Abstract
A 57-year-old woman presented with swelling and thickening of the skin of the lower extremities. Three months prior to presentation, patient had MRI with gadolinium as part of an evaluation for suspected pancreatic malignancy. Creatinine levels at the time of gadolinium exposure were 0.9–1.2 mg/dL, with a corresponding estimated glomerular filtration rate of 64 mL/min/1.73m2 by modification of diet in renal disease equation. Twenty-four-hour urine creatinine clearance was performed as an outpatient following development of symptoms. This revealed a creatinine clearance of 23 mL/min, suggestive of advanced chronic kidney disease despite an estimated glomerular filtration rate of 64 mL/min/1.73m2. Skin biopsy was positive for sclerosing dermopathy. These findings, in addition to the temporal association with gadolinium exposure, led to the diagnosis of nephrogenic systemic fibrosis.
Keywords: dermatology, contraindications and precautions, renal system
Background
Nephrogenic systemic fibrosis (NSF) is a rare systemic disease caused by gadolinium exposure in patients with reduced estimated glomerular filtration rate (eGFR) resulting in fibrosis, primarily of the skin, often with involvement of other organs such as the lungs, oesophagus, heart and skeletal muscles.1 There is currently no standard treatment available to cure this debilitating and potentially fatal disease.2–5 Hence, prevention is of the utmost importance.1 According to the current guidelines, gadolinium is contraindicated in patients with an eGFR below 30 mL/min/1.73 m2 and cautious use is recommended in patients with moderately reduced kidney function of 30–60 mL/min/1.73 m2.6 In clinical practice, eGFR is calculated by equations that incorporate measured serum creatinine levels.4 6 In select patients with poor muscle mass, cachexia, liver disease and amputation with low body creatinine, eGFR is overestimated.4 7 We present such a rare case of NSF in a 57-year-old thin-built woman who received gadolinium based on initial inaccurate eGFR calculation.
Case presentation
A 57-year-old African-American woman with history of hypertension, chronic pancreatitis and malnutrition presented to the office on 6 January 2015 with worsening lower extremity swelling, severe burning pain, and leathery, shiny skin with loss of hair over 2–3 weeks. She had chronic lower extremity oedema due to hypoalbuminaemia from malnutrition.
On examination, her weight was 31.752 kg (70 lb) and body mass index (BMI) was 13.23 kg/m2. She appeared frail, thin built with shiny, leather-like tender skin isolated to the distal lower extremities (figure 1). Ankle motion was severely restricted resulting in dramatic gait impairment. Cardiovascular, pulmonary and gastrointestinal examinations were unremarkable.
Figure 1.
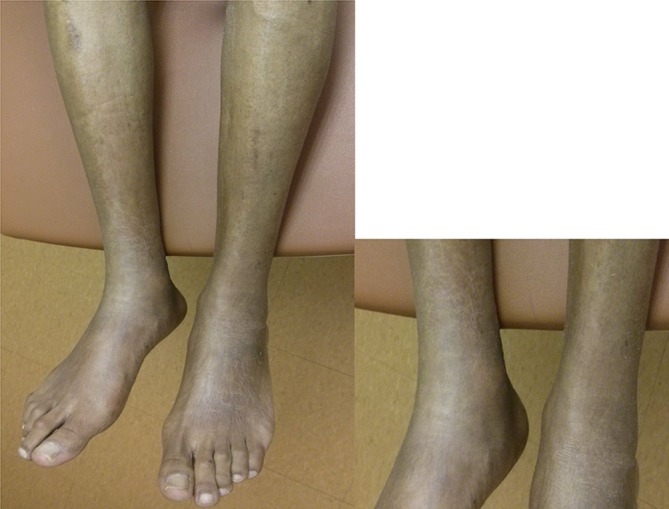
Photograph of distal lower extremities revealing bilateral, hyperpigmented lesions with marked induration and linear banding. Alopecia and mild sclerodactyly are present. Coarse, fibrotic changes obscure distinct borders of medial malleolus.
Prior medical history was most notable for chronic diarrhoea, nausea, vomiting, anorexia and unintentional weight loss of 120 lb in 3 years. For this, she required extensive evaluation and hospitalisations. Most recent hospitalisation was 3 months prior to the presenting illness in November 2014, for persistent diarrhoea and weight loss. During this admission on 5 November 2014, she had MRI of the abdomen with gadolinium-based contrast—gadobenate dimeglumine (MultiHance).
There was no history of gadolinium exposure. Creatinine levels during hospitalisation (when receiving gadolinium contrast) were 0.9–1.2 (0.60–1.4 mg/dL). The patient’s serum creatinine of 1.07 mg/dL at the time of gadolinium exposure corresponded with a calculated eGFR of 64 mL/min/1.73m2 (table 1). The eGFR was calculated using the modification of diet in renal disease (MDRD) equation which included patient’s race as a part of the calculation (equation 1).
Table 1.
Laboratory values
| Reference | ||
| Labs at the time of gadolinium exposure | ||
| Creatinine | 1.07 mg/dL | 0.6–1.4 mg/dL |
| eGFR | 64 mL/min/1.73 m² | >59 mL/min/1.73 m2 |
| Bicarbonate (HCO3) | 14 mmol/L | 21–31 mmol/L |
| Anion gap | 10 | 5–17 |
| Phosphorus | 1.6 mg/dL | 2.3–4.3 mg/dL |
| Sodium | 131 mmol/L | 135–145 mmol/L |
| Potassium | 3.1 mmol/L | 3.5–5.2 mmol/L |
| Chloride | 107 | 95–107 mmol/L |
| Labs during office visit | ||
| eGFR | 52 mL/min/1.73 m² | >59 mL/min/1.73 m2 |
| Creatinine | 1.27 mg/dL | 0.6–1.6 mg/dL |
| White blood cell count | 10.7 bil/L | 3.3–10.7 x109/L |
| Absolute neutrophil count | 7.0 bil/L | 1.6–7.2 x109/L |
| Lymphocytes | 2.4 bil/L | 1.1–4.0 x109/L |
| Eosinophils | 0.3 bil/L | 0.0–0.5 x109/L |
| Monocytes | 1.0 bil/L | 0.0–0.8 x109/L |
| Haemoglobin | 10.1 g/dL | 12.1–15 g/dL |
| Platelets | 191 bil/L | 150–400 bil/L |
| Albumin | 2.6 g/dL | 3.5–5.1 g/dL |
| Prealbumin | <5.0 mg/dL | 18–45 mg/dL |
| Erythrocyte sedimentation rate (ESR) | 15 mm/hour | |
| Antinuclear antibodies (ANA) | Negative | Negative |
| Anti-dsDNA | Negative | Negative |
| Smith antibody | 7.0 | <100AU/mL |
| Ribonucleoprotein (RNP) antibody | 10.0 | <100 AU/mL |
| Scleroderma (SCL-70) antibody IgG | 17.0 | <100 AU/mL |
| Thyroid-stimulating hormone (TSH) | 1.73 | 0.5–5.20 mcIU/mL |
eGFR, estimated glomerular filtration rate.
Equation 1: MDRD equation
GFR (mL/min/1.73 m2)=175 × (Serum creatinine)−1.154 × (Age)−0.203 × (0.742 if female) × (1.212 if African-American)
Additional workup at that time included normal hormonal evaluation (gastrin, motilin, pancreatic polypeptide, somatostatin and vasoactive intestinal peptide); oesophagoduodenoscopy which revealed chronic gastritis; and CT abdomen that showed a soft tissue nodule within the mesentery, below the level of the pancreas, measuring 1.1×1.3 cm, and pancreatic calcifications suggestive of chronic pancreatitis.
Investigations
Subsequently, when she presented to the office, a 24-hour urine creatinine clearance (CC) done on 16 February 2015 was only 23 mL/min (70–140 mL/min), suggestive of advanced chronic kidney disease (CKD). Serum creatinine done at the same time was 1.27 mg/dL with corresponding calculated eGFR by MDRD equation for African-American of 52 mL/min/1.73 m2. Twenty-four-hour urine CC was calculated as illustrated in equation 2.
Equation 2: 24-hour urine CC formula
Creatinine clearance (CrCl)=38.6 mg/dL (urine creatinine) × 1300 mL (volume of urine)÷1.27 mg/dL (serum creatinine) × 29 hours (1740 min){collection time}=22.7 mL/min
Comprehensive laboratory testing was normal (table 1). Workup for autoimmune diseases was negative (refer to table 1 for lab work at the time of office visit). Deep skin biopsy obtained from left dorsal foot skin revealed focal positivity for dermal spindle cell proliferation in the dermis with haphazardly arranged collagen bundles in the deep dermis suggestive of fibrosis of dermis (figure 2A,B) with factor XIII expression (figure 3A) and CD34 expression suggestive of spindle cell proliferation (figure 3B), consistent with nephrogenic sclerosing dermopathy.
Figure 2.
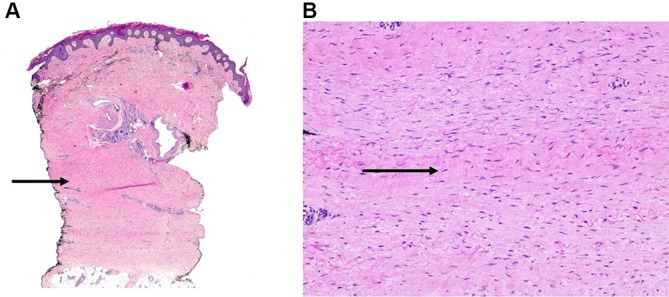
(A) Low power view demonstrating fibrosis of the dermis with haphazardly arranged collagen bundles in the deep dermis. (B) High power view showing increased number of spindle-shaped fibroblasts with increased surrounding mucin deposition.
Figure 3.
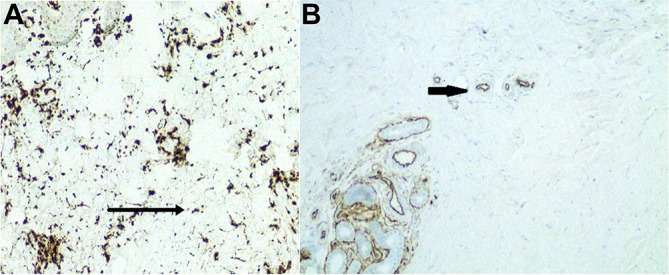
(A) Focal positivity for factor XIII and CD34 in the spindle cell proliferation present in the deep dermis. (B) Focal positivity for CD34 in the spindle cell proliferation present in the deep dermis.
Differential diagnosis
Based on the clinical presentation following gadolinium exposure in this patient with stage IV CKD and skin biopsy results showing fibrosing dermopathy, she was diagnosed as having NSF.
Differential diagnoses included systemic sclerosis (scleroderma), eosinophilic fasciitis, vasculitis and myxoedema. However, normal erythrocyte sedimentation rate, eosinophils, thyroid-stimulating hormone, negative antinuclear antibodies and SCL-70 antibodies made other differentials less likely.
Treatment
In addition to extensive physical therapy, the patient was referred to a dermatologist at a centre where extracorporeal photopheresis therapy is available.
Outcome and follow-up
The patient had minimal improvement in the skin findings, without further progression. There is no other known visceral organ involvement to date. She has residual skin tightness and difficulty with ambulation. She is currently undergoing physical rehabilitation to prevent contractures.
Discussion
NSF is a debilitating and sometimes fatal condition without a definitive cure. Symptoms and signs may develop and progress rapidly, with some affected patients developing contractures and joint immobility.1 It is estimated that patients with end-stage renal disease or severe CKD (stage IV, eGFR 15–29 mL/min/1.73 m2) have a 1%–7% chance of developing NSF after one or more exposures to gadolinium.1 The patient in our case had an eGFR of 64 mL/min/1.73 m2 at the time of gadolinium exposure. However, the absolute CC measured later with 24-hour urine creatinine was severely reduced at 23 mL/min, corresponding with CKD stage IV, placing her at an increased risk for development of NSF.8
Gadolinium interferes with intracellular enzymes and cell membranes by the process of transmetallation which is a phenomenon in which gadolinium replaces endogenous metals such as zinc and copper.6 This occurs more easily when gadolinium-based contrast remains in the body for a longer period of time as in patients with advanced renal disease.6 This is more common with less stable gadolinium-based contrast agents like gadodiamide, gadopentetate dimeglumine and/or gadoversetamide.6 9 The patientreceived gadobenate dimeglumine (MultiHance) which has been studied to have less risk for NSF.10 There were no prior gadolinium exposures confounding this clinical presentation.
The typical clinical presentation involves gradual swelling of distal extremities followed by severe skin induration associated with pain and contractures, which was the clinical presentation and course for our patient.5 6 Symptoms most commonly manifest within weeks to months after receiving gadolinium.6 However, this period may be longer as suggested by one case report of NSF developing 10 years after gadolinium exposure.11 Our patient presented 3 months after gadolinium exposure. The induration can extend to involve the thighs, forearms, lower abdomen and organs such as the heart, lungs, liver and muscles making this a systemic process.6 Our patient did not exhibit visceral organ involvement.
High doses of erythropoietin, metabolic acidosis, chronic inflammation, hypercoagulability, recent surgery, renal transplant failure and increased phosphate levels have been associated as cofactors contributing to the development of NSF.6 Our patient had a non-anion gap metabolic acidosis from bicarbonate loss due to diarrhoea. This may have contributed to disease development.
The definitive diagnosis is made after deep skin biopsy.6 The typical histological findings are infiltration with dermal spindle cells, which are characterised by the surface markers CD45RO, CD34 and procollagen I which are immunologic markers for fibrocytes; the cells active in wound healing.6 In our case, the physical exam findings, in relation to the timing of gadolinium exposure and negative workup for connective tissue disorders, suggested a diagnosis of NSF. This was supported by characteristic biopsy findings of fibroblast proliferation with dendritic cells positive for CD34 and factor XIII expression on immunohistochemistry.
The use of gadolinium-based contrast agents is not recommended with eGFR<30 mL/min/m2 and is avoided in clinical practice in these patients with renal impairment.6 8 Most eGFR equations are based on measured creatinine levels, of which the MDRD study equation is most commonly used.6 7 12 Measurement of serum creatinine, however, is not satisfactory because more than 25% of older patients have normal serum creatinine levels but reduced eGFR.6 A single determination of the eGFR also does not exclude acute renal insufficiency.6 Low serum creatinine levels overestimate eGFR in patients with poor muscle mass, that is, malnourished, advanced age, amputees and chronic illness.7
The most interesting part of our case is that despite an eGFR deemed low risk, our patient developed an iatrogenic complication conferring significant morbidity because of the unique patient circumstances. Our patient was very thin built with poor muscle mass (BMI of 13.23 kg/m2), leading to low creatinine levels that falsely overestimated the GFR. Studies have shown inaccuracies with MDRD and CKD epidemiology collaboration (CKD-EPI) for BMI<20 kg/m2.7 13 14 Hence, the true GFR should be measured in such patients when contrast agents need to be given and medication dosages need to be calculated.7 15 Other measures of glomerular filtration, such as 24-hour CC and cystatin C-based eGFR, if available, have been reported to be more accurate in patients with poor muscle mass.4 7 8 The equations using combined creatinine and cystatin C are more accurate than creatinine alone and should be used when the creatinine-based equations are less accurate.8
There is no proven treatment to date for NSF. Treatment with extracorporeal photopheresis, sodium thiosulfate, pentoxifylline, sirolimus, rapamycin and plasmapheresis has been reported in the literature but has failed to show major improvement.2–5 16 17 Physical therapy to prevent contractures is crucial.18 The use of phototherapy using ultraviolet A has been discussed in the literature.19 Our patient underwent extracorporeal photopheresis as treatment, which has also been reported in a case report by Läuchli et al, and is the treatment option with most supporting data.20 The progression of NSF is decreased with improvement in renal function. Renal transplantation has been shown to have some benefit in halting progression.19
Prevention of NSF by avoidance of gadolinium cannot be overemphasised.19 This requires cautious use of the gadolinium-based contrast agents in patients with marginal renal function as well as use of more accurate calculations to estimate the GFR. Alternate imaging modalities such as CT should be used when possible. When MRI is unavoidable, risks should be explained to the patient and use of a decreased contrast load is recommended. Macrocyclic chelated gadolinium formulations are the preferred agent in reducing the risk of NSF.8 Once NSF develops, halting progression with stabilizing renal function and preventing future exposure to gadolinium is paramount.18
Learning points.
Gadolinium is contraindicated with an estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73m2.
Low creatinine levels in patients with poor muscle mass can lead to overestimation of GFR values. This may lead to inadvertent exposure to gadolinium-based contrast agents in patients with substantially worse renal function, increasing their risk of developing nephrogenic systemic fibrosis (NSF).
NSF is preventable with cautious use of gadolinium with accurate GFR estimation in patients with poor muscle mass. Spot creatinine clearance and creatinine-based calculations can be misleading in such patients. Twenty-four-hour urine creatinine clearance and cystatin C-based measurements are more accurate estimations of GFR in such patients; their use should be considered when possible.
Footnotes
Contributors: SL, JG, AS and AH have substantially contributed to the intellectual content and design of the manuscript including acquisition, interpretation of the data, drafting, and revision of the manuscript. All coauthors agree to be accountable for all aspects of the manuscript and questions related to accuracy and integrity of any part of the manuscript. We assure that all the authors included in the paper fulfill the criteria of authorship. We also assure that there is no one else who fulfills the criteria that have been excluded as an author. SL: designing of the manuscript including the content, drafting, data acquisition, analysis and revision. JG: designing, data acquisition, and revision. AS: intellectual content, revision, data acquisition and interpretation. AH: designing, planning, drafting, intellectual content and revision.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.American College of Radiology Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. 10th ed Reston, VA: American College of Radiology, 2015. [Google Scholar]
- 2.Swaminathan S, Arbiser JL, Hiatt KM, et al. Rapid improvement of nephrogenic systemic fibrosis with rapamycin therapy: possible role of phospho-70-ribosomal-S6 kinase. J Am Acad Dermatol 2010;62:343–5. 10.1016/j.jaad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 3.Ross C, De Rosa N, Marshman G, et al. Nephrogenic systemic fibrosis in a gadolinium-naïve patient: successful treatment with oral sirolimus. Australas J Dermatol 2015;56:e59–e62. 10.1111/ajd.12176 [DOI] [PubMed] [Google Scholar]
- 4.Schieren G, Wirtz N, Altmeyer P, et al. Nephrogenic systemic fibrosis-a rapidly progressive disabling disease with limited therapeutic options. J Am Acad Dermatol 2009;61:868–74. 10.1016/j.jaad.2009.03.040 [DOI] [PubMed] [Google Scholar]
- 5.Grobner T. Gadolinium-a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21:1104–8. 10.1093/ndt/gfk062 [DOI] [PubMed] [Google Scholar]
- 6.Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): a late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging 2007;7:130–7. 10.1102/1470-7330.2007.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyman U, Björk J, Bäck SE, et al. Estimating GFR prior to contrast medium examinations-what the radiologist needs to know!. Eur Radiol 2016;26:425–35. 10.1007/s00330-015-3842-9 [DOI] [PubMed] [Google Scholar]
- 8.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease: National Guideline Clearinghouse (NGC), 2013. [DOI] [PubMed] [Google Scholar]
- 9.Morcos SK, Thomsen HS. Nephrogenic systemic fibrosis: more questions and some answers. Nephron Clin Pract 2008;110:c24–c32. 10.1159/000151228 [DOI] [PubMed] [Google Scholar]
- 10.Martin DR, Krishnamoorthy SK, Kalb B, et al. Decreased incidence of NSF in patients on dialysis after changing gadolinium contrast-enhanced MRI protocols. J Magn Reson Imaging 2010;31:440–6. 10.1002/jmri.22024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson KN, Gagnon AL, Darling MD, et al. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol 2015;151:1117–20. 10.1001/jamadermatol.2015.0976 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyman U, Grubb A, Larsson A, et al. The revised Lund-Malmö GFR estimating equation outperforms MDRD and CKD-EPI across GFR, age and BMI intervals in a large Swedish population. Clin Chem Lab Med 2014;52:815-24 10.1515/cclm-2013-0741 [DOI] [PubMed] [Google Scholar]
- 14.Björk J, Jones I, Nyman U, et al. Validation of the Lund-Malmö, Chronic Kidney Disease Epidemiology (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) equations to estimate glomerular filtration rate in a large Swedish clinical population. Scand J Urol Nephrol 2012;46:212–22. 10.3109/00365599.2011.644859 [DOI] [PubMed] [Google Scholar]
- 15.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5:303–11. [PubMed] [Google Scholar]
- 16.Linfert DR, Schell JO, Fine DM. Treatment of nephrogenic systemic fibrosis: limited options but hope for the future. Semin Dial 2008;21:155–9. 10.1111/j.1525-139X.2007.00407.x [DOI] [PubMed] [Google Scholar]
- 17.Poisson JL, Low A, Park YA. The treatment of nephrogenic systemic fibrosis with therapeutic plasma exchange. J Clin Apher 2013;28:317–20. 10.1002/jca.21253 [DOI] [PubMed] [Google Scholar]
- 18.Knopp EA, Cowper SE. NSF: What we know and what we need to know: Nephrogenic Systemic Fibrosis: Early Recognition and Treatment. Semin Dial 2008;21:123–8. 10.1111/j.1525-139X.2007.00399.x [DOI] [PubMed] [Google Scholar]
- 19.Tran KT, Prather HB, Cockerell CJ, et al. UV-A1 therapy for nephrogenic systemic fibrosis. Arch Dermatol 2009;145:1170-4 10.1001/archdermatol.2009.245 [DOI] [PubMed] [Google Scholar]
- 20.Läuchli S, Zortea-Caflisch C, Nestle FO, et al. Nephrogenic fibrosing dermopathy treated with extracorporeal photopheresis. Dermatology 2004;208:278–80. 10.1159/000077321 [DOI] [PubMed] [Google Scholar]


