Abstract
Purpose
Adjuvant therapy for intermediate-risk and high-risk localized prostate cancer decreases the number of deaths from this disease. Surrogates for overall survival (OS) could expedite the evaluation of new adjuvant therapies.
Methods
By June 2013, 102 completed or ongoing randomized trials were identified and individual patient data were collected from 28 trials with 28,905 patients. Disease-free survival (DFS) and metastasis-free survival (MFS) were determined for 21,140 patients from 24 trials and 12,712 patients from 19 trials, respectively. We evaluated the surrogacy of DFS and MFS for OS by using a two-stage meta-analytic validation model by determining the correlation of an intermediate clinical end point with OS and the correlation of treatment effects on both the intermediate clinical end point and OS.
Results
Trials enrolled patients from 1987 to 2011. After a median follow-up of 10 years, 45% of 21,140 men and 45% of 12,712 men experienced a DFS and MFS event, respectively. For DFS and MFS, 61% and 90% of the patients, respectively, were from radiation trials, and 63% and 66%, respectively, had high-risk disease. At the patient level, Kendall’s τ correlation with OS was 0.85 and 0.91 for DFS and MFS, respectively. At the trial level, R2 was 0.86 (95% CI, 0.78 to 0.90) and 0.83 (95% CI, 0.71 to 0.88) from weighted linear regression of 8-year OS rates versus 5-year DFS and MFS rates, respectively. Treatment effects—measured by log hazard ratios—for the surrogates and OS were well correlated (R2, 0.73 [95% CI, 0.53 to 0.82] for DFS and 0.92 [95% CI, 0.81 to 0.95] for MFS).
Conclusion
MFS is a strong surrogate for OS for localized prostate cancer that is associated with a significant risk of death from prostate cancer.
INTRODUCTION
Each year, there are approximately 1.1 million newly diagnosed cases of prostate cancer, with more than 300,000 deaths worldwide.1 Treatment of intermediate-risk and high-risk localized disease with adjuvant systemic therapy is associated with fewer deaths from prostate cancer.1-4 Advances in understanding prostate cancer biology and drug development have resulted in new therapies that have prolonged the lives of some men with metastatic castration-resistant prostate cancer.5 Use of these therapies in the adjuvant setting—when micrometastases, if present, are more sensitive to therapies—may actually eradicate the disease and further decrease the number of men who die from prostate cancer; however, adjuvant clinical trials in prostate cancer take more than a decade to reach the irrefutable end point of overall survival (OS). Whereas disease-free survival (DFS) has proven to be a surrogate for OS and is used as a primary end point in adjuvant trials of colon cancer,6 no intermediate clinical end points (ICEs) are accepted as robust surrogates for OS in prostate cancer trials.
An ICE can serve as a good surrogate for OS when there is no curative salvage therapy for relapsed disease and/or substantial risk of dying of the disease.7-10 Prior preliminary attempts that have used single studies to identify ICEs as surrogates for OS in localized prostate cancer have included the following: time to biochemical failure, prostate-specific antigen (PSA) doubling time, PSA nadir, end of treatment PSA, DFS, and metastasis-free survival (MFS).11 We hypothesized that DFS and/or MFS may be surrogates for OS, as they track more closely with death from prostate cancer than a PSA-based ICE.11,12 A major proportion of patients with intermediate-risk or high-risk localized prostate cancer are cured and, even if these patients experience relapse, often die of causes other than prostate cancer; therefore, we also investigated the surrogacy of time to disease recurrence (TDR) and time to metastasis (TTM) for disease-specific survival (DSS), where nonprostate cancer deaths were not counted as an event.
METHODS
Search Strategy and Selection Criteria
To enable a meta-analysis of individual patient data (IPD) from randomized controlled trials in localized prostate cancer, we conducted a systematic review of studies that followed the PRISMA guidelines.11 Eligible trials included randomized controlled trials for localized disease that were closed to accrual and conducted in Australia, New Zealand, Canada, Europe or the United States. Trials with primary end points other than efficacy—for example, safety, toxicity, quality of life, feasibility, dosimetry, and patient decision-making—without systematic long-term follow-up were excluded.
At the time of project initiation—June 2013—102 trials were identified as potentially eligible, of which 43 (42%) of 102 trials had both data that were suitable for use and a study group that agreed to participate. This resulted in possible IPD from 28,905 patients. For this analysis, IPD were provided for 28 (65%) of 43 trials with 22,825 patients. Not all trials collected all end points of interest; therefore, for DFS and MFS analysis, 21,140—from 24 (56%) of 43 trials—and 12,712 patients—from 19 (44%) of 43 trials—were included, respectively. Trials that did not document data on these end points were excluded. The selection process and reasons for exclusion are given in the Data Supplement.
Statistical Analyses
Definition of end points.
DFS was measured from the date of random assignment to the date of first evidence of recorded clinical recurrence—local/regional recurrence and/or distant metastases confirmed by imaging or histologic evidence—or death from any cause or was censored at the date of last follow-up. MFS was defined the same as DFS but did not include local/regional recurrence. TDR and TTM were defined analogously to DFS and MFS, but nonprostate cancer deaths without prior progression were censored or were counted as competing risk. OS was measured from the date of random assignment to death from any cause, censored at the date of last follow-up in patients who were alive. DSS was defined the same as OS, but nonprostate deaths were censored or considered as competing risk in sensitivity analyses. Local recurrence and cause of death were based on trial-defined events (Data Supplement).
Surrogacy criteria.
We evaluated the surrogacy of DFS and MFS with OS by using a widely accepted13 meta-analytic two-stage validation model where two conditions must hold to claim that an ICE is a surrogate for OS14,15 (Data Supplement). Condition 1 requires that the ICE and OS be correlated. Condition 2 requires that the treatment effects on both end points also be correlated. The validity of the surrogate is reflected by the strength of the correlations. To be consistent with other surrogacy assessments in oncology, we defined a priori a clinically relevant surrogacy of an R2 value of ≥ 0.7.11
Condition 1 was tested at both the patient level and trial level. At the patient level, associations of OS with DFS and MFS were evaluated via a bivariate copula model (Data Supplement) fitted on IPD.16 Kendall’s τ (range, 0 to 1) quantified the correlation between end points. At the trial level, we first obtained Kaplan-Meier estimates of 5-year DFS or MFS rates and 8-year OS rates for each treatment arm within each trial. We then performed weighted linear regression (WLR) analyses between trial and arm-specific OS rates at 8 years versus DFS and MFS rates at 5 years. These time points were chosen as they are frequently reported in the literature. Regressions were weighted by inverse variances of the 5-year estimates of the ICE. R2 was used to quantify the proportion of variance that was explained by the regressions.
To test condition 2, we performed Cox proportional hazards regression models to obtain the study-specific treatment effects—that is, the natural log (hazard ratio [HR])—on the ICE and OS. We then fit a WLR model between the effects of treatment on OS versus the effects of treatment on DFS or MFS. Regressions were weighted by inverse variances of the natural log (HR) on the ICE, and R2 was used to quantify the proportion of variance that was explained by the regressions. This approach was also applied to the surrogacy analysis of TDR and TTM for DSS (nonprostate cancer deaths were censored).
Subgroup and sensitivity analysis.
Given the heterogeneous population and treatment in the localized disease setting, we conducted preplanned subgroup analyses by types of primary therapy (radical prostatectomy [RP] versus radiation therapy [RT]); within RT-trials: duration of androgen deprivation therapy (ADT; ≤ 6 or > 6 months); and patient risk groups defined by the National Comprehensive Cancer Network, D’Amico, or pathologic features. Because a large proportion of TDR and TTM end points are censored as a result of nonprostate cancer deaths, we performed a sensitivity analysis to estimate trial-level correlation between cumulative incidence estimates of TDR/TTM and DSS and between the subdistribution treatment effect HR estimates for TDR/TTM and DSS from competing risk models17 for which nonprostate cancer deaths were considered as the competing risk for each end point. Model accuracy was assessed by leave-one-out cross validation (Data Supplement).
Surrogate threshold effect.
Surrogate threshold effect (STE) is defined as the minimum treatment effect (HR) on the surrogate necessary to predict a nonzero treatment effect—that is, HR different from 1—on OS in a future trial.18 To obtain STE, we constructed the 95% prediction limits for the regression line of the effect of treatment on OS versus the effect of treatment on the surrogate, accounting for the mean weights of current trials. The intersection of the upper 95% prediction limit with the horizontal line—representing an HR of 1 for OS—was defined as STE, which corresponded to no treatment effect on OS.
All analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC ) and R packages (www.r-project.org).
RESULTS
Trial and Patient Characteristics
For analysis, 21,140 patients from 24 trials and 12,712 patients from 19 trials had documented data on DFS and MFS analysis, respectively (Data Supplement). Five trials were split according to the type of primary therapy or experimental arm, which resulted in 31 and 21 study units for DFS and MFS analysis, respectively (Data Supplement).
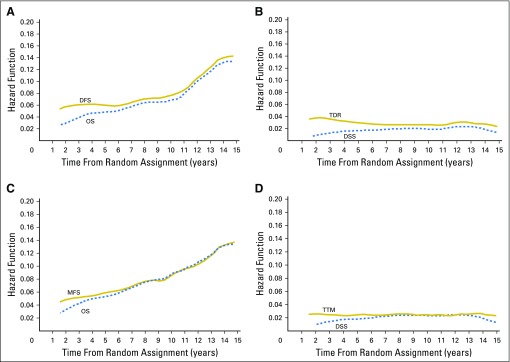
Trials enrolled patients from 1987 to 2011 and median follow-up was 10 years (range, < 0.1 to approximately 22.7 years). More than 80% of patients were age < 75 years (Data Supplement). For DFS and MFS analysis, 61% and 90% of patients, respectively, were in radiation trials, and 63% and 66% had high-risk disease, respectively. Observed 5-year rates were 76% for DFS, 79% for MFS, and 84% for OS. Figure 1 shows the Kaplan-Meier distributions of end points. Estimated hazard function by years since random assignment for each end point is shown in Fig 2.
Fig 1.
(A-D) Kaplan-Meier estimates of end points (A) overall survival (OS) and disease-free survival (DFS), (B) disease-specific survival (DSS) and time to disease recurrence (TDR), (C) OS and metastasis-free survival (MFS), and (D) DSS and time to metastasis (TTM). Median follow-up was 10 years.
Fig 2.
Estimated hazard across times (A) overall survival (OS) and disease-free survival (DFS), (B) disease-specific survival (DSS) and time to disease recurrence (TDR), (C) OS and metastasis-free survival (MFS), and (D) DSS and time to metastasis (TTM).
Surrogacy Condition 1: Correlation Between ICE and OS
At the individual patient level, the correlation with OS was 0.85 (95% CI, 0.85 to 0.86) and 0.91 (95% CI, 0.91 to 0.91) for DFS and MFS, respectively, as measured by Kendall’s τ from a copula model. When nonprostate cancer deaths were censored, the correlation with DSS was 0.68 (95% CI, 0.67 to 0.69) for TDR and 0.91 (95% CI, 0.91 to 0.92) for TTM. The tight correlation between end points is reflected by the tight correlation between the trial and arm-specific Kaplan-Meier estimates of OS or DSS at 8 years versus the Kaplan-Meier estimates of the surrogates at 5 years (Fig 3). From the WLR, R2 was 0.86 (95% CI, 0.78 to 0.90) and 0.83 (95% CI, 0.71 to 0.88) between 8-year OS rates versus 5-year DFS and MFS rates, respectively. When nonprostate cancer deaths were censored, there was still a high correlation of 8-year DSS rates (R2, 0.80 [95% CI, 0.70 to 0.85] with 5-year TDR; and R2, 0.86 [95% CI, 0.75 to 0.90] for 5-year TTM; Table 1).
Fig 3.
Overall survival (OS) or disease-specific survival (DSS) rate at 8-year versus surrogate end points at 5 years: (A) 8-year OS versus 5-year disease-free survival (DFS), (B) 8-year disease-specific survival (DSS) versus 5-year time to disease recurrence (TDR), (C) 8-year OS versus 5-year metastasis-free survival (MFS), (D) 8-year DSS versus 5-year time to metastasis (TTM). All rates were Kaplan-Meier estimates by trial and treatment arm. Circle size and regression were weighed by inverse variance of the 5-year rate estimate for the surrogates.
Table 1.
Two-Condition Surrogacy Analysis

Surrogacy Condition 2: Correlation Between Treatment Effect on ICE and OS
At the trial level, trial-specific treatment effects—measured by HR for each end point—are shown in forest plots in the Data Supplement. R2 was 0.73 (95% CI, 0.53 to 0.82) from the WLR of log(HR)-OS versus log(HR)-DFS and was reduced to 0.63 (95% CI, 0.36 to 0.75) with nonprostate cancer deaths censored. There was a strong correlation between log(HR)-OS and log(HR)-MFS across trials (R2, 0.92 [95% CI, 0.81 to 0.95]), and the high correlation remained when nonprostate cancer deaths were censored (R2, 0.89 [95% CI, 0.72 to 0.93]; Fig 4). The estimated WLR equation for each end point is listed in Table 1.
Fig 4.
Treatment effects (hazard ratio [HR]) on overall survival (OS) or disease-specific survival (DSS) versus treatment effects on surrogates: (A) OS HR versus disease-free survival (DFS) HR, (B) DSS HR versus time to disease recurrence (TDR) HR, (C) OS HR versus metastasis-free survival (MFS) HR, (D) DSS HR versus time to metastasis (TTM) HR. HRs were estimated from Cox proportional hazards regression model for each study, and values were natural logarithm transformed. Circle size and regression were weighed by inverse variance of log(HR) estimates for surrogates. STE, surrogate threshold effect.
Subgroup and Sensitivity Analysis
Overall, results were consistent when analysis was restricted to the high-risk population only or in a subgroup analysis by type of primary therapy and by exposure to ADT within RT-based trials at both the patient level and trial level (Data Supplement). At the patient level, Kendall’s τ correlation between OS and DFS was 0.91 (95% CI, 0.90 to 0.92) and 0.84 (95% CI, 0.83 to 0.84) in RP-based and RT-based trials, respectively. At the trial level, R2 from the WLR of log(HR)-OS versus log(HR)-DFS was 0.87 (95% CI, 0.31 to 0.93) for RP trials and 0.75 (95% CI, 0.48 to 0.84) for RT trials. For MFS end point, no separate analysis was conducted for RP-based trials as 90% of patients were from radiation trials. The correlation between OS or DSS and each ICE was slightly stronger in those who received > 6 months of adjuvant ADT compared with those who received no or short-term neoadjuvant/adjuvant ADT (Data Supplement).
Results were also consistent in a WLR analysis of trial-level correlations when nonprostate cancer deaths were treated as competing risk (Data Supplement) and in leave-one-out cross-validation (Data Supplement).
STE and Implications for Trial Designs
STE on OS was an HR(DFS) of 0.67 and an HR(MFS) of 0.88, which indicates that a risk reduction of 33% and 12%, respectively, would predict a nonzero effect on OS (Fig 4). In addition, STE on DSS was an HR(TDR) of 0.49 and an HR(TTM) of 0.74; thus, a larger treatment effect on TDR would be required to predict a treatment benefit on DSS.
Given the strong correlation between MFS and OS, clinical trials can be designed using MFS as primary end point instead of OS (Data Supplement). Historically, trials have been designed with an OS HR that ranges from 0.71 to 0.75. These trials have a study duration of 11.5 to 16.2 years with 1,000 patients enrolled over 5 years (Data Supplement). Clearly, study durations would be shorter if the same treatment effects were assumed for MFS (Data Supplement). WLR analysis (Table 1, Fig 4) predicts that for OS HRs that range from 0.71 to 0.75, and corresponding MFS HRs would range from 0.65 to 0.7 (Data Supplement), so the benefit of using MFS instead of OS could be even greater; however, the surrogate threshold effect, which is an MFS HR of 0.88, implies that a future trial would require an upper limit of the CI for the estimated HR(MFS) to fall below the STE to predict a significant effect on OS. Hence, depending on the assumed HRs and the number of patients, the duration of the trial may favor choosing MFS or OS as the primary end point (Fig 5). MFS would be the preferred primary end point for an HR(OS) of < 0.7, whereas OS would be the preferred primary end point for an HR(OS) of > 0.72. For example, a trial with 1,000 patients that was designed to detect a treatment effect of an HR(MFS) of 0.6 would have a total study duration of 7.7 years. The associated predicted HR(OS) is 0.67, and a trial designed to detect this effect would have a total study duration of 8.8 years.
Fig 5.
Total study duration required in the study designs using metastasis-free survival (MFS) hazard ratios (HRs) and testing surrogate threshold effect (STE; solid line) or using predicted overall survival (OS) HRs from weighted linear regression (dashed line). MFS would be the preferred primary end point for HR(OS) of < 0.70, whereas OS would be the preferred primary end point for HR(OS) of > 0.72 (vertical solid lines). Design assumptions include 5-year MFS and OS rates of 0.79 and 0.84 (hazard, 0.04714 and 0.03487 under exponential distribution), respectively, 5 years of accrual period, and type I error of 0.025 (one-sided) and type II error of 0.20.
DISCUSSION
In a cohort of patients with prostate cancer with an approximate15% chance of dying of prostate cancer over a 10-year period, DFS and MFS are valid surrogates for OS. As the estimated hazard across times curves (Fig 2) depict, early prostate cancer recurrences are associated with death from prostate cancer before dying from a competing comorbidity in a patient population, 80% of whom are age < 75 years and fit for enrollment in a clinical trial.
The practical output for surrogacy work includes being able to complete trials in a more expeditious manner. The advantage of using a surrogate, such as MFS, rather than OS is the ability to observe the number of required events earlier, but there is some uncertainty as to how well the surrogate predicts the effect on the true end point. However, this uncertainty is captured by the STE, which is the minimum treatment effect required on the surrogate to predict a significant treatment effect on the true end point. In short, use of MFS can allow an expeditious evaluation of a new therapy if it has a meaningful treatment effect on MFS. Of note, an HR(MFS) of 0.6 has been observed in adjuvant trials of testosterone suppression plus radiation versus radiation in high-risk localized disease and resulted in improvements in OS.3,4,19-22 There are possibly other health economic benefits for preventing morbidity and adverse effects of treatment associated with a metastatic event.11 Defining these benefits is part of the ongoing work being conducted by the ICECaP Working Group.
Use of IPD was critical in conducting this analysis and allowed a side-by-side comparison of DFS and MFS as surrogates for OS. There were more patients and trials that were suitable for DFS than MFS analyses, as some studies did not record events beyond first clinical progression and are only viable for DFS analysis. As such, systematic follow-up until first distant recurrence is required in future studies to capture MFS events. The lower correlation of 0.7 for DFS versus 0.9 for MFS results in a lower STE for DFS (0.67 v 0.88) as the prediction intervals for DFS are wider, thus a need for a greater treatment effect. This is presumably a result of local recurrences that are possibly indolent and/or cured with salvage therapy. Sensitivity analysis demonstrated that the MFS correlation with OS was maintained whether the primary localized therapy was surgery or radiation on the basis of whether adjuvant ADT was used (Data Supplement). Of note, the five trials in which MFS was not collected (n = 8,428 patients) were all trials of adjuvant hormonal therapy. In addition, early metastatic relapse is associated with death from prostate cancer, and sensitivity analyses have shown that this is regardless of receipt of ADT in the adjuvant setting and that this may also be the same for biochemical or metastatic disease. Moreover, subgroup analysis by duration of ADT could only be performed at the IPD level, as most trials were designed to compare duration of ADT.
IPD also provides unique insight into the natural history of prostate cancer. Figure 2 indicates that a constant rate of relapses and late relapses have less impact on OS than relapses before 7 years in this cohort, with a median OS of 12.7 years. Presumably, there is an increase of nonprostate cancer deaths in later years and later relapses have a more indolent course.
The cross-validation, subgroup, and sensitivity analyses provide additional reassurance that the results are robust. There were some limitations of our study. First, the DFS end point incorporated local recurrence as an event defined by the trials with variations in the definition of local recurrence. Second, we could not provide a separate analysis for MFS for surgery because there were a limited number of surgery-based trials.
Whereas it would be preferable to have an earlier end point than 5-year DFS or MFS, this time point was chosen as it was associated with enough events to allow robust analysis. Correlation with a 10-year OS was thwarted as some trials did not have enough follow-up, which resulted in a smaller number of units and fewer patients at risk and, presumably, a greater impact from other causes of death. As such, the 8-year OS rate was more reliable. In addition, as OS data require long-term follow-up, most trials that were included commenced before 2005. Our ongoing work with recently completed trials will investigate the reproducibility of our findings in an era with new therapies that prolong OS in men with metastatic hormone-sensitive prostate cancer and castration-resistant prostate cancer; however, because these have a modest improvement in OS and do not cure disease, it is anticipated that surrogacy will still persist.
In conclusion, MFS is a strong surrogate for OS in clinically localized prostate cancer in a patient population with an approximate 15% chance of dying of prostate cancer over 10 years despite potentially curative local therapy. Surrogacy is independent of primary local interventions and type of adjuvant therapy. Linear regression graphs used to generate the STE can be used to define relative improvements in MFS that are associated with clinically meaningful improvements in OS.
ACKNOWLEDGMENT
We thank Victoria Wong, Frontier Science & Technology Research Foundation, for computer programming.
Appendix
ICECaP Working Group Members (in alphabetical order)
Ove Andren, John Armstrong, Donald Berry, Michel Bolla, Marc Buyse, Joseph Chin, Simon Chowdhury, Noel Clarke, Laurence Collette, Matthew Cooperberg, Jim Denham, James Dignam, Savino Mauro Di Stasi, Mario Eisenberger, Karim Fizazi, Boris Freidlin, Silke Gillessen, Martin Gleave, Muriel Habibian, Susan Halabi, Nicholas James, Jonathan Jarow, Philip Kantoff, Nancy Keating, Gary Keloff, Laurence Klotz, Himu Lukka, Malcolm Mason, Andrea Miyahira, Nicolas Mottet, Mari Nakabayashi, Wendy R. Parulekar, Meredith Regan, Howard Sandler, Oliver Sartor, Peter Scardino, Howard Scher, Richard Simon, Jonathan Simons, Eric Small, Howard Soule, Christopher J. Sweeney, Matthew Sydes, Catherine Tangen, Ian Thompson, Bertrand Tombal, Anders Widmark, Thomas Wiegel, Manfred Wirth, Scott Williams, Wanling Xie, Eric Yeoh, Almudena Zapatero
Footnotes
Funded by the Prostate Cancer Foundation Challenge Award and grants from Astellas Pharma, Medivation, Janssen Pharmaceuticals, Millennium Pharmaceuticals, Sotio, and Sanofi.
Processed as a Rapid Communication manuscript.
This study was in collaboration with the ICECaP Working Group. The Dana-Farber Cancer Institute coordinating center had full access to the data, and independent working group members oversaw statistical analysis plan development and interpretation of the data. The corresponding author had final responsibility for the decision to submit for publication. The final report was shared with the pharmaceutical companies that provided financial support as investigator-initiated grants but had no input on the design or interpretation of the results.
AUTHOR CONTRIBUTIONS
Conception and design: Wanling Xie, Meredith M. Regan, Marc Buyse, Susan Halabi, Philip W. Kantoff, Oliver Sartor, Howard Soule, Noel W. Clarke, Laurence Collette, James J. Dignam, Wendy R. Paruleker, Howard M. Sandler, Matthew R. Sydes, Bertrand Tombal, Scott G. Williams, Christopher J. Sweeney
Financial support: Christopher J. Sweeney
Administrative support: Christopher J. Sweeney
Provision of study materials or patients: James J. Dignam, Karim Fizazi, Howard M. Sandler, Matthew R. Sydes, Christopher J. Sweeney
Collection and assembly of data: Wanling Xie, Meredith M. Regan, James J. Dignam, Wendy R. Paruleker, Howard M. Sandler, Christopher J. Sweeney
Data analysis and interpretation: Wanling Xie, Meredith M. Regan, Susan Halabi, Oliver Sartor, Laurence Collette, Karim Fizazi, Wendy R. Paruleker, Bertrand Tombal, Christopher J. Sweeney
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Wanling Xie
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals
Meredith M. Regan
Consulting or Advisory Role: Merck, Ipsen (Inst)
Research Funding: Veridex (Inst), OncoGenex (Inst), Pfizer (Inst), Ipsen (Inst), Novartis (Inst), Merck (Inst), Ferring (Inst), Celgene (Inst), AstraZeneca (Inst), Pierre Fabre (Inst)
Marc Buyse
Employment: International Drug Development Institute
Stock or Other Ownership: International Drug Development Institute
Susan Halabi
Travel, Accommodations, Expenses: Dendreon
Philip W. Kantoff
Stock or Other Ownership: Bellicum Pharmaceuticals, Placon, Tarveda Therapeutics
Consulting or Advisory Role: Bavarian Nordic, Janssen Pharmaceuticals, Millennium Pharmaceuticals, MorphoSys, Pfizer, Astellas Pharma, Bellicum Pharmaceuticals, BIND Biosciences, Endocyte, Metamark Genetics, Medivation, Merck, MTG Therapeutics, OncoCellMDX, Oncogenex, Sotio, Sanofi, Tokai Pharmaceuticals, Bayer, Genentech, Cristal Therapeutics, Ipsen, Omnitura, GTx, Tarveda Therapeutics, Druggablity Technologies, Progenity
Research Funding: Medivation (Inst), Sanofi (Inst), Oncogenex (Inst), Aragon Pharmaceuticals (Inst), Amgen (Inst), Astellas Pharma (Inst), Bayer (Inst), Bavarian Nordic (Inst), Dendreon (Inst), Exelixis (Inst), Janssen Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Method for predicting the risk of prostate cancer morbidity and mortality, predicting and treating prostate cancer, methods for predicting likelihood of responding to treatment, chromosome copy number gain as a biomarker of urothelial carcinoma lethality, drug combinations to treat cancer, somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma (patent), Up-to-Date royalties, Wolters-Kluwer royalties
Expert Testimony: Sanofi, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Sanofi, Janssen Pharmaceuticals, BIND Biosciences, Bavarian Nordic, Millennium Pharmaceuticals
Oliver Sartor
Consulting or Advisory Role: Bayer, Bellicum Pharmaceuticals, Johnson & Johnson, Medivation, Oncogenex, Sanofi, Tokai Pharmaceuticals, AstraZeneca (Inst), Progenics (Inst), Dendreon
Research Funding: Bayer (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Endocyte (Inst), Innocrin Pharma (Inst), Progenics (Inst)
Travel, Accommodations, Expenses: Bayer, Bellicum Pharmaceuticals, Johnson & Johnson, Medivation, Oncogenex, Sanofi, Tokai Pharmaceuticals, AstraZeneca, Progenics
Howard Soule
Leadership: WindMIL
Consulting or Advisory Role: Compugen
Travel, Accommodations, Expenses: Compugen, Sanofi
Noel W. Clarke
Honoraria: Janssen-Cilag, Astellas Pharma, Sanofi, Bayer, AstraZeneca, Ferring, Ipsen
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Ferring, Bayer, Sanofi
Speakers' Bureau: Janssen-Cilag, Janssen-Cilag, Astellas Pharma
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Jannsen-Cilag, Astellas Pharma,Ferring, Bayer, Sanofi, Astra Zeneca
Laurence Collette
No relationship to disclose
James J. Dignam
No relationship to disclose
Karim Fizazi
Honoraria: Janssen Pharmaceuticals, Sanofi, Astellas Pharma, Takeda, Merck, Amgen
Consulting or Advisory Role: Janssen Oncology, Bayer, Astellas Pharma, Sanofi, Orion Pharma, Curevac, AstraZeneca, ESSA, Genentech, Clovis Oncology
Travel, Accommodations, Expenses: Amgen
Wendy R. Paruleker
No relationship to disclose
Howard M. Sandler
Consulting or Advisory Role: Janssen Pharmaceuticals, Blue Earth Diagnostics, Ferring, NantHealth, Dendreon
Other Relationship: Caribou Publishing
Matthew R. Sydes
Research Funding: Astellas Pharma, Janssen-Cilag, Pfizer, Novartis, Sanofi, Clovis Oncology
Bertrand Tombal
Honoraria: Amgen, Astellas Pharma, Bayer, Ferring, Sanofi, Janssen Pharmaceuticals
Consulting or Advisory Role: Astellas Pharma, Bayer, Ferring, Janssen Pharmaceuticals, Takeda, Steba Biotech, Sanofi
Speakers' Bureau: Amgen, Janssen Pharmaceuticals
Research Funding: Ferring (Inst)
Travel, Accommodations, Expenses: Amgen, Astellas Pharma, Bayer, Ferring, Janssen Pharmaceuticals, Sanofi
Scott G. Williams
No relationship to disclose
Christopher J. Sweeney
Stock or Other Ownership: Leuchemix
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer, Genentech, AstraZeneca, Pfizer
Research Funding: Janssen Biotech (Inst), Astellas Pharma (Inst), Sanofi (Inst), Bayer (Inst), Sotio (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix: Parthenolide, dimethylaminoparthenolide; Exelixis: Abiraterone plus cabozantinib combination
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. : Global cancer statistics, 2012. CA Cancer J Clin 65:87-108, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin 66:7-30, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, Gonzalez D, Warde P, et al. : Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 337:295-300, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Denham JW, Steigler A, Lamb DS, et al. : Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12:451-459, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Attard G, Parker C, Eeles RA, et al. : Prostate cancer. Lancet 387:70-82, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Sargent DJ, Wieand HS, Haller DG, et al. : Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23:8664-8670, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Hamdy FC, Donovan JL, Lane JA, et al. : 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 375:1415-1424, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Bill-Axelson A, Holmberg L, Garmo H, et al. : Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 370:932-942, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crook JM, O’Callaghan CJ, Duncan G, et al. : Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med 367:895-903, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross AE, Yousefi K, Davicioni E, et al. : Utility of risk models in decision making after radical prostatectomy: Lessons from a natural history cohort of intermediate- and high-risk men. Eur Urol 69:496-504, 2016 [DOI] [PubMed] [Google Scholar]
- 11.ICECaP Working Group. Sweeney C, Nakabayashi M, Regan M, et al. : The development of Intermediate Clinical Endpoints in Cancer of the Prostate (ICECaP). J Natl Cancer Inst 107:djv261, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweizer MT, Zhou XC, Wang H, et al. : Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol 24:2881-2886, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciani O, Davis S, Tappenden P, et al. : Validation of surrogate endpoints in advanced solid tumors: systematic review of statistical methods, results, and implications for policy makers. Int J Technol Assess Health Care 30:312-324, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Buyse M, Michiels S, Squifflet P, et al. : Leukemia-free survival as a surrogate end point for overall survival in the evaluation of maintenance therapy for patients with acute myeloid leukemia in complete remission. Haematologica 96:1106-1112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buyse M, Molenberghs G, Burzykowski T, et al. : The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1:49-67, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Burzykowski T, Molenberghs G, Buyse M, et al. : Validation of surrogate end points in multiple randomized clinical trials with failure time endpoints. Appl Stat 50:405-422, 2001 [Google Scholar]
- 17.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 18.Burzykowski T, Buyse M: Surrogate threshold effect: An alternative measure for meta-analytic surrogate endpoint validation. Pharm Stat 5:173-186, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Shipley WU, Seiferheld W, Lukka HR, et al. : Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 376:417-428, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messing EM, Manola J, Sarosdy M, et al. : Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med 341:1781-1788, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Messing EM, Manola J, Yao J, et al. : Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 7:472-479, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Bolla M, Van Tienhoven G, Warde P, et al. : External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11:1066-1073, 2010 [DOI] [PubMed] [Google Scholar]