Abstract
Cancer is the second leading cause of death globally, and use of therapeutic peptides to target and kill cancer cells has received considerable attention in recent years. Identification of anticancer peptides (ACPs) through wet-lab experimentation is expensive and often time consuming; therefore, development of an efficient computational method is essential to identify potential ACP candidates prior to in vitro experimentation. In this study, we developed support vector machine- and random forest-based machine-learning methods for the prediction of ACPs using the features calculated from the amino acid sequence, including amino acid composition, dipeptide composition, atomic composition, and physicochemical properties. We trained our methods using the Tyagi-B dataset and determined the machine parameters by 10-fold cross-validation. Furthermore, we evaluated the performance of our methods on two benchmarking datasets, with our results showing that the random forest-based method outperformed the existing methods with an average accuracy and Matthews correlation coefficient value of 88.7% and 0.78, respectively. To assist the scientific community, we also developed a publicly accessible web server at www.thegleelab.org/MLACP.html.
Keywords: anticancer peptides, hybrid model, machine-learning parameters, random forest, support vector machine
INTRODUCTION
Cancer is a heterogeneous group of several complex diseases, rather than a single disease, which is characterized by uncontrolled cell growth and the ability to rapidly spread or invade other parts of the body. This inherent complexity and heterogeneous nature of cancer has proven to be a major hurdle for the development of effective anticancer therapies [1]. Conventional methods for cancer treatment, including radiotherapy and chemotherapy, are expensive and often exhibit deleterious side effects on normal cells. Additionally, cancer cells are capable of developing resistance to current anticancer chemotherapeutic drugs [2, 3]. Therefore, it is necessary to continually develop novel anticancer drugs to attenuate cancer cell proliferation. Peptide-based therapy has several advantages over the use of other small molecules due to their high specificity, increased capability for tumor penetration, and minimal toxicity under normal physiological conditions [4].
Anticancer peptides (ACPs) are peptides capable of use as therapeutic agents to treat various cancers. Recent studies showed that ACPs are selective toward cancer cells without affecting normal physiological functions, making them a potentially valuable therapeutic strategy [5, 6]. ACPs contain between 5-30 amino acids and exhibit cationic amphipathic structures capable of interacting with the anionic lipid membrane of cancer cells, thereby enabling selective targeting [7, 8]. In the previous decade, multiple peptide-based therapies against various tumor types have been evaluated and are currently undergoing evaluation in various phases of preclinical and clinical trials [9], confirming the importance of developing novel ACPs for cancer treatment.
Experimental identification and development of novel ACPs represent extremely expensive and often time-consuming processes. Therefore, development of sequence-based computational methods is necessary to allow the rapid identification of potential ACP candidates prior to their synthesis. To this end, computational methods, including AntiCP, iACP, and that described by Hajisharifi et al (2014), have been developed for ACP prediction [10–13]. Existing methods separately use properties, such as amino acid composition (AAC), binary profile, dipeptide composition (DPC), and Chou's pseudo-amino acid composition (PseAAC), extracted from the primary sequence as input features to a support vector machine (SVM) for the development of a prediction model. Surprisingly, all of these methods use the same machine-learning (ML) method, with the two methods [that of Hajisharifi et al (2014) and iACP] using the same dataset for prediction-model development. These methods produced encouraging results, and iACP and AntiCP remain the only publically available programs for assisting the scientific community [14–16].
Although, the existing methods have specific advantages for ACP prediction, it remains necessary to improve prediction accuracy. In this study, we developed ML-based methods [SVM and random forest (RF); named SVMACP and RFACP, respectively] to predict ACPs (MLACP) using combinations of features calculated from the peptide sequence, including AAC, DPC, atomic composition (ATC), and physicochemical properties (PCP). When tested upon benchmarking datasets, our proposed methods outperformed the existing ones in predicting ACPs. Moreover, we developed a web tool to assist the scientific community working in the field of ACP therapeutics and biomedical research.
RESULTS
Dataset construction
A detailed description of dataset construction is given in the ‘materials and methods’ section. An overview of our methodology is shown in Figure 1. Briefly, we generated three different datasets, namely Tyagi-B dataset, Hajisharifi-Chen (HC), and LEE dataset. The histogram of peptide-length distribution of these datasets is shown in Figure 2. Most of the ACPs contain <35 amino acid residues and non-ACPs have a wider size distribution in Tyagi-B dataset (Figure 2A), which was utilized in the development of a prediction model. HC and LEE datasets were treated as benchmarking datasets. Among these, HC showed similar distribution between ACPs and non-ACPs (Figure 2B), whereas, in LEE dataset, most of the ACPs contained <25 amino acid residues and non-ACPs showed a wider distribution (Figure 2C).
Figure 1. Flowchart showing steps involved in the development of prediction model (MLACP methodology).
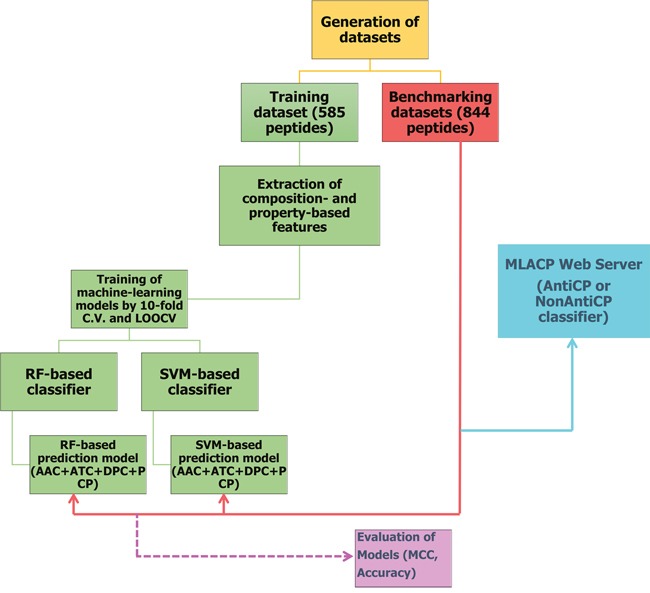
Figure 2. Histogram of the peptide-length distribution of ACPs and non-ACPs.
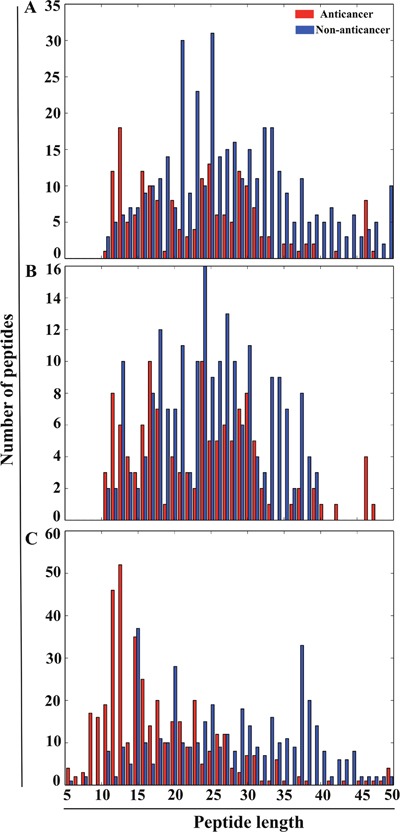
X- and Y-axes represent peptide length and number of peptides. (A) Tyagi-B dataset. (B) HC dataset. (C) LEE dataset.
Compositional analysis
To perform compositional analysis of ACPs and non-ACPs, AAC, DPC, PCC, and ATC frequencies were calculated using the Tyagi-B and HC datasets. AAC analysis revealed that certain residues, including A, F, K, L, and W, were dominant in ACPs, whereas D, E, G, N, and Q were dominant in non-ACPs (Welch's t test; p < 0.01). PCP analysis indicated that only two properties (hydrophobicity and residue mass) were dominant in ACPs, whereas the remaining nine properties were dominant in non-ACPs. ATC analysis revealed that hydrogen and carbon content dominated at a slightly higher level in ACPs as compared with non-ACPs (Figure 3A). Moreover, DPC analyses revealed that 104 out of 400 dipeptides were differentially present in ACPs and non-ACPs (p < 0.01). Our analyses also revealed that the 10 most abundant dipeptides in ACPs and non-ACPs were KK, AK, KL, AL, KA, KW, LA, LK, FA, and LF and KG, GL, GV, LD, GI, DL, LS, SG, LV, and TL, respectively (Figure 3B).
Figure 3. Comparison of AAC, ATC, PCP, and DPC features between ACPs and non-ACPs.
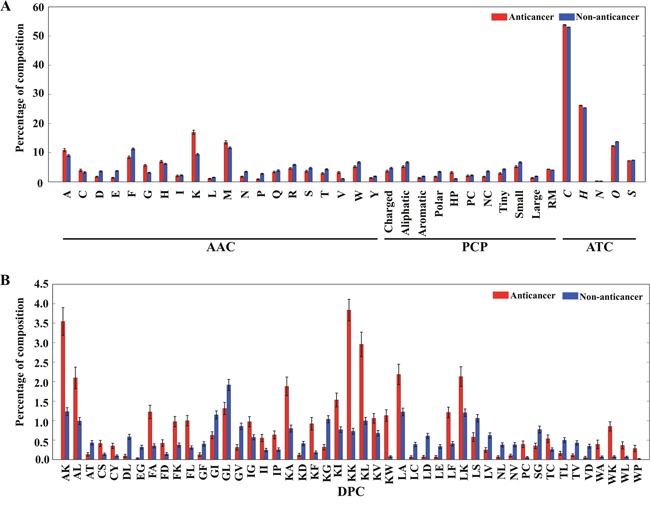
(A) Three different compositions (AAC, PCP, and ATC). For PCPs, HP, PC, NC, and RM represent hydrophobic, positively charged, negatively charged residues and residue mass, respectively. To discriminate element in ATC from AAC, we have shown in italics. Similarly, for PCP to discriminate from DPC. (B) For DPC, we showed only dipeptides exhibiting the absolute differences between ACP and non-ACP is greater than 0.25.
Based on these findings, it was evident that the most abundant dipeptides in ACPs consisted primarily of pairs of positively charged-aromatic or –aliphatic amino acids, positively charged-positively charged amino acids, or aliphatic-aromatic amino acids, whereas the most abundant dipeptides in the non-ACPs were pairs of aliphatic–negatively charged amino acids and aliphatic–hydroxyl-group-containing amino acids. As expected, these results agreed with AAC analysis, which showed that positively charged and aromatic amino acids were abundant in ACPs, whereas negatively charged and hydroxyl-group-containing amino acids were the most abundant in non-ACPs.
Construction of SVMACP and RFACP
In this study, we considered two most commonly used ML methods (i.e. RF and SVM) to predict ACPs. One of the most important steps in ML method is feature selection. Here, we considered both composition- and property-based features (Figure 4). AAC, DPC, ATC, and PCP contained 20, 400, 5, and 11 features, respectively. First, we developed a prediction model based on an individual composition and subsequently developed hybrid models based on the combination of all possible compositions. For each model, we optimized the ML parameters (SVM: C and γ; RF: ntree, mtry, and nsplit) by using 10-fold cross-validation on Tyagi-B dataset. During 10-fold cross-validation, the Tyagi-B dataset was randomly divided into 10 parts (with ~10% ACPs and non-ACPs resident in each part), from which nine parts were used for training, and the 10th part was used for testing. This process was repeated until all the parts were used at least once as a test set, and the overall performance on all 10 parts was evaluated. The optimal parameters which gave the highest MCC was selected as the final one. It should be noted that we performed ten independent 10-fold cross-validations to verify the robustness of the ML parameters.
Figure 4. Overview of feature extraction.
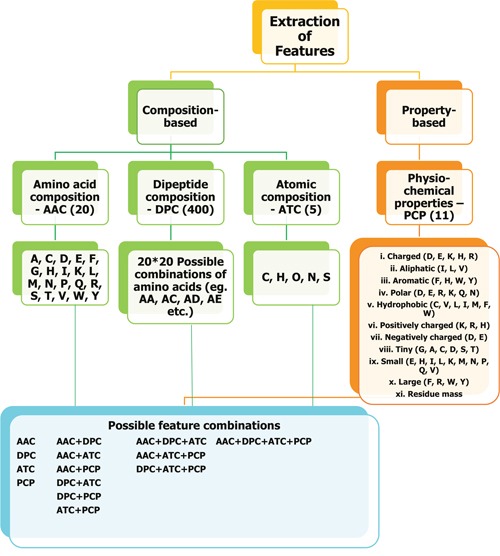
We used both composition-based and property-based information from a given peptide sequence and used as input feature to ML method. AAC, DPC, ATC, and PCP contained 20, 400, 5, and 11 features, respectively.
The following subsections describe the development of different models and the criteria used for final-model selection.
AAC-based models
Previous studies showed that AAC-based ML methods had been developed for the classification of different classes of peptides [14, 16]. During compositional analysis, we found significant differences between ACPs and non-ACPs (Figure 3). Therefore, we utilized these differences to classify peptides as ACPs or non-ACPs using ML models. Table 1 shows that the SVM model produced the best classification, with an accuracy of 0.858 and an MCC of 0.664, while the corresponding values for the RF model were 0.868 and 0.689, respectively.
Table 1. Performance of various prediction models on training dataset.
| Features | MCC | Accuracy | Sensitivity | Specificity | ||||
|---|---|---|---|---|---|---|---|---|
| SVM | RF | SVM | RF | SVM | RF | SVM | RF | |
| AAC | 0.664 | 0.689 | 0.858 | 0.868 | 0.695 | 0.706 | 0.935 | 0.945 |
| ATC | 0.519 | 0.587 | 0.802 | 0.826 | 0.503 | 0.658 | 0.942 | 0.905 |
| PCP | 0.420 | 0.553 | 0.759 | 0.814 | 0.524 | 0.599 | 0.869 | 0.915 |
| DPC | 0.653 | 0.644 | 0.853 | 0.850 | 0.706 | 0.599 | 0.922 | 0.967 |
| AAC+ATC+PCP+DPC | 0.697 | 0.698 | 0.872 | 0.872 | 0.706 | 0.722 | 0.95 | 0.942 |
| AAC+PCP+DCP | 0.693 | 0.661 | 0.870 | 0.856 | 0.706 | 0.620 | 0.947 | 0.967 |
| AAC+PCP+ATC | 0.685 | 0.698 | 0.867 | 0.872 | 0.695 | 0.727 | 0.947 | 0.940 |
| AAC+PCP | 0.681 | 0.681 | 0.865 | 0.865 | 0.695 | 0.695 | 0.945 | 0.945 |
| AAC+ATC | 0.664 | 0.673 | 0.858 | 0.862 | 0.695 | 0.642 | 0.935 | 0.965 |
| AAC+DCP | 0.673 | 0.657 | 0.862 | 0.855 | 0.701 | 0.61 | 0.937 | 0.970 |
| PCP+ATC+DCP | 0.661 | 0.669 | 0.856 | 0.86 | 0.711 | 0.631 | 0.925 | 0.967 |
| PCP+ATC | 0.595 | 0.664 | 0.831 | 0.858 | 0.615 | 0.685 | 0.932 | 0.940 |
| PCP+DCP | 0.661 | 0.661 | 0.856 | 0.856 | 0.701 | 0.620 | 0.93 | 0.967 |
| ATC+DCP | 0.657 | 0.661 | 0.855 | 0.856 | 0.701 | 0.620 | 0.927 | 0.967 |
The first column represents the features. The second, the third, the fourth and the fifth respectively represent the MCC, accuracy, specificity and sensitivity. Columns 2-5 subdivided into two parts namely SVM- (normal font) and RF-based (underlined) performances. AAC: amino acid composition; ATC: atomic composition; PCP: physiochemical properties; DPC: dipeptide composition. Features that gave the highest MCC is shown in bold.
DPC-based models
DPC provides additional information regarding the global and local arrangement of residues in a sequence as compared with AAC. DPC-based ML methods have been previously utilized to classify different classes of peptides [17–19]. Therefore, in this study, we developed RF- and SVM-based models using DPCs. The SVM model produced the best classification, with an accuracy of 0.853 and an MCC of 0.653, whereas the corresponding values for the RF-based model were 0.850 and 0.644, respectively. The performance of the DPC-based model was similar to that of the AAC-based model.
ATC-based models
We calculated a set of ATCs from the given peptides, because these were previously shown to be useful for the prediction of antihypertensive peptides [17–19]. Therefore, in this study, we developed RF- and SVM-based models using ATC. Our results showed that the SVM-based model produced the best classification, with an accuracy of 0.802 and an MCC of 0.519, whereas the corresponding values for the RF-based model were 0.826 and 0.587, respectively. However, the performance of the ATC-based model was slightly worse relative to that of the AAC- and DPC-based models (Table 1).
PCP-based models
For each dataset, we calculated a set of PCPs for each peptide, because these were previously shown to be useful for the prediction of different classes of proteins [19]. Therefore, in this study, we developed SVM- and RF-based models using PCPs. Results indicated that the SVM-based model produced the best classification, with an accuracy of 0.759 and an MCC of 0.420, whereas the corresponding values for the RF-based model were 0.814 and 0.553, respectively. However, the performance of this model was worse relative to that of each of the other three models (Table 1).
The hybrid model
Although individual composition-based models showed good or acceptable performance, to further improve the collective performance, we combined these features using all possible combinations to construct hybrid models. This approach has been widely applied in different peptide- and protein-composition-based classification methods [20, 21]. Table 1 shows that a hybrid model containing all of the composition- and property-based features produced the best classification among the different SVM-based hybrid models. Figure 5A shows the profile of classification accuracy verses the variations of parameters C and γ using all composition- and property-based features. The best classification accuracy of 0.872 peaked at (ln(C), ln(γ)) = (0.778, 2.178) was selected as the final model (SVMACP). Moreover, Table 1 shows that an RF-based hybrid model containing all of the features and a model containing only three features (excluding DCP) produced the same results. Notably, adding DCP features into the three combined features did not detract from the predictive performance; therefore, we selected the model containing all of the composition- and property-based features as the final prediction model (RFACP). Figure 5B shows the profile of classification accuracy verses variations in the parameters ntree and mtry using all composition- and property-based features. The best classification accuracy of 0.872 peaked at (ntree, mtry) = (450, 3) was selected as the final RF-based model.
Figure 5. Accuracies obtained from 10-fold cross-validation using various parameters.
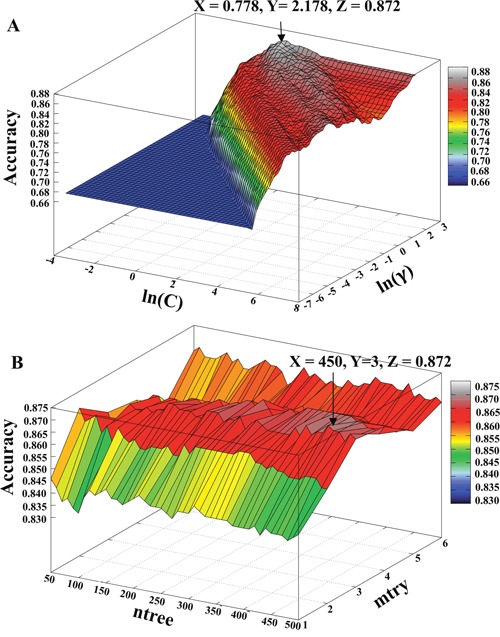
(A) The X- and Y-axes represent the SVM parameters C and γ on a natural logarithmic scale. The Z-axis represents the accuracy with respect to variations in C and γ. (B) The X- and Y-axes represent the RF parameters ntree and mtry. The Z-axis represents the accuracy with respect to variations in the parameters ntree and mtry. The arrow represents the maximum accuracy.
Performance of our methods against AntiCP using the HC dataset
We evaluated the performance of our methods (SVMACP and RFACP) against that of the AntiCP (model_1 and model_2) using the HC dataset, with the results shown in Table 2. The methods in the Table 2 are ranked according to the accuracy, which reflects the prediction capability of the method. For comparison, we also included iACP and the methods presented by Hajisharifi et al (2014) results, wherein the authors used the same dataset for their prediction model development [22]. Among the methods evaluated using the HC dataset, RFACP ranked at the top, with MCC, accuracy, sensitivity, and specificity values of 0.885, 0.946, 0.889, and 0.981, respectively. Additionally, RFACP performance was significantly better than that of AntiCP models, which exhibited ~8% and ~54% decreases in model_2 and model_1 accuracy, respectively, and SVMACP, which exhibited an ~6% decrease in accuracy. Furthermore, comparison of RFACP relative to iACP and that of Hajisharifi et al (2014) showed that RFACP results were slightly better than those of the method presented by Hajisharifi et al (2014), which exhibited a decrease in accuracy of ~2%, and similar to iACP results. Table 2 shows that SVMACP ranked second among all of the methods, exceeding the performance of the AntiCP models, which exhibited ~1% and ~48% decreases in accuracy for model_2 and model_1, respectively. When comparing both AntiCP models, it was observed that model_1 predicted almost all of the given peptides as potential ACPs, suggesting that model_2 performance is better in ACP prediction.
Table 2. Performance of various methods on the HC dataset.
| Method | MCC | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|
| iACP* | 0.897 | 0.951 | 0.899 | 0.985 |
| RFACP | 0.885 | 0.946 | 0.889 | 0.981 |
| Hajisharifi et al*. | 0.784 | 0.927 | 0.897 | 0.852 |
| SVMACP | 0.750 | 0.882 | 0.841 | 0.907 |
| AntiCP (Model_2) | 0.719 | 0.869 | 0.813 | 0.902 |
| AntiCP (Model_1) | 0.062 | 0.402 | 0.976 | 0.049 |
The first column represents the method name. The second, the third, the fourth, and the fifth respectively represent the MCC, accuracy, sensitivity and specificity. For comparison, we also included iACP and Hajisharifi et al. results, which is based on the training dataset results (*). Bold font denotes the best result.
Performance of our methods and other existing methods using the LEE dataset
We evaluated the performance of our methods (SVMACP and RFACP), and the existing methods including iACP, and AntiCP (model_1 and model_2) on the LEE dataset. Notably, our LEE dataset contained 844 peptides, which was ~3-fold larger than previously used benchmark datasets. Table 3 shows that RFACP was ranked at the top, with MCC, accuracy, sensitivity, and specificity values of 0.674, 0.827, 0.706, and 0.948, respectively. Additionally, the performance of RFACP was slightly better than that of SVMACP, which showed a ~1% decrease in accuracy, and significantly better than AntiCP models, which exhibited ~7.5% and ~30% decreases in accuracy for model_2 and model_1, respectively, and iACP, which exhibited ~12% decreases in accuracy. SVMACP ranked second in performance, which was significantly better than AntiCP models, which exhibited ~6% and 28.7% decreases in accuracy for model_2 and model_1, respectively, and iACP, which exhibited 11% decreases in accuracy. AntiCP model_2 and iACP occupied the third and fourth positions, respectively, with AntiCP model_1 exhibiting the worst performance. This evaluation clearly showed that RFACP and SVMACP exceeded the performance of the existing methods. Interestingly, although SVMACP and RFACP produced the same results (MCC: 0.697 and 0.872, respectively) on the training dataset, RFACP performance was slightly better on the benchmarking datasets (~6% better on the HC dataset and ~1% better on the LEE dataset) relative to that of SVMACP. This result showed that the RF-based method was more effective than the SVM for ACP prediction. A previous study reported successful application of RF for many biomedical classification problems [14, 15, 23]. Moreover, a detailed comparison of our methods and the existing methods in terms of methodology is provided in Table 4, showing that our methodology exceeded current methods while using a slightly larger training dataset, different ML methods, additional features, and larger benchmarking datasets.
Table 3. Performance of various methods on the LEE dataset.
| Method | MCC | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|
| RFACP | 0.674 | 0.827 | 0.706 | 0.948 |
| SVMACP | 0.630 | 0.814 | 0.775 | 0.853 |
| AntiCP (Model_2) | 0.505 | 0.752 | 0.744 | 0.761 |
| iACP | 0.412 | 0.706 | 0.697 | 0.716 |
| AntiCP (Model_1) | 0.096 | 0.527 | 0.938 | 0.116 |
The first column represents the method name. The second, the third, the fourth, and the fifth respectively represent the MCC, accuracy, sensitivity and specificity. Bold font denotes the best result.
Table 4. A comparison of anticancer peptide prediction methods.
| Method | Choice of ML method | Cross-validation | Training dataset size | Benchmarking dataset size | Features |
|---|---|---|---|---|---|
| AntiCP | SVM | 10-fold cross-validation (10-fold CV) | 450 | 200 | AAC, DPC, and binary profile |
| iACP | SVM | Leave-one-out cross-validation (LOOCV) | 344 | 300 | one-gap DPC |
| Hajisharifi et al. | SVM | LOOCV | 344 | 22 | Chou's PseAAC |
| MLACP | SVM and RF | 10-fold CV | 585 | 332 and 603 | AAC, DPC, ATC, and PCP |
The first column represents the method name. The second column represents the choice of ML methods used for their method development. The third column represents the cross-validation procedure used for the optimization of ML parameters. The fourth and fifth column respectively represent the size of the training dataset and benchmarking dataset. The final column represents the total number of compositional features considered by each method. AAC: amino acid composition; ATC: atomic composition; PCP: physiochemical properties; DPC: dipeptide composition.
The MLACP online prediction server
As mentioned in a series of publications [20, 24–30], a prediction method along with its web server would be practically useful to the experimentalists [31–37]. To this end, an online prediction server called MLACP was developed to allow ACP prediction using the methods presented here. The prediction server is freely accessible at the following link: www.thegleelab.org/MLACP.html. Users can paste or upload query peptide sequences in the FASTA format, and after submitting peptide sequences, retrieve results in a separate interface. To enable the reproducibility of our findings, all datasets used in this study can be downloaded from the MLACP web server.
DISCUSSION
Anticancer peptides exhibit a broad spectrum of activity, including the ability to kill cancer cells, destroy primary tumors, prevent metastasis, and perform these actions at adequate concentrations without damaging normal cells or vital organs [38]. To identify highly efficient ACPs, an experimentalist should screen a peptide from the existing peptide libraries or scan the entire protein in overlapping-window patterns associated with areas of peptide chains, and test each segment for its potential anticancer activity, which seems laborious and time-consuming. Therefore, the development of sequence-based computational methods capable of determining ACP candidates will be helpful to researchers, who are keen to rapidly screen ACPs prior to its synthesis, thereby accelerating ACP-based research. Here, we developed two MLACP methods, RFACP and SVMACP.
AAC, DPC, ATC, and PCP analyses revealed that ACPs most often consist of positively charged, aromatic, and hydrophobic residues. Previous studies showed that peptide hydrophobicity plays an important role in membrane permeabilization and/or anticancer activity [9, 39]. Furthermore, we observed a significant difference in residue preference between ACPs and non-ACPs, which prompted us to use these as input features to ML methods to encourage improved classification. The major advantage of ML methods is their capability to consider multiple features simultaneously, often capturing hidden relationships [40–46].
In this study, we employed two different ML algorithms, SVM and RF, for ACP prediction, whereas existing methods use only SVM [14, 16]. This is the first application of an RF-based method in ACP prediction, with systematic approaches employed to select between SVMACP- and RFACP-based prediction models. Notably, MLACP represents the only method utilizing a combination of all composition- and property-based features as inputs; however, other existing methods [AntiCP, iACP, and that of Hajishari et al (2014)] utilize only one of the following properties, AAC, DPC, binary profile, or PseAAC, separately as an input feature to SVM in order to develop their prediction models [14–16]. Although, AAC and DPC features were used in earlier studies, this is the first study describing the use of PCP and ATC features for ACP prediction. To show the effect of including PCP and ATC in MLACP (i.e. RFACP and SVMACP), we evaluated a prediction model (which contains only AAC and DCP as input features) on LEE datasets. Supplementary Information 1 shows that improvement of both ML-based methods is found by adding PCP and ATC into MLACP.
We used two benchmarking datasets (HC and LEE) to evaluate the performance of our methods along with the existing methods. Using the HC dataset, RFACP and SVMACP, respectively, ranked as the first and second most effective predictors, with significantly better performances than the existing AntiCP methods (model_2 and model_1). Interestingly, RFACP accuracy was better than that of the method described by Hajisharifi et al (2014) using the same training set. Recently, Chen et al (2016) evaluated their method along with the AntiCP method using a smaller benchmarking dataset (300 peptides). Indeed, this was the first instance where ACP-prediction methods were evaluated using standard benchmarking dataset. However, the LEE dataset constructed in this study was almost 3-fold larger than previously reported benchmarking datasets. Such a large-sized benchmarking dataset is sufficient to evaluate the performance of various methods, with our benchmarking results showing that RFACP significantly outperformed existing methods (AntiCP and iACP) both in terms of accuracy and MCC. SVMACP ranked as the second most effective ACP predictor, with performance still significantly better than those of the other existing methods. The improved performance of our methods is primarily due to the larger size of training dataset, rigorous optimization procedures to select ML parameters, inclusion of new features, the combination of various properties, and the choice of ML method. However, a limitation of this method is that the prediction might not be accurate for longer peptides (length > 50 amino acids) due to their exclusion from the training dataset. Although, our current method is focused on the sequence-based prediction, further studies focused on structure-based membrane-peptide interaction is needed
Consensus algorithms combine output from different predictors popular tools used in various fields of bioinformatics; however, these methods remain in the early stages of development for use in ACP prediction. To generate higher confidence in ACP prediction, we have presented the option of considering consensus results from RFACP and SVMACP methods. Similar approaches were recently implemented via generation of consensus results to predict ACPs from Achatina fulica mucus for further experimentation [14–16].
The comparatively low cost and minimal time required for the in silico identification of ACPs when compared to the tedious and expensive experimental procedures make these computational tools more attractive among the scientific community. In this study, we developed a novel method to predict ACPs from the sequence information and our results showed that the prediction accuracy is significantly higher than the existing methods. Our developed MLACP tool is freely available for research use as a web server. We hope that our method will be useful to both experimentalists and computational biologists.
MATERIALS AND METHODS
As demonstrated by a series of recent publications [24, 47–51] in compliance with Chou's 5-step rule [52], to establish a really useful sequence-based statistical predictor for a biological system, we should follow the following five guidelines: (a) construct or select a valid dataset to train and test the predictor; (b) formulate the biological sequence samples with an effective mathematical expression that can truly reflect their intrinsic correlation with the target to be predicted; (c) introduce or develop a powerful algorithm (or engine) to operate the prediction; (d) properly perform cross-validation tests to objectively evaluate the anticipated accuracy of the predictor; (e) establish a user-friendly web-server for the predictor that is accessible to the public. Below, we are to describe how to deal with these steps one-by- one.
Dataset collection
Training dataset
We utilized the Balanced 1 (B1) and Balanced 2 (B2) datasets described previously [53] to generate a new dataset called the Tyagi-B dataset. In total, we obtained 450 ACPs (225 each from B1 and B2) and 450 non-ACPs (225 each from B1 and B2) by combining both the B1 and B2 datasets. Additionally, we applied the following screening procedures on B1 and B2 datasets: 1) peptides that contained non-natural amino acid residues, 2) peptides with length >50 amino acid residues, and 3) redundant and/or similar peptides defined by the CD-HIT program ( http://www.bioinformatics.org/cd-hit/) by applying a 90% sequence-identity cut-off. It should be noted that similar peptides were removed only from the training dataset and not from the benchmarking dataset. To avoid overfitting in the prediction model, we excluded redundant or similar peptides. Since very few peptides have length greater than 50 amino acid residues, we also excluded these peptides to avoid outlier in the prediction model. After the screening procedure, we obtained 187 ACPs and 398 non-ACPs (Tyagi-B dataset) for use in developing the prediction model.
Benchmarking datasets
To compare our methods with existing methods, we generated two datasets: 1) one based on the dataset reported from previous studies and 2) another based on our own search against the existing databases. We named the first and second datasets as Hajisharifi-Chen (HC) and LEE datasets, respectively. It should be noted that Hajisharifi et al (2014) and Chen et al (2016) developed their prediction models using the same dataset, which contained 138 ACPs and 206 non-ACPs. After applying the screening procedure described in the previous section, we obtained 126 ACPs and 205 non-ACPs (HC dataset).
Construction of the LEE dataset proceeded as follows. We applied the screening procedure described in the previous section to an independent dataset (ACPs and non-ACPs: 150 peptides each) reported by Chen et al (2016), obtaining 140 ACPs and 94 non-ACPs. Furthermore, we extracted 229 and 53 experimentally validated ACPs from CancerPPDB (http://crdd.osdd.net/raghava/cancerppd/) and APD3 (http://aps.unmc.edu/AP/database/antiC.php), respectively [16]. Because few experimentally determined non-ACPs are present in the LEE dataset, we obtained 98 non-ACPs from the Tyagi independent datasets and generated 234 random peptides from Swiss-Prot (http://web.expasy.org/docs/swiss-prot_guideline.html), with these representing a set of non-ACPs for the LEE dataset. This strategy for creating a negative-control dataset was implemented in previous studies [54, 55]. In total, we generated 844 peptides (422 ACPs and 422 non-ACPs; LEE dataset). We note here that the peptides in the LEE dataset are unique (i.e., they are present neither in our training dataset nor the prediction models used by previous methods).
Feature generation
The aim of this experiment was to train either an SVM or RF model to accurately map input features extracted from a peptide primary sequence to predict its class (i.e., ACP or non-ACP), which is considered a classification problem. The most crucial part of this task is extraction of a set of relevant features. All possible features used in this study are shown in Figure 4, and the definition of each composition-based feature is provided below.
AAC
AAC is defined as the fraction of each amino acid present in a given peptide sequence. AAC can be calculated by using the following equation:
| (1) |
where i can be any natural amino acid. The AAC has a fixed length of 20 features.
Atomic composition (ATC)
Recently, Kumar et al (2015) reported the number and types of atoms present in naturally occurring amino acids. In this study, we utilized those data and calculated the frequency of each atom (C, H, N, O, and S) present in the given peptide sequence. The ATC has a fixed length of five features.
DPC
DPC represents the total number of dipeptides normalized by all the possible combinations of dipeptides present in the given peptide sequence. DPC has a fixed length of 400 (20 × 20) features which can be calculated using the following equation:
| (2) |
where DPC(j) is one of 400 possible dipeptides.
PCP
PCP represents the physicochemical class of residues present in a given peptide sequence. We calculated the percentage composition of polar (D, E, R, K, Q, N), hydrophobic (C, V, L, I, M, F, W), charged (D, E, K, H, R), aliphatic (I, L, V), aromatic (F, H, W, Y), positively charged (H, K, R), negatively charged (D, E), tiny (A, C, D, G, S, T), small (E, H, I, L, K, M, N, P, Q, V), and/or large (F, R, W, Y) amino acid residues, as well as peptide mass [14, 16, 17, 56], and used these eleven properties as an input feature.
To the best of our knowledge, this is the first study where all four properties have been considered in ACP prediction. Notably, PCC and ATC have never been considered prior to this, whereas DPC and AAC have been utilized in existing ML-based methods for ACP prediction [57, 58].
Methodology
We employed RF- and SVM-based ML methods to develop a prediction model using the Tyagi-B dataset. The description of the two ML methods is provided below.
RF
RF is an ensemble technique utilizing hundreds or thousands of independent decision trees to perform classification and regression [43, 59, 60] and that has been used for numerous biological applications. A detailed description of the RF algorithm has been reported elsewhere [61]. The three most influential parameters of this algorithm, including the number of trees (ntree), number of variables randomly chosen at each node split (mtry), and minimum number of samples required to split an internal node (nsplit), require optimization. We optimized these parameters using a grid search within the following ranges: ntree from 10 to 500, with a step size of 10; m from 1 to 7, with a step size of 1; and nsplit from 2 to 10, with a step size of 1.
SVM
The SVM is a well-known supervised-ML technique used for developing both classification and regression models, and a detailed description of an SVM has been reported elsewhere [14–16, 23, 62, 63]. In this study, we experimented with several common kernels, including a linear, a Gaussian radial-basis function (RBF), and a polynomial. Among these, RBF worked best for our purposes. A RBF-SVM requires optimization of two critical parameters: γ, which controls how peaked Gaussians are centered on the support vectors; and C, which controls the trade-off between training error and margin size [45, 46, 63]. These two parameters were optimized using a grid search within the following ranges: C from 2−15 to 210 and γ from 2−10 to 210 in log2 scale.
In this study, we used SVM and RF as implemented in the scikit-learn package [64–66].
Cross-validation
In statistical prediction, the following three cross-validation methods are often used to examine a predictor for its effectiveness in practical application: independent dataset test, subsampling test, and jackknife test. However, of the three test methods, the jackknife test is deemed the least arbitrary that can always yield a unique result for a given dataset as elaborated in [21] and demonstrated by Eqs.28-30 in [52]. Accordingly, the jackknife test has been widely recognized and increasingly used by investigators to examine the quality of various predictors [51, 67–69]. However, to reduce the computational time, we adopted the 10-fold cross-validation in this study was done by many investigators [16, 63].
Evaluation metrics
To measure prediction quality, we used the following four metrics: sensitivity, specificity, accuracy, and the Matthews correlation coefficient (MCC). Since, the conventional formulae of these metrics lacking intuitiveness and not easy-to-understand for most biologist, particularly MCC. Chen et al [25, 70] derived a new set of equations for the above-mentioned metrics based on Chou's symbols used in studying protein signal peptide cleavage site [71]. The new formulae for these metrics are given in equation (3).
| (3) |
where N+ represents the total number of ACPs investigated, N−+ represents the number of ACPs incorrectly predicted as non-ACPs, N− represents the total number of non-ACPs investigated and N+− represents the number of non-ACPs incorrectly predicted as ACPs. The formulae given in eq (3) is more intuitive and easy-to-understand, particularly for the meaning of MCC, as concurred by a series of studies published recently [25, 29, 48, 50, 72–74]. The set of metrics is valid only for the single-label systems. For the multi-label systems, whose existence has become more frequent in system biology [75] and system medicine [20, 47, 76], a completely different set of metrics is needed as defined in [77].
Development of a prediction server
An online prediction server was also developed using hypertext markup language and Java script, with a Python script executing in the backend upon submission of peptide sequences in the FASTA format. Users can submit single or multiple sequences containing only standard amino acid residues in FASTA format, after which the MLACP web server outputs the results of RFACP and SVMACP for a given peptide sequence.
Statistical analysis
The differences in AAC, ATC, PCP, and DPC between ACPs and non-ACPs were analyzed using Welch's t test. The data are presented as mean ± standard error (SE). Statistical differences were considered significant at p < 0.01, indicates that the difference is statistically meaningful. All statistical analysis was performed using our own script.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (2015R1D1A1A09060192), Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093826), Mid-Career Researcher Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2017R1A2B4010084) (to S. Choi) and the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1904392). The authors would like to thank Dr. Sathiyamoorthy Subramaniyam for his assistance in web server development.
Abbreviations
- AAC
Amino acid composition
- ACP
Anticancer peptide
- ATC
Atomic composition
- DPC
Dipeptide composition
- HC
Hajisharifi-Chen
- MCC
Matthews correlation coefficient
- ML
Machine-learning
- MLACP
Machine-learning-based prediction of anticancer peptides
- PCP
Physico-chemical properties
- PseAAC
Pseudo amino acid composition
- RF
Random forest
- RFACP
Random forest based anticancer peptide prediction
- SVM
Support vector machine
- SVMACP
Support vector machine based anticancer peptide prediction
Author contributions
Conceived and designed the experiments: BM, SC, GL. Performed the experiments: BM. Analyzed the data: BM, SB, THS. Contributed reagents/materials/software tools: THS, SC, MOK. Wrote paper: BM, GL.
CONFLICTS OF INTEREST
The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Choi S, Macalino SJ, Cui M, Basith S. Expediting the design, discovery, and development of anticancer drugs using computational approaches. Curr Med Chem. 2016 doi: 10.2174/0929867323666160902160535. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. https://doi.org/10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. https://doi.org/10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Harris F, Dennison SR, Singh J, Phoenix DA. On the selectivity and efficacy of defense peptides with respect to cancer cells. Med Res Rev. 2013;33:190–234. doi: 10.1002/med.20252. https://doi.org/10.1002/med.20252. [DOI] [PubMed] [Google Scholar]
- 5.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010;15:40–56. doi: 10.1016/j.drudis.2009.10.009. https://doi.org/10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Thundimadathil J. Cancer treatment using peptides: current therapies and future prospects. J Amino Acids. 2012;2012:967347. doi: 10.1155/2012/967347. https://doi.org/10.1155/2012/967347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaspar D, Veiga AS, Castanho MA. From antimicrobial to anticancer peptides. A review. Front Microbiol. 2013;4:294. doi: 10.3389/fmicb.2013.00294. https://doi.org/10.3389/fmicb.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan M, Liu Q. Differentiation therapy: a promising strategy for cancer treatment. Chin J Cancer. 2016;3(5: 3) doi: 10.1186/s40880-015-0059-x. https://doi.org/10.1186/s40880-015-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boohaker RJ, Lee MW, Vishnubhotla P, Perez JM, Khaled AR. The use of therapeutic peptides to target and to kill cancer cells. Curr Med Chem. 2012;19:3794–804. doi: 10.2174/092986712801661004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deplanque G, Madhusudan S, Jones PH, Wellmann S, Christodoulos K, Talbot DC, Ganesan TS, Blann A, Harris AL. Phase II trial of the antiangiogenic agent IM862 in metastatic renal cell carcinoma. Br J Cancer. 2004;91:1645–50. doi: 10.1038/sj.bjc.6602126. https://doi.org/10.1038/sj.bjc.6602126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregorc V, De Braud FG, De Pas TM, Scalamogna R, Citterio G, Milani A, Boselli S, Catania C, Donadoni G, Rossoni G, Ghio D, Spitaleri G, Ammannati C, et al. Phase I study of NGR-hTNF, a selective vascular targeting agent, in combination with cisplatin in refractory solid tumors. Clin Cancer Res. 2011;17:1964–72. doi: 10.1158/1078-0432.CCR-10-1376. https://doi.org/10.1158/1078-0432.CCR-10-1376. [DOI] [PubMed] [Google Scholar]
- 12.Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, Basche M, Gore L, Zang C, O'Bryant CL, Baron A, Gallemann D, Colevas D, et al. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol. 2007;18:1400–7. doi: 10.1093/annonc/mdm140. https://doi.org/10.1093/annonc/mdm140. [DOI] [PubMed] [Google Scholar]
- 13.Khalili P, Arakelian A, Chen G, Plunkett ML, Beck I, Parry GC, Donate F, Shaw DE, Mazar AP, Rabbani SA. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol Cancer Ther. 2006;5:2271–80. doi: 10.1158/1535-7163.MCT-06-0100. https://doi.org/10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Ding H, Feng P, Lin H, Chou KC. iACP: a sequence-based tool for identifying anticancer peptides. Oncotarget. 2016;7:16895–909. doi: 10.18632/oncotarget.7815. https://doi.org/10.18632/oncotarget.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajisharifi Z, Piryaiee M, Mohammad Beigi M, Behbahani M, Mohabatkar H. Predicting anticancer peptides with Chou's pseudo amino acid composition and investigating their mutagenicity via Ames test. J Theor Biol. 2014;341:34–40. doi: 10.1016/j.jtbi.2013.08.037. https://doi.org/10.1016/j.jtbi.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Tyagi A, Kapoor P, Kumar R, Chaudhary K, Gautam A, Raghava GP. In silico models for designing and discovering novel anticancer peptides. Sci Rep. 2013;3:2984. doi: 10.1038/srep02984. https://doi.org/10.1038/srep02984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautam A, Chaudhary K, Kumar R, Sharma A, Kapoor P, A; Tyagi. Open source drug discovery consortium, Raghava GP. In silico approaches for designing highly effective cell penetrating peptides. J Transl Med. 2013;11:74. doi: 10.1186/1479-5876-11-74. https://doi.org/10.1186/1479-5876-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, Sharma AK, Jaiswal SK, Sharma VK. Prediction of biofilm inhibiting peptides: an in silico approach. Front Microbiol. 2016;7:949. doi: 10.3389/fmicb.2016.00949. https://doi.org/10.3389/fmicb.2016.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R, Chaudhary K, Singh Chauhan J, Nagpal G, Kumar R, Sharma M, Raghava GP. An in silico platform for predicting, screening and designing of antihypertensive peptides. Sci Rep. 2015;5:12512. doi: 10.1038/srep12512. https://doi.org/10.1038/srep12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng X, Zhao SG, Xiao X, Chou KC. iATC-mHyb: a hybrid multi-label classifier for predicting the classification of anatomical therapeutic chemicals. Oncotarget. 2017;8:58494–503. doi: 10.18632/oncotarget.17028. https://doi.org/10.18632/oncotarget.17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou KC, Shen HB. Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nat Protoc. 2008;3:153–62. doi: 10.1038/nprot.2007.494. https://doi.org/10.1038/nprot.2007.494. [DOI] [PubMed] [Google Scholar]
- 22.Mishra NK, Chang J, Zhao PX. Prediction of membrane transport proteins and their substrate specificities using primary sequence information. PLoS One. 2014;9:e100278. doi: 10.1371/journal.pone.0100278. https://doi.org/10.1371/journal.pone.0100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manavalan B, Subramaniyam S, Tae Hwan S, Kim MO, Lee G. MLCPP: machine-learning based prediction of cell-penetrating peptides with improved accuracy. Bioinformatics. 2017 doi: 10.1021/acs.jproteome.8b00148. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Feng P, Yang H, Ding H, Lin H, Chou KC. iRNA-AI: identifying the adenosine to inosine editing sites in RNA sequences. Oncotarget. 2017;8:4208–17. doi: 10.18632/oncotarget.13758. https://doi.org/10.18632/oncotarget.13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W, Tang H, Ye J, Lin H, Chou KC. iRNA-PseU: identifying RNA pseudouridine sites. Mol Ther Nucleic Acids. 2016;5:e332. doi: 10.1038/mtna.2016.37. https://doi.org/10.1038/mtna.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia J, Zhang L, Liu Z, Xiao X, Chou KC. pSumo-CD: predicting sumoylation sites in proteins with covariance discriminant algorithm by incorporating sequence-coupled effects into general PseAAC. Bioinformatics. 2016;32:3133–41. doi: 10.1093/bioinformatics/btw387. https://doi.org/10.1093/bioinformatics/btw387. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Wu H, Chou KC. Pse-in-One 2.0: an improved package of Web servers for generating various modes of pseudo components of DNA, RNA, and protein sequences. Nat Sci. 2017;9:67. doi: 10.1093/nar/gkv458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z, Xiao X, Yu DJ, Jia J, Qiu WR, Chou KC. pRNAm-PC: predicting N(6)-methyladenosine sites in RNA sequences via physical-chemical properties. Anal Biochem. 2016;497:60–7. doi: 10.1016/j.ab.2015.12.017. https://doi.org/10.1016/j.ab.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Qiu WR, Jiang SY, Xu ZC, Xiao X, Chou KC. iRNAm5C-PseDNC: identifying RNA 5-methylcytosine sites by incorporating physical-chemical properties into pseudo dinucleotide composition. Oncotarget. 2017;8:41178–88. doi: 10.18632/oncotarget.17104. https://doi.org/10.18632/oncotarget.17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang CJ, Tang H, Li WC, Lin H, Chen W, Chou KC. iOri-Human: identify human origin of replication by incorporating dinucleotide physicochemical properties into pseudo nucleotide composition. Oncotarget. 2016;7:69783–93. doi: 10.18632/oncotarget.11975. https://doi.org/10.18632/oncotarget.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basith S, Manavalan B, Gosu V, Choi S. Evolutionary, structural and functional interplay of the IkappaB family members. PLoS One. 2013;8:e54178. doi: 10.1371/journal.pone.0054178. https://doi.org/10.1371/journal.pone.0054178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basith S, Manavalan B, Govindaraj RG, Choi S. In silico approach to inhibition of signaling pathways of Toll-like receptors 2 and 4 by ST2L. PLoS One. 2011;6:e23989. doi: 10.1371/journal.pone.0023989. https://doi.org/10.1371/journal.pone.0023989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindaraj RG, Manavalan B, Basith S, Choi S. Comparative analysis of species-specific ligand recognition in Toll-like receptor 8 signaling: a hypothesis. PLoS One. 2011;6:e25118. doi: 10.1371/journal.pone.0025118. https://doi.org/10.1371/journal.pone.0025118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Govindaraj RG, Manavalan B, Lee G, Choi S. Molecular modeling-based evaluation of hTLR10 and identification of potential ligands in Toll-like receptor signaling. PLoS One. 2010;5:e12713. doi: 10.1371/journal.pone.0012713. https://doi.org/10.1371/journal.pone.0012713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manavalan B, Basith S, Choi YM, Lee G, Choi S. Structure-function relationship of cytoplasmic and nuclear IkappaB proteins: an in silico analysis. PLoS One. 2010;5:e15782. doi: 10.1371/journal.pone.0015782. https://doi.org/10.1371/journal.pone.0015782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manavalan B, Govindaraj R, Lee G, Choi S. Molecular modeling-based evaluation of dual function of IkappaBzeta ankyrin repeat domain in toll-like receptor signaling. J Mol Recognit. 2011;24:597–607. doi: 10.1002/jmr.1085. https://doi.org/10.1002/jmr.1085. [DOI] [PubMed] [Google Scholar]
- 37.Manavalan B, Murugapiran SK, Lee G, Choi S. Molecular modeling of the reductase domain to elucidate the reaction mechanism of reduction of peptidyl thioester into its corresponding alcohol in non-ribosomal peptide synthetases. BMC Struct Biol. 2010;10:1. doi: 10.1186/1472-6807-10-1. https://doi.org/10.1186/1472-6807-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Statnikov A, Aliferis CF. Are random forests better than support vector machines for microarray-based cancer classification? AMIA Annu Symp Proc. 2007:686–90. [PMC free article] [PubMed] [Google Scholar]
- 39.Leuschner C, Hansel W. Membrane disrupting lytic peptides for cancer treatments. Curr Pharm Des. 2004;10:2299–310. doi: 10.2174/1381612043383971. [DOI] [PubMed] [Google Scholar]
- 40.Cao R, Adhikari B, Bhattacharya D, Sun M, Hou J, Cheng J. QAcon: single model quality assessment using protein structural and contact information with machine learning techniques. Bioinformatics. 2017;33:586–8. doi: 10.1093/bioinformatics/btw694. https://doi.org/10.1093/bioinformatics/btw694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao R, Bhattacharya D, Hou J, Cheng J. DeepQA: improving the estimation of single protein model quality with deep belief networks. BMC Bioinformatics. 2016;17:495. doi: 10.1186/s12859-016-1405-y. https://doi.org/10.1186/s12859-016-1405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao R, Cheng J. Protein single-model quality assessment by feature-based probability density functions. Sci Rep. 2016;6:23990. doi: 10.1038/srep23990. https://doi.org/10.1038/srep23990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manavalan B, Lee J, Lee J. Random forest-based protein model quality assessment (RFMQA) using structural features and potential energy terms. PLoS One. 2014;9:e106542. doi: 10.1371/journal.pone.0106542. https://doi.org/10.1371/journal.pone.0106542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uziela K, Menendez Hurtado D, Shu N, Wallner B, Elofsson A. ProQ3D: improved model quality assessments using deep learning. Bioinformatics. 2017 doi: 10.1093/bioinformatics/btw819. https://doi.org/10.1093/bioinformatics/btw819. [DOI] [PubMed] [Google Scholar]
- 45.Uziela K, Shu N, Wallner B, Elofsson A. ProQ3: improved model quality assessments using Rosetta energy terms. Sci Rep. 2016;6:33509. doi: 10.1038/srep33509. https://doi.org/10.1038/srep33509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manavalan B, Kuwajima K, Joung I, Lee J. Structure-based protein folding type classification and folding rate prediction. 2015 IEEE International Conference on Bioinformatics and Biomedicine (BIBM); 2015. pp. 1759–61. [Google Scholar]
- 47.Cheng X, Zhao SG, Xiao X, Chou KC. iATC-mISF: a multi-label classifier for predicting the classes of anatomical therapeutic chemicals. Bioinformatics. 2017;33:341–6. doi: 10.1093/bioinformatics/btw644. https://doi.org/10.1093/bioinformatics/btw644. [DOI] [PubMed] [Google Scholar]
- 48.Feng P, Ding H, Yang H, Chen W, Lin H, Chou KC. iRNA-PseColl: identifying the occurrence sites of different RNA modifications by incorporating collective effects of nucleotides into PseKNC. Mol Ther Nucleic Acids. 2017;7:155–63. doi: 10.1016/j.omtn.2017.03.006. https://doi.org/10.1016/j.omtn.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan M, Hayat M, Khan SA, Iqbal N. Unb-DPC: identify mycobacterial membrane protein types by incorporating un-biased dipeptide composition into Chou's general PseAAC. J Theor Biol. 2017;415:13–9. doi: 10.1016/j.jtbi.2016.12.004. https://doi.org/10.1016/j.jtbi.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, Yang F, Chou KC. 2L-piRNA: a two-layer ensemble classifier for identifying piwi-interacting RNAs and their function. Mol Ther Nucleic Acids. 2017;7:267–77. doi: 10.1016/j.omtn.2017.04.008. https://doi.org/10.1016/j.omtn.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meher PK, Sahu TK, Saini V, Rao AR. Predicting antimicrobial peptides with improved accuracy by incorporating the compositional, physico-chemical and structural features into Chou's general PseAAC. Sci Rep. 2017;7:42362. doi: 10.1038/srep42362. https://doi.org/10.1038/srep42362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou KC. Some remarks on protein attribute prediction and pseudo amino acid composition. J Theor Biol. 2011;273:236–47. doi: 10.1016/j.jtbi.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.EK T, Thongararm P, Roytrakul S, Meesuk L, Chumnanpuen P. Prediction of anticancer peptides against MCF-7 breast cancer cells from the peptidomes of Achatina fulica mucus fractions. Comput Struct Biotechnol J. 2016;14:49–57. doi: 10.1016/j.csbj.2015.11.005. https://doi.org/10.1016/j.csbj.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyagi A, Tuknait A, Anand P, Gupta S, Sharma M, Mathur D, Joshi A, Singh S, Gautam A, Raghava GP. CancerPPD: a database of anticancer peptides and proteins. Nucleic Acids Res. 2015;43:D837–43. doi: 10.1093/nar/gku892. https://doi.org/10.1093/nar/gku892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–93. doi: 10.1093/nar/gkv1278. https://doi.org/10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders WS, Johnston CI, Bridges SM, Burgess SC, Willeford KO. Prediction of cell penetrating peptides by support vector machines. PLoS Comput Biol. 2011;7:e1002101. doi: 10.1371/journal.pcbi.1002101. https://doi.org/10.1371/journal.pcbi.1002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chothia C. Structural invariants in protein folding. Nature. 1975;254:304–8. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- 58.Kumar M, Thakur V, Raghava GP. COPid: composition based protein identification. In Silico Biol. 2008;8:121–8. [PubMed] [Google Scholar]
- 59.Lee J, Lee K, Joung I, Joo K, Brooks BR, Lee J. Sigma-RF: prediction of the variability of spatial restraints in template-based modeling by random forest. BMC Bioinformatics. 2015;16:94. doi: 10.1186/s12859-015-0526-z. https://doi.org/10.1186/s12859-015-0526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J, Gross SP, Lee J. Improved network community structure improves function prediction. Sci Rep. 2013;3:2197. doi: 10.1038/srep02197. https://doi.org/10.1038/srep02197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 62.Cao R, Wang Z, Wang Y, Cheng J. SMOQ: a tool for predicting the absolute residue-specific quality of a single protein model with support vector machines. BMC Bioinformatics. 2014;15:120. doi: 10.1186/1471-2105-15-120. https://doi.org/10.1186/1471-2105-15-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manavalan B, Lee J. SVMQA: support-vector-machine-based protein single-model quality assessment. Bioinformatics. 2017 doi: 10.1093/bioinformatics/btx222. https://doi.org/10.1093/bioinformatics/btx222. [DOI] [PubMed] [Google Scholar]
- 64.Scholkopf B, Smola AJ. MIT Press: Cambridge; 2001. Learning with kernels: support vector machines, regularization, optimization, and beyond. [Google Scholar]
- 65.Drucker H, Burges C, Kaufman L, Smola A, Vapnik V. Support vector regression machines, advances in neural information processing systems 9. MIT Press: Cambridge; 1997. [Google Scholar]
- 66.Vapnik VN, Vapnik V. Statistical learning theory. Wiley; New York: 1998. [Google Scholar]
- 67.Behbahani M, Mohabatkar H, Nosrati M. Analysis and comparison of lignin peroxidases between fungi and bacteria using three different modes of Chou's general pseudo amino acid composition. J Theor Biol. 2016;411:1–5. doi: 10.1016/j.jtbi.2016.09.001. https://doi.org/10.1016/j.jtbi.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Khan ZU, Hayat M, Khan MA. Discrimination of acidic and alkaline enzyme using Chou's pseudo amino acid composition in conjunction with probabilistic neural network model. J Theor Biol. 2015;365:197–203. doi: 10.1016/j.jtbi.2014.10.014. https://doi.org/10.1016/j.jtbi.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Tripathi P, Pandey PN. A novel alignment-free method to classify protein folding types by combining spectral graph clustering with Chou's pseudo amino acid composition. J Theor Biol. 2017;424:49–54. doi: 10.1016/j.jtbi.2017.04.027. https://doi.org/10.1016/j.jtbi.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 70.Chen W, Feng PM, Lin H, Chou KC. iRSpot-PseDNC: identify recombination spots with pseudo dinucleotide composition. Nucleic Acids Res. 2013;41:e68. doi: 10.1093/nar/gks1450. https://doi.org/10.1093/nar/gks1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chou KC. Prediction of protein signal sequences. Curr Protein Pept Sci. 2002;3:615–22. doi: 10.2174/1389203023380468. [DOI] [PubMed] [Google Scholar]
- 72.Jia J, Liu Z, Xiao X, Liu B, Chou KC. iCar-PseCp: identify carbonylation sites in proteins by Monte Carlo sampling and incorporating sequence coupled effects into general PseAAC. Oncotarget. 2016;7:34558–70. doi: 10.18632/oncotarget.9148. https://doi.org/10.18632/oncotarget.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia J, Liu Z, Xiao X, Liu B, Chou KC. pSuc-Lys: predict lysine succinylation sites in proteins with PseAAC and ensemble random forest approach. J Theor Biol. 2016;394:223–30. doi: 10.1016/j.jtbi.2016.01.020. https://doi.org/10.1016/j.jtbi.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 74.Liu B, Wang S, Long R, Chou KC. iRSpot-EL: identify recombination spots with an ensemble learning approach. Bioinformatics. 2017;33:35–41. doi: 10.1093/bioinformatics/btw539. https://doi.org/10.1093/bioinformatics/btw539. [DOI] [PubMed] [Google Scholar]
- 75.Chou KC, Wu ZC, Xiao X. iLoc-Hum: using the accumulation-label scale to predict subcellular locations of human proteins with both single and multiple sites. Mol Biosyst. 2012;8:629–41. doi: 10.1039/c1mb05420a. https://doi.org/10.1039/c1mb05420a. [DOI] [PubMed] [Google Scholar]
- 76.Qiu WR, Sun BQ, Xiao X, Xu ZC, Chou KC. iPTM-mLys: identifying multiple lysine PTM sites and their different types. Bioinformatics. 2016;32:3116–23. doi: 10.1093/bioinformatics/btw380. https://doi.org/10.1093/bioinformatics/btw380. [DOI] [PubMed] [Google Scholar]
- 77.Chou KC. Some remarks on predicting multi-label attributes in molecular biosystems. Mol Biosyst. 2013;9:1092–100. doi: 10.1039/c3mb25555g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


