Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a serious life-threatening disease if not recognised early. In patients with HIV/AIDS, this association has been reported following acute opportunistic infections, including histoplasmosis. However, optimal treatment is not known. We describe a male aged 46 years with AIDS who developed HLH following acute disseminated histoplasmosis. Presenting symptoms included fever, hepatosplenomegaly and pancytopenia. Bone marrow biopsy confirmed HLH. Initially, he was refractory to the treatment with amphotericin B, antiretroviral therapy and intravenous immunoglobulin (IVIG). Anakinra, an interleukin-1 receptor antagonist, and dexamethasone were initiated. He improved clinically, did not exhibit any harmful effects and ultimately was discharged from the hospital. This, we believe, is the first reported treatment of HLH with anakinra in a patient with AIDS and acute disseminated histoplasmosis.
Keywords: haematology (incl blood transfusion), hiv / aids, biological agents, rheumatology
Background
Hemophagocytic lymphohistiocytosis (HLH) is a rare, but often fatal, syndrome of hyperactivation of the immune system consistent with a cytokine storm.1 The HLH-2004 criteria for diagnosis require either a molecular diagnosis or five of the eight following criteria: fever, hyperferritinemia, splenomegaly, cytopenias affecting at least two of three lineages, hypertriglyceridemia and/or hypofibrinogenemia, low or absent natural killer (NK)-cell activity, elevated soluble CD25 and confirmatory evidence of hemophagocytosis in bone marrow, spleen or lymph nodes.2 Multiple genetic mutations have been found to cause primary HLH, which is usually diagnosed in children.1 Secondary HLH, which is more common in adults, is a hyperactive process of the immune system in association with infection, immunodeficiency, neoplasm, autoimmunity or other idiopathic triggers.1 It has been previously reported in patients with HIV with and without disseminated infections and is a challenging diagnosis to make.3–5 Additionally, secondary HLH is a known complication of acute disseminated histoplasmosis in patients with and without HIV infection.6 7 In these cases, therapy mainly relies on acute treatment of histoplasmosis and HIV, combined with corticosteroids, immune globulins and chemotherapeutic agents, such as etoposide.1 However, the efficacy of these treatment modalities has not been adequately studied. Immune-modulating therapy with the interleukin (IL)-1 receptor antagonist anakinra has been reported to successfully treat HLH secondary to cytomegalovirus (CMV).8 This and other biologic agents with reported efficacy for HLH have not been used in patients with HIV/AIDS complicated with disseminated histoplasmosis, likely due to concern of further suppression of the immune system in an already immunocompromised patient. We describe a case of HLH in a patient with disseminated histoplasmosis and AIDS, who initially had worsening cytopenia despite antifungal, highly active antiretroviral therapy (HAART) and IVIG therapy, but was successfully treated with anakinra and dexamethasone.
Case presentation
A man aged 49 years originally from Guyana and recently diagnosed with HIV/AIDS presented with fever, encephalopathy, 8.16 kg. weight loss and generalised weakness. He was not on HAART. His encephalopathy manifested as slow cognitive processing and confusion. Otherwise, his review of systems was negative. He had no prior history of medical illness, surgeries, opportunistic infections or acute retroviral syndrome. However, he had not sought physician consultation in many years. Physical exam was significant for a temperature of 103°F, heart rate of 110, slow cognitive processing and answering of questions, hepatosplenomegaly and peripheral oedema in the upper and lower extremities.
Investigations
Initial complete blood count revealed white blood cell count (WBC) of 2900 (reference range 4100–9300 per mm3), haemoglobin of 5.9 g/dL (reference range 11–14.7 g/dL), platelets of 89 000 (reference range 1 30 000–3 50 000 per mm3). Sodium was 131 mEq/L (reference range 135–145 mEq/L), blood urea nitrogen was 30 mg/dL (reference range 7–22 mg/dL), creatinine was 1.9 mg/dL (reference range 0.6–1.2 mg/dL) and aspartate aminotransferase was 113 IU/L (reference range 5–45 IU/L). Ferritin levels were at 18 860 ng/dL (reference range 24–336 ng/dL). CD4 cell count was 7 (reference range 500–1500 per mm3) and HIV viral load was 1 28 000 copies/mL. Hepatitis B core antibody was positive, but surface antigen and antibody were negative.
Infectious workup was significant for blood cultures growing filamentous fungi consistent with Histoplasma capsulatum. Sputum and urine cultures were negative. Cryptococcus, tuberculosis, CMV and cerebral spinal fluid studies were negative for Gram stain, acid-fast bacilli, viruses, fungi and mycobacteria. Chest X-ray, CT scan of the head and MRI of the brain were unrevealing. Abdominal ultrasound was significant for hepatosplenomegaly.
Differential diagnosis
Differential diagnoses at this time included untreated HIV/AIDS, fulminant opportunistic infection, parvovirus B19, disseminated mycobacterium avium intercellulare infection, lymphoma or other neoplastic process or granulomatous infiltration of the bone marrow.
Treatment
The patient developed spontaneous epistaxis and bleeding haemorrhoids complicating the anaemia and thrombocytopenia, for which he required 6 units of red blood cells, 10 units of platelets and 7 units of fresh frozen plasma transfusions. Anaemia and thrombocytopenia became refractory to transfusions secondary to the development of human leukocyte antigen (HLA) antibodies. IVIG was given with only minor improvement in blood cell counts.
Initial treatment began with liposomal amphotericin B (350 mg intravenous daily, 5 mg/kg/day) and HAART (abacavir, emtricitabine and raltegravir), as well azithromycin and atovaquone for prophylaxis. After 15 days of therapy, response was minimal, and pancytopenia did not improve (WBC 0.8 per mm3, haemoglobin 6.9 g/dL, platelets 17 000/µL).
Due to pancytopenia and elevated ferritin, a bone marrow biopsy was performed. The results were significant for H. capsulatum (figure 1) and hemophagocytosis (figure 2). Etoposide was considered. However, due to the medication’s propensity to cause pancytopenia, the decision was made to not administer it. The main reasoning was that if further myelosuppression occurred, it would be difficult to then discriminate the cause, and further myelosuppression would unlikely be tolerated by this patient. Alternative therapies without the side effect of pancytopenia were considered. In particular, anakinra, an IL-1 receptor antagonist, was considered due to its prior known efficacy in treatment of HLH, and its safety in sepsis.9 10 The patient was started on anakinra 100 mg subcutaneously daily and dexamethasone 10 mg intravenously daily.
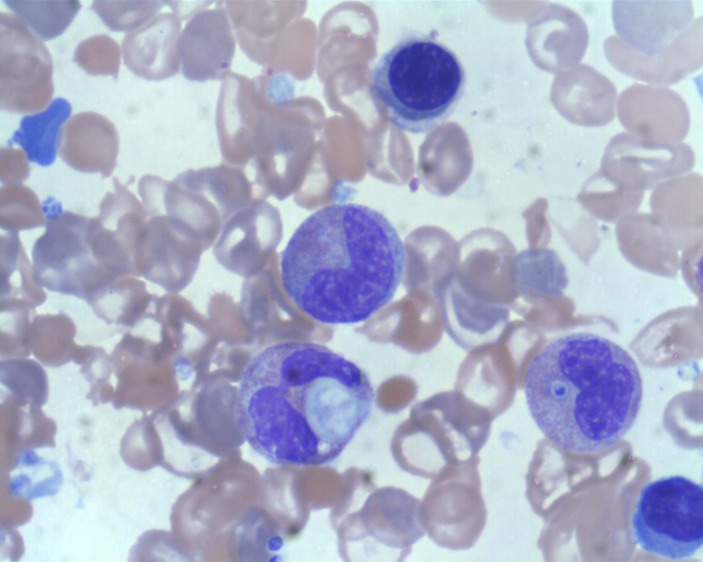
Figure 1.
Intracellular inclusion of histoplasmosis seen on bone marrow aspirate. 100x magnification.
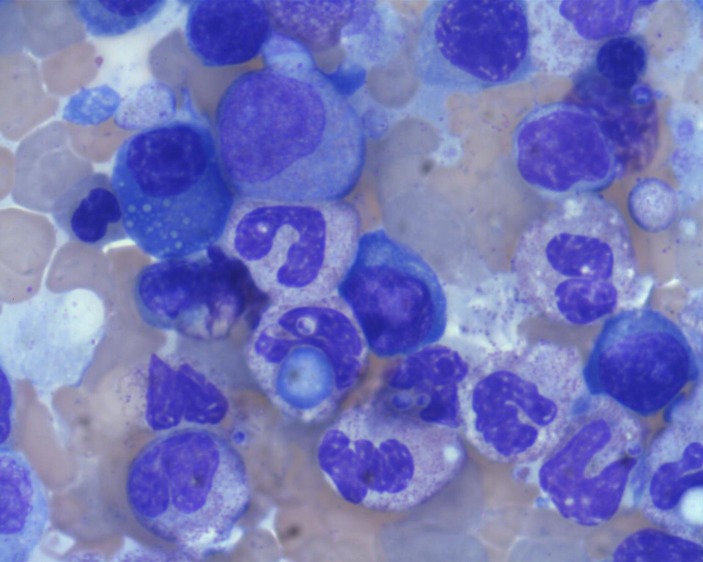
Figure 2.
Hemophagocytosis noted on bone marrow aspirate. 100x magnification.
Outcome and follow-up
Within days, defervescence was achieved concurring with improved cognitive status. Blood counts plateaued and improved. The ferritin level dropped to 1391 mg/dL by the end of the third week. He did not demonstrate any adverse side effects during the treatment. Four weeks after discharge, anakinra and dexamethasone were discontinued, and amphotericin was transitioned to oral itraconazole.
Discussion
To the best of our knowledge, we describe for the first time the use of anakinra, an IL-1 receptor antagonist, along with dexamethasone for treatment of secondary HLH in a patient with disseminated histoplasmosis and HIV/AIDS, who was refractory to other treatments. The patient went into full remission without any adverse effects. As far as we are aware, anakinra has not been used in a patient with HIV/AIDS and disseminated histoplasmosis.
Anakinra is a recombinant competitive antagonist of IL-1 receptor. It has been safely used to treat a variety of autoimmune and/or rheumatological disorders including rheumatoid arthritis, systemic autoinflammatory diseases, Schnitzler’s syndrome, Behçet’s disease, adult-onset Still’s disease, systemic juvenile idiopathic arthritis, gout and diabetes mellitus.11 Serious infection is the dreaded complication of the medication. However, reports of such cases remain low. A meta-analysis of 2771 patients who received anakinra for rheumatoid arthritis found serious infections occurred in only 1.4%, with the majority having multiple medical comorbidities.12 Furthermore, a recent prospective trial using anakinra in patients with sepsis associated with macrophage activating syndrome showed it to be well tolerated and to reduce mortality.13 To the best of our knowledge, there have been no studies or trials of anakinra use in patients with HIV/AIDS. The role of IL-1 is an active area in HIV research, especially in the realm of HIV-related neurological diseases.14 Additionally, glucocorticoids also are known to suppress IL-1.15 Thus, combined therapy with anakinra and dexamethasone likely has a synergistic effect on overall suppression. However, the HIV/AIDS population is particularly prone to opportunistic infections and the use of immunosuppressing agents warrants caution.
The role of IL-1, its ligands and receptors is not fully defined in HLH. Secondary HLH causes a significant inflammatory response with dysregulation of the cytotoxic capabilities of NK cells and CD8+ T cells as well as uncontrolled activation of macrophages.1 IL-1 is thought to be central to this response.8 Other cytokines, including IL-6, IL-18, tumour necrosis factor-α and interferon-γ, likely play a role in an inflammatory cascade.16 Further research is necessary to fully understand the cytokine pathways and elicit new targets for treatment.
Learning points.
Anakinra, an interleukin (IL)-1 receptor antagonist, and dexamethasone effectively treated secondary hemophagocytic lymphohistiocytosis (HLH) in a patient with HIV/AIDS and disseminated histoplasmosis that was refractory to concurrent antifungal, highly active antiretroviral therapy and IVIG.
IL-1 appears to play an important role in the inflammatory response in patients with HLH and HIV/AIDS.
Anakinra was associated with full remission without any adverse effects.
Footnotes
Contributors: When this patient was admitted to the hospital, there were four people involved in the patient’s case. AJO was the medical resident who was taking care of the patient on the floor. BDB was the rheumatology fellow involved in the patient care while the patient was admitted to the hospital. RAP was the rheumatology attending overseeing BDB and the patient when he was admitted to the hospital. CM was the HIV-infectious disease specialist involved in the care of the patient in both the inpatient and outpatient setting. All the authors were involved in writing this case report. All the authors were involved in writing the summary, background, the case presentation, investigations, differential diagnosis, treatment, outcome and discussion/learning point. All the authors approved the final version of the draft to be submitted. All the authors are responsible for the overall content as guarantors. All the authors do not have any financial disclosures.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hayden A, Park S, Giustini D, et al. . Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: a systematic scoping review. Blood Rev 2016;30:411–20. 10.1016/j.blre.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 2.Henter JI, Horne A, Aricó M, et al. . HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. 10.1002/pbc.21039 [DOI] [PubMed] [Google Scholar]
- 3.Azevedo L, Gerivaz R, Simões J, et al. . The challenging diagnosis of haemophagocytic lymphohistiocytosis in an HIV-infected patient. BMJ Case Rep 2015. bcr2015211817 10.1136/bcr-2015-211817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Joncour A, Bidegain F, Ziol M, et al. . Hemophagocytic lymphohistiocytosis associated With bartonella henselae infection in an HIV-infected patient. Clin Infect Dis 2016;62:804–6. 10.1093/cid/civ999 [DOI] [PubMed] [Google Scholar]
- 5.Akenroye AT, Madan N, Mohammadi F, et al. . Hemophagocytic lymphohistiocytosis mimics many common conditions: case series and review of literature. Eur Ann Allergy Clin Immunol 2017;49:31–41. [PubMed] [Google Scholar]
- 6.Subedee A, Van Sickels N. Hemophagocytic syndrome in the setting of AIDS and disseminated histoplasmosis: case report and a review of literature. J Int Assoc Provid AIDS Care 2015;14:391–7. 10.1177/2325957415570740 [DOI] [PubMed] [Google Scholar]
- 7.Townsend JL, Shanbhag S, Hancock J, et al. . Histoplasmosis-induced hemophagocytic syndrome: a case series and review of the literature. Open Forum Infect Dis 2015;2:ofv055 10.1093/ofid/ofv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Divithotawela C, Garrett P, Westall G, et al. . Successful treatment of cytomegalovirus associated hemophagocytic lymphohistiocytosis with the interleukin 1 inhibitor - anakinra. Respirol Case Rep 2016;4:4–6. 10.1002/rcr2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher CJ, Dhainaut JF, Opal SM, et al. . Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase iii rhil-1ra sepsis syndrome study group. JAMA 1994;271:1836–43. [PubMed] [Google Scholar]
- 10.Wohlfarth P, Agis H, Gualdoni GA, et al. . Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. J Intensive Care Med 2017:088506661771138 10.1177/0885066617711386 [DOI] [PubMed] [Google Scholar]
- 11.Lopalco G, Rigante D, Giannini M, et al. . Safety profile of anakinra in the management of rheumatologic, metabolic and autoinflammatory disorders. Clin Exp Rheumatol 2016;34:531–8. [PubMed] [Google Scholar]
- 12.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis 2009;68:25–32. 10.1136/ard.2007.083188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakoory B, Carcillo JA, Chatham WW, et al. . Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase iii trial. Crit Care Med 2016;44:275–81. 10.1097/CCM.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka M, Bradley WG, Shapshak P, et al. . Role of immune activation and cytokine expression in HIV-1-associated neurologic diseases. Adv Neuroimmunol 1995;5:335–58. 10.1016/0960-5428(95)00012-Q [DOI] [PubMed] [Google Scholar]
- 15.Knudsen PJ, Dinarello CA, Strom TB. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol 1987;139:4129–34. [PubMed] [Google Scholar]
- 16.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med 2015;66:145–59. 10.1146/annurev-med-061813-012806 [DOI] [PMC free article] [PubMed] [Google Scholar]