Abstract
Purpose
Despite advances in childhood cancer care, some patients die soon after diagnosis. This population is not well described and may be under-reported. Better understanding of risk factors for early death and scope of the problem could lead to prevention of these occurrences and thus better survival rates in childhood cancer.
Methods
We retrieved data from SEER 13 registries on 36,337 patients age 0 to 19 years diagnosed with cancer between 1992 and 2011. Early death was defined as death within 1 month of diagnosis. Socioeconomic status data for each individual’s county of residence were derived from Census 2000. Crude and adjusted odds ratios and corresponding 95% CIs were estimated for the association between early death and demographic, clinical, and socioeconomic factors.
Results
Percentage of early death in the period was 1.5% (n = 555). Children with acute myeloid leukemia, infant acute lymphoblastic leukemia, hepatoblastoma, and malignant brain tumors had the highest risk of early death. On multivariable analysis, an age younger than 1 year was a strong predictor of early death in all disease groups examined. Black race and Hispanic ethnicity were both risk factors for early death in multiple disease groups. Residence in counties with lower than median average income was associated with a higher risk of early death in hematologic malignancies. Percentages of early death decreased significantly over time, especially in hematologic malignancies.
Conclusion
Risk factors for early death in childhood cancer include an age younger than 1 year, specific diagnoses, minority race and ethnicity, and disadvantaged socioeconomic status. The population-based disease-specific percentages of early death were uniformly higher than those reported in cooperative clinical trials, suggesting that early death is under-reported in the medical literature. Initiatives to identify those at risk and develop preventive interventions should be prioritized.
INTRODUCTION
Childhood cancer outcomes have improved significantly over the past decades in the United States.1 However, there remains a group of children with cancer who do not survive long enough to start treatment, or they die early in the treatment process. Although this group is relatively small, it represents a significant number of childhood cancer deaths, many of which may be preventable. The characteristics of these individuals and the reasons for their poor outcomes are not well understood. Most of our knowledge of pediatric oncology outcomes is derived from clinical trials; we hypothesized that early death may be under-reported in clinical trial reports because many of these children and adolescents die before enrollment or may be ineligible as a result of critical illness at presentation.
Two prior population-based studies have explored this topic. An analysis of pediatric cancer deaths within 1 month of diagnosis between 1967 and 1998, using the Childhood Cancer Registry of Piedmont in Italy, revealed that risk factors for early death included an age younger than 1 year, disseminated disease at diagnosis, diagnosis earlier in the study period, and specific diseases, such as acute myeloid leukemia (AML), non-Hodgkin lymphoma, CNS tumors, and liver tumors.2 A second study analyzed early deaths in the United States from 1973 to 1995,3 using data from the SEER registry. Greater risk was noted for infants overall and for children with leukemia, CNS tumors, liver tumors, or neuroblastoma. This study did not find race or ethnicity to be associated with early death risk. Several studies have shown an impact of socioeconomic status on diagnosis and outcomes in pediatric cancer,4-10 a topic summarized in a recent systematic review11; however, its relationship with risk for early death was not addressed.
In our population-based study, we sought to define the scope and characteristics of this poorly characterized group of children with cancer who die shortly after diagnosis, using SEER data to describe their demographics, disease, and socioeconomic status characteristics to identify groups at risk for which targeted interventions should be developed.
METHODS
Study Design
This was a retrospective cohort study using population-based data.
Data Source and Study Cohort
Individuals age 0 to 19 years with any childhood cancer (International Classification of Childhood Cancer [ICCC] groups I to XII) diagnosed in the period from 1992 to 2011 were selected from SEER 13 registries through SEER*Stat software (version 8.1.5). Only the first matching record for each individual was included, to exclude second malignancies in those children and adolescents with multiple primary tumors. The SEER program is run by the National Cancer Institute as a source of information on cancer incidence and survival. SEER 13 encompasses the following cancer registries: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, Utah, Los Angeles, San Jose–Monterey, rural Georgia, and the Alaska Native Tumor Registry. Although it covers only 13.4% of the US population, it is designed to represent both its geographic and social diversity.
Outcome
Early death was defined as survival duration < 1 month from the time of diagnosis and vital status recode dead. One month was chosen as the time point of interest because this represented the shortest time interval available for analysis in the SEER database. All other patients were classified as nonearly death, including all living children and adolescents and those who died ≥ 1 month from diagnosis.
Individual-Level Demographic Variables
The early death and nonearly death groups were compared on the basis of the following individual-level variables: age, sex, race (white, black, or other), and ethnicity (Hispanic or non-Hispanic). Individuals were divided into age categories at diagnosis (younger than 1, 1 to 4, 5 to 9, 10 to 14, and 15 to 19 years).
Year of Diagnosis
Year of diagnosis was used to create a variable with five periods: 1992 to 1995, 1996 to 1999, 2000 to 2003, 2004 to 2007, and 2008 to 2011.
Census-Based Socioeconomic Status Variables
The early death and nonearly death groups were also compared using the following socioeconomic status indicators (based on Census 2000) for each individual’s county of residence: percentage of families in poverty, percentage of people with less than high school education, percentage of unemployed people, percentage of families in language isolation, percentage of foreign-born people, and median household income. For each of these attributes, patients were grouped into two categories separated by the median value for the study cohort (Appendix Table A1, online only).
Cancer Subtypes
The ICCC third edition (ICCC-3)12 was used to define three broad tumor categories: non-CNS solid tumors (ICCC-3 groups IV to XII), hematologic malignancies (ICCC-3 groups I to II), and CNS tumors (ICCC-3 group III). We also analyzed the risk of early death based on the 12 main ICCC-3 categories using neuroblastoma, a common childhood cancer that fell near the center for early death risk, as the reference group. The diagnoses were then subdivided into more-specific ICCC-3 categories to determine the risk of early death resulting from these more-specific diseases, also in relation to neuroblastoma. The usual age cutoff of younger than 1 year at diagnosis was used for infant acute lymphoblastic leukemia (ALL). Low-grade gliomas were defined as grade 1 to 2 and high grade as 3 to 4.
Staging
Disease stage at diagnosis was evaluated by categorizing patients’ disease as locoregional versus distant using SEER historic stage A; children and adolescents without a documented stage at diagnosis in this classification were excluded from this specific analysis. Because individuals with leukemia were categorized as having distant disease, and because staging information for CNS tumors was available for only 4.3% of the cohort, staging information was analyzed only for patients with solid tumors.
Statistical Analysis
Two-tailed χ2 or Fisher’s exact test was used to study the association of categorical variables with occurrence of early death. Logistic regression was used to identify independent predictors. For each disease type, a hierarchic model was built including all variables that achieved a P value < .25 in the univariable analysis. The Hosmer-Lemeshow test was used to check goodness of fit. Crude and adjusted odds ratios (ORs) and corresponding 95% CIs were estimated. All statistical tests were performed using STATA 13 software (STATA, College Station, TX), and α = .05 was used to define statistical significance. Data were exported into Joinpoint (version 4.2.0.1; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD) to examine changes in the early death percentages over time and estimate annual percentage changes (APCs) using a Joinpoint regression model.
RESULTS
Study Groups and Disease Categories
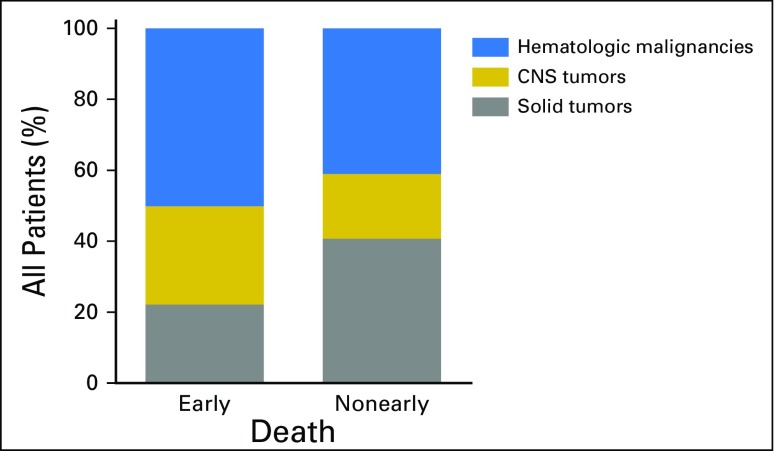
Our cohort included 36,337 children and adolescents with cancer diagnosed from 1992 to 2011. Early death was registered for 555 individuals (1.5%). The total number of deaths over this period was 7,403, so the early death group represented 7.5% of total deaths. Compared with the nonearly death group, the early death group had higher proportions of hematologic malignancies (49.9% v 40.9%) and CNS tumors (27.9% v 18.3%) and a lower proportion of solid tumors (22.2% v 40.7%; Appendix Fig A1, online only). In comparison with children and adolescents with solid tumors (0.8% of whom experienced early deaths), those with hematologic malignancies (early death, 1.8%; OR, 2.23; 95% CI, 1.80 to 2.76) or CNS tumors (early death, 2.3%; OR, 2.80; 95% CI, 2.21 to 3.56) had a significantly higher risk of early death (Appendix Table A2, online only). Given these differences in the percentages of early death resulting from disease, we divided our univariable and multivariable analyses of predictors of early death along these three disease groups.
Early Death Risk by Cancer Subtype
When cancer diagnoses were divided into the 12 large ICCC-3 categories, liver tumors, leukemias, and CNS tumors conveyed significantly increased risk of early death when compared with neuroblastoma as a standard, whereas renal and epithelial tumors carried significantly lower risk (Appendix Table A2).
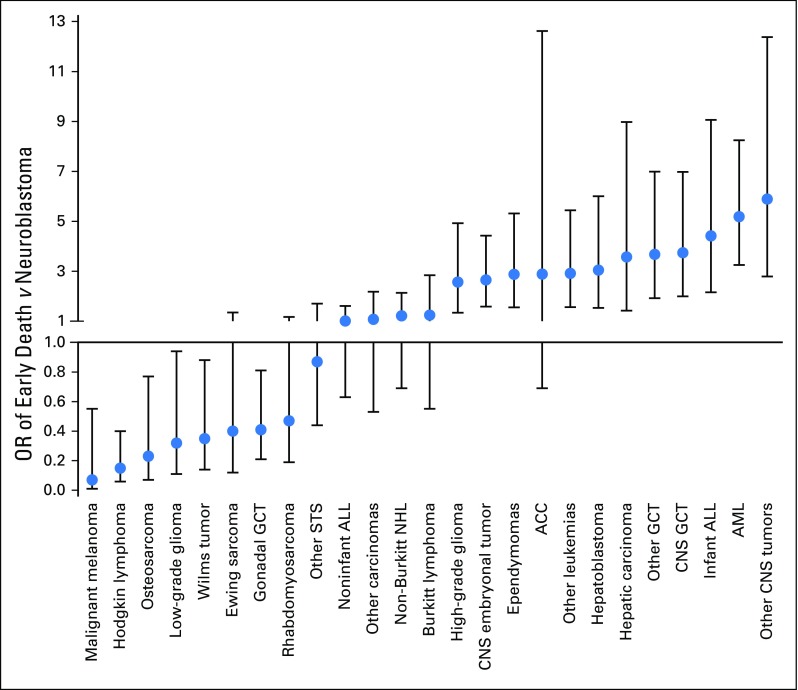
Next, the early death risk for a set of more specific diagnoses was also compared with that for neuroblastoma as a standard (Fig 1; Appendix Table A2). AML, infant ALL, hepatoblastoma, and several types of malignant CNS tumors were among the cancers with a significantly higher OR of early death. Malignant melanoma, Hodgkin lymphoma, osteosarcoma, low-grade glioma, and Wilms tumor showed a significantly lower OR of early death.
Fig 1.
Odds ratios (ORs) with 95% CIs of early death for patients with a series of specific childhood cancers compared with patients with neuroblastoma. ACC, adrenocortical carcinoma; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; GCT, germ cell tumor; NHL, non-Hodgkin lymphoma; STS, soft tissue sarcoma.
Demographic and Socioeconomic Predictors of Early Death: Univariable Analysis
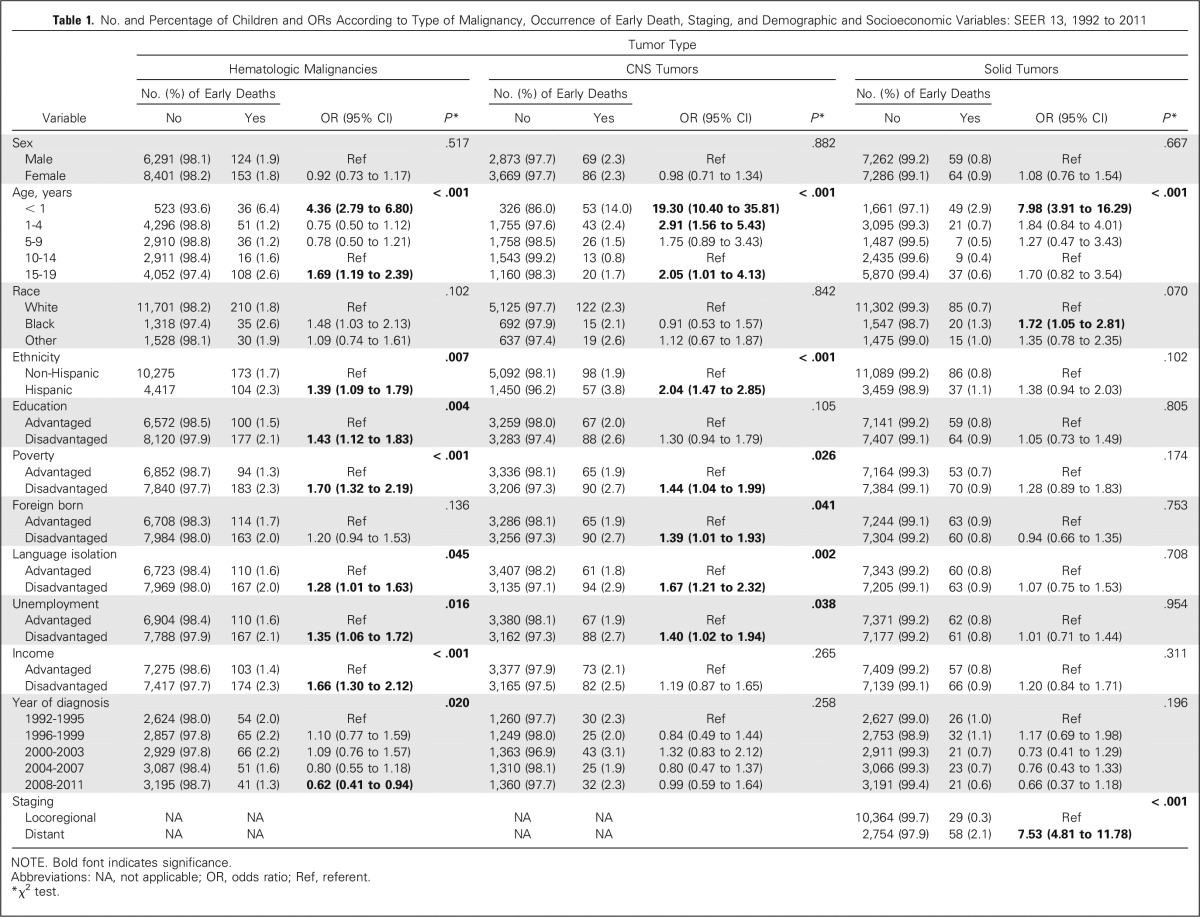
Demographic and socioeconomic predictors of early death identified in the univariable analysis are listed in Table 1.
Table 1.
No. and Percentage of Children and ORs According to Type of Malignancy, Occurrence of Early Death, Staging, and Demographic and Socioeconomic Variables: SEER 13, 1992 to 2011
Hematologic malignancies.
Infants (age younger than 1 year; OR, 4.36; 95% CI, 2.79 to 6.80) and adolescents (age 15 to 19 years; OR, 1.69; 95% CI, 1.19 to 2.39) had significantly increased risks of early death compared with individuals in the 10- to 14-year age group. Black race was associated with a higher risk of early death compared with white race (OR, 1.48; 95% CI, 1.03 to 2.13), and Hispanics had a higher risk of early death compared with non-Hispanics (OR, 1.39; 95% CI, 1.09 to 1.79). There was no difference in early death risk by sex. With the exception of foreign-born status, all county-based social disadvantages were associated with significantly higher risks of early death, including those related to education (OR, 1.43; 95% CI, 1.12 to 1.83), poverty (OR, 1.70; 95% CI, 1.32 to 2.19), language isolation (OR, 1.28; 95% CI, 1.01 to 1.63), unemployment (OR, 1.35; 95% CI, 1.06 to 1.72), and income (OR, 1.66; 95% CI, 1.30 to 2.12). Diagnosis during the last study period (2008 to 2011) was associated with a lower early death risk than during the first period (1992 to 1995; OR, 0.62; 95% CI, 0.41 to 0.94).
CNS tumors.
A statistically significant association was observed between age group and early death: infants (OR, 19.30; 95% CI, 10.40 to 35.81), children age 1 to 4 years (OR, 2.91; 95% CI, 1.56 to 5.43), and adolescents age 15 to 19 years (OR, 2.05; 95% CI, 1.01 to 4.13) had significantly increased early death risk compared with individuals age 10 to 14 years. No significant differences in early death rates were noted by race or sex. Hispanic children and adolescents had an increased early death risk compared with non-Hispanics (OR, 2.04; 95% CI, 1.47 to 2.85). Individuals living in disadvantaged areas in terms of poverty (OR, 1.44; 95% CI, 1.04 to 1.99), foreign-born status (OR, 1.39; 95% CI, 1.01 to 1.93), language isolation (OR, 1.67; 95% CI, 1.21 to 2.32), and unemployment (OR, 1.40; 95% CI, 1.02 to 1.94) also had significantly increased early death risks compared with those living in advantaged areas. Income and education were not significantly associated with early death in this group.
Solid tumors.
Infants were the only age group that significantly differed from the reference category of patients age 10 to 14 years regarding early death risk (OR, 7.98; 95% CI, 3.91 to 16.29). No differences in percentage of early death were noted by ethnicity or sex. Black race was associated with an increased early death risk compared with white (OR, 1.72; 95% CI, 1.05 to 2.81). Distant disease at diagnosis was associated with occurrence of early death (OR, 7.53; 95% CI, 4.81 to 11.78). None of the socioeconomic status characteristics were significantly associated with early death risk in this group.
Demographic and Socioeconomic Predictors of Early Death: Multivariable Analysis
Demographic and socioeconomic predictors of early death identified in the multivariable analysis are listed in Table 2.
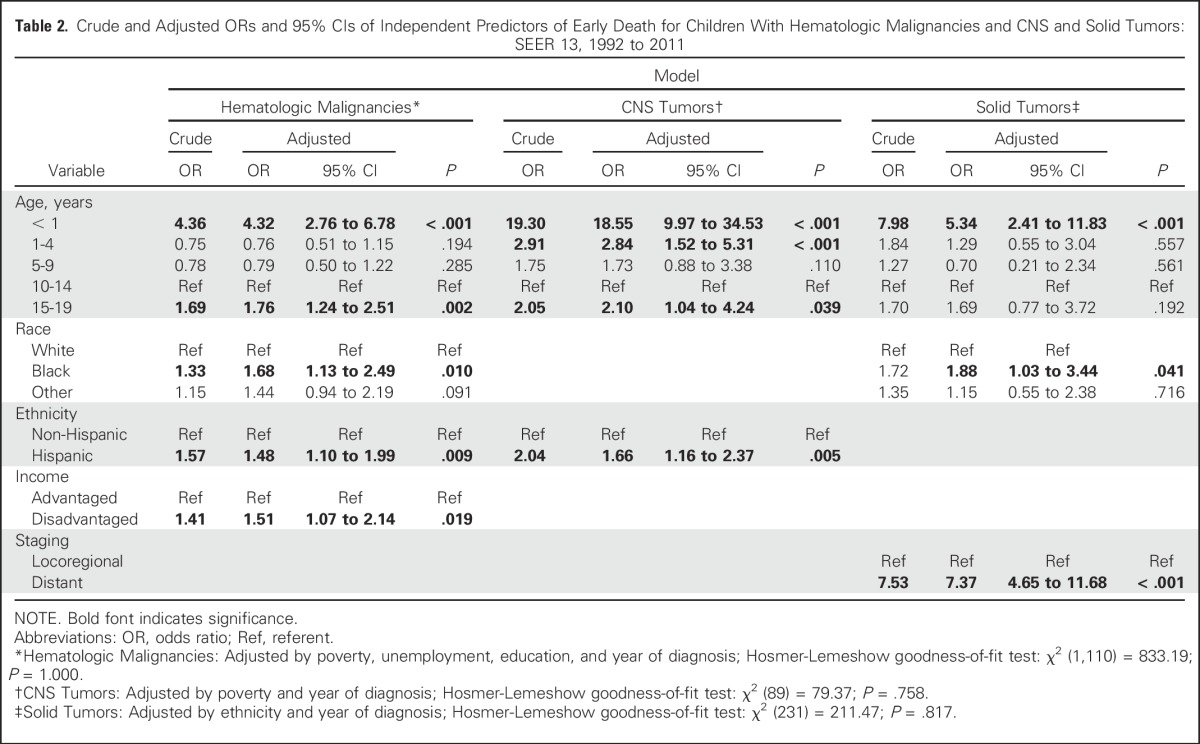
Table 2.
Crude and Adjusted ORs and 95% CIs of Independent Predictors of Early Death for Children With Hematologic Malignancies and CNS and Solid Tumors: SEER 13, 1992 to 2011
Hematologic malignancies.
In the multivariable model, adjusting for poverty, unemployment, education, and year of diagnosis, age younger than 1 year (OR, 4.32; 95% CI, 2.76 to 6.78) or 15 to 19 years (OR, 1.76; 95% CI, 1.24 to 2.51), Hispanic ethnicity (OR, 1.48; 95% CI, 1.10 to 1.99), black race (OR, 1.68; 95% CI, 1.13 to 2.49), and income (OR, 1.51; 95% CI, 1.07 to 2.14) remained as independent predictors of early death.
CNS tumors.
After controlling for poverty and year of diagnosis, age younger than 1 year (OR, 18.55; 95% CI, 9.97 to 34.53), 1 to 4 years (OR, 2.84; 95% CI, 1.52 to 5.31), and 15 to 19 years (OR, 2.10; 95% CI, 1.04 to 4.24) and Hispanic ethnicity (OR, 1.66; 95% CI, 1.16 to 2.37) were the only independent predictors of early death for children and adolescents with CNS tumors.
Solid tumors.
An age younger than 1 year (OR, 5.34; 95% CI, 2.41 to 11.83) was also identified as an independent significant early death predictor for individuals with solid tumors, as were black race (OR, 1.88; 95% CI, 1.03 to 3.44) and distant spread of disease (OR, 7.37; 95% CI, 4.65 to 11.68), after the model was adjusted for ethnicity and year of diagnosis.
Time Trends in Early Death Rates
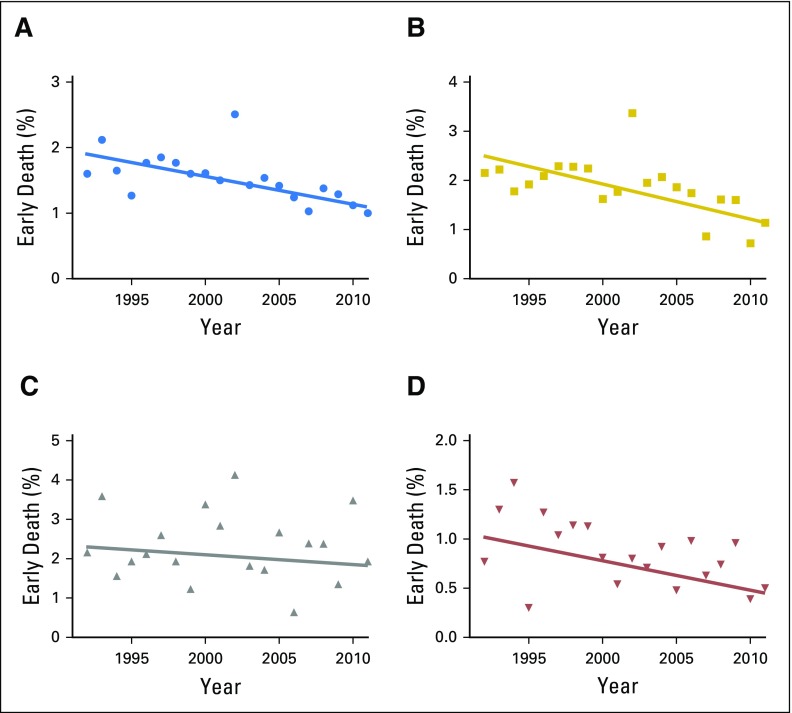
Over the 20-year study period, a significant overall decrease in early death percentages was observed (APC, −2.5; 95% CI, −3.9 to −1.1). When the analysis was stratified by tumor type, we observed that only hematologic malignancies showed a statistically significant decrease in early death occurrence (APC, −3.5; 95% CI, −5.7 to −1.3). Changes in early death rates for solid tumors (APC, −2.9; 95% CI, −6.1 to 0.3) and CNS tumors (APC, −0.8; 95% CI, −4.3 to 2.8), although demonstrating declining trends over time, did not achieve statistical significance (Fig 2).
Fig 2.
Trends in percentage of all patients with childhood cancer experiencing early death over the time period of the study, with best-fit lines, for (A) all patients, (B) hematologic malignancies, (C) CNS tumors, and (D) solid tumors.
DISCUSSION
In this population-based study, we have characterized the population of children and adolescents with cancer who die within 1 month of diagnosis, which represents 7.5% of all deaths in this cohort. This group is important to study because these individuals die too soon to benefit fully from the therapeutic advances that have been achieved in pediatric oncology over the last generation. Infants and older adolescents are at a disproportionately high risk of early death, as are individuals with specific diagnoses such as AML, infant ALL, malignant CNS tumors, and hepatoblastoma. Although these findings in part confirm those from prior studies of early death based on SEER and Italian Childhood Cancer Registry of Piedmont databases,2,3 we have provided additional evidence that in some cases contradicts that of prior studies, including the significant impact of race, ethnicity, and socioeconomic status on early death risk. Finally, we have shown that overall rates of early death seem to be decreasing.
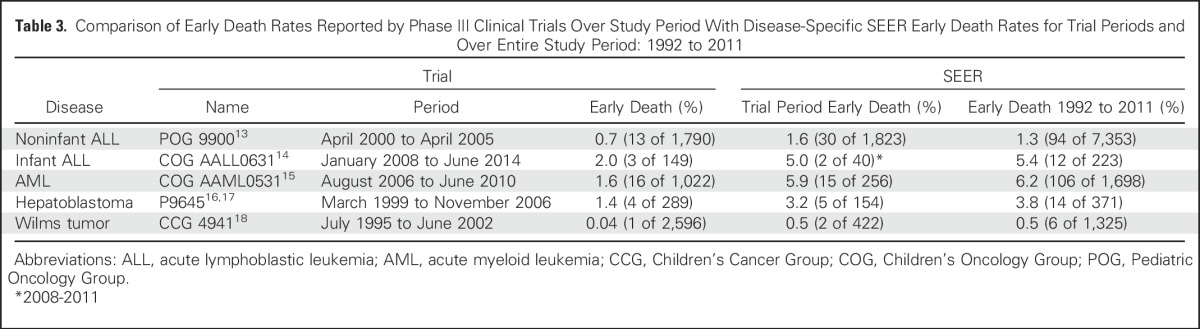
Despite the decrease in early death rates observed over time, we hypothesized that the overall scope of early deaths in childhood cancer would be greater than that reported in the clinical trial literature, by far the most prominent source of childhood cancer outcome data. Five multi-institutional phase III trials conducted by the Children’s Oncology Group or its predecessors during our study period opened enrollment to all patients from one disease group examined in this study and included data on deaths within the first month after initiation of therapy.13-18 When we compared the early death percentages from these trials with those from SEER, both over the time period of the trial and over the entire time period of our study, we found that the percentages of early deaths in the SEER database were consistently higher than the early death rates reported in the trials (Table 3). The early death percentages in SEER ranged from approximately twice the value observed in noninfant ALL to greater by a factor of 12.5 in Wilms tumor. One limitation of SEER is that we cannot know when during the first month from diagnosis death occurred. We interpreted the increased early deaths in SEER versus clinical trials as likely having occurred before the chance for clinical trial enrollment and thus a result of disease as opposed to early treatment toxicity. This disparity introduces an important bias in our overall interpretation of clinical trial results. Socioeconomically disadvantaged patients are also less likely to enroll in clinical trials,19 which may further magnify the disparity.
Table 3.
Comparison of Early Death Rates Reported by Phase III Clinical Trials Over Study Period With Disease-Specific SEER Early Death Rates for Trial Periods and Over Entire Study Period: 1992 to 2011
A major limitation of SEER is the limited availability of the underlying cause of death; the explanation for the increased early death risk in certain disease types is therefore not completely clear. For some diseases, individuals may present with life-threatening acute illness. For example, leukostasis and hemorrhage have been documented as causes of early death in children with AML20; this may explain the significantly higher percentages of early death for children and adolescents with this diagnosis found in our study. With advances in anticancer treatment and supportive care, the early death percentage is decreasing over time; this is the result in greatest part of decreases in early death for individuals with hematologic malignancies, probably reflecting improvements in infection control and supportive care.21
As advances through clinical and translational research continue to increase the percentage of children and adolescents with cancer who can be cured, we must readily address the under-reported problem of early death and assure that all individuals have the opportunity to receive the best available treatments; as shown here, up to 1.5% of children with cancer die before they can fully benefit from modern therapies, accounting for 7.5% of childhood cancer deaths. We advocate a two-pronged approach in addressing this problem. First, prospective registration studies at the cooperative group level should be considered that could capture individual-level socioeconomic status characteristics, as well as clinical data, including timing of diagnosis and death and underlying, intermediate, and direct causes of death, independent of clinical trial enrollment. Prospective studies such as Project Every Child by the Children’s Oncology Group could potentially fill this gap in knowledge by prospectively collecting outcome data and could create future study opportunities by storing cancer samples in a biorepository for subsequent analysis. This could pave the way for future research on understanding which patients are at risk for early death, causes of early death, and how to prevent early death. Second, specific initiatives targeting risk groups identified here should be developed now to allow earlier diagnosis and treatment, to make care more equitable for all children and adolescents with cancer.
Appendix
Table A1.
Category Parameters for Census-Based Socioeconomic Status Variables
Table A2.
No. and Percentage of Children and ORs According to Occurrence of Early Death and Cancer Type: SEER 13, 1992 to 2011
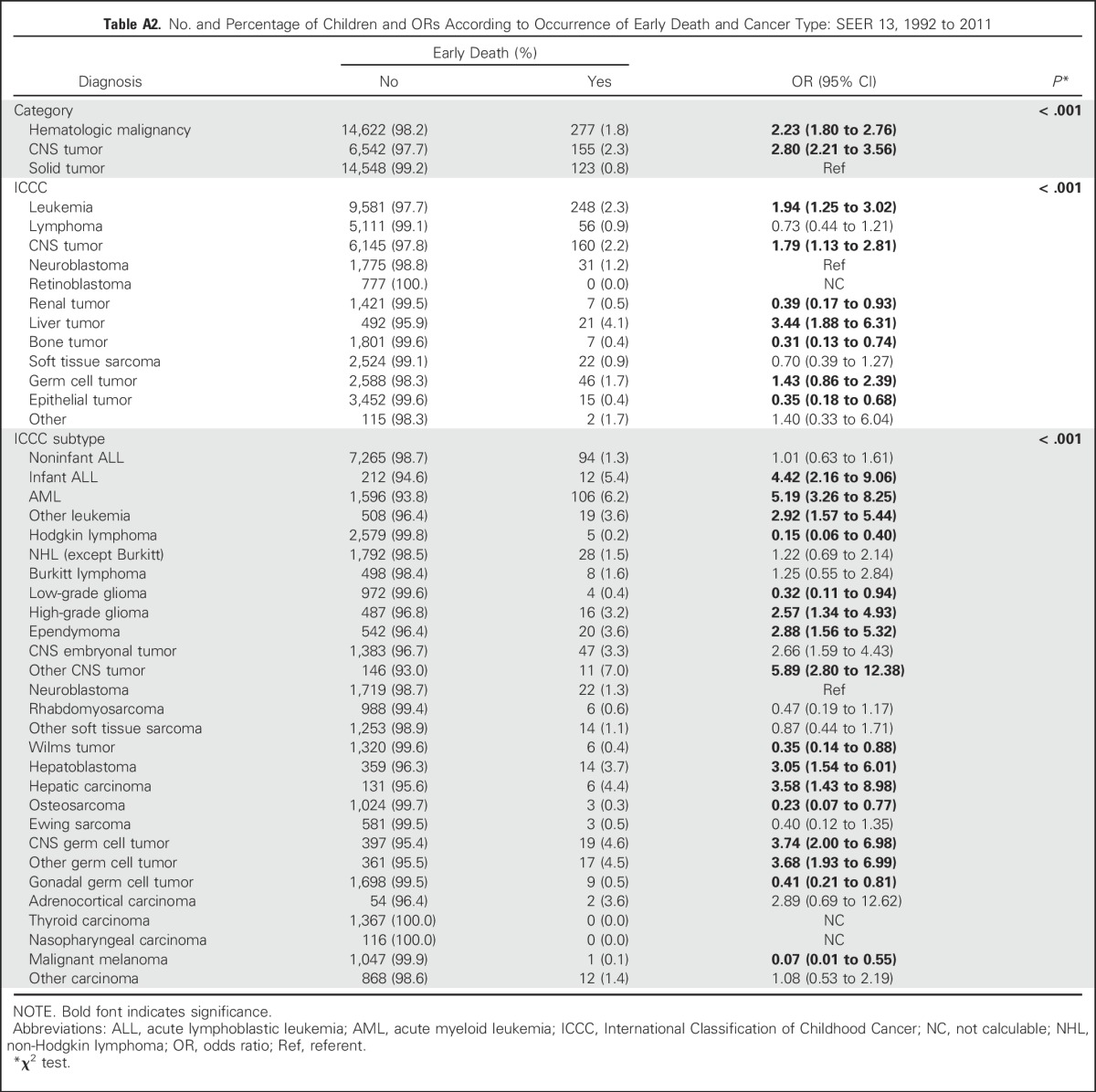
Fig A1.
Percentage of patients from early death and nonearly death groups in each of the three major cancer categories.
Footnotes
Supported by the Luke’s Army Pediatric Cancer Research Fund St. Baldrick’s Fellowship and a Hyundai Hope on Wheels Young Investigator Award (A.L.G.).
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
AUTHOR CONTRIBUTIONS
Conception and design: Adam L. Green, Karina Braga Ribeiro, Carlos Rodriguez Galindo
Collection and assembly of data: Adam L. Green, Elissa Furutani, Karina Braga Ribeiro
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Death Within 1 Month of Diagnosis in Childhood Cancer: An Analysis of Risk Factors and Scope of the Problem
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Adam L. Green
No relationship to disclose
Elissa Furutani
No relationship to disclose
Karina Braga Ribeiro
No relationship to disclose
Carlos Rodriguez Galindo
Honoraria: Novimmune
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Pastore G, Viscomi S, Mosso ML, et al. Early deaths from childhood cancer: A report from the Childhood Cancer Registry of Piedmont, Italy, 1967-1998. Eur J Pediatr. 2004;163:313–319. doi: 10.1007/s00431-004-1425-x. [DOI] [PubMed] [Google Scholar]
- 3.Hamre MR, Williams J, Chuba P, et al. Early deaths in childhood cancer. Med Pediatr Oncol. 2000;34:343–347. doi: 10.1002/(sici)1096-911x(200005)34:5<343::aid-mpo5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Kent EE, Sender LS, Largent JA, et al. Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes Control. 2009;20:1409–1420. doi: 10.1007/s10552-009-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin S, Ulrich C, Munsell M, et al. Delays in cancer diagnosis in underinsured young adults and older adolescents. Oncologist. 2007;12:816–824. doi: 10.1634/theoncologist.12-7-816. [DOI] [PubMed] [Google Scholar]
- 6.Smith EC, Ziogas A, Anton-Culver H. Association between insurance and socioeconomic status and risk of advanced stage Hodgkin lymphoma in adolescents and young adults. Cancer. 2012;118:6179–6187. doi: 10.1002/cncr.27684. [DOI] [PubMed] [Google Scholar]
- 7.Sharib J, Horvai A, Gray Hazard FK, et al. Comparison of Latino and non-Latino patients with Ewing sarcoma. Pediatr Blood Cancer. 2014;61:233–237. doi: 10.1002/pbc.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koohbanani B, Han G, Reed D, et al. Ethnicity and age disparities in Ewing sarcoma outcome. Fetal Pediatr Pathol. 2013;32:246–252. doi: 10.3109/15513815.2012.721480. [DOI] [PubMed] [Google Scholar]
- 9.Worch J, Matthay KK, Neuhaus J, et al. Ethnic and racial differences in patients with Ewing sarcoma. Cancer. 2010;116:983–988. doi: 10.1002/cncr.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong B, Green AL, Friedrich P, et al. Ethnic, racial, and socioeconomic disparities in retinoblastoma. JAMA Pediatr. 2015;169:1096–1104. doi: 10.1001/jamapediatrics.2015.2360. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Wilejto M, Pole JD, et al. Low socioeconomic status is associated with worse survival in children with cancer: A systematic review. PLoS One. 2014;9:e89482. doi: 10.1371/journal.pone.0089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steliarova-Foucher E, Stiller C, Lacour B, et al: International Classification of Childhood Cancer, third edition. Cancer 103:1457-1467, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Winick N, Borowitz MJ, Devidas M, et al: Changes in the delivery of standard chemotherapeutic agents during Induction affect early measures of minimal residual disease (MRD): POG 9900 for patients with B-precursor low and standard risk ALL. Presented at the 48th Annual Meeting of the American Society of Hematology Annual Meeting, Orlando, FL, December 9-12, 2006. [Google Scholar]
- 14.Salzer WL, Jones TL, Devidas M, et al. Decreased induction morbidity and mortality following modification to induction therapy in infants with acute lymphoblastic leukemia enrolled on AALL0631: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62:414–418. doi: 10.1002/pbc.25311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: Results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malogolowkin MH, Katzenstein H, Krailo MD, et al. Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol. 2006;24:2879–2884. doi: 10.1200/JCO.2005.02.6013. [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.1002/cncr.24667. Katzenstein HM, Chang KW, Krailo M, et al: Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: A report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children’s Oncology Group. Cancer 115:5828-35, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms’ tumor: Results from the fifth National Wilms’ Tumor Study. J Clin Oncol. 2006;24:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 19.Parsons HM, Harlan LC, Seibel NL, et al. Clinical trial participation and time to treatment among adolescents and young adults with cancer: Does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29:4045–4053. doi: 10.1200/JCO.2011.36.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creutzig U, Zimmermann M, Reinhardt D, et al. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: Analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22:4384–4393. doi: 10.1200/JCO.2004.01.191. [DOI] [PubMed] [Google Scholar]
- 21.Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998–3003. doi: 10.1182/blood.v97.10.2998. [DOI] [PubMed] [Google Scholar]