Abstract
The cancer immunotherapy field has had many promising developments in recent years. Checkpoint inhibitors are good examples of that. This new class of medications comes with a new constellation of side effects that require early recognition and management. Here we present a patient with metastatic adenocarcinoma on pembrolizumab who was admitted to the hospital for colitis. This was found to be an immune-related adverse event from pembrolizumab. We discuss our work-up and approach to the diagnosis, then highlight important treatment pearls for internal medicine physicians who are increasingly taking care of such patients.
Keywords: unwanted effects / adverse reactions, immunological products and vaccines, malignant disease and immunosuppression
Background
Checkpoint inhibitors (CPI) have been recently introduced in cancer therapy and their use is gradually increasing due to improved outcomes and durable response. Although generally well tolerated, patients may suffer from a wide variety of adverse effects that can insidiously manifest with common symptoms. Many would warrant initial evaluation by an internal medicine physician who may not be fully aware of this new class of medications. Moreover, some of these adverse effects can be severe and lead to devastating complications if not recognised and treated early.
Case presentation
A 74-year-old Caucasian woman, with medical history of metastatic gallbladder cancer, presented to our emergency room with diffuse abdominal pain of 4 days’ duration. It has gradually worsened over time and is associated with non-bloody diarrhoea (6 stools/24 hours). No fevers or chills. No sick contacts or unusual exposures. No unusual food intake or recent travel. No similar previous episodes.
As for her gallbladder cancer, this was diagnosed a year ago; and is currently known to have multiple liver and bony metastases. She has been on different chemo protocols for her malignancy (nine cycles of gemcitabine, one cycle of paclitaxel, one dose of FOLFOX (folinic acid, fluorouracil and oxaliplatin)) and currently she is on pembrolizumab (last dose was administered 6 weeks ago). No over-the-counter medications or non-steroidal anti-inflammatory drugs (NSAID) use.
On physical examination, she appeared dehydrated with dry mucous membranes. Her abdomen was soft but mildly tender to deep palpation with no guarding or rebound tenderness. Bowel sounds were slightly hypoactive.
Investigations
Her laboratory data showed leucocytosis with neutrophilic predominance, chronic normocytic anaemia and acute-on-chronic renal failure. Lipase was normal. Blood and urine cultures were negative. A stool sample was sent for a gastrointestinal (GI) pathogen panel (table 1); no pathogen was detected. Finally, a CT scan of the abdomen and pelvis was done and revealed circumferential wall thickening involving the ascending colon, suggestive of colitis (figure 1).
Table 1.
Gastrointestinal pathogen panel results. Stool samples were tested using PCR
| Campylobacter species | Negative |
| Clostridium difficile toxin | Negative |
| Plesiomonas shigelloides | Negative |
| Salmonella species | Negative |
| Vibrio species | Negative |
| Vibrio cholerae | Negative |
| Yersinia species | Negative |
| Enteroaggregative Escherichia coli | Negative |
| Enteropathogenic E. coli (EPEC) | Negative |
| Enterotoxigenic E. coli (ETEC) | Negative |
| Shiga toxin-producing E. coli | Negative |
| Shigella/enteroinvasive E. coli | Negative |
| Cryptosporidium species | Negative |
| Cyclospora cayetanensis | Negative |
| Entamoeba histolytica | Negative |
| Giardia | Negative |
| Adenovirus F40/41 | Negative |
| Astrovirus | Negative |
| Norovirus Genogroup I (GI)/GII | Negative |
| Rotavirus | Negative |
| Sapovirus | Negative |
Figure 1.
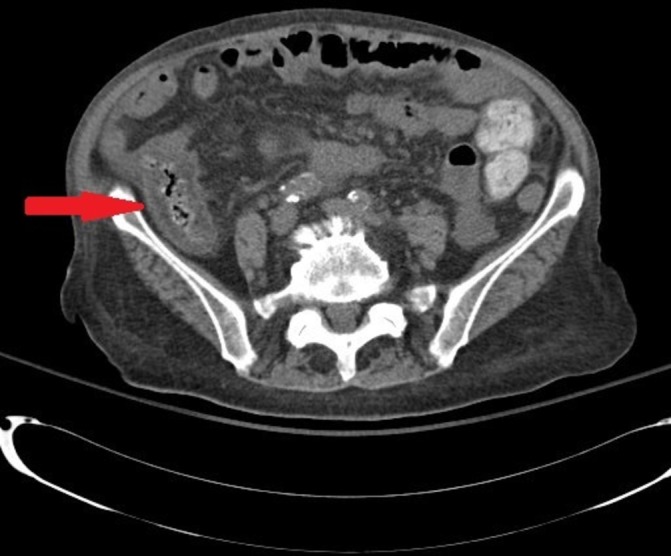
CT scan of the abdomen and pelvis on admission, showing circumferential wall thickening involving the ascending colon (arrow), suggestive of colitis.
Differential diagnosis
Infectious colitis was high on the differential list initially, given the leucocytosis and immunosuppression state. Stool samples were tested for a variety of GI pathogens, all of which however came back negative, making this diagnosis less likely. Ischaemic colitis is possible but our patient did not have haematochezia or risk factors such as heart failure or hypotension. Also, the colitis distribution on CT did not involve the usual watershed areas that are typically at higher risk for ischaemia such as the splenic flexure and the rectosigmoid junction. Multiphasic CT angiography was not done initially given the acute-on-chronic renal failure. This was not pursued later either given the dramatic clinical improvement with oral steroids. Diverticulitis is another diagnosis that was ruled out based on the CT findings. Inflammatory bowel diseases such as ulcerative colitis were also unlikely given the age, onset of symptoms and location of lesions on CT.
Medication-induced colitis is an inflammation of the colon that has most commonly been attributed to NSAIDs.1 The increasing use of CPIs however has caused an increase in cases of immune-related colitis. Our patient received a dose of pembrolizumab 6 weeks prior to the onset of her symptoms; this fits well with pembrolizumab clinical trials that reported 6 weeks as the average time interval between the initiation of the drug and the development of colitis-associated symptoms.2
Treatment
Pembrolizumab-induced colitis is an immune-related adverse effect. Management strategies depend on the grade of this specific adverse effect. For example, grade 1 colitis (<4 stools/day over baseline) is managed conservatively with diet modifications and antimotility agents. Grade 2 colitis (4–6 stools/day) is treated with corticosteroids, budesonide or prednisolone. Grade 3 or 4 colitis (≥7 stools/day) is a serious condition requiring immediate discontinuation of CPIs and initiation of intravenous steroids (prednisolone 1–2 mg/kg/day followed by 4–6 weeks of tapering). Infliximab is the preferred drug for cases of steroid-resistant colitis that do not respond after 3 days of steroid administration.3 Our patient’s symptoms were classified as grade 2 colitis; thus, she received oral budesonide 9 mg/24 hours for 3 days with a dramatic improvement in her symptoms. Oncology team was involved in her care as well during this hospital stay and she continued to follow-up after discharge.
Outcome and follow-up
Our patient responded quite well to budesonide with improvement of both abdominal pain and diarrhoea. Her oral intake improved and she was discharged home in a stable condition. A CT scan of the abdomen and pelvis was done 10 days later and showed improvement in the ascending colon thickening (figure 2).
Figure 2.
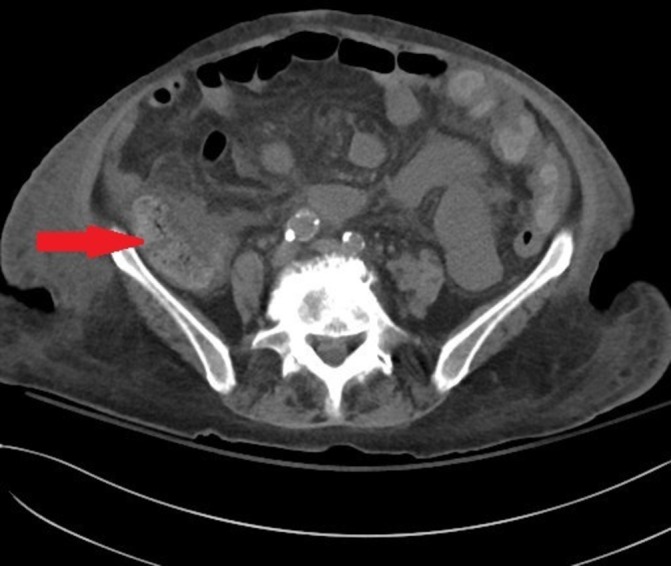
CT scan of the abdomen and pelvis 10 days after treatment. The previously seen thickening of the ascending colon has decreased (arrow).
Discussion
CPIs are novel monoclonal antibodies that restore the antitumour immune response of the immune system, thereby producing a therapeutic effect in a variety of malignancies. Since introduced, their use has been widely increasing due to improved outcomes and durable response.4 Their unique mechanism of action, however, has led to the development of a distinct set of adverse effects that are challenging for the internist to recognise and manage.
CPIs target two key receptors: programmed cell death (PD-1/PD-L1) and cytotoxic T-lymphocyte antigen (CTLA-4). Pembrolizumab is an anti-PD-1 humanised monoclonal antibody approved for patients with metastatic melanoma, non-small cell lung cancer, Hodgkin’s lymphoma, head and neck squamous cell carcinoma, urothelial carcinoma and microsatellite instability-high cancer.5 It has demonstrated clinical activity in several other tumour types and is being studied in a wide range of phase III clinical trials.6
Unrestrained T cell activation with CPIs may induce toxicity in the form of immune-mediated adverse events.7 Specifically, pembrolizumab use is linked to cardiovascular, endocrine and GI adverse effects (table 2). Pembrolizumab-mediated colitis has been described in many clinical trials with an average incidence of 2%. The rate is significantly higher in patients treated with combination of two antibodies (9.3%).8
Table 2.
Selected immune and non-immune-related adverse effects of some CPIs, based on their frequency
| >10% | 1%–10% | <1% | |
| CTLA-4 inhibitors (Ipilimumab) |
Diarrhoea
Pruritus |
Intestinal perforation Insomnia |
ARDS Encephalitis |
| PD-1 inhibitors (Pembrolizumab) |
Hyperglycaemia Skin rash |
Colitis Pneumonitis |
Hypophysitis Myocarditis |
| PD-L1/2 inhibitors (Atezolizumab) |
Fatigue
Urinary tract infections |
Hypothyroidism Pneumonitis |
Adrenal insufficiency
Diabetes mellitus |
ARDS, acute respiratory distress syndrome; CPI, checkpoint inhibitor; CTLA, cytotoxic T-lymphocyte antigen; PD-1, programmed cell death-1; PD-L1/2, programmed cell death ligand-1/2.
As for diagnostic colonoscopy with biopsy, intestinal perforation remains a serious complication in high-risk patients with inflamed colonic tissues, as occurs in immune-related colitis. Importantly, CT scan has an excellent positive predictive value of correctly diagnosing immune-related colitis when compared with colonoscopy with biopsy.9 Endoscopic assessment, however, is still indicated if diarrhoea is persistent or worsening despite supportive treatment and corticosteroids.3 10
While GI symptoms are common in patients with cancer undergoing treatment, clinicians should have a high level of suspicion of immune-mediated adverse effects, as some of these can be life threatening and require prompt diagnosis and management. The good news is that CPI-induced colitis is often reversible if rapidly treated. Management plan depends on the severity and grade as mentioned earlier. Overall, treatment is not different from that of any other autoimmune process. Immunosuppression with corticosteroids and discontinuation of CPIs are the mainstay of treatment, whereas infliximab and anti-tumour necrosis factor-α agents are recommended in steroid-refractory cases.7 Surgical consultation should occur early in very severe cases as colectomy might be needed.
Learning points.
Special consideration should be given to patients receiving checkpoint inhibitors.
Immune-mediated adverse events should be recognised promptly as early interventions can alleviate devastating complications.
Working with subspecialty teams is integral to the success of managing these complex cases.
Footnotes
Contributors: IM and MI participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis and writing of the manuscript. They both made substantial contributions to the work reported in the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Philpott HL, Nandurkar S, Lubel J, et al. Republished: drug-induced gastrointestinal disorders. Postgrad Med J 2014;90:411–9. 10.1136/postgradmedj-2013-100316rep [DOI] [PubMed] [Google Scholar]
- 2.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015;35:76–83. 10.14694/EdBook_AM.2015.35.76 [DOI] [PubMed] [Google Scholar]
- 3.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2016;2:1346 10.1001/jamaoncol.2016.1051 [DOI] [PubMed] [Google Scholar]
- 4.Bersanelli M, Buti S. From targeting the tumor to targeting the immune system: transversal challenges in oncology with the inhibition of the PD-1/PD-L1 axis. World J Clin Oncol 2017;8:37 10.5306/wjco.v8.i1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramamurthy C, Godwin JL, Borghaei H. Immune checkpoint inhibitor therapy: what line of therapy and how to choose? Curr Treat Options Oncol 2017;18:33 10.1007/s11864-017-0476-y [DOI] [PubMed] [Google Scholar]
- 6.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904–11. 10.1002/cncr.30642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Neuer M, Marmarelis ME, Jangi SR, et al. Diagnostic comparison of CT scans and colonoscopy for immune-related colitis in ipilimumab-treated advanced melanoma patients. Cancer Immunol Res 2017;5:286–91. 10.1158/2326-6066.CIR-16-0302 [DOI] [PubMed] [Google Scholar]
- 10.Prieux-Klotz C, Dior M, Damotte D, et al. Immune checkpoint inhibitor-induced colitis: diagnosis and management. Target Oncol 2017;12:301–8. 10.1007/s11523-017-0495-4 [DOI] [PubMed] [Google Scholar]


