Abstract
Introduction
We summarise an ethically approved protocol for the development of an experimental human challenge colonisation model. Globally Bordetella pertussis is one of the leading causes of vaccine-preventable death. Many countries have replaced whole cell vaccines with acellular vaccines over the last 20 years during which pertussis appears to be resurgent in a number of countries in the developed world that boast high immunisation coverage. The acellular vaccine provides relatively short-lived immunity and, in contrast to whole cell vaccines, may be less effective against colonisation and subsequent transmission. To improve vaccine strategies, a greater understanding of human B. pertussis colonisation is required. This article summarises a protocol and does not contain any results.
Methods and analysis
A controlled human colonisation model will be developed over two phases. In phase A, a low dose of the inoculum will be given intranasally to healthy participants. This dose will be escalated or de-escalated until colonisation is achieved in approximately 70% (95% CI 47% to 93%) of the exposed volunteers without causing disease. The colonisation period, shedding and exploratory immunology will be assessed during a 17-day inpatient stay and follow-up over 1 year. The dose of inoculum that achieves 70% colonisation will then be confirmed in phase B, comparing healthy participants exposed to B. pertussis with a control group receiving a sham inoculum.
Ethics and dissemination
This study has been approved by the ethical committee reference: 17/SC/0006, 24 February 2017. Findings will be published in peer-reviewed open access journals as soon as possible.
Keywords: bordetella pertussis, human challenge study, whooping cough, colonisation, vaccine development
Strengths and limitations of this study.
Close inpatient observation after Bordetella pertussis exposure allows close monitoring of volunteer safety, prevents transmission to people at risk of pertussis disease and provides the opportunity to assess shedding of B. pertussis.
The proposed dose escalation schedule is designed to optimise volunteer safety.
As part of the Periscope consortium, it will be possible to compare the immunology and microbiology results of this human challenge study with vaccine-induced immunity and results of animal challenge studies.
The individual variation and the low number of participants might influence the external validity of the results.
Aiming for 70% colonisation may be suboptimal for future vaccine efficacy studies using this model.
Introduction
Pertussis, also called whooping cough, is an acute bacterial infection caused by Bordetella pertussis (Bp), an exclusively human pathogen. Although it can affect people of all ages, young unimmunised infants are the most vulnerable group with the highest rates of complications and death.1 In 1999, there were an estimated 48.5 million cases with pertussis in children worldwide and 295 000 deaths,2 but based on seroepidemiological prevalence studies, the number of asymptomatic Bp infections may be much higher.3 By 2013, mortality fell to about 60 600 (IQR 22 300–136 800) children per year, still making it one of the leading causes of vaccine-preventable death.4
Pertussis vaccines have been included in National Immunisation Programmes since their introduction in the 1940s–1950s. Acellular pertussis (aP) vaccines have a favourable reactogenicity profile in comparison with whole-cell pertussis (wP) vaccines and are currently mostly used in industrialised countries to immunise against pertussis.5 However, many countries in the developed world that boast high immunisation coverage have seen an increase in the incidence of pertussis over the past 20 years.6 Five main hypotheses have been proposed to contribute to this resurgence: (1) rapid waning of immunity following vaccination, especially with aP,7 (2) the very different immune response profile induced by aP compared with wP vaccines,8 (3) adaptation of Bp to escape protective immunity, (4) low vaccine coverage and (5) less effective reduction of transmission from infected individuals vaccinated with aPs.9 The latter is supported by baboon studies that have demonstrated that vaccination with aP prevents severe disease but does not prevent asymptomatic infection, that is, colonisation.10 Studies in mice are consistent with these findings and demonstrate that protective immunity is more effective and persistent when induced by natural infection or wP than by aP.11 To study the pathogenesis of pertussis, a variety of animal models have been used, including mice, rabbits, guinea pigs and newborn piglets.12 However, there are still important knowledge gaps relating to human immunity to Bp, and it is not clear to what extent these observations in animal models translate into clinical practice. This paucity of knowledge hampers the development of improved vaccines and the design of better vaccination strategies against pertussis in infants, adolescents and adults.
The deliberate infection of human volunteers with micro-organisms has contributed uniquely to our understanding of the pathogenesis, immune responses and the treatment and prevention of numerous microbial diseases including pneumococcal disease,13 influenza, cholera, typhoid and hepatitis.14 We aim to develop a safe human challenge colonisation model to allow a more thorough understanding of the immune response against wild-type Bp and to facilitate development of bioassays and next-generation pertussis vaccines.
Methods
This is a controlled human infection study consisting of two phases: phase A: development of a Bp human challenge colonisation model and phase B: development of a modified Bp human challenge colonisation model in which we will compare participants receiving the standard inoculum (SI) with a control group receiving a sham inoculum. This article summarises the protocol for phase A.
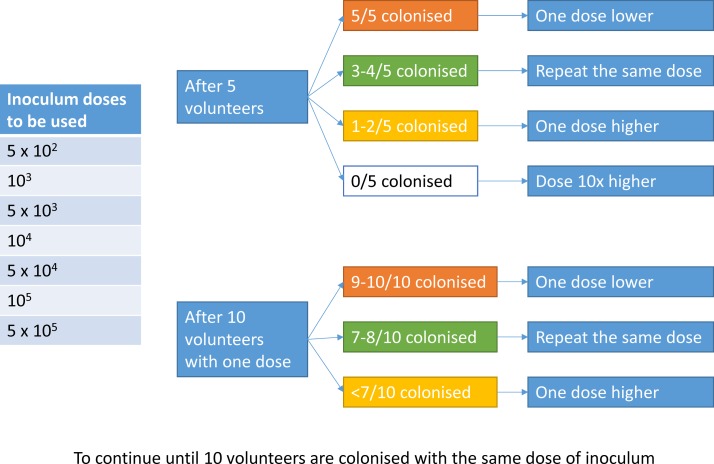
Phase A will be a dose-finding phase in which the dose of inoculum will be escalated or de-escalated to find the SI, defined as the dose of Bp that will safely cause colonisation in approximately 70% (95% CI 47% to 93%) of the exposed participants. Colonisation will be defined as a positive culture of Bp from the nasal washspecimen between day 0 and 14. After five participants have received the inoculum, colonisation will be assessed, and the following five participants will receive either the same dose or an escalated or decreased dose (figure 1). Once a dose of inoculum achieves a colonisation rate of 70%, then that dose will be used to inoculate further participants until a total of 10 participants have been colonised. This will require inoculation of approximately 14 participants with that dose, which will then be defined as the SI dose.
Figure 1.
Escalating or de-escalating the dose of the inoculum according to colonisation frequency.
The various samples (nasopharyngeal swab, throat swab, nasal wash and nasal fluid sample) will be compared to identify the technique that yields the highest sensitivity of Bp detection to inform the design of phase B. The seroconversion rate will be measured comparing preinoculation and postinoculation serum samples of participants infected after receiving the SI. Seroconversion will be defined as a threefold rise in anti-pertussis toxin (PT) IgG titre (IU/mL) from day 0 to day 28. We will then identify the ‘colonisation period’, which is defined as the earliest day after inoculation on which colonisation of the nasopharynx (as detected by the most sensitive technique) is observed in 100% of those volunteers who subsequently seroconvert at day 28. The colonisation period will be used in the protocol for phase B to minimise the duration of infection prior to eradication of Bp by antibiotics.
Study objectives
The primary objective of phase A of this study is to determine the dose of the SI in participants who do not have evidence of recent Bp exposure. The SI is defined as the dose of Bp causing colonisation in approximately 70% of the participants between day 0 and 14 without causing Bp disease after being challenged with this dose at day 0. An endpoint of 70% is used to avoid, if possible, a ‘saturating dose’ that results in non-physiological colonisation by Bp in participants but still induces sufficiently high colonisation rates to enable potential future vaccine efficacy trials. Secondary endpoints will explore the ability to dose the inoculum accurately, the prechallenge and postchallenge Bp-specific immunity in healthy participants and the environmental shedding of Bp following nasal inoculation (see table 1). Feedback and recommendations from Patient and Public Involvement, in addition to participant questionnaires from previous malaria human challenge studies performed in Southampton, have been incorporated into this study.
Table 1.
Objectives and endpoints of the study
| Objectives | Endpoints | |
| 1A | To determine the dose of the standard inoculum in participants who do not have evidence of recent Bp exposure—safety | Safety endpoints: - Occurrence of possible or confirmed Bp disease within the study period - Occurrence of unsolicited adverse events within the study period - Occurrence of serious adverse events within the study period |
| 1B | To determine the dose of the standard inoculum in participants who do not have evidence of recent Bp exposure—70% colonisation | Microbiologically proven Bp colonisation by positive culture of Bp from a nasal wash sample taken between time points day 0 and 14 after being challenged on day 0 |
| 2x | To evaluate accuracy of the inoculum dosing | Estimation of the actual challenge dose in comparison with the prescribed challenge dose by viable count (cfu/mL) of residual inoculum following inoculation of each participant |
| 3 | To describe the human physiological response to Bp challenge in those developing or not developing infection | Description of the clinical course after challenge using, for example: Clinical and laboratory observations such as temperature (°C), systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), heart rate (beats/min), respiratory rate (breaths/min), O2 saturation in blood (%), CRP (mg/L), WCC (109/L), lymphocyte count (109/L) at various time points |
| 4 | To determine the colonisation period: the earliest day after inoculation at which colonisation of the nasopharynx (as detected by culture) is observed in 100% of those participants who subsequently seroconvert at day 28 | A threefold rise in anti-PT IgG titre (IU/mL) from day 0 to day 28 will be used as a marker of seroconversion. Colonisation will be detected by positive culture of Bp from a nasopharyngeal swab taken between time points day 0 and 14 |
| 5 | To determine the characteristics of bacterial dynamics after challenge | Microbiological assays to detect and characterise Bp dynamics after challenge in nasopharyngeal swabs (culture, qPCR and microbiome analyses), nasal wash (culture including semiquantitative method using cfu count/mL, and precision quantification with qPCR) and sequencing of isolates at various time points |
| 6 | To assess environmental shedding of Bp following nasal inoculation of healthy participants with Bp. | Daily microbiological assays from day 0 to 16 to detect Bp on surface contact (culture and PCR), air sampling (PCR) fingertip culture (culture Bp and PCR), cough box (culture Bp, particle size during various activities: talking, coughing and singing) |
| 7 | To determine the eradication frequency of Bp after a 3-day course of azithromycin | Microbiological assays after eradication in nasopharyngeal swabs (culture, qPCR and microbiome analyses), nasal wash (culture including semiquantitative method using cfu count/mL, qPCR) on days 15 and 16 |
| 8 | To describe the human immune response to challenge, including innate, humoural, cell-mediated and mucosal responses. | Immunological assays to measure innate, humoural, cell-mediated and mucosal responses to challenge in blood (anti-PT IgG (IU/mL), anti-FHA IgG (IU/mL), anti-PRN IgG (IU/mL), anti-FIM IgG (IU/mL), nasal washes (T cell/B cell analyses), nasal fluid samples (cytokines) and saliva (Bp-specific IgA (IU/mL)) samples, comparing day 0 with days 1, 3, 4, 7, 9, 11 and 14 and weeks 4, 8, 26 and 52. |
Bp, B. pertussis; cfu, colony-forming units; CRP, C reactive protein; FIM, fimbriae; FHA, filamentous haemagglutinin; IU, international units; PCR, polymerase chain reaction; PRN: pertactin; PT, pertussis toxin; qPCR, quantitative PCR; WCC: white cell count.
Challenge strain
The Bp isolate to be used in this human colonisation model is B1917, which is representative of current isolates in Europe.15 16 The strain, isolated in 2000 from a Dutch patient with Bp disease, is characterised as ptxP3-ptxA1-prn2-fim3-2, fim2-1 MLVA27, PFGE BpSR11 and expresses pertactin, PT and filamentous haemagglutinin. This strain has been extensively characterised in the mouse model as well as by proteomics and transcriptomics and has a closed genome available.16 It is fully sensitive to azithromycin in vitro.
The inoculum has been prepared by Q Biologicals (Ghent, Belgium) according to good manufacturing practice (GMP) standard in licenced GMP facilities and using a process free of animal-derived products. The identity and purity of the cell bank have been confirmed, in addition to any other quality specifications agreed within the consortium and needed for compliance with regulatory requirements. Before intranasal inoculation, there will be no further culture of the challenge inoculum at the clinical site. The dose and purity of the inoculum will be determined after inoculation for quality assessment.
Study setting
This is one of several other clinical and preclinical studies of the Periscope consortium (http://periscope-project.eu/consortium/), which brings together internationally renowned scientists with many years of experience in Bp research, clinical trials, bioinformatics, immunology and public health. The aim of the consortium is to promote scientific and technological innovation in pertussis vaccine development and to foster the creation of a laboratory and scientific network that facilitates the testing and helps expedite the development of novel pertussis vaccines in Europe.
The study will be conducted by the University of Southampton in the National Health Institute for Health Research Clinical Research Facility (CRF) Southampton.
Recruitment
Participants will be recruited via the following:
Southampton CRF and Periscope websites—information about the study will be available on the website with a downloadable volunteer information sheet
posters in public places, including buses and trains, university campus, student bars, halls of residence, health centres and so on with the agreement of the owner/proprietor
newspapers or other literature for circulation
press release
Clinical Research Update magazine
a post on a Twitter, Facebook or Gumtree account owned and operated by our group
email distribution to individuals who have already expressed an interest in taking part in any clinical trial at the CRF Southampton
Southampton CRF Database of Healthy Volunteers: individuals from this database have previously expressed an interest in receiving information about future studies for which they may be eligible.
A recruitment and volunteer management plan has been formulated to prioritise and coordinate these strategies. Potential volunteers who are interested will be sent the volunteer information sheet and will be invited for a screening visit. During this visit, they will be given an opportunity to discuss the study questions and complete a preconsent questionnaire to ensure they understand the study. The informed consent procedure includes specific infection prevention information regarding the measures that are taken to prevent transmission during admission to the research unit. Once consent has been given, their eligibility will be assessed. This will include a general health questionnaire, which is a screening tool to identify common psychiatric conditions. The medical history will be checked with the general practitioner. Participants will be offered reimbursement for their time, travel and inconvenience.
Eligibility criteria
Healthy volunteers, men and women, aged 18–45 years without known or suspected recent pertussis infection (anti-PT IgG >20 IU/mL) will be recruited for phase A. Care will be taken not to recruit from vulnerable groups (mental health or other impaired capacity issues). Specific inclusion and exclusion criteria can be found in the online supplementary table 1. Female volunteers are required to use an effective form of contraception for the duration of their participation in this study. These criteria should minimise the risk of complicated disease or transmission of Bp to risk groups and aim to select a homogenous group that will be able to adhere to the scheduled admission and follow-up visits.
bmjopen-2017-018594supp001.pdf (355.4KB, pdf)
Interventions
The start of volunteer participation is defined as the screening visit. The end of volunteer participation is defined as the last visit. The duration of involvement in this study from screening will be approximately 56 weeks. A detailed schedule of the interventions is shown in table 2.
Table 2.
Scheduled events and interventions
| Day | −30 | −7 | 0 | 1 | 2 | 3 | 4 | 5 | 7 | 9 | 11 | 14 | 15 | 16 | 28 | 56 | 183 | 365 |
| Visit | x | x | x | x | x | x | ||||||||||||
| Admission | ||||||||||||||||||
| Inoculum | x | |||||||||||||||||
| Bloods | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||
| Urine | x | x | ||||||||||||||||
| ECG | x | |||||||||||||||||
| Nasal wash | x | x | x | x | x | x | x | x | x | x | x | |||||||
| Nasal fluid sample | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | 2x | ||||
| Nasopharyngeal swab | x | x | x | x | x | x | x | x | x | x | x | |||||||
| Throat swab | x | x | x | x | x | x | x | x | x | |||||||||
| Saliva | x | x | x | x | x | x | x | x | x | |||||||||
| Antibiotic therapy | x | x | x |
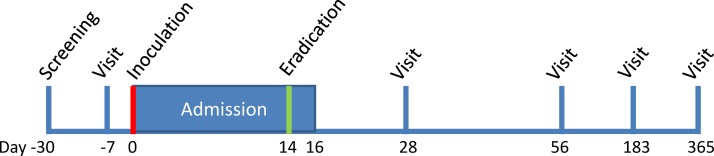
A week before the challenge, nasal samples will be collected (see figure 2). On the day of the challenge, the participant will be admitted to a designated area in the CRF and will have access to a dedicated individual bedroom, shared toilet, shower and recreational areas during their stay in the facility. Participants will be required to wear a surgical mask covering their nose and mouth when outside their personal room, for example, in the recreational room, unless outside in open air. Participants will be allowed to leave the CRF for a maximum of 2 hours twice a day, during the daytime. When outside the CRF, participants will be asked to adhere to infection prevention rules based on Public Health England guidelines. These include avoiding contact with people at risk of pertussis, avoiding direct face-to-face contact and wearing a surgical face mask when inside. When they leave or come back to the designated area, they will be escorted by a member of the study team. The Bp inoculum will be prepared and administered following study-specific standard operating procedures that are based on previous human challenge studies using nasal inoculation with Neisseria lactamica.17 18 One phial of inoculum will be removed from the freezer, thawed and diluted to the required inoculum dose. The participant will be positioned supine with neck extended, mouth open and breathing normally through their mouth. An inoculum of 0.5 mL will be gently expelled into each nostril, while the participant is positioned with mouth open, neck extended and breathing normally through their mouth. After the inoculum is administered, the inoculum residuum will be diluted and cultured for 5 days on Bordetella selective medium for determination and viable counts of Bp and on non-selective medium to assess purity of the inoculum.
Figure 2.
Visits and admission design.
During admission, clinical observations and symptoms of possible early pertussis disease such as rhinorrhoea, nasal congestion, epistaxis, sneezing, ear pain, eye pain, sore throat, cough, dyspnoea, feeling generally unwell, tiredness and headache will be reviewed six times per day. Regular nasal, mouth, throat and blood samples will be taken to assess the microbial dynamics and the host response to the challenge. Environmental samples will be taken to assess shedding. These include cultures and quantitative polymerase chain reaction of: face masks, contact areas in the personal room, air sampling of the personal room and during aerosol producing activities. Nasopharyngeal samples will be requested from CRF staff members with participant contact to monitor transmission of viable Bp.
In phase A, azithromycin 500 mg will be given once a day for 3 days to eradicate possible Bp colonisation on day 14 or to treat possible early stage Bp disease before day 14 following national guidelines.19 After start of the treatment, participants will remain in the hospital for another 48 hours to assess eradication success and environmental shedding after eradication.
A nasopharyngeal swab will be taken prior to discharge, which will be 48 hours after the start of eradication therapy. If this sample is positive for Bp within 5 days, the course of azithromycin will be repeated. Follow-up visits will take place at week 4, 8, 26 and 52.
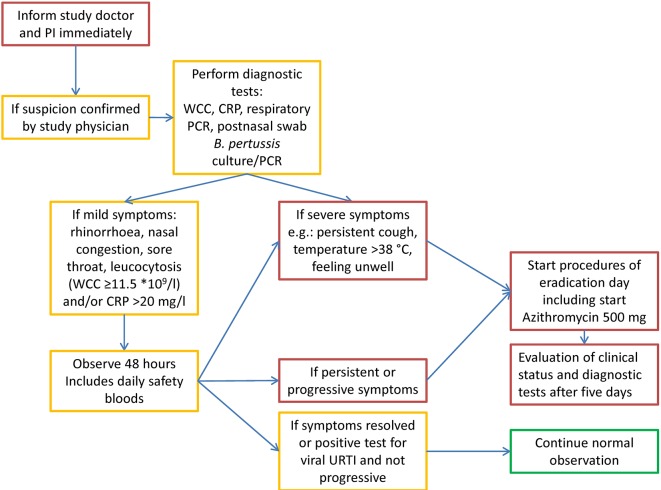
If, following inoculation, the participant develops symptoms of early pertussis disease, the participant will be given azithromycin according to Public Health England treatment guidelines. This will be done following a predefined treatment algorithm (see figure 3), which is designed to exclude trivial viral infection or transient and non-specific upper respiratory tract symptoms. After treatment, the participant will continue to be included in the study, and specimens will be collected as if they had received eradication treatment at day 14 per protocol.
Figure 3.
Actions to be taken when symptoms of early Bordetella pertussis disease are suspected. CRP, C reactive protein; PI, principal investigator; URTI, upper respiratory tract infection; WCC, white cell count.
Safety
Our priority is to develop this model without causing harm to the individual or the environment. An important facet of a safe model will be to limit participant exposure to Bp to the minimum dose and number of days required to signal successful colonisation. Safety considerations in the protocol include:
The dose of the inoculum will start low and will be modified to the lowest dose required to effectively establish colonisation with regular reviews of the available safety data by the study steering committee and the external safety committee.
The timing of eradication will be modified to optimise the safety of the participants by exposing them to the minimal effective period of colonisation.
The incubation time for the catarrhal phase is unknown but is estimated to be 7–22 days. After inoculation of the participants, there will therefore be a moderate risk of symptom development because eradication therapy will initially be given at day 14.
If a participant shows signs of possible disease, there will be a low threshold to eradicate colonisation.
Bp disease in adults is often atypical and relatively mild. Treatment is not thought to reduce the duration of coughing in natural infection, but adults with natural infection will not usually receive treatment until a late stage of the disease, if at all. Early treatment (as proposed in this study) is considered more likely to reduce symptoms quickly.
The likelihood of severe disease is extremely low, and the risk of harm to the participants, staff and others can be minimised by admitting the participants as inpatients in the Southampton CRF, which is an National Institute for Health Research (NIHR) facility specifically funded to conduct higher risk experimental medicine in a safe National Health Service environment. Household contacts will not be asked to provide informed consent, but volunteers with household members within the risk groups for pertussis disease will be excluded from the study.
All healthcare workers involved in this study will be vaccinated against Bp at least 2 weeks before working with participants that have received the inoculum unless they have been vaccinated against Bp in the last 5 years.20
An external safety committee of independent infectious disease experts will assess the safety of study after every five participants and advise on the continuation of the study.
Sample size
Phase A is a dose-finding study, and the sample size has been estimated with the assumption that we will colonise no, or only a few, participants at the initial low dose, allowing for escalation of the dose based on colonisation frequency and safety parameters. We are aiming to achieve colonisation and seroconversion in 10 participants with the SI dose of Bp.
Statistical analysis
Formal statistical comparisons of colonisation/seroconversion will not be carried out in phase A. In this dose-finding phase, decisions to escalate or de-escalate the dose will be based on the results accumulating over the progression of the study.
Ethics and dissemination
In the United Kingdom and the European Union, an experimental human challenge study with a wild-type bacterium falls outside the European Clinical Trials Directive.14 Participants in challenge trials are healthy volunteers who do not obtain direct health benefit from participation. Potential volunteers will be informed of all conceivable risks and have adequate time to decide on their individual participation in relation to the risks involved and financial compensation for the time and inconvenience of taking part. Medical ethicists have argued that it is a healthy adult’s right to self-determine their participation in such trials in relation to the risk (Controlled human infection studies in the development of vaccines & therapeutics, Wellcome Trust Scientific Conference, January 9–11, 2013, University of Cambridge, UK).
The protocol has been reviewed by independent peer reviewers including experts from Public Health England and worldwide experts on pertussis within the Periscope consortium (www.periscope-project.eu) who have assessed the safety and the quality of the study, the study design and the feasibility of the study objectives. The protocol has been reviewed and approved by the South Central – Oxford A Research Ethics Committee (REC reference: 17/SC/0006, 24 February 2017) and the UK Health Research Authority (IRAS project ID: 219496, 1 March 2017). Findings will be published in peer-reviewed open access journals as soon as possible. The final protocol for phase B will be presented as a substantial protocol amendment, because it will be based on the SI and colonisation period identified in phase A as well as the outcome of the exploratory immune assays performed during this phase.
Discussion
To the best of our knowledge, this will be the first adult human challenge study with wild type Bp. A paediatric Bp human challenge study was performed in 1933 in which two naive and two vaccinated children were exposed to 140 cfu of intranasal Bp.21 While the vaccinated children did not develop whooping cough, the naive children developed severe disease including anorexia, fever, severe paroxysmal cough, leucocytosis, lymphocytosis and positive cough plate cultures. They received no treatment and improved by day 35. This study confirmed that pertussis disease is caused by the bacillus of Bordet and Gengou and could be prevented by the experimental Bp vaccine that was used.
In a recent first in human study, healthy volunteers were given a live attenuated Bp strain as a nasal vaccine.22 The inoculated strain was genetically modified; dermonecrotic toxin and tracheal cytotoxin were removed, and PT was genetically detoxified by two independent mutations, removing the toxic activity of PT without affecting its immunogenic properties. Three groups, each of 12 participants, were inoculated with 103, 105 or 107 colony forming units, respectively. Colonisation was seen in one subject in the low dose, one in the medium dose and five in the high dose group. Adverse events occurred in similar frequency in all groups, including the placebo group and were found to be trivial. The effect of the genetic changes on the ability to colonise are unknown, but it is expected to have a significant effect on colonisation.
The inoculum required to colonise previously vaccinated adults exposed to natural infection with Bp is unknown but is assumed to be higher than that used in the paediatric challenge study because previously exposed and vaccinated adults are included. The initial inoculum dose used in our study (1000 cfu) has been chosen as a dose low enough to be assumed to be safe but high enough to allow accurate monitoring.
This model presumes that Bp disease is preceded by colonisation. Direct evidence for this has proven hard to obtain in surveillance studies.23 24 Potential reasons for this are the use of inadequate sampling methods or insufficiently sensitive assays, or that colonisation is a very short phenomenon and often missed in a cross-sectional study. Seroprevalence studies do show there is a natural boosting of anti-PT IgG levels, suggesting asymptomatic infection/colonisation is quite frequent.25–27
Results of two previous studies demonstrated statistically significant correlations between protection against pertussis disease and the presence of anti-PT IgG in pre-exposure sera.28 29 Because of this, we will exclude volunteers with a high anti-PT IgG in phase A, although there is no evidence that high anti-PT IgG levels correlate with protection against colonisation. In phase B, this will not be an exclusion criterion, and the correlation between anti-PT IgG levels and protection against colonisation will be assessed.
To minimise the risk for the participant, this controlled infection study is not a disease model, as was the case with many other challenge studies,30 31 but a colonisation model. The risk of symptom development in phase A is moderate rather than low because eradication therapy is not planned to be given until day 14 to allow an adequate immune response to develop. Eradication therapy will be given earlier if symptoms develop that are suggestive of pertussis disease. This approach is considered to be acceptable because the risk of severe disease in healthy adults, who are previously vaccinated and probably naturally exposed to Bp, is extremely low.32 33 Antibiotic treatment is known to be effective at eradicating Bp if given early after diagnosis but does not alter the subsequent clinical course of the illness,34 especially if administered beyond 2–3 weeks after the onset of symptoms. In this study, treatment will be given at an early stage of the infection, which we predict will have a more positive effect on the disease course.
We have implemented comprehensive transmission prevention measures, including an inpatient stay of 17 days. The shedding of Bp, the colonisation frequency and colonisation period will be evaluated during phase A alongside the host immune response to inform a more directed approach in phase B.
The target colonisation frequency of 70% was chosen partly to be appropriate for future studies requiring a comparison of colonised participants with non-colonised/protected participants, for example, studies aiming to identify protective biomarkers. This colonisation frequency might not be appropriate for studies designed to assess the efficacy of novel vaccines in prevention of colonisation where a near 100% colonisation frequency would be preferable. Therefore, the SI dose may be increased in future studies, depending on the results of this initial study.
We recognise that this method of intranasal inoculation to induce colonisation/infection differs from the natural course of infection. However, this model will provide the opportunity to study the systemic and local immune response to exposure to Bp in a way that would not be feasible in natural human exposure or accurately represented by an animal model of exposure. Because of the atypical and often late presentation of pertussis in adults, knowledge about the features of presymptomatic and early infection is lacking. This study will give a unique insight in the initial interaction between bacteria and host during the first 2 weeks after initial exposure. The development of a safe human challenge model of pertussis, in conjunction with the recently developed baboon model of pertussis, has the potential to provide a path forward for answering critical questions about pertussis pathogenesis and host responses and will likely aid in the development of next-generation pertussis vaccines.35 Within the Periscope consortium, we aim to compare the results of this study with animal studies, vaccination studies with acellular and whole cell vaccines and natural infection studies with Bp.
Supplementary Material
Footnotes
Contributors: HDG has written the protocol and paper. DG, AG, DD, KK and SF assisted and advised writing the protocol and paper. RR has written the protocol and paper.
Funding: This work was supported by Periscope. Periscope has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 115910. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and European Federation of Pharmaceutical Industries and Associations (EFPIA) and Bill and Melinda Gates Foundation (BMGF).
Competing interests: HDG hasreceived sponsorship from Abbvie to attend a clinical paediatric rheumatology course. SF acts on behalf of the University ofSouthampton/University Hospital Southampton NHS Foundation trust as chief and principal investigator for clinical trials Sponsored by vaccine andantimicrobial manufacturers but receives no personal payments for the work.
Ethics approval: South Central – Oxford A Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Because this is a protocol publication, no data are available yet. Findings will be published in peer-reviewed open access journals as soon as possible.
References
- 1. Public Health England. Factsheet Pertussis: Public Health England, 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/323000/Factsheet_for_healthcare_professionals.pdf.
- 2. Crowcroft NS, Stein C, Duclos P, et al. How best to estimate the global burden of pertussis? Lancet Infect Dis 2003;3:413–8. 10.1016/S1473-3099(03)00669-8 [DOI] [PubMed] [Google Scholar]
- 3. de Greeff SC, Mooi FR, Westerhof A, et al. Pertussis disease burden in the household: how to protect young infants. Clin Infect Dis 2010;50:1339–45. 10.1086/652281 [DOI] [PubMed] [Google Scholar]
- 4. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang L, Prietsch SO, Axelsson I, et al. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst Rev 2014;9:Cd001478 10.1002/14651858.CD001478.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol Infect 2014;142:672–84. 10.1017/S0950268812003093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 2012;367:1012–9. 10.1056/NEJMoa1200850 [DOI] [PubMed] [Google Scholar]
- 8. Brummelman J, Wilk MM, Han WG, et al. Roads to the development of improved pertussis vaccines paved by immunology. Pathog Dis 2015;73:ftv067 10.1093/femspd/ftv067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 2014;111:787–92. 10.1073/pnas.1314688110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warfel JM, Papin JF, Wolf RF, et al. Maternal and neonatal vaccination protects newborn baboons from pertussis infection. J Infect Dis 2014;210:604–10. 10.1093/infdis/jiu090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgs R, Higgins SC, Ross PJ, et al. Immunity to the respiratory pathogen Bordetella pertussis. Mucosal Immunol 2012;5:485–500. 10.1038/mi.2012.54 [DOI] [PubMed] [Google Scholar]
- 12. Elahi S, Holmstrom J, Gerdts V. The benefits of using diverse animal models for studying pertussis. Trends Microbiol 2007;15:462–8. 10.1016/j.tim.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 13. Ferreira DM, Jambo KC, Gordon SB. Experimental human pneumococcal carriage models for vaccine research. Trends Microbiol 2011;19:464–70. 10.1016/j.tim.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 14. The Academy of Medical Science. Microbial Challenge Studies of Human Volunteers, 2005. [Google Scholar]
- 15. Bart MJ, Zeddeman A, van der Heide HG, et al. Complete Genome Sequences of Bordetella pertussis Isolates B1917 and B1920, Representing Two Predominant Global Lineages. Genome Announc 2014;2:e01301-14 10.1128/genomeA.01301-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bart MJ, van der Heide HG, Zeddeman A, et al. Complete Genome Sequences of 11 Bordetella pertussis Strains Representing the Pandemic ptxP3 Lineage. Genome Announc 2015;3:e01394-15 10.1128/genomeA.01394-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deasy A, Guccione E, Dale A, et al. Nasal Inoculation of the Commensal Neisseria lactamica Inhibits Carriage of Neisseria meningitidis by Young Adults: A Controlled Human Infection Study. Clin Infect Dis 2015;May 15;60(10):1512-20. [DOI] [PubMed] [Google Scholar]
- 18. Evans CM, Pratt CB, Matheson M, et al. Nasopharyngeal colonization by Neisseria lactamica and induction of protective immunity against Neisseria meningitidis. Clin Infect Dis 2011;52:70–7. 10.1093/cid/ciq065 [DOI] [PubMed] [Google Scholar]
- 19. Public Health England. Guidelines for the Public Health Management of Pertussis in England London, 2016. Available from https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/541694/Guidelines_for_the_Public_Health_Management_of_Pertussis_in_England.pdf.
- 20. Public Health England.. Guidance Whooping cough (pertussis): immunisation of healthcare workers. London, 2013. https://www.gov.uk/guidance/whooping-cough-pertussis-immunisation-of-healthcare-workers. [Google Scholar]
- 21. Macdonald H, Macdonald EJ. Experimental Pertussis. J Infect Dis 1933;53:328–30. 10.1093/infdis/53.3.328 [DOI] [Google Scholar]
- 22. Thorstensson R, Trollfors B, Al-Tawil N, et al. A phase I clinical study of a live attenuated bordetella pertussis vaccine-BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One 2014;9:e83449 10.1371/journal.pone.0083449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linnemann CC, Bass JW, Smith MH. The carrier state in pertussis. Am J Epidemiol 1968;88:422–7. 10.1093/oxfordjournals.aje.a120903 [DOI] [PubMed] [Google Scholar]
- 24. Zhang Q, Yin Z, Li Y, et al. Prevalence of asymptomatic Bordetella pertussis and Bordetella parapertussis infections among school children in China as determined by pooled real-time PCR: a cross-sectional study. Scand J Infect Dis 2014;46:280–7. 10.3109/00365548.2013.878034 [DOI] [PubMed] [Google Scholar]
- 25. Mertsola J, Ruuskanen O, Eerola E, et al. Intrafamilial spread of pertussis. J Pediatr 1983;103:359–63. 10.1016/S0022-3476(83)80403-X [DOI] [PubMed] [Google Scholar]
- 26. Huygen K, Rodeghiero C, Govaerts D, et al. Bordetella pertussis seroprevalence in Belgian adults aged 20-39 years, 2012. Epidemiol Infect 2014;142:724–8. 10.1017/S0950268813002458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He H, Yao P, Zhou Y, et al. Is Pertussis Infection Neglected in China? Evidence from a Seroepidemiology Survey in Zhejiang, an Eastern Province of China. PLoS One 2016;11:e0155965 10.1371/journal.pone.0155965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Storsaeter J, Hallander HO, Gustafsson L, et al. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998;16:1907–16. 10.1016/S0264-410X(98)00227-8 [DOI] [PubMed] [Google Scholar]
- 29. Cherry JD, Gornbein J, Heininger U, et al. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 1998;16:1901–6. 10.1016/S0264-410X(98)00226-6 [DOI] [PubMed] [Google Scholar]
- 30. McCullagh D, Dobinson HC, Darton T, et al. Understanding paratyphoid infection: study protocol for the development of a human model of Salmonella enterica serovar Paratyphi A challenge in healthy adult volunteers. BMJ Open 2015;5:e007481 10.1136/bmjopen-2014-007481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rampling T, Ewer KJ, Bowyer G, et al. Safety and High Level Efficacy of the Combination Malaria Vaccine Regimen of RTS,S/AS01B With Chimpanzee Adenovirus 63 and Modified Vaccinia Ankara Vectored Vaccines Expressing ME-TRAP. J Infect Dis 2016;214:772–81. 10.1093/infdis/jiw244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farizo KM, Cochi SL, Zell ER, et al. Epidemiological features of pertussis in the United States, 1980-1989. Clin Infect Dis 1992;14:708–19. 10.1093/clinids/14.3.708 [DOI] [PubMed] [Google Scholar]
- 33. De Serres G, Shadmani R, Duval B, et al. Morbidity of pertussis in adolescents and adults. J Infect Dis 2000;182:174–9. 10.1086/315648 [DOI] [PubMed] [Google Scholar]
- 34. Altunaiji S, Kukuruzovic R, Curtis N, et al. Antibiotics for whooping cough (pertussis). The Cochrane database of systematic reviews 2007;3:CD004404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Merkel TJ, Halperin SA. Nonhuman primate and human challenge models of pertussis. J Infect Dis 2014;209:S20–S23. 10.1093/infdis/jit493 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018594supp001.pdf (355.4KB, pdf)