Abstract
A 61-year-old man with relapsing chronic lymphocytic leukaemia, status post allogeneic stem cell transplant and multiple chemotherapy regimens presented to the emergency room after suffering a grand mal seizure. His evaluation revealed a 1.5–2 cm ring-enhancing left temporal lobe brain lesion on the CT scan. This brain lesion was resected and the histopathology revealed an invasive fungal organism resembling mucormycosis. Amplification and sequencing of the 28S ribosomal RNA gene identified the organism as Rhizomucor pusillus. The patient was treated with liposomal amphotericin B 5 mg/kg every 24 hours for 4 weeks, and then was transitioned to oral posaconazole. Serial brain imaging at 1 and 3 months, while on therapy, showed significant improvement.
Keywords: infectious diseases, infection (neurology)
Background
We report a rare case of isolated cerebral mucormycosis due to unusual Mucorales sp, Rhizomucor pusillus, in an immunocompromised host. Diagnosis was established by histopathological examination of the resected mass showing fungal element consistent with mucormycosis. The causative organism was identified with amplification and sequencing gene targets that lead to the final identification of R. pusillus. The patient had excellent outcome with combined surgical and medical therapy.
Case presentation
A 61-year-old man presented to the emergency department with acute grand mal seizure activity. He had a complicated medical history most remarkable for relapsing chronic lymphocytic leukaemia (CLL), diagnosed 20 years prior to his current presentation, for which he underwent multiple treatments, including an allogeneic peripheral blood stem cell transplant 3 years prior to his presentation. His post-transplant was complicated by chronic cutaneous graft versus host disease (GVHD) for which he was maintained on tacrolimus and prednisone along with photopheresis. After he experienced a CLL relapse, he was started on ibrutinib. He was kept on trimethoprim–sulfamethoxazole for pneumocystis prophylaxis and fluconazole for fungal prophylaxis, and received monthly intravenous immunoglobulin (Ig) for hypogammaglobulinaemia. The patient had no prior history of seizures and was afebrile at presentation. He had no history of intravenous drug use. He lived in the Midwest region of USA and wintered in Arizona state.
On neurological examination, patient displayed expressive aphasia but had no other focal or global neurological deficits. The skin examination had findings consistent with cutaneous GVHD. Respiratory, cardiovascular, gastrointestinal and genitourinary examinations were unremarkable.
Investigations
Initial laboratory evaluations included a complete blood count revealing anaemia (haemoglobin of 8.5 g/dL and haematocrit of 26.8%), lymphopaenia (absolute lymphocyte count of 0.28×109/L) and thrombocytopaenia (platelet count of 149/L). Serum creatinine at presentation level was 1 mg/dL. Additional tests included normal chemistries, prostate-specific antigen, lipids and thyroid-stimulating hormone level. Blood PCR for cytomegalovirus was negative. 1–3 Beta-D-glucan testing was performed and was negative (36 pg/mL; ref: <60 pg/mL=negative).
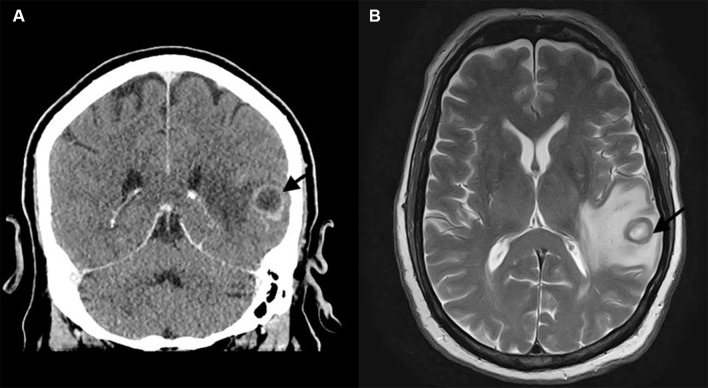
A CT scan of the head revealed a 1.5–2 cm ring enhancing lesion in the left temporal lobe (figure 1). Subsequent MRI showed a 1.8 cm ring-enhancing lesion in the left temporal lobe with vasogenic oedema, which was thought to be an abscess or malignancy. CT of the chest, sinuses and abdomen–pelvis revealed no evidence of extracranial disease. Bacterial and fungal blood cultures remained negative after 5 and 30 days of incubation, respectively, and extensive serological workup for infectious aetiologies was negative, including serum Cryptococcus antigen, Histoplasma complement fixation and immunodiffusion antibodies, Histoplasma urine antigen, Blastomyces antibody enzyme immunoassay (EIA), Coccidioides antibody EIA and serum galactomannan.
Figure 1.
(A) Head CT with contrast, coronal section, showing a left temporal lobe ring-enhancing lesion surrounded by vasogenic oedema (arrow head). (B) Brain MRI, flair sequence, showing left temporal lobe mass surrounded by vasogenic oedema.
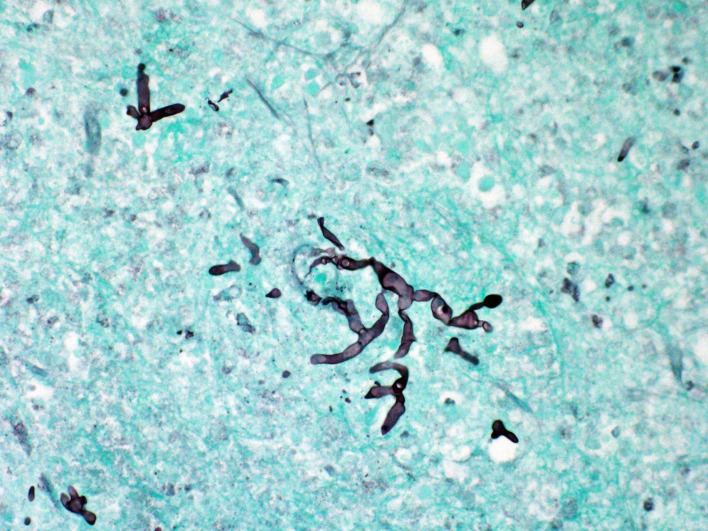
A left temporal craniotomy with mass resection was performed and the initial intraoperative impression was of a metastatic tumour. Consequently, no tissue was sent to microbiology for culture. However, histopathological examination of the tissue showed necrosis with acute and chronic inflammation. Grocott-Gomori methenamine silver (GMS) stain highlighted numerous broad pauciseptate fungal hyphae, suggestive of a Mucorales sp (figure 2). To confirm the identity of the causative pathogen, amplification and sequencing of the 28S ribosomal RNA gene and the internal transcribed spacer 1 and 2 (ITS1 and 2) gene regions were performed on the formalin-fixed paraffin-embedded (FFPE) tissue and sequencing results identified the organism as R. pusillus.
Figure 2.
Brain tissue with GMS stain. Numerous broad, ribbon-like, pauciseptate or pauciseptate hyphae are stained, consistent with Mucorales sp. GMS, Grocott-Gomori methenamine silver.
Treatment
The patient received induction therapy with liposomal amphotericin B 5 mg/kg every 24 hours for 4 weeks, and then was transitioned to oral posaconazole delayed-release tablets. Serial brain imaging at 1 and 3 months, while on therapy, showed significant improvement with decreased oedema and further contraction of the surgical cavity.
Outcome and follow-up
Clinically, patient made almost complete recovery and was maintained on oral posaconazole for secondary prophylaxis given the need for ongoing immunosuppression for GVHD. At 1-year follow-up, the patient was doing well and there were no signs of disease recurrence.
Discussion
The term mucormycosis is often used to imply an angioinvasive infection caused by moulds that belong to the order Mucorales, a ubiquitous environmental mould found in decaying organic substrates. Members of the Mucorales order important in human disease include Rhizopus sp, Mucor sp, Rhizomucor sp, Lichtheimia sp and Cunninghamella sp, and are characterised by pauciseptate, irregularly branching, ribbon-like hyphae. In a review of 929 reported cases of confirmed mucormycosis, 47% were attributed to the genus Rhizopus, 18% by pathogens belonging to the genus Mucor, 7% by the genus Cunninghamella and 4% by pathogens in the genus Rhizomucor.1
Mucormycosis is most often associated with immunocompromised hosts as an opportunistic infection. Risk factors for the development of invasive mucormycosis include diabetic ketoacidosis, neutropaenia, corticosteroid use, haematological malignancy and solid organ or bone marrow transplantation. Invasive mucormycosis in immunocompetent individuals has been reported following trauma associated with natural disasters. Mucormycosis can be fatal in up to 96% of disseminated cases and delays in microbial diagnosis may contribute to high mortality. Site of infection, underlying predisposing factors and the species involved are known predictors for mortality as well.1
The genus Rhizomucor, first described in 1978, is a thermophilic saprophytic fungus in the order Mucorales. R. pusillus, R. miehei and R. variabilis are the pathogenic species associated with human disease. Due to the small size (3–5 µm), the spores of Rhizomucor are easily disseminated via the airborne route and aerosolised sporangiospores are readily inhaled. R. pusillus has a wide geographic distribution and, although a frequent cause of invasive Mucorales infection in animals, has been rarely associated with human disease.2
R. pusillus is reported to be less virulent and have lower minimal inhibitory concentrations (MIC) to antifungal agents compared with more common Mucorales sp, such as Cunninghamella, which is associated with increased mortality.2 However, infection with R. pusillus causes significant morbidity and mortality, and patients have a 30% risk for disseminated infection at presentation and 46% risk of mortality.2 Individuals with haematological malignancies are at highest risk of acquiring this infection.2
Diagnosis of invasive mucormycosis may be clinically challenging. The Mucorales are associated with disease presentation involving multiple organ systems, including pulmonary, rhinocerebral, cutaneous, central nervous system (CNS) and the gastrointestinal tract.
Rhinocerebral disease is characterised by rapid invasion into surrounding facial structures and is classically associated with poorly controlled diabetes and ketoacidosis. Pulmonary mucormycosis is more commonly described in patients with neutropaenia. Cutaneous mucormycosis is most often associated with traumatic disruption of the skin barrier. Skin is also a common site of disseminated infection.
Cerebral mucormycosis is most often diagnosed in the context of concomitant sinus disease or disseminated disease. However, isolated cerebral mucormycosis may account for up to 8% of all cases of mucormycosis.1 Intravenous drug use is the most important risk factor for isolated cerebral disease.1 3 Radiographic features cannot distinguish between mucormycosis and other pyogenic cerebral infections. However, a rapidly enlarging brain abscess in the basal ganglia in the context of intravenous drug use should raise the suspicion for isolated cerebral mucormycosis.4 5
The diagnosis of mucormycosis often relies on histopathological examination of surgically removed tissue and visualisation of broad, ribbon-like, pauciseptate hyphae with wide-angle branching suggests a diagnosis of Mucorales. Angioinvasion is common in Mucorales infections, and hyphae may be visualised within blood vessels. Acute inflammation with neutrophilic predominance is often present, but may be diminished in patients with neutropaenia.
Distinguishing between Aspergillus and Mucorales may be complicated when hyphae are not abundant in tissue. Artefactual folds or breakdown of hyphae may also be confused with septations, leading to an incorrect identification. Additionally, histopathological identification of mucormycosis does not provide genus and species information or susceptibility data and has limited ability to detect mixed or dual fungal infections.6 7
Culture of Mucorales from infected tissue is important for species identification, yet the recovery of Mucorales in culture is often difficult. In a large case series of histopathologically confirmed mucormycosis, cultures were positive in only 50% (465/929) of cases.1
Processing in the microbiology laboratory may damage the fragile pauciseptate hyphae, decreasing culture yield from tissue sources, and mincing of tissue, rather than grinding, is recommended. Growth is rapid (1–7 days) on routine fungal selective media (eg, Inhibitory mould agar) and isolates often have erect aerial mycelium reaching the lid of the petri dish, leading to the colloquial designation of this group as ‘lid lifters’. Despite the angioinvasive nature of Mucorales, blood cultures are rarely positive.8
Molecular methods are increasingly used for detection and identification of fungi in fresh and FFPE tissue and, most recently, in plasma specimens. Broad-range fungal PCR, using primers targeting the ITS gene regions or the 28S or 18S ribosomal RNA gene regions, followed by sequencing has demonstrated good agreement with culture results, and sensitivity is increased when applied to fresh over FFPE tissue. Molecular detection may allow for the identification of the infecting organism to the genus and species level, a distinct advantage over histopathological diagnosis, which may facilitate the choice of appropriate antimicrobial therapy based on known susceptibility patterns.8 9
Advances in the diagnosis of Mucorales are needed to accurately and rapidly diagnosis patients with invasive disease. PCR assays targeting the most common species of Mucorales have been designed for use in tissue samples or serum/plasma. In one study, frozen plasma specimens from a cohort of 44 patients with proven or probable mucormycosis were retrospectively tested using a set of three Mucorales PCR assays targeting Rhizomucor sp, Lichtheimia sp and Mucor/Rhizopus sp. Interestingly, 81% (36/44) of patients had at least one positive PCR, often preceding the date of radiological findings or microbiological confirmation.10 Clinical use of molecular assays for the detection of
Mucorales in plasma specimens may allow for earlier diagnosis and initiation of effective therapy in patients. While promising, evaluation of the performance of these assays in larger prospective studies is needed.
Early diagnosis and initiation of Mucorales active antifungal therapy in known to improve mortality, and delay in therapy of >1 week has been associated with increased mortality.11 Antifungal agents active against the Mucorales include amphotericin B, isavuconazole and posaconazole, and the two active azoles are commonly used as maintenance therapy after initial treatment with amphotericin B. Surgical resection, if feasible, and modification of immunosuppression are also known to improve mortality.12
Learning points.
Diagnosis of mucormycosis may be challenging, and often requires a high index of clinical suspicion and histopathological visualisation of broad, ribbon-like, pauciseptate hyphae with wide-angle branching in biopsy specimens.
Tissue processing at the microbiology laboratory can affect the yield of cultures. Early communication with laboratory staff regarding clinical suspicion of mucormycosis may permit modified tissue processing steps to optimise culture yield.
Molecular methods may help to established the diagnosis and identifying causative species, especially when fungal cultures are not sent or are negative.
Early diagnosis and initiation of antifungal therapy along with surgery, if feasible, are associated with improved patient survival.
Footnotes
Contributors: SF and MRS: case report planning. SF, OAS andRL: crafting of the manuscript. SF, OAS and MRS: critical revision of themanuscript for important intellectual content. MRS: case report supervision. Allauthors: acquisition of data; analysis and interpretation of data.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Roden MM, Zaoutis TE, Buchanan WL, et al. . Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 2.Gomes MZ, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev 2011;24:411–45. 10.1128/CMR.00056-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma A, Brozman B, Petito CK. Isolated cerebral mucormycosis: report of a case and review of the literature. J Neurol Sci 2006;240:65–9. 10.1016/j.jns.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 4.Hopkins RJ, Rothman M, Fiore A, et al. . Cerebral mucormycosis associated with intravenous drug use: three case reports and review. Clin Infect Dis 1994;19:1133–7. 10.1093/clinids/19.6.1133 [DOI] [PubMed] [Google Scholar]
- 5.Sundaram C, Mahadevan A, Laxmi V, et al. . Cerebral zygomycosis. Mycoses 2005;48:396–407. 10.1111/j.1439-0507.2005.01167.x [DOI] [PubMed] [Google Scholar]
- 6.Sangoi AR, Rogers WM, Longacre TA, et al. . Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: a ten-year retrospective review at a single institution. Am J Clin Pathol 2009;131:364–75. 10.1309/AJCP99OOOZSNISCZ [DOI] [PubMed] [Google Scholar]
- 7.Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011;24:247–80. 10.1128/CMR.00053-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lackner M, Caramalho R, Lass-Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol 2014;9:683–95. 10.2217/fmb.14.23 [DOI] [PubMed] [Google Scholar]
- 9.Bialek R, Zelck UE. [PCR-based diagnosis of mucormycosis in tissue samples]. Pathologe 2013;34:511–8. 10.1007/s00292-013-1831-9 [DOI] [PubMed] [Google Scholar]
- 10.Millon L, Herbrecht R, Grenouillet F, et al. . Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clinical Microbiology and Infection 2016;22:810.e1–810.e8. 10.1016/j.cmi.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 11.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2008;47:503–9. 10.1086/590004 [DOI] [PubMed] [Google Scholar]
- 12.Farmakiotis D, Kontoyiannis DP. Mucormycoses. Infect Dis Clin North Am 2016;30:143–63. 10.1016/j.idc.2015.10.011 [DOI] [PubMed] [Google Scholar]