Abstract
Formaldehyde (FA) is an environmental chemical classified as a human carcinogen. It is highly reactive and can bind covalently with hemoglobin (Hb) to produce Hb adducts. Measurement of these Hb adducts provides valuable information about exposure to this chemical. We developed a robust, ultraperformance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) method for quantifying FA-Hb adducts in red blood cells. The method measures the FA-VHLTPEEK peptide after trypic digestion. The peptide is a FA adduct at the N-terminus of the beta chain of human Hb. Method mean (±SD) accuracy, determined by recovery in quality control and blank material was 103.2% ± 8.11. The mean among-day and within-day coefficients of variation determined at three concentration levels (%CV) were 9.2% (range: 7.2–10.2%) and 4.9% (range 3.1–7.3%), respectively. The limit of detection was 3.4 nmol/g Hb. This method was applied to the analysis of 135 human blood samples, and FA-VHLTPEEK was detected in all study samples. FA-VHLTPEEK concentrations were not significantly different between smokers and nonsmokers. This work is the first validated UPLC–MS/MS method in which a FA peptide derived from a FA-Hb adduct could be used to monitor exposure to FA in population studies.
Graphical Abstract

1. INTRODUCTION
Formaldehyde (FA) is a chemical frequently used in the preparation of building materials and furniture,1 and it is a major byproduct of combustion processes including tobacco smoke.2,3 Inhalation of FA released from these materials and processes is assumed to be one of the exposure paths in the general population.4 FA is also produced in the body as part of normal metabolism of serine, glycine, and choline5 from the demethylation of N,O- and S-methyl xenobiotics such as p-methoxyacetophenone, and dimethylsulfate, and from methanol oxidation by ADH1.3,6 The International Agency for Research on Cancer (IARC) classified FA as a human carcinogen (Group 1),3 and the National Toxicology Program (NTP) listed it as “known to be a human carcinogen”.4 Research studies found FA exposure is associated with increased risks of nasopharyngeal, sinonasal, and lymphohematopoietic cancers4 and increased mortality rate from certain types of leukemia.7 However, the role of endogenous FA on cancer risk as well as the exact mode of action and toxicokinetics of FA from inhalation exposure is still subject to discussions.8 One reason is the lack of analytical methods for measuring FA exposure in humans.
In the human body, FA is metabolized to formate and then excreted. However, it is highly reactive and also forms covalent bonds with DNA and proteins (so-called “adducts”). FA adducts with albumin,9 insulin,10 and hemoglobin (Hb)11,12 were reported. Hb adducts can accumulate in the blood during the life span of the red blood cells (RBCs), allowing for an exposure assessment covering the past three months.13 These adducts, especially adducts at the N-terminus, have been used successfully as biomarkers of exposure to reactive chemicals such as acrylamide, butadiene, ethylene oxide, and acrylonitrile.13–18 The N-terminal FA adduct to Hb showed a positive correlation with occupational exposure to FA, suggesting that such adducts are suitable biomarkers for assessing exposure to FA.19,20 However, the adducts reported in these studies are known to be susceptible to hydrolysis19–21 and require stabilization by reduction with NaBH4 to N-methylenvaline. The stabilized adduct must then undergo a modified Edman reaction prior to analysis. We recently identified an imidazolidone ring formed as a result of the reaction of FA with the N-terminal valine of Hb and described the fragmentation patterns by presenting the MS/MS spectra of the N-terminal peptide control and the FA–peptide adduct.22 This adduct is very stable and does not require additional chemical modifications for stabilization. However, it will not undergo the Edman reaction and requires a new analytical approach for analysis. Analytical methods based on enzymatic digestion of Hb and subsequent measurement of the resulting N-terminal peptide adduct by liquid chromatography–tandem mass spectrometry (LC–MS/MS) have been described for acetaldehyde,23 1,2:3,4-diepoxybutane,24,25 and isoprene diepoxide.16 These methods provide an alternative approach for the quantitative analysis of N-terminal adducts, especially for adducts not reacting with the Edman reagents.
In this study, a new analytical method is presented for quantifying the stable imidazolidone ring Hb adduct in human blood samples. The method is based on the digestion of lyzed RBCs with trypsin followed by UPLC–MS/MS analysis of the N-terminus, the FA-modified octapeptide (FA-VHLTPEEK). To our knowledge, this is the first method using UPLC–MS/MS for the determination of this new FA-Hb adduct in RBCs.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
Methanol and water, both LC–MS grade, and isotonic saline solution were obtained from Fisher Scientific (Waltham, MA). Formic acid 98% was purchased from Sigma-Aldrich (St. Louis, MO). Bovine, porcine, horse, mouse, and human EDTA-whole blood were purchased from Bioreclamation IVT (Westbury, NY). 2,2,2-Trifluoroethanol (TFE) for protein denaturation was procured from Acros Organics (Morris, NJ). Acetic acid, ACS grade, was purchased from Fisher Scientific (Waltham, MA). Solvents and reagents were tested for potential impurities that can produce FA adducts by processing a VHLTPEEK peptide solutions in the same manner as a patient sample. No formation of adducts was observed. Trypsin, MS grade, was obtained from Promega (Fitchburg, WI). Hb Reagent Set (HRS) for the quantitative determination of Hb in RBCs by the cyanmethemoglobin method was obtained from Teco Diagnostics (Anaheim, CA). FA-VHLTPEEK (purity 98.6%, peptide content 70%) and the isotopically labeled internal standard (IS) FA-V (13C5, 15N) HLTPEEK (purity 98.6%, peptide content 76%) were synthesized by CPC Scientific (Sunnyvale, CA). All other chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO). RBC samples (135) from individuals 19–67 years of age were obtained from Bioreclamation IVT (Westbury, NY). The company had IRB approval to collect blood and urine and obtained informed consent from donors. CDC’s use of the blood and urine was consistent with the IRB approval and donor consent. No personal identifiers were provided to CDC.
2.2. Preparation of Standards
Eight calibrator solutions of the peptide FA-VHLTPEEK were prepared with deionized (DI) water at concentrations ranging from 103–1091 nmol/L. The IS solutions (465 nmol/L) contained FA-V (13C5, 15N) HLTPEEK. All calibrator and IS solutions were stored at −70 °C. Calibration curves were prepared daily by adding the calibrator solutions to bovine RBCs as calibrator sample matrix.
For measuring total Hb in the sample solution, an eight point calibration curve with Hb concentrations ranging from 71.7–3582.8 μmol/L was prepared according to manufacturer’s instructions using a lyophilized Hb linearity standard from Analytical Control Systems (Fishers, IN). FA-adduct measurement results are calculated by combining the results from the adduct measurement and the total hemoglobin measurement and are expressed as nmol FA adduct per gram hemoglobin (nmol/g Hb).
2.3. Preparation of Quality Control Materials
Quality control (QC) materials were prepared by selecting human RBCs isolated from EDTA-whole blood with different human FA-Hb adduct concentrations. The QC materials cover the concentration range typically observed in the general population. We analyzed QC samples in duplicate over 20 days and calculated target values and limits using statistical procedures described previously.26 Three QC materials were with concentrations of 60.71, 84.85, and 108.35 nmol FA-VHLTPEEK/g Hb, respectively. For total Hb measurements, commercially available QCs from Pointe Scientific (Canton, MI) were used.
2.4. Sample Preparation
RBCs were isolated from human and bovine EDTA-whole blood by centrifugation and washed three times with isotonic saline solution. RBCs were lyzed with equal amounts of DI water before storage at −70 °C. Samples were thawed before analysis, and the total Hb content in the aliquot used for adduct measurements was determined spectrophotometrically as cyanmethemoglobin.
Human and bovine RBCs including QCs were processed as outlined in Figure 1. In brief, samples were diluted with saline to a total Hb concentration of approximately 1 g/dL. Fifty microliter aliquots of either diluted human or bovine RBCs were transferred to a 96-well plate. To denature the proteins and increase the efficiency of digestion, 80 μL of TFE was added to all RBCs samples, and the plate was incubated in a Thermomixer from Eppendorf (Westbury, NY) for 1 h at 70 °C and 700 rpm. The TFE was evaporated under vacuum in a Genevac centrifugal evaporator from SP Scientific (Gardiner, NY).
Figure 1.

Flowchart for sample preparation and FA-Hb adducts measurement procedures.
Eighty microliters of the calibrator solutions was added to the wells containing bovine RBCs, and 80 μL DI water was added to all samples and QC materials. Eighty microliters of 200 mM ammonium bicarbonate, 50 μL of IS solutions, and 20 μL of trypsin solution (1 μg/μL in 50 mM acetic acid) were added to all samples, QC materials, and calibrator solutions. Digestion was performed in a Thermomixer for 48 h at 48 °C and 700 rpm and was stopped by addition of 10 μL of formic acid. The plate was transferred to the UPLC–MS/MS for analysis.
2.5. UPLC–MS/MS Analysis
Chromatographic separation of the analytes was accomplished using an UPLC pump system and autosampler from Thermo Fisher Scientific (Sunnyvale, CA), equipped with a Luna C18(2), 100 × 2.1 mm, 3 μm column from Phenomenex (Torrance, CA) operated at 30 °C. The mobile phase consisted of 1% formic acid in water (solvent A) and 1% formic acid in methanol (solvent B). Peptides were eluted with a gradient from 10% to 40% B in 5 min at a flow rate of 0.4 mL/min. At the end of the gradient, solvent B was increased to 100% for 1 min and held at 100% for additional 3 min, after which the system was re-equilibrated to the starting conditions (total run time: 12 min). Samples were stored in the autosampler at 5 °C. The analytes were monitored using a TSQ Vantage MS equipped with an electrospray ionization (ESI) probe from Thermo Fisher Scientific (Sunnyvale, CA). Instrument parameters were as follows: spray voltage, 4100 V; sheath gas, 40 psi; aux gas, 12 psi; ESI vaporization temperature, 450 °C; capillary temperature, 270 °C; Q2 gas, 1.2 mTorr. The MS was operated in positive ion multiple reaction monitoring (MRM) mode with quadrupole mass filters Q1 and Q3 at unit resolution, and with argon as the collision-activated dissociation gas.
The doubly charged ions for FA-VHLTPEEK (m/z 482.9) and IS (m/z 485.9) were used as precursor ions. Two transitions were monitored for both peptides. For the analyte, the transition was m/z 482.9 → 221.1 for quantitation (QI) and m/z 482.9 → 716.5 for the confirmation ion (CI). For the IS, the transition was m/z 485.9 → 227.2 for QI and m/z 485.9 → 716.4 for CI. The MS/MS spectra from which these transitions are derived were described recently.22 These transitions correspond to fragments that appear to extend from the N-terminal ([M+2H]2+ → a2) and the C-terminal ([M+2H]2+ → y6) of the FA-VHLTPEEK and the IS peptides.
2.6. Method Validation
To evaluate among day precision, low, medium, and high QC materials (60.71, 84.85, and 108.35 nmol FA-VHLTPEEK/g Hb) were analyzed in duplicate over 20 days. Within-day precision was evaluated by analyzing these three QC materials in eight replicates each.
The limit of detection (LOD) and the limit of quantitation (LOQ) were determined as described earlier.27 Serial dilutions of the low QC material using bovine RBCs as diluent were created and analyzed in multiple replicates as described above. The SD values for each dilution level were plotted against the concentration, and the data points were extrapolated to zero concentration (S0). The LOD was defined as 3S0 and the LOQ as 10S0.
The accuracy was evaluated by “spike” recovery28 because no certified reference materials are available for this analyte. Bovine RBCs and medium QC material were mixed with 0, 1.4, and 5.6 nmol of FA-VHLTPEEK per mL of RBCs, and each level was analyzed in five replicates. The recovery in percent was calculated as the difference between the samples after and before the addition of the FA-VHLTPEEK solutions, divided by the nominal amount added and multiplied by 100. The overall accuracy was defined as the mean accuracy calculated across bovine RBC, QC material, spiked levels (1.4 and 5.6 nmol of FA-VHLTPEEK per mL of RBCs), and replicates.
To assess analyte loss during the digestion step, we performed an analyte recovery experiment by comparing two sets of eight replicates of low and high QC materials. The first set was spiked with IS before enzymatic digestion, and the second set was spiked with IS after enzymatic digestion. The difference in analyte concentration between both experiments was calculated as percent of analyte recovered during this process.
Matrix effects (ME) and cross-talk for this method were evaluated according to the procedures described by Matuszewski et al.29,30 The impact of the matrix on signal intensity was evaluated using bovine, pig, horse, and mouse RBCs. These nonhuman RBCs contain Hb with amino acid sequences different from the human Hb and do not produce the peptides used in our method for analysis. These RBCs can be assumed to sufficiently mimic a human blood sample. The sample ME was evaluated by comparing the area ratios of each calibration point prepared in the alternative matrix to the matrix-free calibration curve.
Cross-talk experiments were conducted to identify contributions from the analyte signal to the IS signal and vice versa. Three samples with high levels of FA-VHLTPEEK and no IS were analyzed in eight replicates. In a separate experiment, eight samples containing only IS were analyzed.
The mean QI/CI ratio was calculated for each run using the calibrator samples. QI/CI ratios in individual study samples outside ±20% of the mean values from the calibrators indicates the presence of interferences (CLSI C50-A).31 Identification and validity of the analyte and IS signals were performed based on retention times and the QI/CI ratios.
The linearity of the calibration curve was determined for a range of 12 nmol/g Hb to 199.7 nmol/g Hb by assessing whether polynomial terms in a regression equation are statistically significant as outlined in CLSI document EP6-A.32 Nine concentration levels were created by mixing a RBC sample with 199.7 nmol FA-VHLTPEEK/g Hb with one sample that contained 12 nmol FA-VHLTPEEK/g Hb at different volume ratios. Area ratios of the analyte to IS were plotted against the concentration levels. Linearity calculations were performed using Analyze-it for Microsoft Excel software version 4.60.4 (Leeds, UK). We considered several unweighted and weighted calibration curves and the best fit was obtained using an unweighted linear curve.
The method robustness was evaluated by determining the impact of pH 8.0, 8.5, and 9.0; ammonium bicarbonate concentrations of 150, 200, and 250 mM; trypsin amounts with 12.5, 16.7, and 23.5 μg/sample; and hemoglobin amounts with 425, 500, and 575 mg using the low QC material. The optimum enzymatic digestion time was determined by performing digestions of seven replicates per sample for 4, 6, 20, 22, 24, 28, 30, 44, and 50 h. The stability of the analyte was evaluated by measuring the same sample at different conditions and time points (−70 °C fresh and after 1 year, 20 °C fresh and after 4 h, and 4 °C fresh and after 48 h) using unprocessed low and high QC materials, calibrator stock solutions, and the digested samples.
2.7. Data Analysis
Data were acquired and integrated using Xcalibur software version 2.2 SP1.48. An instrument suitability standard was analyzed at the beginning and the end of each sequence to confirm acceptable chromatography, retention time, peak shapes, and mass spectral sensitivity. The calibration curve was obtained by plotting the peak area ratios of the analyte to IS over the amount of analyte in the calibrator solutions. This calibration curve was used to calculate the amount of analyte in the samples. The final adduct concentrations were obtained by adjusting for the amount of Hb used in each sample.
A two-way ANOVA model was used to test for an overall effect of different times between sample preparation and analysis time, the effect of the pH and concentration of ammonium bicarbonate, the effect of varying the trypsin amount and the amount of Hb used for the experiment. A p value of <0.05 was considered significant for all tests. SAS/STAT software version 9.3 from SAS Institute (Cary, NC) was used for two-way ANOVA.
2.8. Applicability to Human Samples
The method was applied to the measurement of FA adducts in 135 human RBCs obtained from 89 self-reported smokers and 46 self-reported nonsmokers. We measured the QI/CI ratio in each blood sample and evaluated for possible interferences as described above. FA adduct levels were assessed for self-reported smokers and nonsmokers.
3. RESULTS AND DISCUSSION
The stable imidazolidone ring formed from the reaction of FA with the N-terminal amino acid of Hb is a potential biomarker of FA exposure. However, this Hb adduct will not undergo the Edman degradation commonly used for other Hb adducts. Therefore, we developed a new approach based on trypsin digestion of Hb and analysis of the resulting adduct, FA-VHLTPEEK, using isotope dilution tandem-mass spectrometry. In this method, the analytes are analyzed directly in the digestion solution without further isolation.
3.1. Analytical Method Performance
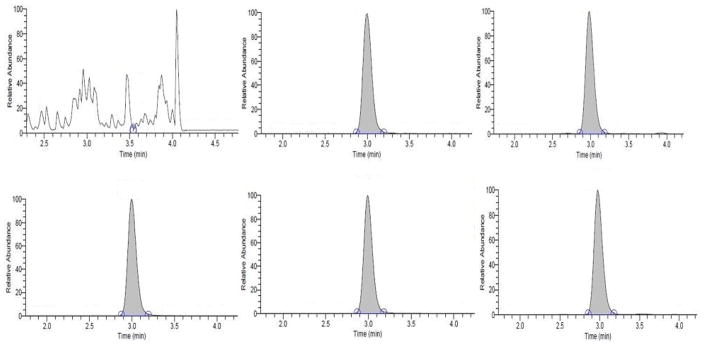
The method is highly specific for the measured FA adduct. No peaks were observed at the retention time and transition of the IS neither when a sample with high FA-VHLTPEEK concentration was digested (78 nmol FA-VHLTPEEK/g Hb) nor when analyzing the IS in bovine RBCs. Therefore, no cross-talk was observed from the analyte to the IS and vice versa. The chromatographic conditions employed in this method resulted in symmetrical peaks with no potentially interfering compounds detected in the chromatogram (Figure 2). The mean retention time of 2.9 min and variability, expressed as %CV, of 4.2% determined from daily injections of QC materials over 44 days suggest that the chromatographic procedure provides highly consistent and reliable separation. The fragment ions used for QI were selected (a2 at m/z 221.1 and 227.1) to contain the N-terminus of the peptide with the FA modification. These ions resulted from fragmentation of the amide backbone and the loss of CO. The y6 ions (m/z 716.5) used for CI contain the unmodified C-terminus of the peptide. Using QI and CI ions from opposite ends of the same peptide provides an additional level of specificity. The differences between the mean QI/CI ratio for the calibrators and individual samples were within 3.5%, well within the suggested criterion (20%).31 These findings demonstrate that no interferences were detectable in human blood samples.
Figure 2.
Multiple reaction monitoring ion chromatograms of FA-VHLTPEEK and IS. Shown on the top are the QI transitions for the analyte (482.8 → 221.1) and on the bottom the stable isotope labeled internal standard peptide (485.8 → 227.1). From left to right: reagent blank containing bovine RBCs and IS, calibrator at 103 nmol/L spiked into bovine RBCs, and the low QC material at 60.7 nmol/g Hb.
The method is sufficiently precise and accurate for measuring FA adducts in epidemiological studies. The among-day precision, expressed as %CV ranged between 7.2% and 10.2%, while the within-day precision ranged between 3.1% and 7.3% (Table 1). Mean (SD) method accuracy was 103.2% (8.11) ranging from 84.1–114.0%, which demonstrated that the loss of analyte during sample preparation is negligible. The linear range and LOD were adequate for measuring FA adduct concentrations commonly observed in the general population. The analytical measurement from nine concentration levels of FA-VHLTPEEK showed a linear relationship between instrument response and the analyte concentration for the range of 12 nmol/g Hb to 199.7 nmol/g Hb with no significant higher order (polynomial) relationship detected. The LOD was 3.4 nmol FA-VHLTPEEK/g Hb, and the LOQ was 11.3 nmol FA-VHLTPEEK/g Hb. FA adducts were clearly detectable in all human blood samples, and adduct values were all within the linear range of the calibration curve.
Table 1.
Among- and within-Day Precision, Expressed as Percent Coefficient of Variation, Determined in Low, Medium, and High QC Materials in Duplicates over 20 Days
| QC material | concentration FA-VHLTPEEK (nmol/g Hb) | among-day precision (%CV) | within-day precision (%CV) |
|---|---|---|---|
| low | 60.71 | 7.2 | 4.4 |
| medium | 84.85 | 10.1 | 3.1 |
| high | 108.35 | 10.2 | 7.3 |
The effects of sample matrix on the final measurement results can be considered negligible. Because of the unavailability of FA adduct-free human RBCs, we determined ME using calibration curves prepared in RBCs from several animals to mimic the effects of salts, lipids, proteins, peptides, and small organic molecules present in human blood samples. We found a mean ion suppression of 44.4% (range: 40.3–50.4%) and 45.9% (range: 42.2–53.0%) for FA-VHLTPEEK and IS, respectively (Table 2). Even though the ion suppression can be considered high, the IS-normalized ME is still considered low, and %CV of ME is 4.7% (Table 2). This can be explained with the high signal intensity obtained in the analytical measurement range and the use of an isotopically labeled IS that is similarly affected by the ME. The MEs observed with RBCs from different animals and among different lots of RBCs from the same animal were very similar (Table 3). We selected bovine RBCs as blank sample and calibrator matrix because of availability.
Table 2.
Matrix Effect (ME) at Eight Calibration Points Using Aqueous (Matrix-Free) Solutions and RBCs from Bovine (Bovine)
| pmol | mean peak area | area ratio | ratioa | ME% | IS | IS-compensated ME | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| analyte | IS | matrix free | bovine | analyte | |||||||
|
|
|
||||||||||
| matrix free | bovine | matrix free | bovine | ||||||||
| Cal A | 8.29 | 63 105 | 31 836 | 259 384 | 137 543 | 0.24 | 0.23 | 0.24 | 50.4 | 53.0 | 1.0 |
| Cal B | 10.91 | 89 904 | 38 023 | 262 692 | 110 915 | 0.34 | 0.34 | 0.34 | 42.3 | 42.2 | 1.0 |
| Cal C | 16.58 | 132 366 | 58 094 | 268 016 | 117 422 | 0.49 | 0.49 | 0.49 | 43.9 | 43.8 | 1.0 |
| Cal D | 21.83 | 191 236 | 77 117 | 272 020 | 119 664 | 0.70 | 0.64 | 0.67 | 40.3 | 44.0 | 0.9 |
| Cal E | 33.16 | 281 336 | 118 103 | 266 962 | 119 057 | 1.05 | 0.99 | 1.02 | 42.0 | 44.6 | 0.9 |
| Cal F | 43.66 | 389 684 | 168 554 | 274 876 | 120 404 | 1.42 | 1.40 | 1.41 | 43.3 | 43.8 | 1.0 |
| Cal G | 66.32 | 566 098 | 254 415 | 266 594 | 123 205 | 2.12 | 2.06 | 2.09 | 44.9 | 46.2 | 1.0 |
| Cal H | 87.32 | 787 933 | 380 060 | 269 786 | 135 215 | 2.92 | 2.81 | 2.88 | 48.2 | 50.4 | 1.0 |
| 7.6b | 8.2b | 4.7b | |||||||||
Average ratio of matrix free analyte/IS and analyte/IS in bovine.
Coefficient of variation (%CV).
Table 3.
Calibration Curves in Different Matrices Including Three Different Lots of Bovine RBCs Compared to Neat Solutions
| neat | matrix (lot)
|
mean | mean ± 2SD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| bovine.1 | bovine.2 | bovine.3 | pig.1 | horse.1 | mouse.1 | ||||
| slope | 0.033 | 0.034 | 0.033 | 0.031 | 0.033 | 0.032 | 0.032 | 0.032 | 0.030–0.035 |
| R2 | 0.996 | 0.998 | 0.999 | 0.998 | 0.999 | 1.000 | 0.999 | 0.999 | 0.997–1.000 |
For the cross-talk experiments, we did not see any peaks at the IS transition and their corresponding retention time in samples without IS; therefore, there is no contribution to the IS trace from the analyte. The results show the IS is contributing to the analyte signal with approximately 0.4 to 0.5 pmol of FA-VHLTPEEK in each sample. However, when subtracting the blank sample, this contribution has no effects on quantitation.
Assessments of the impact of modifications on sample processing and storage conditions show that the method is highly robust (Table 4). Of the parameter modifications tested, only a variation in digestion buffer resulted in changes in measurement results by 10% when the digestion buffer concentration was increased from 200 to 250 mM. The samples appeared stable after digestion when stored at 4 °C for up to 30 days. The mean values from the replicates in stability testing results were within 15% of the established values for each QC material or stock solution. The unprocessed samples, calibrator stock solutions, and processed samples did not show any detectable changes in analyte concentration over the time intervals tested (unprocessed samples and stock solutions: 1 year at −70 °C, unprocessed samples over 4 h at 20 °C, processed sample at 4 °C over 48 h).
Table 4.
Robustness Testinga
| method parameters | current method parameter | result at current level (nmol/g Hb) | parameter at lower level | result at lower level (nmol/g Hb) | parameter at higher level | result at higher level (nmol/g Hb) |
|---|---|---|---|---|---|---|
| pH of digest buffer | 8.5 | 51.1 ± 3.2 | 8.0 | 50.6 ± 4.9 | 9.0 | 49.9 ± 2.7 |
| concentration of digest buffer (mM) | 200 | 51.1 ± 3.2 | 150 | 52.3 ± 2.9 | 250 | 45.1 ± 1.6b |
| digestion time (h) | 48 | 51.5 ± 3.3 | 44 | 49.2 ± 2.9 | 50 | 51.0 ± 1.6 |
| amount of trypsin (μg) | 16.7 | 43.0 ± 3.7 | 12.5 | 40.3 ± 2.5 | 23.5 | 45.6 ± 3.4 |
| amount of hemoglobin (mg) | 500 | 38.9 ± 4.4 | 425 | 36.5 ± 2.7 | 575 | 37.5 ± 2.9 |
FA-VHLTPEEK concentrations values at sample preparation conditions above and below those used in the method.
Significantly different from values obtained with current method parameter, p < 0.05 using two-way ANOVA model.
3.2. Measurements in Human Samples
FA-VHLTPEEK was measured in 135 individual human RBCs samples (age range from 19–67 years) and was detected in all samples, with concentrations ranging from 59.27–130.57 nmol/g Hb (mean, SD: 104.8 nmol/g Hb, 13.4). The observed values are lower than those reported for a different FA adduct (N-methylenvaline) measured at the N-terminal valine of the alpha- and beta-chains of Hb.19,20 These differences in adduct levels can be explained by the different analytes measured in these studies. N-Methylenvaline is formed at the alpha- and beta- chains of Hb, while the described method measures FA adducts only on the beta-chain. Therefore, the FA adduct concentrations reported by these authors are expected to be higher than those reported in our study. The range of hemoglobin adduct levels observed in this study (ratio highest value/lowest value: 2.2) appears to be higher or similar to the ranges reported in other studies (ratios ranged between 1.4 and 2.4).19,33,34 This observation suggests that the range of individual exposures in this study is similar to those reported in other studies.
FA-adducts levels were similar among self-reported smokers and nonsmokers. The mean (SD) concentrations of FA-VHLTPEEK were 103.5 (12.5) nmol/g Hb (fifth to 95th percentile: 68.7–118.7 nmol/g Hb,) in nonsmokers and 105.4 (13.8) nmol/g Hb (fifth to 95th percentile: 65.8–119.8 nmol/g Hb) in smokers (Table 5). While tobacco smoke as well as use of e-cigarettes is a known source of FA exposure,2,3,35,36 findings about FA-adduct levels in smokers and nonsmokers are inconsistent.19,20 Our findings appear consistent with those reported in a study using DNA-adducts of FA as exposure biomarker.37 The authors report that FA exposure from inhalation only reached the nose but not tissues distant to the site of exposure. Larger studies with well-characterized participants and well-defined use of tobacco products are needed to better assess the impact of smoking and use of other tobacco products on FA-adduct levels.
Table 5.
FA-VHLTPEEK Concentrations in Blood from 135 Individual Donors by Self-Reported Smoking Status
| FA-VHLTPEEK (nmol/g Hb)
|
||
|---|---|---|
| smokers | nonsmokers | |
| median | 107.3 | 106.3 |
| mean | 105.4 | 103.5 |
| 5th percentile | 65.8 | 68.7 |
| 95th percentile | 119.8 | 118.7 |
The adduct concentrations measured with this method do not provide information about FA exposure sources but do provide information about the overall exposure of FA, including endogenous production of FA. The method allows for processing of samples in 96-well plates using automated sample handling systems, which enables measuring FA adducts in large population studies.
4. CONCLUSIONS
The presented new isotope dilution UPLC–MS/MS method is highly reproducible, specific, and accurate, with high throughput and thus is suitable for measuring FA adducts in the general population. The analytical performance of this method is in agreement with those suggested for bioanalytical methods.38,39 The use of tryptic digestion of RBCs followed by measurement of N-terminal peptides containing the FA adduct offers an alternative to methods using conventional Edman degradation procedures.
Acknowledgments
Funding
This work was supported by the Division of Laboratory Sciences from the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, and the U.S. Department of Health and Human Services.
We thank Tasia Nabors, Khadija Omri, Gerhard Kummerow, and Alina Costin, for their support in the laboratory measurements. We thank Larry Moore, Heather Kuiper, and Uliana Danilenko for their review and suggestions (all with the National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia).
ABBREVIATIONS
- FA
formaldehyde
- Hb
hemoglobin
- UPLC–MS/MS
ultra-performance liquid chromatography–tandem mass spectrometry
- RBCs
red blood cells
- FA-VHLTPEEK
FA-modified octapeptide
- TFE
2,2,2-trifluoroethanol
- IS
isotopically labeled internal standard
- DI
deionized
- QC
quality control
- MRM
multiple reaction monitoring
- QI
quantitation
- CI
confirmation
- LOD
limit of detection
- LOQ
limit of quantitation
- ME
matrix effects
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Pala M, Ugolini D, Ceppi M, Rizzo F, Maiorana L, Bolognesi C, Schiliro T, Gilli G, Bigatti P, Bono R, Vecchio D. Occupational exposure to formaldehyde and biological monitoring of Research Institute workers. Cancer Detect Prev. 2008;32:121–126. doi: 10.1016/j.cdp.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Godish T. Formaldehyde exposures from tobacco smoke: a review. Am J Public Health. 1989;79:1044–1045. doi: 10.2105/ajph.79.8.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Formaldehyde, 2-Butoxyethanol, and 1-tert-Butoxypro-pan-2-ol. Vol. 88. International Agency for Research on Cancer; Lyon, France: 2006. [PMC free article] [PubMed] [Google Scholar]
- 4.National Toxicology Program. NTP 12th Report on Carcinogens, Introduction and NTP Report on Carcinogen Review Process. U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 5.Lutz WK. Endogenous formaldehyde does not produce detectable DNA-protein crosslinks in rat liver. Toxicol Pathol. 1986;14:462–465. doi: 10.1177/019262338601400413. [DOI] [PubMed] [Google Scholar]
- 6.Dahl AR, Hadley WM. Formaldehyde production promoted by rat nasal cytochrome P-450-dependent monooxygenases with nasal decongestants, essences, solvents, air pollutants, nicotine, and cocaine as substrates. Toxicol Appl Pharmacol. 1983;67:200–205. doi: 10.1016/0041-008x(83)90225-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Steinmaus C, Eastmond DA, Xin XK, Smith MT. Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat Res, Rev Mutat Res. 2009;681:150–168. doi: 10.1016/j.mrrev.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council. Review of the Environmental Protection Agency’s Draft IRIS Assessment of Formaldehyde. The National Academies Press; Washington, DC: 2011. [Google Scholar]
- 9.Bogdanffy MS, Morgan PH, Starr TB, Morgan KT. Binding of formaldehyde to human and rat nasal mucus and bovine serum albumin. Toxicol Lett. 1987;38:145–154. doi: 10.1016/0378-4274(87)90122-6. [DOI] [PubMed] [Google Scholar]
- 10.Metz B, Kersten GF, Baart GJ, de Jong A, Meiring H, ten Hove J, van Steenbergen MJ, Hennink WE, Crommelin DJ, Jiskoot W. Identification of formaldehyde-induced modifications in proteins: reactions with insulin. Bioconjugate Chem. 2006;17:815–822. doi: 10.1021/bc050340f. [DOI] [PubMed] [Google Scholar]
- 11.Guthe KF. The formaldehyde–hemoglobin reaction. J Biol Chem. 1959;234:3169–3173. [PubMed] [Google Scholar]
- 12.Hoberman HD, San George RC. Reaction of tobacco smoke aldehydes with human hemoglobin. J Biochem Toxicol. 1988;3:105–119. doi: 10.1002/jbt.2570030205. [DOI] [PubMed] [Google Scholar]
- 13.Tornqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B: Anal Technol Biomed Life Sci. 2002;778:279–308. doi: 10.1016/s1570-0232(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 14.Osterman-Golkar S, Peltonen K, Anttinen-Klemetti T, Landin HH, Zorcec V, Sorsa M. Haemoglobin adducts as biomarkers of occupational exposure to 1,3-butadiene. Mutagenesis. 1996;11:145–149. doi: 10.1093/mutage/11.2.145. [DOI] [PubMed] [Google Scholar]
- 15.Boogaard PJ, Rocchi PS, van Sittert NJ. Biomonitoring of exposure to ethylene oxide and propylene oxide by determination of hemoglobin adducts: correlations between airborne exposure and adduct levels. Int Arch Occup Environ Health. 1999;72:142–150. doi: 10.1007/s004200050353. [DOI] [PubMed] [Google Scholar]
- 16.Fred C, Kautiainen A, Athanassiadis I, Tornqvist M. Hemoglobin adduct levels in rat and mouse treated with 1,2:3,4-diepoxybutane. Chem Res Toxicol. 2004;17:785–794. doi: 10.1021/tx034214g. [DOI] [PubMed] [Google Scholar]
- 17.Schettgen T, Broding HC, Angerer J, Drexler H. Hemoglobin adducts of ethylene oxide, propylene oxide, acrylonitrile and acrylamide-biomarkers in occupational and environmental medicine. Toxicol Lett. 2002;134:65–70. doi: 10.1016/s0378-4274(02)00164-9. [DOI] [PubMed] [Google Scholar]
- 18.Vesper HW, Ospina M, Meyers T, Ingham L, Smith A, Gray JG, Myers GL. Automated method for measuring globin adducts of acrylamide and glycidamide at optimized Edman reaction conditions. Rapid Commun Mass Spectrom. 2006;20:959–964. doi: 10.1002/rcm.2396. [DOI] [PubMed] [Google Scholar]
- 19.Bono R, Vincenti M, Schiliro T, Scursatone E, Pignata C, Gilli G. N-Methylenvaline in a group of subjects occupationally exposed to formaldehyde. Toxicol Lett. 2006;161:10–17. doi: 10.1016/j.toxlet.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Bono R, Romanazzi V, Pirro V, Degan R, Pignata C, Suppo E, Pazzi M, Vincenti M. Formaldehyde and tobacco smoke as alkylating agents: the formation of N-methylenvaline in pathologists and in plastic laminate workers. Sci Total Environ. 2012;414:701–707. doi: 10.1016/j.scitotenv.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 21.Kautiainen A, Tornqvist M, Svensson K, Osterman-Golkar S. Adducts of malonaldehyde and a few other aldehydes to hemoglobin. Carcinogenesis. 1989;10:2123–2130. doi: 10.1093/carcin/10.11.2123. [DOI] [PubMed] [Google Scholar]
- 22.Ospina M, Costin A, Barry AK, Vesper HW. Characterization of N-terminal formaldehyde adducts to hemoglobin. Rapid Commun Mass Spectrom. 2011;25:1043–1050. doi: 10.1002/rcm.4954. [DOI] [PubMed] [Google Scholar]
- 23.Conduah Birt JE, Shuker DE, Farmer PB. Stable acetaldehyde–protein adducts as biomarkers of alcohol exposure. Chem Res Toxicol. 1998;11:136–142. doi: 10.1021/tx970169z. [DOI] [PubMed] [Google Scholar]
- 24.Kautiainen A, Fred C, Rydberg P, Tornqvist M. A liquid chromatography tandem mass spectrometric method for in vivo dose monitoring of diepoxybutane, a metabolite of butadiene. Rapid Commun Mass Spectrom. 2000;14:1848–1853. doi: 10.1002/1097-0231(20001015)14:19<1848::AID-RCM106>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Basile A, Ferranti P, Pocsfalvi G, Mamone G, Miraglia N, Caira S, Ambrosi L, Soleo L, Sannolo N, Malorni A. A novel approach for identification and measurement of hemoglobin adducts with 1,2,3,4-diepoxybutane by liquid chromatography/electrospray ionisation mass spectrometry and matrix-assisted laser desorption/ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:527–540. doi: 10.1002/rcm.263. [DOI] [PubMed] [Google Scholar]
- 26.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 27.Taylor JK. Quality Assurance of Chemical Measurements. CRC Press; Boca Raton, FL: 1987. [Google Scholar]
- 28.ICH. Guidance for Industry: Q2B Validation of Analytical Procedures: Methodology. U.S. Department of Health and Human Services; 1996. [Google Scholar]
- 29.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 30.Matuszewski BK. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. J Chromatogr B: Anal Technol Biomed Life Sci. 2006;830:293–300. doi: 10.1016/j.jchromb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 31.CLSI. Mass Spectrometry in the Clinical Laboratory: General Principles and Guidance; Approved Guideline in CLSI document C50-A. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. [Google Scholar]
- 32.NCCLS. NCCLS document EP6-A. 16. Vol. 23. NCCLS; Wayne, PA: 2003. Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. [Google Scholar]
- 33.Bader M, Lewalter J, Angerer J. Analysis of N-alkylated amino acids in human hemoglobin: evidence for elevated N-methylvaline levels in smokers. Int Arch Occup Environ Health. 1995;67:237–242. doi: 10.1007/BF00409405. [DOI] [PubMed] [Google Scholar]
- 34.Heck HD, Casanova-Schmitz M, Dodd PB, Schachter EN, Witek TJ, Tosun T. Formaldehyde (CH2O) concentrations in the blood of humans and Fischer-344 rats exposed to CH2O under controlled conditions. Am Ind Hyg Assoc J. 1985;46:1–3. doi: 10.1080/15298668591394275. [DOI] [PubMed] [Google Scholar]
- 35.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372:392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 36.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu R, Lai Y, Hartwell HJ, Moeller BC, Doyle-Eisele M, Kracko D, Bodnar WM, Starr TB, Swenberg JA. Formation, Accumulation, and Hydrolysis of Endogenous and Exogenous Formaldehyde-Induced DNA Damage. Toxicol Sci. 2015;146:170–182. doi: 10.1093/toxsci/kfv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. U.S. Food and Drug Administration; 2001. [Google Scholar]
- 39.European Medicines Agency. Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev.1, Corr.2. European Medicines Agency; London, United Kingdom: 2011. [Google Scholar]