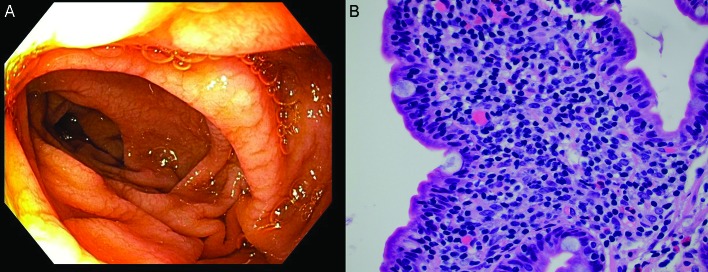
Abstract
Common variable immunodeficiency (CVID), characterised by disordered B cell function, is one of the most common primary immunodeficiency disorders. Patients with CVID are at lifelong risk of recurrent infections, particularly of the respiratory and gastrointestinal tracts. Paradoxically, given their immunocompromised state, patients with CVID are also at significantly increased risk of autoimmune disorders, which are seen in almost 25% of cases. The authors report a 24-year-old female patient with CVID, manifested as severe hypogammaglobulinaemia with recurrent sinopulmonary infections and enterocolitis, who presented with transaminitis, chronic diarrhoea and haematemesis. No infectious aetiologies were identified. She was diagnosed with coeliac disease after a small bowel biopsy and positive response to gluten-free diet. Haematemesis was attributed to portal hypertension due to liver cirrhosis, which was confirmed via liver biopsy. Coeliac disease can be a cause of diarrhoea in patients with immunodeficiency disorders and is often underdiagnosed. It can also be the underlying source of liver disease and is an often under-recognised cause of cirrhosis. The case presented emphasises the paradoxical and challenging relationship that patients with CVID face between immunodeficiency and autoimmune disorders, and also highlights that coeliac disease is an under-recognised cause of liver disease.
Keywords: gi bleeding, infection(gastroenterology), cirrhosis, portal hypertension, coeliac disease
Background
Patients with common variable immunodeficiency (CVID) have a complicated natural history and require lifelong treatment and disease surveillance. While almost 95% of patients with CVID will suffer recurrent infectious complications, paradoxically about 25% will additionally have autoimmune disorders.1 The underlying pathogenesis of autoimmune disease in patients with CVID is not completely understood. Additionally, due to the profound hypogammaglobulinaemia, diagnosis of autoimmune disorders such as coeliac disease is more challenging in these patients as the autoantibodies typically assessed for, such as tissue transglutaminase, will often be absent even in the presence of disease, giving a false-negative report. Although gastrointestinal autoimmune disorders in patients with CVID have been reported, coeliac disease presenting as a likely cause of liver cirrhosis for a young patient is very rare. Additionally, non-alcoholic liver cirrhosis in a 24-year-old patient is quite uncommon. We present a unique case here which has all the above elements.
Case presentation
A 24-year-old Caucasian woman presented to the hospital with haematemesis. Her medical history was notable for CVID with severe hypogammaglobulinaemia for which she received monthly intravenous immunoglobulin (IVIG), chronic transaminitis, thrombocytopaenia and diarrhoea. She had recently been taking high-dose ibuprofen for several days for a headache. Her social history was significant for history of daily use of marijuana. She had a history of homelessness but was currently working as a student and part-time teacher and in a stable living situation. She had a history of smoking cigarettes one pack per week for 2 years but quit smoking 1 year ago. She had a 3-year-old son. Her family history was significant for asthma in her sister, as well as stroke and heart attack in other family members. There was no family history of immunodeficiency diseases or liver diseases. Prior work-up for her chronic diarrhoea had included antitissue transglutaminase antibodies and broad analysis for stool microbial pathogens, all of which were negative except for prior cytomegalovirus (CMV) enteritis for which she was on suppressive valganciclovir. For her transaminitis, she had been checked for hepatitis A, B and C viruses, which were negative, and additionally antismooth muscle antibody to assess for autoimmune hepatitis, which had also been negative. On initial presentation to the hospital, vital signs were notable for temperature of 98.8°F (36.9°C), heart rate of 90 beats per minute, blood pressure of 92/52 mm Hg and respiratory rate of 18 breaths per minute. Physical examination was notable for epigastric tenderness.
Investigations
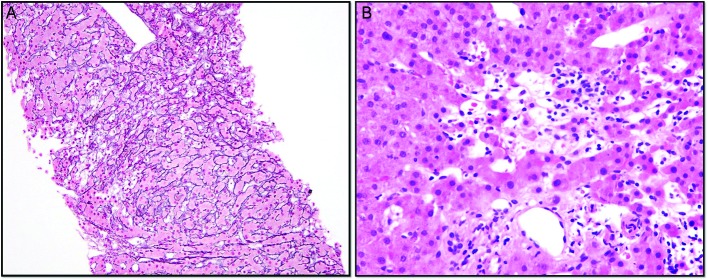
Laboratory tests were notable for alanine transaminase 73 IU/L, aspartate aminotransferase 49 IU/L, alkaline phosphatase 216 IU/L, total bilirubin 0.7 mg/dL, international normalized ratio (INR) 1.5, haemoglobin 10.4 g/dL (from a baseline of 13 g/dL), haematocrit 25.5 (around 10-point drop in haematocrit from 36.7) and platelet count of 85 000 x109/L. Evaluation of T cell subsets showed CD3 as 665 (90%), CD4 291 (40%), CD8 378 (51%), CD4/CD8 0.77, CD19 0.63% and CD20 0.26%. IgG trough values were 300–357 units with monthly IVIG (Gammagard 30 g). Urgent oesophagogastroduodenoscopy showed non-bleeding grade 3 oesophageal varices with moderate portal gastropathy and nodularity of the duodenum (figure 1). Her haematemesis was felt to be due to the gastropathy, related to recent ibuprofen use, although the large varices were very concerning (figure 1). Duodenal endoscopy and biopsies were obtained and showed small intestinal mucosa with diffuse subtotal villous shortening and markedly increased intraepithelial lymphocytes, consistent with coeliac disease in the appropriate clinical and serological settings (figure 4) (figure 2A,B). Given the concern for advanced liver disease in this young patient with oesophageal varices, transaminitis, coagulopathy and thrombocytopaenia, a liver biopsy was obtained, which showed bridging fibrosis consistent with cirrhosis (figure 3A,B).
Figure 1.
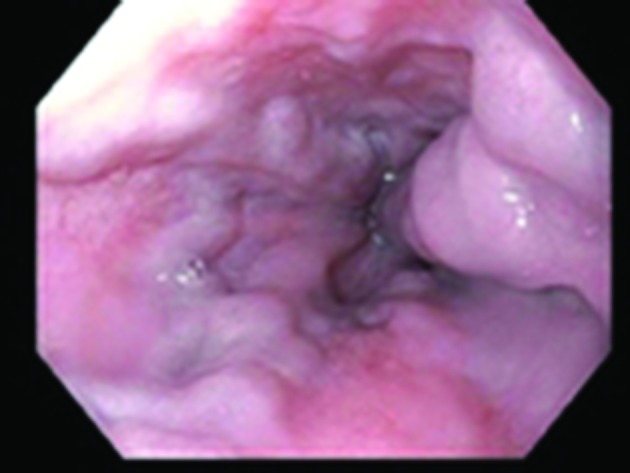
Oesophagogastroduodenoscopy showing non-bleeding grade 3 varices.
Figure 4.
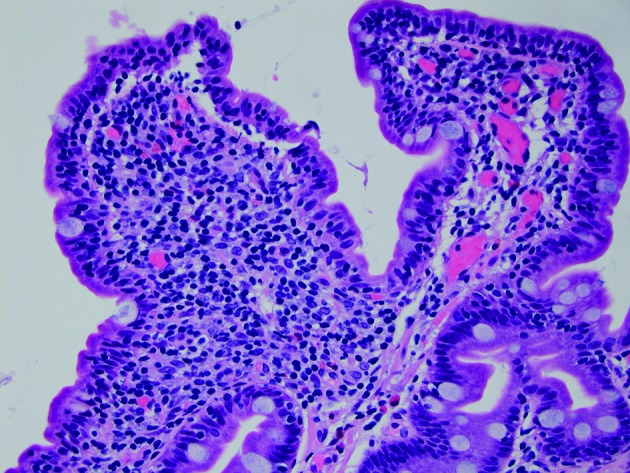
Sections demonstrate small intestinal mucosa with shortened villi with increased intraepithelial lymphocytes, a histological picture most commonly seen in coeliac disease but also seen with common variable immunodeficiency (H&E stain, 400× magnification). (Picture contributed by Dr Robert M Najarian, MD, Consultant in Gastrointestinal, Hepatobiliary and Pancreatic Pathology Central Pathologist, Pancreatic Disease Registry and Biorepository, Beth Israel Deaconess Medical Center, Department of Pathology, Boston, Massachusetts, USA.)
Figure 2.
(A) Macroscopic duodenal view. (B) A high magnification view of the lamina propria of the small intestinal mucosa demonstrating a lack of definite plasma cells, a feature typical of common variable immunodeficiency (H&E stain, 600× magnification) (Picture contributed by Dr Robert M Najarian, MD, Consultant in Gastrointestinal, Hepatobiliary and Pancreatic Pathology Central Pathologist, Pancreatic Disease Registry and Biorepository, Beth Israel Deaconess Medical Center, Department of Pathology, Boston, Massachusetts, USA.)
Figure 3.
(A) Core needle liver biopsy with reticulin and trichrome stains highlighting foci of hepatocyte dropout and collapse with areas of nodularity. Focal mild portal and sinusoidal fibrosis are present. (B) Core needle liver biopsy showing primarily lymphocytic inflammation with few eosinophils, neutrophils and macrophages, with an absence of basal plasmocytes.
Differential diagnosis
The diarrhoea of the patient can be due to inflammatory bowel disease, irritable bowel syndrome, infectious causes such as Clostridium difficile, yersiniosis, tuberculosis, Entamoeba, CMV, herpes simplex virus, Escherichia coli infection, giardia, salmonella, typhoid, cholera, or HIV, Whipple disease, laxatives, small bowel bacterial overgrowth, malabsorption syndrome, autoimmune causes such as coeliac sprue, tropical sprue, hepatobiliary disorders, carcinoid tumour, lymphoma or colon cancer.
Haematemesis can be due to gastric or duodenal ulcers, oesophagogastric varices, erosive oesophagitis, erosive gastritis, erosive duodenitis, portal hypertensive gastropathy, angiodysplasia, mass lesions such as polyps, gastric or oesophageal malignancies, Mallory-Weiss tear, aortoenteric fistula or ectopic varices.
Treatment
The patient was initially treated with intravenous pantoprazole and an octreotide drip for upper gastrointestinal bleed. Upper endoscopy was performed, which elicited grade 3 oesophageal varices and portal gastropathy. A duodenal biopsy was also performed to further investigate the aetiology of diarrhoea. She was treated with ciprofloxacin 400 mg twice daily for prophylaxis of spontaneous bacterial peritonitis. She was stabilised by hospital day 2. She was transfused with 1 unit of platelets before the liver biopsy since she has chronic history of thrombocytopaenia. Liver biopsy was conducted and liver cirrhosis was diagnosed (figure 3A,B). When duodenal biopsy suggested the possibility of coeliac disease (figure 4), she was started on a diagnostic and potentially therapeutic strict gluten-free diet.
Outcome and follow-up
After 7 days of hospitalisation, the patient was feeling better. There were no further episodes of haematemesis and her diarrhoea improved. Her liver enzymes remained elevated. On discharge, the patient was advised to follow a completely gluten-free diet, as well as to avoid taking non steroidal anti inflammatory drugs (NSAIDS) and acetaminophen. At 2-month follow-up, the patient had been well-adherent to a gluten-free diet, and had gained 20 pounds, with improving appetite. Her diarrhoea completely resolved and she now had only one or two formed bowel movements per day, with report of feeling significantly better. She will receive liver ultrasound every 6 months to assess for hepatocellular carcinoma and continues to receive IVIG once monthly.
Discussion
CVID is a heterogeneous primary immune deficiency disorder characterised by impaired B cell differentiation with resultant defective immunoglobulin production, resulting in increased susceptibility to infectious diseases but also paradoxically to autoimmune disorders such as coeliac disease.1 2 The prevalence of CVID is estimated at 1 in 50 000 among Caucasians. Gastrointestinal track involvement is frequent in patients with CVID, estimated to occur in 9%–20% of such cases, and is often clinically challenging with significant morbidity and mortality.3 Many of the gastrointestinal manifestations in CVID have protean manifestations resembling inflammatory bowel disease or coeliac disease. Furthermore, on duodenal biopsy patients with CVID can have villous atrophy mimicking coeliac disease, although avoidance of gluten provides no benefit.4 It can be challenging to differentiate villous atrophy and chronic diarrhoea from CVID versus the same findings from coeliac disease; as patients with CVID are by definition immunoglobulin-deficient, typical testing for coeliac disease such as antibodies to tissue transglutaminase or gliadin is often unhelpful and expected to be negative.5 Response to an empiric gluten-free diet of several months in duration is often needed to distinguish between the two.
In this case, our patient likely had previously undiagnosed coeliac disease, based on her symptoms, duodenal biopsy results and positive response to a gluten free-diet. A gluten-free diet is helpful in approximately 20% of patients with CVID with a coeliac-like syndrome, and the efficacy of gluten avoidance seems slightly higher in subjects with the coeliac-associated genotype Human Leukocyte Antigen DQ2 (HLA DQ2) and Human Leukocyte Antigen DQ8 (HLA DQ8).1 This relatively low response rate highlights the significant histological similarities between CVID-associated villous atrophy and coeliac disease. Some underinvestigated strategies that could be helpful in diagnosing coeliac disease in patients with CVID include gliadin challenge of duodenal explant culture, which has shown expression modification of duodenal mucosal T cell immune response markers and also PY99 and Human Leukocyte Antigen DR (HLADR) phosphotyrosine antibody (PY99) expression markers.6 Another strategy is demonstration of gluten-dependent TG2-specific IgA deposits in the small bowel mucosa.7 However, such strategies are not yet widely available for clinical practice. Our patient was found to have cirrhosis, which was surprising in this 24-year-old woman without a history of alcohol abuse.8 Liver disease, including primary biliary cirrhosis and autoimmune hepatitis, occurs in CVID for unknown reasons, although liver biopsy was not consistent with these diagnoses.5 Coeliac disease is often under-recognised as a cause of cirrhosis. Between 15% and 55% of patients with coeliac disease may have liver damage, ranging from mild hepatic abnormalities to severe liver disease, and among the early signs are transaminitis and thrombocytopaenia.9 The pathogenesis of liver injury is due to two causes, either a cryptogenic injury or concomitant autoimmune hepatitis, which is seen at increased rates in patients with coeliac disease.9
Liver involvement of patients with CVID includes elevated transaminases and alkaline phosphatase, nodular regenerative hyperplasia (NRH) or liver cirrhosis. NRH is defined as hepatocellular nodules of less than 3 mm diameter, not surrounded by marked fibrosis.10 NRH can be clinically silent for many years in patients with CVID, only associated with elevated alkaline phosphatase or can result in significant portal hypertension.11 12 There was no evidence for NRH on biopsy from our patient.
Management of coeliac disease in CVID includes strict gluten-free diet and regular follow-up and monitoring with multidisciplinary team including physician and dietitian. In case of refractory coeliac disease, steroids and immunomodulators have been used, although it is a challenge to differentiate from CVID-associated coeliac-like disease.13 14
Patients with CVID are paradoxically at increased risk for autoimmune disorders, including coeliac disease, and resultant liver injury. Given the vast array of pathology these patients can suffer from, a broad differential diagnosis is necessary when approaching a patient with CVID.
Learning points.
Patients with common variable immunodeficiency (CVID) are at increased risk of infection.
Paradoxically, patients with CVID are also at increased risk of autoimmune disorders, including coeliac disease.
Coeliac disease is often under-recognised as a cause of liver disease and cirrhosis.
Patients with coeliac disease should be monitored for other autoimmune diseases.
Providers must maintain a broad differential when caring for patients with CVID.
Acknowledgments
The authors would like to thank Dr. Daryl Lau and Dr. Sanjiv Chopra for their feedback about the case report and Dr. Robert M. Najarian for providing the histopathological images.
Footnotes
Contributors: ATS was involved in the conception or design of the work, data collection, data analysis and interpretation, drafting the article, critical revision of the article, and final approval of the version to be published. AH was involved in the conception or design of the work, data collection, data analysis and interpretation, critical revision of the article, and final approval of the version to be published.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Uzzan M, Ko HM, Mehandru S, et al. . Gastrointestinal disorders associated with Common Variable Immune Deficiency (CVID) and Chronic Granulomatous Disease (CGD). Curr Gastroenterol Rep 2016;18:17 10.1007/s11894-016-0491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward C, Lucas M, Piris J, et al. . Abnormal liver function in common variable immunodeficiency disorders due to nodular regenerative hyperplasia. Clin Exp Immunol 2008;153:331–7. 10.1111/j.1365-2249.2008.03711.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gathmann B, Mahlaoui N, Gérard L, et al. . Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 2014;134:116–26. 10.1016/j.jaci.2013.12.1077 [DOI] [PubMed] [Google Scholar]
- 4.Khodadad A, Aghamohammadi A, Parvaneh N, et al. . Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci 2007;52:2977–83. 10.1007/s10620-006-9736-6 [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol 2013;11:1050–63. 10.1016/j.cgh.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tortora R, Russo I, De Palma GD, et al. . In vitro gliadin challenge: diagnostic accuracy and utility for the difficult diagnosis of celiac disease. Am J Gastroenterol 2012;107:111–7. 10.1038/ajg.2011.311 [DOI] [PubMed] [Google Scholar]
- 7.Salmi TT, Collin P, Korponay-Szabó IR, et al. . Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut 2006;55:1746–53. 10.1136/gut.2005.071514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novick DM, Enlow RW, Gelb AM, et al. . Hepatic cirrhosis in young adults: association with adolescent onset of alcohol and parenteral heroin abuse. Gut 1985;26:8–13. 10.1136/gut.26.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zali MR, Rostami Nejad M, Rostami K, et al. . Liver complications in celiac disease. Hepat Mon 2011;11:333–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Lleo A, Yang GX, et al. . Common variable immunodeficiency and liver involvement. Clin Rev Allergy Immunol 2017. 10.1007/s12016-017-8638-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.León R, Sánchez-Martínez R, Palazón JM, et al. . [Nodular regenerative hyperplasia associated with common variable immunodeficiency and other comorbidities]. Med Clin 2016;146:263–6. 10.1016/j.medcle.2016.05.005 [DOI] [PubMed] [Google Scholar]
- 12.Venhoff N, Emmerich F, Neagu M, et al. . The role of HLA DQ2 and DQ8 in dissecting celiac-like disease in common variable immunodeficiency. J Clin Immunol 2013;33:909–16. 10.1007/s10875-013-9892-3 [DOI] [PubMed] [Google Scholar]
- 13.Imperatore N, Tortora R, Gerbino N, et al. . Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) mimicking refractory celiac disease. Dig Liver Dis 2017;49:1061–2. 10.1016/j.dld.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 14.Agarwal S, Cunningham-Rundles C. Autoimmunity in common variable immunodeficiency. Curr Allergy Asthma Rep 2009;9:347–52. 10.1007/s11882-009-0051-0 [DOI] [PMC free article] [PubMed] [Google Scholar]