.1111/j.1468-2982.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 4.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 5.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 6.Itoh T, Hirota K, Hisano T, Namba T, Fukuda K. The volatile anesthetics halothane and isoflurane differentially modulate proinflammatory cytokine-induced p38 mitogen-activated protein kinase activation. J Anesth. 2004;18:203–209. doi: 10.1007/s00540-004-0237-5. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Aono M, Lee Y, Massey G, Pearlstein RD, Warner DS. Effects of volatile anesthetics on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cultures. Anesthesiology. 2001;95:756–765. doi: 10.1097/00000542-200109000-00031. [DOI] [PubMed] [Google Scholar]
- 8.Martin DC, Plagenhoef M, Abraham J, Dennison RL, Aronstam RS. Volatile anesthetics and glutamate activation of N-methyl-D-aspartate receptors. Biochem Pharmacol. 1995;49:809–817. doi: 10.1016/0006-2952(94)00519-r. [DOI] [PubMed] [Google Scholar]
- 9.Roughan JV, Laming PR. Large slow potential shifts occur during halothane anaesthesia in gerbils. J Comp Physiol [A] 1998;182:839–848. doi: 10.1007/s003590050228. [DOI] [PubMed] [Google Scholar]
- 10.Toda N, Toda H, Hatano Y. Anesthetic modulation of immune reactions mediated by nitric oxide. J Anesth. 2008;22:155–162. doi: 10.1007/s00540-007-0590-2. [DOI] [PubMed] [Google Scholar]
- 11.Wentlandt K, Samoilova M, Carlen PL, El Beheiry H. General anesthetics inhibit gap junction communication in cultured organotypic hippocampal slices. Anesth Analg. 2006;102:1692–1698. doi: 10.1213/01.ane.0000202472.41103.78. [DOI] [PubMed] [Google Scholar]
- 12.Wise-Faberowski L, Pearlstein RD, Warner DS. NMDA-induced apoptosis in mixed neuronal/glial cortical cell cultures: the effects of isoflurane and dizocilpine. J Neurosurg Anesthesiol. 2006;18:240–246. doi: 10.1097/00008506-200610000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Wieseler J, Ellis A, Sprunger D, Brown K, McFadden A, Mahoney J, Rezvani N, Maier SF, Watkins LR. A novel method for modeling facial allodynia associated with migraine in awake and freely moving rats. J Neurosci Methods. 2009;185:236–245. doi: 10.1016/j.jneumeth.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 15.Milligan ED, Maier SF, Watkins LR. Sciatic inflammatory neuropathy in the rat: surgical procedures, induction of inflammation, and behavioral testing. Methods Mol Med. 2004;99:67–89. doi: 10.1385/1-59259-770-X:067. [DOI] [PubMed] [Google Scholar]


