Abstract
Posterior reversible encephalopathy syndrome (PRES), first introduced in 1996, is a neurotoxic state characterised by seizures, headache, vision change, paresis, nausea and altered mental status. Risk factors include hypertension, eclampsia/pre-eclampsia, infection/sepsis and cancer chemotherapy. Although exposure to toxic agents is a common occurrence in patients who develop PRES, oxaliplatin has rarely been associated with it, with only 10 cases reported worldwide. We present the case of an oxaliplatin-induced PRES in a 23-year-old male patient who was started on oxaliplatin/capecitabine as adjuvant chemotherapy for anal canal adenocarcinoma. The patient developed symptoms of headache, slurred speech and left-sided facial weakness on the ninth day after the first dose of oxaliplatin that lasted for 6–8 hours. The patient experienced another episode next day with similar symptoms that lasted for 8 hours. Oxaliplatin was withheld and the patient was discharged on capecitabine only. The patient had no new episodes since discharge on follow-up.
Keywords: oncology, carcinogenesis, chemotherapy, colon cancer, unwanted effects / adverse reactions
Background
Posterior reversible encephalopathy syndrome (PRES) was first introduced by Hinchey et al in 1996.1 It is more common in women, with a mean incidence of age at around 40–50 years.2 3 Hypertension is the most common condition associated with PRES, being present in 6%–72% of cases.4 5 Other comorbidities associated with PRES include pre-eclampsia/eclampsia, infections/sepsis, autoimmune disease and cancer chemotherapy. Although exposure to toxic agents is a common occurrence in patients who develop PRES, association of oxaliplatin with PRES is a very uncommon occurrence with only a handful of data for reference. We report the case of a 23-year-old man diagnosed with adenocarcinoma of anal canal who developed symptoms of slurred speech and left-sided facial weakness on the ninth day after receiving first dose of intravenous oxaliplatin while he was on oral capecitabine.
Case presentation
A 23-year-old man was diagnosed with adenocarcinoma of anal canal, stage T4, N1, M0. The patient had completed neoadjuvant chemoradiation therapy with capecitabine and undergone abdominal perineal resection for his anal canal adenocarcinoma. The patient was started on adjuvant chemotherapy with XELOX consisting of intravenous oxaliplatin (187 mg) and oral capecitabine (1440 mg twice daily). After receiving one dose of oxaliplatin and 9 days on capecitabine, the patient presented with headache, slurred speech and left-sided facial weakness. The patient denied having seizures.
On general physical examination, there was apparent facial asymmetry noted with drooping on the left side of the face. Marked motor dysfunction was noted on the left side of the face, more pronounced on the lower side of the face. Sensation was intact in the affected area. The rest of the neurological and musculoskeletal exam was normal.
Investigations
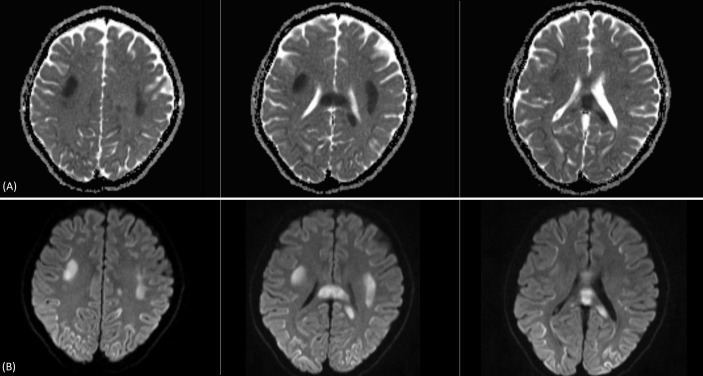
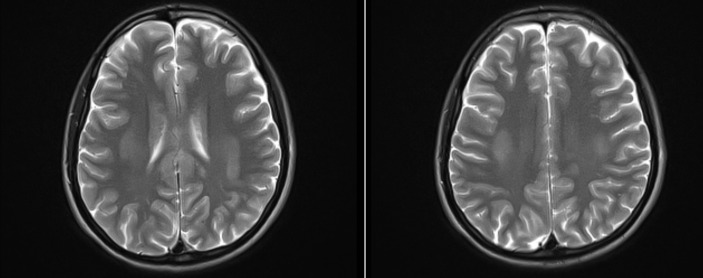
The patient was admitted to the hospital for further management. Laboratory work-up including coagulation studies and HIV work-up were normal. MRI was done and revealed bilateral symmetric areas of restricted diffusion on diffusion weighted imaging (DWI) with reduced values on apparent diffusion coefficient (ADC) mapping involving the bilateral centrum semiovale and subcortical regions of the frontoparietal, temporal and occipital convexities. Restricted diffusion on DWI with reduced ADC values was also seen in the corpus callosum predominantly involving the genu anteriorly and mildly expanded splenium posteriorly and mildly expanding splenium posteriorly (figure 1). These areas returned subtle isointense to intermediate signal on T2 weighted (T2W) and fluid-attenuated inversion recovery (FLAIR) sequences and isointense to slightly low signal on T1 weighted (T1W) images (figure 2). No enhancement was seen in these areas on postcontrast T1W imaging. Findings were slightly more pronounced on the left side in both the cerebrum and the corpus callosum.
Figure 1.
Trace diffusion weighted imaging (DWI) (A) and apparent diffusion coefficient (ADC) map (B).
Figure 2.
T2 weighted (T2W) image.
Differential diagnosis
Considering the patient’s acute clinical picture, transient ischaemic attack (TIA), stroke and severe hypoglycaemia should be considered as top on the differential. With the patient’s history of cancer, possible metastasis to the brain producing motor symptoms should also be entertained. As patient was on chemotherapy before the presentation of symptoms, drug-induced leucoencephalopathy should also be considered, such as PRES. Other diseases that might give similar presentation include gliomatosis cerebri, sagittal sinus thrombosis (asymmetric), cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy, and hypoxic ischaemic encephalopathy.
Treatment
The patient’s episode lasted for 6–8 hours. Chemotherapy was stopped and the patient was monitored. There was another similar episode on the next day which lasted for 8 hours. The patient was discharged on oral capecitabine (1560 mg twice daily) after no new episodes were observed.
Outcome and follow-up
The patient was followed up after 1 month by the oncologist in the outpatient department. The patient did not experience any new episodes and was tolerating Xeloda well.
Discussion
The patient typically presents with seizures, headache, vision change, paresis, hemianopsia, nausea and altered mental status.1 3 4 6 In a study of 38 patients with PRES, the most common symptoms included encephalopathy (92%), clinical seizures (87%), headache (53%) and visual symptoms (39%).3 Although seizures are common at the onset of neurological symptoms, they can develop later in the disease progression.1 4 Most often lethargy and somnolence are first reported by patients.4 On examination, the tendon reflexes are often brisk associated with weakness and incoordination of the limbs.1
Patient presenting with motor symptoms acutely, TIA, stroke or severe hypoglycaemia should be initially excluded as the top differentials. Our patient being young, having no risk factors for stroke (no hypercoagulability), normal serum glucose at presentation and MRI being negative for typical radiological features helped us to exclude all of these conditions. The patient’s history of anorectal cancer also raised the possibility of brain metastasis, but MRI did not reveal any acute brain metastatic disease. The patient’s symptoms resolving immediately after stopping chemotherapy and remaining symptom-free since that time confirmed our diagnosis of PRES. The patient tolerated capecitabine as neoadjuvant chemotherapy, and later when oxaliplatin was stopped the patient received capecitabine as a single agent, making oxaliplatin as the likely culprit behind the patient’s symptoms causing PRES.
The pathophysiology of PRES remains unclear. As hypertensive encephalopathy being the most frequent condition associated with PRES, the currently preferred explanation relates to hypertension, impaired autoregulation and hyperperfusion.1 7 It is postulated that sudden elevations in systemic blood pressure exceed the autoregulatory capability of the brain vasculature resulting in its failure. The autoregulatory failure of the vessels leads to vasodilation of the vessels, ultimately causing increased capillary hydrostatic pressure with subsequent vasogenic oedema.8 Although subsequent resolution of clinical symptoms and radiological oedema is often observed with prompt treatment of hypertension in patients with PRES, other mechanisms seem to be involved in the pathophysiology of PRES as it can occur in normotensive patients as well.9 Another alternative theory that has also surfaced attributes the development of the syndrome to decreased blood flow and ischaemia leading to cytotoxic oedema.10 Association of PRES with chemotherapeutic agents has been well documented, but little is known about its pathophysiology. One plausible theory suggests that cytotoxic drugs have direct toxic effects on vascular endothelial cells, disrupting blood–brain barrier, which overwhelms cerebral autoregulation resulting in breakthrough hyperaemia, producing direct cytotoxic effects causing PRES.11 12
Although exposure to toxic agents is a common occurrence in patients who develop PRES, it is uncommon for oxaliplatin to be associated with it.5 An in-depth literature review was conducted by utilising PubMed’s MEDLINE and Google Scholar databases. We found that there have been 10 cases of PRES associated with oxaliplatin or in combination with other drugs like 5-flourouracil (5-FU) and bevacizumab. The first case was reported by Skelton et al13 of a 19-year-old woman with metastatic adenocarcinoma of the rectum receiving modified FOLFOX (oxaliplatin/5-FU) therapy who went on to develop seizures and altered mental status, later being confirmed as PRES. Since then nine other cases have been reported in various parts of the world identifying oxaliplatin or combination of oxaliplatin with 5-FU or bevacizumab as the possible cause related to the development of PRES in such patients.9 14–21
We found only one case of a 62-year-old woman who developed motor neurological deficits following administration of oxaliplatin, bevacizumab and capecitabine, later confirmed as PRES after radiological investigation was done.14 She initially presented with altered mental status, hypertension and seizures. Neurological exam revealed bilateral lower extremity weakness with right-sided hyper-reflexia and positive Babinski’s sign. The patient’s symptoms resolved after 48 hours and she tolerated therapy when chemotherapy regimen was switched to bevacizumab, and irinotecan identifying oxaliplatin or capecitabine as the principal toxic agent. We found two other cases that identified oxaliplatin as the sole toxic agent, reported by Rahal et al20 and Tang21, but none of these patients developed motor symptoms, although one patient reported seizures.
On review of the 10 cases we found, majority of the patients had colorectal adenocarcinoma (eight patients). Almost all of the patients reported altered mental status on initial presentation of the episode. Seizures were reported in seven of these cases unlike our case. Two patients reported headaches similar to our case. Five patients reported hypertension, although our patient was normotensive when he first arrived at our setting. Majority of the patients had good prognosis with complete resolution of symptoms on cessation of chemotherapy agents, with only one patient reported to deteriorate despite adequate management being done (table 1).
Table 1.
Comparison of symptoms, outcome and treatment in patients with PRES associated with oxaliplatin
| Authors | Patients (age, sex) | Cancer type | Treatment | Symptoms and onset | Outcome |
| Skeleton et al13 | 19 F | Rectal adenocarcinoma | 5-FU and oxaliplatin | Seizures and AMS | CRSSC, death later |
| Pinedo et al14 | 62 F | Rectal adenocarcinoma | Oxaliplatin, capecitabine, bevacizumab | Seizures, AMS, bilateral lower extremity weakness | CRSSC, bevacizumab continued without new symptoms |
| Nagata et al15 | 35 F | Sigmoid adenocarcinoma | Oxaliplatin, capecitabine | AMS, hypertension, seizures, headache, visual defects | CRSSC |
| Sharief and Perry16 | 59 M | Colon adenocarcinoma | Folinic acid, fluorouracil, oxaliplatin | AMS, seizures, status epilepticus | CRSSC |
| Chang et al17 | – | Intrahepatic cholangiocarcinoma | Bevacizumab, gemcitabine, oxaliplatin | AMS, hypertension, proteinuria | – |
| Femia et al18 | 56 M | Colon adenocarcinoma | Oxaliplatin, capecitabine | Seizures, hypertension, AMS, renal impairment | Symptoms deteriorated, death later |
| Truman and Nethercott9 | 73 F | Caecal adenocarcinoma | Oxaliplatin, 5-FU | Severe occipital headache, AMS, after 48 hours develop hypertension, seizures, | CRSSC |
| Porcello et al19 | 27 F | Colorectal adenocarcinoma | Oxaliplatin, 5-FU, leucovorin | Hypertension, renal impairment, AMS | CRSSC, later died due to complications from cancer |
| Rahal et al20 | 50 M | Mixed adenoneuroendocrine carcinoma of appendix | Folinic acid, fluorouracil, oxaliplatin | AMS, seizures | CRSSC, seizures again on the original regimen, on changing to folinic acid, fluorouracil and irinotecan (FOLFIRI) no symptoms |
| Tang21 | 81 M | Colorectal adenocarcinoma | Oxaliplatin and capecitabine | AMS | CRSSC, she tolerated capecitabine only before starting oxaliplatin |
5-FU, 5-flourouracil; AMS, altered mental status; CRSSC, complete resolution of symptoms after stopping chemotherapy; PRES, posterior reversible encephalopathy syndrome.
Typically, MRI is preferred rather than CT scan to diagnose patients with PRES.5 The classic imaging findings are of vasogenic oedema in the subcortical white matter of the parietal and occipital lobes.10 Radiologically there are three primary patterns that are associated with this syndrome: holohemispheric watershed pattern, superior frontal sulcus pattern and dominant parietal-occipital pattern. Rarely incomplete pattern is also visualised on radiological investigations that involve variable expression of these above-mentioned primary patterns. The asymmetric or partial pattern was more frequently recognised in patients with eclampsia and solid organ transplantation.22 ‘Atypical’ lesions in PRES have also been noted in some patients, where frontal lobe was the most common location, followed by temporal lobe and cerebellum.23
It is imperative that investigations should be performed quickly to diagnose PRES promptly, which will allow early identification of the causative factors. Symptomatic treatment should be given immediately and the causative factors corrected without delay. If the patient presented with seizures initially, appropriate antiepileptic treatment should be started according to the electrical and clinical pattern of the patient. Blood pressure should also be monitored and controlled as a significant proportion of patients present with hypertension.5
The prognosis of PRES is generally favourable, as in majority of the patients it resolves spontaneously after correction or removal of the causative agent. Often there is complete resolution of symptoms to baseline with evidence of radiological improvement as well.14 In a study to follow up long-term prognosis in patients with PRES, only 8% of the patients had recurrence, indicating good long-term prognosis.22
In all the cases and literature we reviewed relating to oxaliplatin-induced PRES, the symptoms and signs among patients are variable and diverse to say the least. This diversity of signs and symptoms presents a challenge for the clinicians when trying to figure out the definitive diagnosis in order to provide appropriate management. The risk of stopping administration of a single chemotherapeutic agent must always be balanced with the possibility of establishing another diagnosis that would exclude the possibility of drug-induced PRES in that patient. A wrong decision or diagnosis might prove counterproductive for the patient’s therapeutic progress and prognosis.
Learning points.
Posterior reversible encephalopathy syndrome (PRES) is an uncommon disease typically characterised by headache, seizures, vision change, haemiparesis and altered mental status.
Exclusion of other diagnosis must be made before establishing drug-induced PRES.
Oxaliplatin has been rarely associated with incidence of PRES, with only 10 cases reported worldwide with variable signs and symptoms among patients.
MRI is required to diagnose patients suspected of PRES.
The prognosis is generally favourable and the symptoms resolve spontaneously in majority of the patients.
Footnotes
Contributors: TKJ and MH wrote the manuscript and did the major work in conceptualisation, literature review and initial draft preparation. HKA and NAZ did the final revision and changes.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996;334:494–500. 10.1056/NEJM199602223340803 [DOI] [PubMed] [Google Scholar]
- 2.Lee VH, Wijdicks EF, Manno EM, et al. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 2008;65:205-10 10.1001/archneurol.2007.46 [DOI] [PubMed] [Google Scholar]
- 3.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914–25. 10.1016/S1474-4422(15)00111-8 [DOI] [PubMed] [Google Scholar]
- 4.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008;29:1036–42. 10.3174/ajnr.A0928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legriel S, Pico F, Azoulay E. Understanding posterior reversible encephalopathy syndrome. InAnnual update in intensive care and emergency medicine 2011:631–53. [Google Scholar]
- 6.Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 2010;85:427–32. 10.4065/mcp.2009.0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granata G, Greco A, Iannella G, et al. Posterior reversible encephalopathy syndrome--Insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev 2015;14:830–6. 10.1016/j.autrev.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Brubaker LM, Smith JK, Lee YZ, et al. Hemodynamic and permeability changes in posterior reversible encephalopathy syndrome measured by dynamic susceptibility perfusion-weighted MR imaging. AJNR Am J Neuroradiol 2005;26:825–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Truman N, Nethercott D. Posterior reversible encephalopathy syndrome (PRES) after treatment with oxaliplatin and 5-fluorouracil. Clin Colorectal Cancer 2013;12:70–2. 10.1016/j.clcc.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol 2012;85:1566–75. 10.1259/bjr/25273221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamaki K, Sadoshima S, Baumbach GL, et al. Evidence that disruption of the blood-brain barrier precedes reduction in cerebral blood flow in hypertensive encephalopathy. Hypertension 1984;6(2 Pt 2):I75 10.1161/01.HYP.6.2_Pt_2.I75 [DOI] [PubMed] [Google Scholar]
- 12.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008;29:1043–9. 10.3174/ajnr.A0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skelton MR, Goldberg RM, O’Neil BH. A case of oxaliplatin-related posterior reversible encephalopathy syndrome. Clin Colorectal Cancer 2007;6:386–8. 10.3816/CCC.2007.n.009 [DOI] [PubMed] [Google Scholar]
- 14.Pinedo DM, Shah-Khan F, Shah PC. Reversible posterior leukoencephalopathy syndrome associated with oxaliplatin. J Clin Oncol 2007;25:5320–1. 10.1200/JCO.2007.13.5954 [DOI] [PubMed] [Google Scholar]
- 15.Nagata Y, Omuro Y, Shimoyama T, et al. A case of colon cancer with reversible posterior leukoencephalopathy syndrome following 5-FU and oxaliplatin (FOLFOX regime). Gan To Kagaku Ryoho 2009;36:1163–6. [PubMed] [Google Scholar]
- 16.Sharief U, Perry DJ. Delayed reversible posterior encephalopathy syndrome following chemotherapy with oxaliplatin. Clin Colorectal Cancer 2009;8:163–5. 10.3816/CCC.2009.n.026 [DOI] [PubMed] [Google Scholar]
- 17.Chang Y, Mbeo G, Littman SJ. Reversible posterior leukoencephalopathy syndrome associated with concurrent bevacizumab, gemcitabine, and oxaliplatin for cholangiocarcinoma. J Gastrointest Cancer 2012;43:505–7. 10.1007/s12029-011-9279-8 [DOI] [PubMed] [Google Scholar]
- 18.Femia G, Hardy TA, Spies JM, et al. Posterior reversible encephalopathy syndrome following chemotherapy with oxaliplatin and a fluoropyrimidine: a case report and literature review. Asia Pac J Clin Oncol 2012;8:115–22. 10.1111/j.1743-7563.2012.01544.x [DOI] [PubMed] [Google Scholar]
- 19.Porcello Marrone LC, Marrone BF, Pascoal TA, et al. Posterior Reversible Encephalopathy Syndrome Associated with FOLFOX Chemotherapy. Case Rep Oncol Med 2013;2013:1–3. 10.1155/2013/306983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahal AK, Truong PV, Kallail KJ, et al. Oxaliplatin-Induced Tonic-Clonic Seizures. Case Rep Oncol Med 2015;2015:1–3. 10.1155/2015/879217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang KH. Oxaliplatin-induced posterior reversible encephalopathy syndrome with isolated involvement of pons. J Cancer Res Ther 2015;11:1022 10.4103/0973-1482.146134 [DOI] [PubMed] [Google Scholar]
- 22.Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry 2010;81:773–7. 10.1136/jnnp.2009.189647 [DOI] [PubMed] [Google Scholar]
- 23.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2007;28:1320–7. 10.3174/ajnr.A0549 [DOI] [PMC free article] [PubMed] [Google Scholar]