Abstract
Obesity has been estimated to decrease life expectancy by as little as 0.8 to as much as 7 years being the second leading cause of preventable death in the United States after smoking. Along with the increase in the prevalence of obesity, there has been a dramatic rise of the prevalence of prediabetes and type 2 diabetes among adolescents. Despite that, very little is known about the pathogenesis of these conditions in pediatrics and about how we could detect prediabetes in an early stage in order to prevent full blown diabetes. In this review we summarize the current knowledge on the pathophysiology of prediabetes and type 2 diabetes in adolescents and describe how biomarkers of beta-cell function might help identifying those individuals who are prone to progress from normal glucose tolerance towards prediabetes and overt type 2 diabetes. To better understand and fight this disease, we will need to explore and develop novel therapeutic strategies and individuate more sensitive and specific biomarkers that can allow an earlier detection of the disease.
Keywords: Prediabetes, Disposition Index, TCF7L2
Introduction
Prediabetes in the obese adolescent: the prelude to type 2 diabetes
The increased prevalence of childhood obesity is being accompanied by a rise of the prevalence of type 2 diabetes (T2DM) in pediatrics. The SEARCH for diabetes study estimated that by 2050 the prevalence of T2DM among youth might almost quadruple.1
T2DM onset in pediatrics shows a great heterogeneity in terms of onset and progression. Since the onset of T2DM is a progressive phenomenon, overt diabetes is preceeded by a range of glucose related phenotypes, characterized by a progressive decline of beta-cell function. These conditions, that clinically define a state known as prediabetes, are highly prevalent among obese youth.2 This review will focus on the main elements that alter glucose metabolism early in the course of diabetes development as well as on genetic and developmental factors that predispose to the development of prediabetes and diabetes.
Prevalence of youth onset prediabetes
“Prediabetes” serves as a broad expression describing multiple facets of altered glucose metabolism, including impaired fasting glucose (IFG), impaired glucose tolerance (IGT), elevated HbA1c or combinations of them.3, 4 Each one of these conditions, whether detected in childhood or adulthood, confers greater risk for the development of T2DM over time as well as for the presence of adverse cardiovascular risk factors. Importantly, these conditions are not interchangeable and each one represents specific alterations in glucose metabolism. The emerging rise in the prevalence of prediabetes in children and adolescents parallels the rise in rates of childhood obesity in recent decades.5 The prevalence of prediabetes amongst youth depends on the tools used for screening as well as on the population studied. Upon testing obese children and adolescents, the prevalence of IGT ranges from 1 to 30% 6–8 while the prevalence of IFG in obese children but also amongst the general adolescent population and not specifically within at-risk groups has a broad range.7, 9–12
Estimates of prevalence rates are made more difficult by some differences in classification of prediabetes. For example, there are two glucose cut-offs used to define IFG: the American Diabetes Association suggests 5.6 mmol/L,13 while the World Health Organization promotes 6.1 mmol/L.14 European studies report prevalence rates of IFG in obese children ranging from 1% in Italy15 and 4% in Germany to 17% in Sweden (all using the ADA criteria).16 American studies have reported prevalence rates ranging from 2–9% (WHO criteria) to 15–47% (ADA criteria).17–19 Other countries, including India, China and Mexico, report only a few percent of patients with IFG in the obese pediatric population20–22 whereas 28% of obese adolescents in Taiwan are reported to have IFG.23 The United Arab Emirates (UAE), which has among the highest adult prevalence of T2DM, reports that 12% of overweight and obese children have IFG.24 Several challenges arise when attempting to compare and interpret these prevalence rates since different methods (e.g. population-based vs obesity clinic) were used to recruit the sample populations. Importantly, when performing epidemiological studies assessing the prevalence of IFG in large samples, there is always a possibility that some of the participants are not truly fasting, causing an overestimation of the true prevalence. Similarly, it has been demonstrated that the presence of IGT may be variable upon repeated sampling within weeks.25 This may be due to variations of insulin sensitivity between studies (such as the presence of a minor infection or the phase of the menstrual cycle which may affect whole body insulin sensitivity) yet one can argue that a single detection of IGT, even if not repeated, already indicates that in the face of significant insulin resistance – the individuals’ beta-cell has inadequate function.
HbA1c in healthy blood donors is within the prediabetes range in up to 10% of adolescents. (26) Importantly, the presence of prediabetes is highly dependent on the tools used for its detection, the population studied and the pubertal status of subjects and thus cannot be viewed as a single entity. To this end, few years ago the American Diabetes Association (ADA) published revised recommendations to use HbA1c to diagnose prediabetes and diabetes.1 The decision was based on numerous cross-sectional and longitudinal studies showing the correlation between A1C and diabetes at baseline or long-term association between A1C and risk of diabetes and diabetes-related comorbidities.1–6 The use of HbA1c as diagnostic tool for diabetes has been proven not to overlap completely with the evaluation of diabetes done by an OGTT.27
Even though, HbA1c combined with 2-h glucose, represents the strongest predictor for development of prediabetes or diabetes in at-risk young subjects27 and an independent predictor of cardiovascular risk in non-diabetic adults.28
These findings prevent the utilization of absolute levels of HbA1c as specific single biomarker for the detection of “prediabetes” in youth, yet the combination of several such biomarkers do shed light on the pathogenesis of altered glucose metabolism and its complications in this age group and provide potential targets for intervention.
Pathophysiology of altered glucose metabolism in obese children and adolescents
Glucose levels are normally restricted within a narrow range between fasting and post-prandial conditions. Maintenance of glucose within this narrow range depends upon the delicate interplay of coordinated hormonal (insulin and glucagon), neural and metabolic activity in all organs and tissues involved in glucose metabolism. Insulin and glucagon, secreted from beta and alpha cells respectively govern glucose metabolism. The timely secretion of insulin and its target organ action leads to clearance of glucose from the circulation thus preventing it from reaching hyperglycemic levels and providing mostly muscle but also adipose tissue and the liver with a source of energy for immediate utilization or for storage. Normal glucose metabolism depends on the physiological interplay of insulin secretion and insulin action and in order to develop T2DM – defects in both are usually present.29
The role of insulin secretion
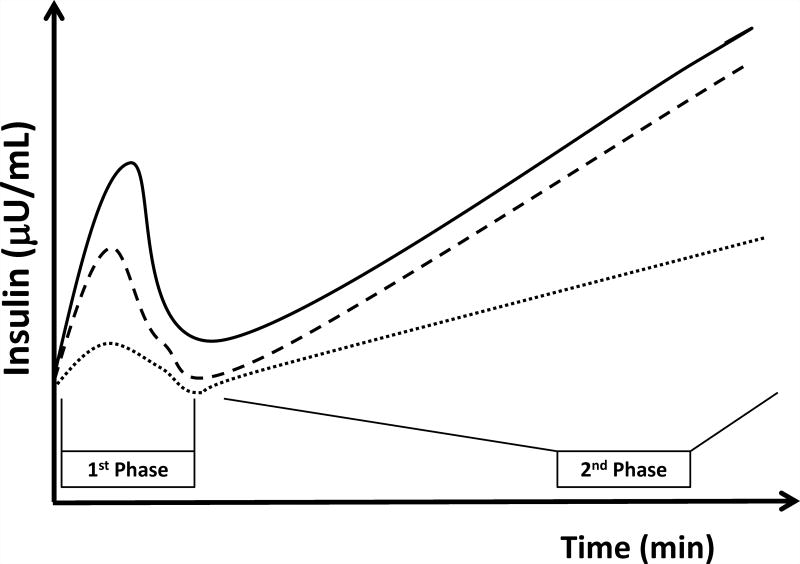
Insulin secretion is bi-phasic (figure 1). First phase secretion is the immediate limited response to increasing plasma glucose and is postulated to consist of pre-packaged insulin from secretory granules. It is measured within the first 10 minutes of a hyperglycemic clamp study. Second phase secretion is the prolonged response to persistent elevated glucose and involves a longer process of trafficking of insulin from the Golgi system to secretory granules and out of the beta-cell. Second phase is measured by most during 10–120 minutes of the hyperglycemic clamp. First and second phase insulin secretion can be accurately quantified only in response to a non-physiological intravenous glucose stimulus (a hyperglycemic clamp or an intra-venous glucose tolerance test). Both phases can be evaluated by modeling of the response to a more physiological oral glucose load. Despite this limitation, measurement of phasic insulin dynamics is crucial as defects in first phase secretion have been shown to precede the development of overt diabetes.30 Obese adolescents with pre-diabetes (IFG, IGT or both) have been shown to have reduced first phase insulin secretion compared to those with normal glucose metabolism.31 Using c-peptide modeling of OGTTs, glucose sensitivity of first-phase insulin secretion has been shown to decline progressively as obese adolescents deteriorate from isolated IFG or IGT to combined IFG/IGT.32 Thus, alterations in second-phase insulin secretion are a somewhat later phenomenon that occurs only in those with combined IFG/IGT or overt T2DM.3 This observation implies that an isolated first phase defect is an early manifestation of pre-diabetes while a combination of first and second phase defects represents profound beta-cell dysfunction which is the prelude of overt T2DM.
Figure 1. Insulin secretion during a hyperglycemic clamp in obese youth.
Those with NGT (solid line) show normal first and second phase insulin secretion. Those with IGT (dashed loine) show reduced first phase along with preserved second phase insulin secretion. Those with T2DM (dotted line) show defects in both first and second phase insulin secretion.
The deterioration of the beta-cell usually occurs faster in youth than in adults. In fact, while in adults the transition toward T2DM takes about 10 years with ~7% per year reduction in beta-cell function, in obese adolescents the beta-cell deteriorates at a rate of ~20–30% per year,34 with a mean transition time from pre-diabetes to overt diabetes of about 2.5 years.35 It is important to remember that the defects in insulin secretion in obese youth emphasize failure to compensate for the profound ambient insulin resistance. Despite this, obese youth demonstrate very high insulin concentrations during oral glucose tolerance tests in comparison to adults. These seemingly supra-physiological insulin concentrations are still less than that required to overcome the marked insulin resistance of these youths.
Whole body insulin sensitivity is defined as the action of the hormone in all insulin responsive tissues and organs. It is well established that whole body insulin sensitivity declines in obese children with normal compared to those with impaired glucose tolerance.36, 37 Importantly, obese children with IGT are uniformly markedly insulin resistant while those with NGT have insulin sensitivity levels ranging from highly sensitive to markedly resistant, similar to those with IGT.38 Reduced insulin sensitivity in obese children is in most cases associated with a typical lipid partitioning profile generally characterized by increased intra-abdominal (visceral), intra-hepatic, and intramyocellular lipid deposition.39 This lipid depot distribution pattern may differ between obese youths of different ethnic backgrounds. The ability of the obese child to compensate for low insulin sensitivity by increasing insulin concentrations is the determinant of actual glucose tolerance. In order to increase circulating insulin levels, two parallel compensatory mechanisms are activated: enhanced insulin secretion and reduced insulin clearance by the liver.40 Children with very low insulin sensitivity most probably reduce hepatic insulin clearance to a trough level beyond which insulin action within the liver may be compromised and are thus left with further increasing insulin secretion as the sole compensation mechanism aimed at maintaining euglycemia.40 This enhanced continuous stress on the beta-cell results in an allostatic price manifesting as slight yet significant increases in glucose levels at fasting and the post absorptive state41 needed to stimulate the beta-cell to secrete adequate amounts of insulin.
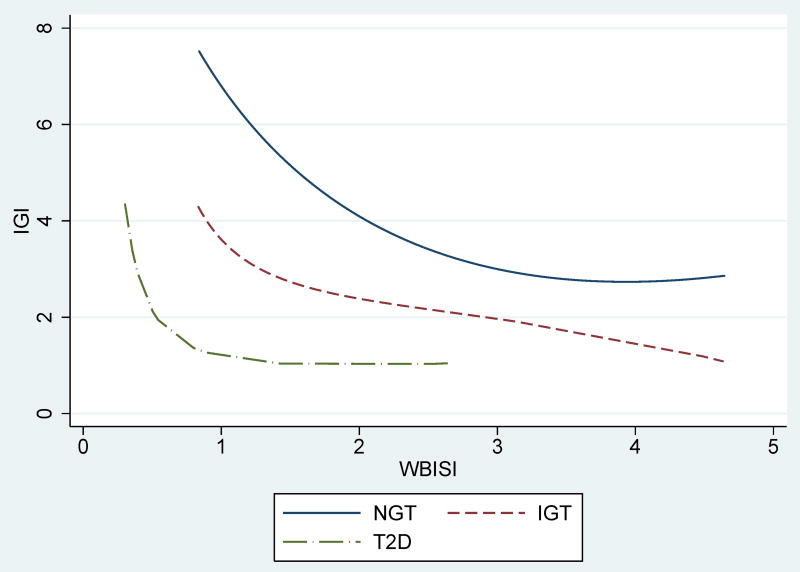
The mathematical relation of insulin sensitivity and secretion is best described as hyperbolic, such that the product of insulin sensitivity X insulin secretion equals a constant (figure 2).42 The product of the two has been named “disposition index” (DI) and reflects beta-cell function in the context of ambient insulin sensitivity. The DI has been shown to be the strongest predictor of the development of diabetes over time.43 Obese children with impaired glucose metabolism have lower DI than their NGT counterparts, reflecting defects in beta-cell function that precede development of diabetic range hyperglycemia.32, 44 Importantly, even appropriate beta-cell compensation requires a continuous stimulus to maintain enhanced insulin secretion. For a given DI, exposure to lower insulin sensitivity (i.e. being on the left of the hyperbolic DI curve) is accompanied by slight yet significant increases in both fasting and 2-hr glucose.41 These may still be within the “normal” glucose tolerance range yet reflect an increased demand upon the stressed beta-cell. It should be noted that “normal glucose tolerance” in obese children represents a continuous spectrum. Specifically, a worsening of the DI accompanies increases in postprandial glucose levels even within in the “normal” range.44 In other words, major defects in beta-cell function may exist in obese youth despite having “high normal” 2-hr glucose values on the OGTT.
Figure 2. The disposition index across glucose tolerance categories.
Data from youths followed at Yale Obesity-Diabetes Clinic. Per given degree of insulin sensitivity, obese youths with NGT (solid line, n=2568, z-score BMI 2.24±0.61) have greater insulin secretion than those with IGT (dashed line, dashed-dotted line, n=693, z-score BMI 2.38±0.48) and those with T2DM (dot-dashed line, n=72, z-score BMI 2.38±0.48). While less prominent in insulin sensitive subjects, at lower levels of insulin sensitivity (greater insulin resistance), these differences are highly significant and insulin secretion is insufficient to maintain normal glucose metabolism. (unpublished data)
In a recent study analyzing a multiethnic cohort of ~1600 obese youths,45 we showed that 36.6% and 43.6% of the obese adolescents in the study had 2-h glucose concentrations within the higher category, while only 19.8% showed 2-h glucose lower than 100 mg/dl.45 Moreover, we observed a decline of insulin sensitivity and secretion across the categories of DI, which in turn is associated with an increased risk of progressing to IGT later in life).45 These observations indicated that beta-cell function relative to insulin sensitivity is impaired even in youth with high 2-h glucose concentrations within the “non-pathologic range”. Notably, at baseline the DI, that is a robust index of beta-cell function in the context of insulin sensitivity, predicted the risk of developing prediabetes or type 2 diabetes over time.45, 46
The DI is likely shaped by genetic/epigenetic factors that limit the ability of the obese child to compensate for insulin resistance. Indeed, it has been shown that a history of gestational diabetes, reflecting exposure to both the genetic background of T2DM and to hyperglycemia, results in a lower DI for a given degree of obesity in childhood and predicts deterioration of glucose tolerance over time.47
The role of insulin resistance
The link between obesity and prediabetes is ectopic fat accumulation. Obesity-related ectopic fat accumulation in key insulin responsive organs like skeletal muscle and liver alters insulin signaling pathway, leading to increased insulin resistance, characterized by defects in the non-oxidative pathway of glucose metabolism, a higher intramyocellular lipid content and greater visceral and hepatic fat content.48 Fat accumulation in the liver is an important trigger of insulin resistance and its severity is associated with the presence of pre-diabetes in adolescents.49 As indicated above, the directionality of the associations between lipid accumulation in insulin-responsive tissues and tissue specific insulin resistance may be bi-directional, ie – it has been shown that acute delivery of free fatty acids causes a significant reduction in muscle insulin sensitivity.50 On the other hand – liver insulin resistance may lead to further hyperinsulinemia and additional hepatic lipid deposition.51 Recent pediatric studies shed light on the role of fatty liver on insulin resistance and metabolic syndrome. In a multiethnic group of obese youth it has been shown that the severity of fatty liver is associated with a decline in beta-cell function and higher rates of pre-diabetes.49 Moreover, it was also shown that increasing amounts of intra-hepatic fat were paralleled by an increased prevalence of the metabolic syndrome, suggesting that fatty liver disease may be a predictive factor of metabolic syndrome in children.49 Importantly, in obese adolescents the negative effect of fatty liver on insulin sensitivity is independent of the degree of visceral fat and intramyocellular lipid content.52 D’Adamo et al. studied 23 obese adolescents with and 20 obese adolescents without fatty liver, matched for age, Tanner stage, BMI z score, and percentage of body fat, visceral fat, and intramyocellular lipid.52 While baseline hepatic glucose production was similar between the groups, the suppression of hepatic glucose production in the face of comparable insulin concentration was significantly lower in those with fatty liver indicating hepatic insulin resistance. Moreover, the group with fatty liver showed a higher degree of muscle insulin resistance, expressed as glucose disposal rate. The investigators also showed a trend for individuals with fatty liver having lower ability to suppress glycerol turnover indicating adipose insulin resistance. These data clearly suggest that intra-hepatic fat accumulation is a major determinant of liver, muscle and adipose tissue insulin resistance.52 Moreover, data from Alderete et al. show that the association of high intra-hepatic fat content and poor beta-cell compensation is more pronounced in African Americans than in other ethnic groups, suggesting that the relationship between fatty liver and insulin sensitivity might be modulated be race/ethnicity.53 A recent longitudinal study has shown that baseline hepatic fat content is associated with changes in glucose metabolism over time and that it correlates with 2-hour glucose, insulin sensitivity and insulin secretion at 2 years follow-up.54 These data indicate that intra-hepatic fat accumulation is more deleterious for glucose metabolism than ectopic fat accumulation elsewhere in the body.55
Of note, a unique property of obese adolescent is their transition through phases of puberty. It is well established that mid puberty (tanner stage III) is characterized by a ~30% reduction in whole body insulin sensitivity.56 This reduction may be completely or partially recovered by the end of puberty (Tanner stage V).57 This issue is of major importance when assessing insulin sensitivity and glucose tolerance in this age group as some obese youth that are tested at mid-puberty may improve their glucose tolerance upon repeated testing at a later pubertal stage.
Glucose effectiveness
Glucose has the ability to facilitate its own uptake via a mass effect in peripheral tissues and to suppress hepatic glucose production depending on basal insulin concentrations.58 This property of glucose is known as “glucose effectiveness” (GE), and it tends to increase with greater insulin concentrations.59 GE is difficult to measure and thus its major role in glucose metabolism tends to be overlooked. The contribution of GE to whole body glucose disposal in fasting conditions (fasting/basal insulin concentrations) is estimated at ~70% of total, while at typical post-absorptive insulin concentrations imposed during a hyperinsulinemic-euglycemic clamp, the contribution of GE to whole body glucose disposal drops to ~30%. It is thus estimated that the contribution of insulin-independent glucose disposal (GE) to the maintenance of glucose homeostasis in typical physiological post absorptive conditions is similar to that of insulin.59
When glucose tolerance deteriorates, GE is impaired and is unable to reduce blood glucose levels via suppression of hepatic glucose production or acceleration of muscle glucose uptake, independent of increased insulin concentration. In combination, the defects in GE and beta-cell insulin secretion promote a further rise in circulating blood sugar. Lower GE has been demonstrated in adult patients with T2DM60 and in children and adolescents with altered glucose metabolism.61 Of note, baseline levels and the dynamics of GE are independent predictors of changes in 2-h glucose levels over time,61 emphasizing the role of this factor in the development of altered glucose metabolism in obese children.
The role of gut-derived incretins and glucagon
Incretins are hormones released from the gastrointestinal tract in response to food intake and regulate islet hormone secretion. The two major incretins known at present are glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). Both potentiate glucose-induced insulin secretion and decrease the release of glucagon from the pancreatic islets.62 This manifests as an enhanced insulin response to oral glucose in comparison to intravenous glucose administration when both are matched for plasma glucose concentrations. Obesity and altered glucose metabolism in obese children are associated with reduced fasting and variable post-prandial GLP-1 concentrations.63 Obese children with IGT and T2DM manifest a significantly reduced incretin effect compared to those with normal glucose metabolism in the face of comparable GIP and GLP-1 concentrations.64 Moreover, obese African American children seem to have a reduced GLP-1 response during an OGTT compared to Caucasians. 65 The role of GIP is less clear in the context of altered glucose metabolism in childhood, as it has been shown to be released in comparable amounts in lean and obese children at euglycemia and in post prandial hyperglycemic conditions.66 Fasting GLP-1 has been associated with increased resting energy expenditure and fat oxidation in adults.67 Further investigation is needed to decipher whether the lower fasting concentrations of GLP-1 observed in obese youth may provide a mechanism for the development of altered glucose metabolism associated with adiposity in childhood and serve as a therapeutic pharmacological target in the future.
Intra-islet communications between beta and alfa cells via adjacent cell junctions and paracrine effects normally ensure coordinated secretion of insulin and glucagon. T2DM is characterized by disinhibited glucagon secretion in the face of relative systemic hyperinsulinemia.29 It has been shown that prior to the development of overt T2DM in obese youths, basal glucagon levels are increased and are less suppressed in the face of hyperinsulinemia in subjects with IGT.68 Moreover, obese children with NGT that were more insulin resistant had greater basal glucagon levels than those who were more insulin sensitive. In this study, deterioration from normal to impaired glucose tolerance over time was accompanied by significantly increased fasting glucagon.
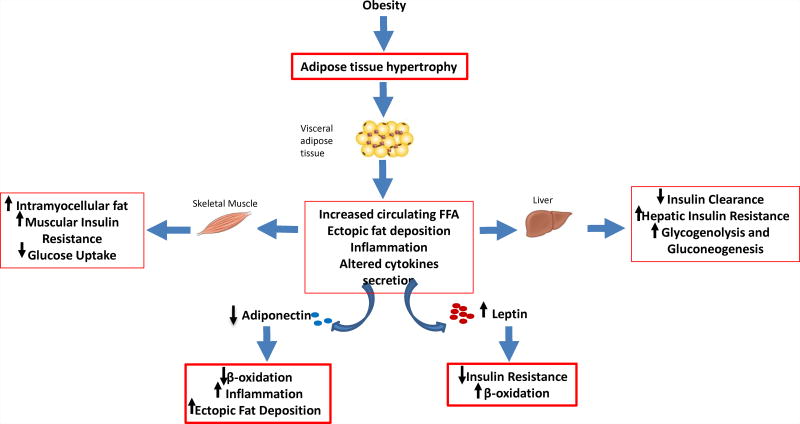
The role of fat derived hormones and cytokines (figure 3)
Figure 3. Interplay between fat derived hormones and cytokines.
The excess of visceral adipose tissue is linked to ectopic fat deposition and abnormal adipokines secretion. Obese patients with higher visceral adiposity show lower adiponectin and relatively low leptin levels despite similar body mass index and body fat percentage. Lower Adiponectin is associated with higher ectopic fat deposition in skeletal muscle and liver. Lower Leptin is associated with lower insulin sensitivity.
Adipose tissue is an active endocrine organ characterized by a unique profile of secreted hormones and cytokines. Adiponectin is a fat derived hormone that is paradoxically at lower concentrations in those with greater degrees of obesity.69 Receptors of this hormone are present in the major insulin responsive tissues related to glucose metabolism and its effects culminate in increased fat oxidation. It is thus not surprising that concentrations of this hormone are negatively related to intramyocellular, 70 intra-hepatic71 and visceral fat depots37 in obese children. Moreover, low concentrations of adiponectin were shown to be associated with higher C-reactive protein (CRP) concentrations and with components of the metabolic syndrome, such as low HDL-cholesterol and a high triglyceride-to-HDL-cholesterol ratio.72 Thus, adiponectin may be one of the signals linking inflammation and obesity. Importantly, there appear to be racial variations in concentrations of adiponectin amongst obese adolescents yet its tight relation with insulin sensitivity, independent of the amount of visceral fat, is consistent.73
Leptin is the also secreted from adipose tissue yet its concentrations increase with greater adipose tissue (in contrast to adiponectin). Leptin concentrations have been shown to be associated with insulin sensitivity in obese youth, independent of body fat.74 Leptin has been shown to induce fatty acid oxidation thus reducing liver75 and beta-cell76 triglyceride concentrations and can thus be considered a “favorable” player in the context of childhood obesity. Lower leptin level has been shown in obese adolescents with T2DM compared to matched equally obese adolescents with normal glucose metabolism, 77 suggesting that relative hypoleptinemia in obese youth may be a biomarker indicating the presence of an adverse metabolic phenotype.
Prediabetes and the Progression to T2DM
Although prediabetes is a high-risk state for developing overt diabetes, many people with prediabetes will not progress to severe glucose intolerance.13, 14 Indeed, the prevalence of prediabetes and diabetes in the obese pediatric population varies dramatically across different countries and ethnicities.17, 18, 20, 78, 79 Rates of progression of IFG to overt T2DM appear to be lower in the pediatric obese population than in adults.80 On the other hand, the transition from IGT to T2DM has been shown to be more rapid in children and adolescents than adults.35 The prediabetic stages IGT and IFG do not necessarily coexist15, 24, 79 which emphasizes that these two conditions are distinct metabolic abnormalities.81 Therefore, subjects with both IFG and IGT have additive metabolic defects and are more likely to progress to overt T2DM.82 Importantly, the repeatability of prediabetes detection using an OGTT in obese children is not ideal yet the presence of elevated post oral load glucose concentrations, even on a single study, probably indicates the presence of substantial defects in glucose metabolism.25
Genetic markers of pediatric prediabetes
Several genome wide association studies (GWAS) have helped highlighting the genetic basis of T2DM and several single nucleotide polymorphisms (SNPs) for example in genes involved in insulin metabolism and the inflammatory response have been discovered to be associated with T2DM.83 The majority of gene variants associated with prediabetes and T2DM are in genes expressed in beta-cells. Because of the lack of large pediatric cohorts, the majority of GWAS have been conducted in adults and information about the genetics of prediabetes in youth is limited. Barker et al. genotyped 16 SNPs, previously found to be associated with diabetes by GWAS, in 6000 children and adolescents and determined their association with fasting glucose concentrations.84 The authors observed that 9 loci were indeed associated with the fasting glucose concentrations, specifically confirming 5 previously discovered SNPs and discovering 4 additional loci. More recently, it has been shown that common variants in or near genes modulating insulin secretion are associated with a high risk for developing prediabetes in youth.85 The co-occurrence of risk alleles in or near genes expressed in the beta-cell is associated with defects of insulin secretion, that may results in the development of prediabetes when severe insulin resistance occurs.85 Although the number of relevant T2DM susceptibility genes has climbed over the past decade, the rs7903146 SNP in the TCF7L2 gene remains the single strongest known T2DM genetic risk factor in adults. Recently we observed in a young cohort, that each copy of the T allele of the rs7903146 SNP is associated with almost 2 fold increased odds of showing IGT (p=0.0001).86 Moreover, our longitudinal data showed that the TCF7L2 risk genotype is associated with a high risk of maintaining IGT or progressing toward T2DM (OR 2.419; 95% CI 1.291–4.532, p=0.006).86 To unravel the mechanisms underlying the genotype/phenotype association we employed the oral minimal model in a large multiethnic cohort of youths, assessed the proinsulin processing by measuring the circulating fasting proinsulin/c-peptide ratio and used the euglycemic clamp coupled with tracer methodologies to more accurately assess hepatic and peripheral insulin sensitivity. We observed that the T allele of TCF7L2 rs7903146 has profound effects on beta-cell function as reflected by a reduced DI (and an altered proinsulin secretory efficiency). The effect of the TCF7L2 rs7903146 probably involves the liver by reducing the ability of insulin to suppress hepatic endogenous glucose production.86
Conclusions and future perspectives
Taken together, altered glucose metabolism in obese children is preceded by early defects in insulin secretion in the face of low insulin sensitivity as well as inadequate suppression of glucagon. These defects can be detected within the “high-normal” range of normal glucose concentrations, emphasizing that glucose tolerance represents a continuous spectrum. A combination of low insulin sensitivity tightly linked to adverse lipid partitioning patterns, along with inadequate beta-cell compensation, impaired glucose effectiveness, and elevated basal glucagon secretion drives deterioration of glucose tolerance that may be progressive and culminate in overt diabetes. Despite the fact that pre-diabetes indicators such as IFG and IGT may be reversible, their detection implicates that the individuals’ beta-cell function reached it maximal capacity and has already failed once and is thus predisposed to fail in the future facing a similar metabolic challenge.
As the role of gut hormones as trophic agents for beta-cells and of inflammation as a major driver of beta-cell destruction and failure, interventions targeting these metabolic pathways are being investigated. These could be used in the future to treat T2DM or more importantly to prevent it in patients with pre-diabetes. The impact of anatomical modifications of the gastrointestinal tract on glucose metabolism, either via hormonal and metabolic effects or by changes in the gut microbiome is another topic of interest. The effects of surgical manipulations of the gut on the prevention of T2DM in obese youth with prediabetes over time need further investigation.
From a therapeutic point of view, several questions remain unanswered. It would be important to understand whether early interventions might help recover the beta cell function and stop the progression from prediabetes towards overt diabetes. Although some multicentric studies are ongoing in adults and adolescents, 7 more studies targeting obese adolescents with prediabetes are needed. Moreover, despite our knowledge in the field of biomarkers has gained in the last few years, so far we have not been able to translate this knowledge in clinical setting. Therefore, the next step in pediatric prediabetes should be to try to understand how we can leverage the current knowledge in this field to develop more sensitive and more specific diagnostic tools.
Key messages of the paper.
Prediabetes in obese children involves inadequate insulin secretion the face of significant insulin resistance.
There are strong associations between lipid partitioning in insulin-responsive tissues (such as the liver and muscle) and whole body insulin resistance
The development of pre-diabetes involves multiple metabolic dfecets that involve gut-hormone profiles, hyperglucegonemia and reduced glucose effectiveness
Genome wide association studies have identified specific SNPs that are associated with tissue specific insulin resistance and/or beta-cell dysfunction making their carriers more prone to the adverse metabolic impact of obesity
Search strategy and selection criteria.
The authors used the Pubmed database using the search terms : prediabetes, impaired fasting glucose and impaired glucose tolerance with the age limits of birth to 18 years of age. Studies included were written in English, preferably using “gold standard” methodology to assess insulin sensitivity and secretion (clamps or IVGTTs). Prevalence studies from across the globe were selected based on the sample size. The data range was between the years 1980 to the April 2017.
Acknowledgments
This work has been made possible by R01-DK111038 and R01-HD028016 to SC. NS is funded by the American Heart Association (AHA) through the 13SDG14640038 and the 16IRG27390002, and by the Allen foundation award to NS. A.G. received a Research Fellowship from the International Society for Pediatric and Adolescent Diabetes (ISPAD This work was also made possible by DK045735 to the Yale Diabetes Research Center and Clinical and Translational Science Awards Grant UL1-RR- 024139 from the National Center for Advancing Translational Sciences, a component of the NIH, and NIH Roadmap for Medical Research. This paper’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declared no conflicts of interest
Contribution statement
All authors participated equally to the manuscript writing. All authors reviewed, commented and approved the final text.
References
- 1.Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes care. 2012;35(12):2515–20. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. The New England journal of medicine. 2002;346(11):802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 3.Morera J, Joubert M, Morello R, Rod A, Lireux B, Reznik Y. Sustained Efficacy of Insulin Pump Therapy in Type 2 Diabetes: 9-Year Follow-up in a Cohort of 161 Patients. Diabetes care. 2016;39(6):e74–5. doi: 10.2337/dc16-0287. [DOI] [PubMed] [Google Scholar]
- 4.Picard S, Hanaire H, Baillot-Rudoni S, Gilbert-Bonnemaison E, Not D, Reznik Y, et al. Evaluation of the Adherence to Continuous Glucose Monitoring in the Management of Type 1 Diabetes Patients on Sensor-Augmented Pump Therapy: The SENLOCOR Study. Diabetes Technol Ther. 2016;18(3):127–35. doi: 10.1089/dia.2015.0240. [DOI] [PubMed] [Google Scholar]
- 5.Harris DL, Battin MR, Weston PJ, Harding JE. Continuous glucose monitoring in newborn babies at risk of hypoglycemia. J Pediatr. 2010;157(2):198–202. doi: 10.1016/j.jpeds.2010.02.003. e1. [DOI] [PubMed] [Google Scholar]
- 6.Breda E, Toffolo G, Polonsky KS, Cobelli C. Insulin release in impaired glucose tolerance: oral minimal model predicts normal sensitivity to glucose but defective response times. Diabetes. 2002;(51 Suppl 1):S227–33. doi: 10.2337/diabetes.51.2007.s227. [DOI] [PubMed] [Google Scholar]
- 7.Consortium R. Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes care. 2014;37(3):780–8. doi: 10.2337/dc13-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronson R, Reznik Y, Conget I, Castañeda JA, Runzis S, Lee SW, et al. Sustained efficacy of insulin pump therapy compared with multiple daily injections in type 2 diabetes: 12-month data from the OpT2mise randomized trial. Diabetes Obes Metab. 2016;18(5):500–7. doi: 10.1111/dom.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236(6):E667–77. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 10.Toffanin C, Messori M, Di Palma F, De Nicolao G, Cobelli C, Magni L. Artificial pancreas: model predictive control design from clinical experience. J Diabetes Sci Technol. 2013;7(6):1470–83. doi: 10.1177/193229681300700607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376(15):1419–29. doi: 10.1056/NEJMoa1610187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.administration USFaD. Symlin (Pramlintide Acetate) Injection - Approval package. [Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21-332_Symlin.cfm.
- 13.Amer Diabet A. Diagnosis and Classification of Diabetes Mellitus AMERICAN DIABETES ASSOCIATION. Diabetes care. 2011;34:S62–S9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Oganization G, Switzerland. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF Consultation. 2006 [Google Scholar]
- 15.Brufani C, Ciampalini P, Grossi A, Fiori R, Fintini D, Tozzi A, et al. Glucose tolerance status in 510 children and adolescents attending an obesity clinic in Central Italy. Pediatric Diabetes. 2010;11(1):47–54. doi: 10.1111/j.1399-5448.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- 16.Hagman E, Reinehr T, Kowalski J, Ekbom A, Marcus C, Holl RW. Impaired fasting glucose prevalence in two nationwide cohorts of obese children and adolescents. Int J Obes (Lond) 2014;38(1):40–5. doi: 10.1038/ijo.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranowski T, Cooper DM, Harrell J, Hirst K, Kaufman FR, Goran M, et al. Presence of diabetes risk factors in a large U.S. eighth-grade cohort. Diabetes care. 2006;29(2):212–7. doi: 10.2337/diacare.29.02.06.dc05-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan GE. Prevalence of diabetes and impaired fasting glucose levels among US adolescents: National Health and Nutrition Examination Survey, 1999–2002. Archives of pediatrics & adolescent medicine. 2006;160(5):523–8. doi: 10.1001/archpedi.160.5.523. [DOI] [PubMed] [Google Scholar]
- 19.Williams DE, Cadwell BL, Cheng YJ, Cowie CC, Gregg EW, Geiss LS, et al. Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics. 2005;116(5):1122–6. doi: 10.1542/peds.2004-2001. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto-Kimura L, Posadas-Romero C, Posadas-Sanchez R, Zamora-Gonzalez J, Cardoso-Saldana G, Mendez Ramirez I. Prevalence and interrelations of cardiovascular risk factors in urban and rural Mexican adolescents. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2006;38(5):591–8. doi: 10.1016/j.jadohealth.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Narayanappa D, Rajani HS, Mahendrappa KB, Prabhakar AK. Prevalence of prediabetes in school-going children. Indian Pediatr. 2011;48(4):295–9. doi: 10.1007/s13312-011-0061-6. [DOI] [PubMed] [Google Scholar]
- 22.Yan WL, Li XS, Wang Q, Huang YD, Zhang WG, Zhai XH, et al. Overweight, high blood pressure and impaired fasting glucose in Uyghur, Han, and Kazakh Chinese children and adolescents. Ethn Health. 2015;20(4):365–75. doi: 10.1080/13557858.2014.921894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CM, Lou MF, Gau BS. Prevalence of impaired fasting glucose and analysis of related factors in Taiwanese adolescents. Pediatric Diabetes. 2014;15(3):220–8. doi: 10.1111/pedi.12081. [DOI] [PubMed] [Google Scholar]
- 24.Al Amiri E, Abdullatif M, Abdulle A, Al Bitar N, Afandi EZ, Parish M, et al. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC public Health. 2015;15:1298. doi: 10.1186/s12889-015-2649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLendon D, Check J, Carteaux P, Michael L, Moehring J, Secrest JW, et al. Implementation of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics. 2003;111(4 Pt 2):e497–503. [PubMed] [Google Scholar]
- 26.Rachman J, Barrow BA, Levy JC, Turner RC. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia. 1997;40(2):205–11. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- 27.Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes care. 2011;34(6):1306–11. doi: 10.2337/dc10-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan Y, Elleri D, Allen JM, Tauschmann M, Wilinska ME, Dunger DB, et al. Pharmacokinetics of diluted (U20) insulin aspart compared with standard (U100) in children aged 3–6 years with type 1 diabetes during closed-loop insulin delivery: a randomised clinical trial. Diabetologia. 2015;58(4):687–90. doi: 10.1007/s00125-014-3483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Prato S, Marchetti P, Bonadonna RC. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes. 2002;(51 Suppl 1):S109–16. doi: 10.2337/diabetes.51.2007.s109. [DOI] [PubMed] [Google Scholar]
- 31.Conget I, Castaneda J, Petrovski G, Guerci B, Racault AS, Reznik Y, et al. The Impact of Insulin Pump Therapy on Glycemic Profiles in Patients with Type 2 Diabetes: Data from the OpT2mise Study. Diabetes Technol Ther. 2016;18(1):22–8. doi: 10.1089/dia.2015.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group TS. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes. 2007;8(5):286–98. doi: 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 33.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, VanWeissenbruch M, Midgley P, et al. Validation of the continuous glucose monitoring sensor in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2013;98(2):F136–40. doi: 10.1136/archdischild-2012-301661. [DOI] [PubMed] [Google Scholar]
- 34.Narasimhan S, Weinstock RS. Youth-onset type 2 diabetes mellitus: lessons learned from the TODAY study. Mayo Clinic proceedings. 2014;89(6):806–16. doi: 10.1016/j.mayocp.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes care. 2005;28(4):902–9. doi: 10.2337/diacare.28.4.902. [DOI] [PubMed] [Google Scholar]
- 36.Asao T, Oki K, Yoneda M, Tanaka J, Kohno N. Hypothalamic-pituitary-adrenal axis activity is associated with the prevalence of chronic kidney disease in diabetic patients. Endocr J. 2015 doi: 10.1507/endocrj.EJ15-0360. [DOI] [PubMed] [Google Scholar]
- 37.Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, et al. The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. The Journal of clinical endocrinology and metabolism. 2005;90(6):3731–7. doi: 10.1210/jc.2004-2305. [DOI] [PubMed] [Google Scholar]
- 38.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. The Journal of clinical endocrinology and metabolism. 2004;89(3):1096–101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 39.Bode BW, Garg SK. THE EMERGING ROLE OF ADJUNCTIVE NONINSULIN ANTIHYPERGLYCEMIC THERAPY IN THE MANAGEMENT OF TYPE 1 DIABETES. Endocr Pract. 2016;22(2):220–30. doi: 10.4158/EP15869.RA. [DOI] [PubMed] [Google Scholar]
- 40.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49(3):571–9. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 41.Weiss R, Cali AM, Dziura J, Burgert TS, Tamborlane WV, Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes care. 2007;30(7):1845–50. doi: 10.2337/dc07-0325. [DOI] [PubMed] [Google Scholar]
- 42.Lönnrot M, Lynch K, Larsson HE, Lernmark Å, Rewers M, Hagopian W, et al. A method for reporting and classifying acute infectious diseases in a prospective study of young children: TEDDY. BMC Pediatr. 2015;15:24. doi: 10.1186/s12887-015-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan O, Chan S, Inouye K, Shum K, Matthews SG, Vranic M. Diabetes impairs hypothalamo-pituitary-adrenal (HPA) responses to hypoglycemia, and insulin treatment normalizes HPA but not epinephrine responses. Diabetes. 2002;51(6):1681–9. doi: 10.2337/diabetes.51.6.1681. [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, et al. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4(158):ra6. doi: 10.1126/scisignal.2001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giannini C, Weiss R, Cali A, Bonadonna R, Santoro N, Pierpont B, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61(3):606–14. doi: 10.2337/db11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cali AM, Man CD, Cobelli C, Dziura J, Seyal A, Shaw M, et al. Primary defects in beta-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes care. 2009;32(3):456–61. doi: 10.2337/dc08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Group TS. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci. 2008;1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(31):12587–94. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49(6):1896–903. doi: 10.1002/hep.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wadwa RP, Kinney GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, et al. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes care. 2005;28(5):1051–6. doi: 10.2337/diacare.28.5.1051. [DOI] [PubMed] [Google Scholar]
- 51.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Adamo E, Cali AM, Weiss R, Santoro N, Pierpont B, Northrup V, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes care. 2010;33(8):1817–22. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alderete TL, Toledo-Corral CM, Desai P, Weigensberg MJ, Goran MI. Liver fat has a stronger association with risk factors for type 2 diabetes in African-American compared with Hispanic adolescents. The Journal of clinical endocrinology and metabolism. 2013;98(9):3748–54. doi: 10.1210/jc.2013-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim G, Giannini C, Pierpont B, Feldstein AE, Santoro N, Kursawe R, et al. Longitudinal effects of MRI-measured hepatic steatosis on biomarkers of glucose homeostasis and hepatic apoptosis in obese youth. Diabetes care. 2013;36(1):130–6. doi: 10.2337/dc12-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(36):15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444–50. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 57.Nisticò L, Iafusco D, Galderisi A, Fagnani C, Cotichini R, Toccaceli V, et al. Emerging effects of early environmental factors over genetic background for type 1 diabetes susceptibility: evidence from a Nationwide Italian Twin Study. J Clin Endocrinol Metab. 2012;97(8):E1483–91. doi: 10.1210/jc.2011-3457. [DOI] [PubMed] [Google Scholar]
- 58.Genuth SM, Backlund JY, Bayless M, Bluemke DA, Cleary PA, Crandall J, et al. Effects of prior intensive versus conventional therapy and history of glycemia on cardiac function in type 1 diabetes in the DCCT/EDIC. Diabetes. 2013;62(10):3561–9. doi: 10.2337/db12-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 60.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–20. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 61.Fendler W, Walenciak J, Mlynarski W, Piotrowski A. Higher glycemic variability in very low birth weight newborns is associated with greater early neonatal mortality. J Matern Fetal Neonatal Med. 2012;25(7):1122–6. doi: 10.3109/14767058.2011.624220. [DOI] [PubMed] [Google Scholar]
- 62.Smith U. TCF7L2 and type 2 diabetes--we WNT to know. Diabetologia. 2007;50(1):5–7. doi: 10.1007/s00125-006-0521-z. [DOI] [PubMed] [Google Scholar]
- 63.Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, Sjögren M, et al. Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes. 2006;55(10):2890–5. doi: 10.2337/db06-0381. [DOI] [PubMed] [Google Scholar]
- 64.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–16. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–90. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40(5):638–45. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336–41. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cloherty P, Eichenwald E, Hansen A, Stark A. Manual of Neonatal Care. 7. Lippincott, editor; [Google Scholar]
- 69.Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiss R, Dufour S, Groszmann A, Petersen K, Dziura J, Taksali SE, et al. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. The Journal of clinical endocrinology and metabolism. 2003;88(5):2014–8. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 71.Cengiz E. Closer to ideal insulin action: ultra fast acting insulins. Panminerva Med. 2013;55(3):269–75. [PubMed] [Google Scholar]
- 72.Chase HP, Lutz K, Pencek R, Zhang B, Porter L. Pramlintide lowered glucose excursions and was well-tolerated in adolescents with type 1 diabetes: results from a randomized, single-blind, placebo-controlled, crossover study. J Pediatr. 2009;155(3):369–73. doi: 10.1016/j.jpeds.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 73.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes care. 2006;29(1):51–6. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 74.Cengiz E. Undeniable need for ultrafast-acting insulin: the pediatric perspective. J Diabetes Sci Technol. 2012;6(4):797–801. doi: 10.1177/193229681200600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casazza K, Phadke RP, Fernandez JR, Watanabe RM, Goran MI, Gower BA. Obesity attenuates the contribution of African admixture to the insulin secretory profile in peripubertal children: a longitudinal analysis. Obesity (Silver Spring) 2009;17(7):1318–25. doi: 10.1038/oby.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26(12):1198–203. doi: 10.1111/j.1464-5491.2009.02841.x. [DOI] [PubMed] [Google Scholar]
- 77.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16(8):1901–7. doi: 10.1038/oby.2008.307. [DOI] [PubMed] [Google Scholar]
- 78.Valerio G, Licenziati MR, Iannuzzi A, Franzese A, Siani P, Riccardi G, et al. Insulin resistance and impaired glucose tolerance in obese children and adolescents from Southern Italy. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2006;16(4):279–84. doi: 10.1016/j.numecd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 79.van Vliet M, Gazendam RP, von Rosenstiel IA, van Zanten AP, Brandjes DP, Beijnen JH, et al. Differential impact of impaired fasting glucose versus impaired glucose tolerance on cardiometabolic risk factors in multi-ethnic overweight/obese children. European journal of pediatrics. 2011;170(5):589–97. doi: 10.1007/s00431-010-1323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagman E, Danielsson P, Brandt L, Ekbom A, Marcus C. Association between impaired fasting glycaemia in pediatric obesity and type 2 diabetes in young adulthood. Nutr Diabetes. 2016;6(8):e227. doi: 10.1038/nutd.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cali AM, Bonadonna RC, Trombetta M, Weiss R, Caprio S. Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. The Journal of clinical endocrinology and metabolism. 2008;93(5):1767–73. doi: 10.1210/jc.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78(3):305–12. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 83.Flannick J, Florez JC. Type 2 diabetes: genetic data sharing to advance complex disease research. Nature reviews Genetics. 2016;17(9):535–49. doi: 10.1038/nrg.2016.56. [DOI] [PubMed] [Google Scholar]
- 84.Barker A, Sharp SJ, Timpson NJ, Bouatia-Naji N, Warrington NM, Kanoni S, et al. Association of genetic Loci with glucose levels in childhood and adolescence: a meta-analysis of over 6,000 children. Diabetes. 2011;60(6):1805–12. doi: 10.2337/db10-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giannini C, Dalla Man C, Groop L, Cobelli C, Zhao H, Shaw MM, et al. Co-occurrence of risk alleles in or near genes modulating insulin secretion predisposes obese youth to prediabetes. Diabetes care. 2014;37(2):475–82. doi: 10.2337/dc13-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cropano C, Santoro N. The rs7903146 Variant in the TCF7L2 Gene Increases the Risk of Prediabetes/Type 2 Diabetes in Obese Adolescents by Impairing beta-Cell Function and Hepatic Insulin Sensitivity. 2017 doi: 10.2337/dc17-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]