Abstract
OBJECTIVE
We assessed dysglycemia and a T1D Diagnostic Index60 (Index60) ≥1.00 (on the basis of fasting C-peptide, 60-min glucose, and 60-min C-peptide levels) as prediagnostic end points for type 1 diabetes among Type 1 Diabetes TrialNet Pathway to Prevention Study participants.
RESEARCH DESIGN AND METHODS
Two cohorts were analyzed: 1) baseline normoglycemic oral glucose tolerance tests (OGTTs) with an incident dysglycemic OGTT and 2) baseline Index60 <1.00 OGTTs with an incident Index60 ≥1.00 OGTT. Incident dysglycemic OGTTs were divided into those with (DYS/IND+) and without (DYS/IND−) concomitant Index60 ≥1.00. Incident Index60 ≥1.00 OGTTs were divided into those with (IND/DYS+) and without (IND/DYS−) concomitant dysglycemia.
RESULTS
The cumulative incidence for type 1 diabetes was greater after IND/DYS− than after DYS/IND− (P < 0.01). Within the normoglycemic cohort, the cumulative incidence of type 1 diabetes was higher after DYS/IND+ than after DYS/IND− (P < 0.001), whereas within the Index60 <1.00 cohort, the cumulative incidence after IND/DYS+ and after IND/DYS− did not differ significantly. Among nonprogressors, type 1 diabetes risk at the last OGTT was greater for IND/DYS− than for DYS/IND− (P < 0.001). Hazard ratios (HRs) of DYS/IND− with age and 30- to 0-min C-peptide were positive (P < 0.001 for both), whereas HRs of type 1 diabetes with these variables were inverse (P < 0.001 for both). In contrast, HRs of IND/DYS− and type 1 diabetes with age and 30- to 0-min C-peptide were consistent (all inverse [P < 0.01 for all]).
CONCLUSIONS
The findings suggest that incident dysglycemia without Index60 ≥1.00 is a suboptimal prediagnostic end point for type 1 diabetes. Measures that include both glucose and C-peptide levels, such as Index60 ≥1.00, appear better suited as prediagnostic end points.
Introduction
The diagnosis of type 1 diabetes frequently occurs after the development of multiple pancreatic autoantibodies and a gradual loss of β-cell function (1) occurring over a period of several years (2–6). Thus, clinical trials (7–9) have determined whether treatments during this period might delay or even prevent the development of the disease. One of the challenges in conducting such trials is the length of time before type 1 diabetes is diagnosed, even in high-risk populations (e.g., autoantibody-positive individuals), which can result in costly trials of long duration. To reduce the length of these trials, interest exists in using prediagnostic end points rather than the occurrence of type 1 diabetes itself.
The objective of this analysis was to assess dysglycemia as a prediagnostic end point in high-risk relatives of patients with type 1 diabetes (two or more autoantibodies) and to compare the performance between dysglycemia and a T1D Diagnostic Index60 (Index60) threshold ≥1.00. Dysglycemia is a known predictor of type 1 diabetes (10) and has been considered for use as a prediagnostic end point. Index60 was developed from a proportional hazards model and combines the log fasting C-peptide level with the 60-min glucose and 60-min C-peptide levels from oral glucose tolerance tests (OGTTs). One analysis suggested that an Index60 threshold ≥2.00 has value as a diagnostic end point for type 1 diabetes (11). Of note, the analysis also suggested the possibility that a lower Index60 threshold could serve as a prediagnostic end point. The comparison of these two potential prediagnostic end points is of particular interest because dysglycemia is a marker for glucose alone, whereas an Index60 threshold is a marker for glucose and C-peptide combined.
Research Design and Methods
Participants
The data used for the analyses were derived from participants in the Type 1 Diabetes TrialNet Pathway to Prevention (PTP) study who had two or more autoantibodies at screening. All participants were between the ages of 1 and 45 years and were relatives of patients with type 1 diabetes. Individuals with diabetic-range baseline OGTT results were excluded from the analysis. The PTP was approved by the institutional review boards of all participating sites (participating institutions can be found in the Supplementary Data), and written informed consent and assent, as appropriate, were obtained from all study participants.
Procedure
The PTP has been previously described (12). Briefly, after autoantibody screening, participants positive for type 1 diabetes–associated autoantibodies are followed with 2-h OGTT surveillance at either 6- or 12-month intervals according to their risk for type 1 diabetes. All participants analyzed for this report were followed at 6-month intervals because the presence of two or more autoantibodies is one of the criteria for such follow-up. Autoantibodies against GAD, IA2, and insulin are initially measured at screening, and if one of these autoantibodies is positive, islet cell cytoplasmic autoantibodies and zinc transporter 8 autoantibodies are then measured. All autoantibodies have been measured from the inception of the PTP except for zinc transporter 8. For the 2-h OGTTs, C-peptide and glucose measurements are obtained at 0, 30, 60, 90, and 120 min after the ingestion of 1.75 g/kg of carbohydrate (maximum 75 g). A diagnosis of type 1 diabetes is made if an OGTT in the diabetic range (fasting glucose ≥126 mg/dL, 2-h glucose ≥200 mg/dL) is confirmed by another OGTT. If the second OGTT is not in the diabetic range, participants continue to be followed at 6-month intervals. A diagnosis could also be made on clinical grounds (e.g., symptomatic with marked hyperglycemia). The glucose oxidase method was used to measure plasma glucose. C-peptide was measured by the Tosoh assay. Methods for autoantibody measurements have been previously described (13).
Data Analysis
Two prediagnostic end points were studied: dysglycemia and Index60 values ≥1.00. A dysglycemic OGTT is a fasting glucose value of 110–125 mg/dL; a 30-, 60-, and/or 90-min glucose value ≥200 mg/dL; and/or a 2-h glucose value of 140–199 mg/dL. Index60 is calculated as 0.3695 × (log fasting C-peptide [ng/mL]) + 0.0165 × 60-min glucose (mg/dL) – 0.3644 × 60-min C-peptide (ng/mL) (11).
Supplementary Fig. 1 is a flow diagram for the analytic design of the study. The cohort selected for studying dysglycemia as a prediagnostic end point had glucose values in the normal glucose range at the baseline OGTT (normoglycemia cohort), whereas the cohort selected for studying Index60 ≥1.00 as an end point had Index60 values <1.00 at the baseline OGTT (Index60 <1.00 cohort). The normoglycemia cohort was followed for the occurrence of the first (incident) dysglycemic OGTT, whereas the Index60 <1.00 cohort was followed for the occurrence of the first (incident) Index60 ≥1.00 OGTT. After the occurrence of the incident OGTT (incident dysglycemia or incident Index60 ≥1.00), each cohort was followed for the development of type 1 diabetes. Among participants with an incident dysglycemic OGTT, those who also had concomitant Index60 ≥1.00 at that time were compared with those who did not. Participants who did not progress to type 1 diabetes were also compared for the Diabetes Prevention Trial Risk Score (DPTRS) (14) at the last OGTT. Similar comparisons were made for those with an incident Index60 ≥1.00 OGTT according to whether concomitant dysglycemia was present at that OGTT.
The DPTRS was developed to predict the occurrence of type 1 diabetes in autoantibody-positive relatives of patients with type 1 diabetes. It was derived from a proportional hazards model that includes log fasting C-peptide, log BMI, age, and the C-peptide sum and glucose sum from 30, 60, 90, and 120 min. The method for converting the DPTRS to risk estimates has been previously described (14). The conversion used for the current study was based on the overall survival curve for a Type 1 Diabetes TrialNet population with two or more autoantibodies at baseline.
By nature of the study design, some participants met criteria for inclusion in both the normoglycemia and the Index60 <1.00 cohorts. Those individuals were excluded from comparisons between incident dysglycemia without concomitant Index60 ≥1.00 (DYS/IND−) and incident Index60 ≥1.00 without concomitant dysglycemia (IND/DYS−).
Log-rank tests were used to compare cumulative incidence curves and were adjusted for age by using a competing risk regression model. Comparison of the distribution of age between groups was done by using the nonparametric Wilcoxon rank sum test. Hazard ratios (HRs) were derived from proportional hazards regression to examine associations of end points with baseline variables. The t and χ2 tests were used to compare groups. Time-dependent analyses for the area under the receiver operating characteristic curve (AUCROC) were based on the method of Uno et al. (15). SAS 9.4 software was used for statistical analyses. P values are two-sided.
Results
The following nomenclature is used for the description of the findings. For the normoglycemia cohort (n = 1,054, mean ± SD age 14.6 ± 11.1 years, BMI z score 0.39 ± 1.0) (Supplementary Table 1):
DYS/IND− = incident dysglycemic OGTT with concomitant Index60 value <1.00
DYS/IND+ = incident dysglycemic OGTT with concomitant Index60 value ≥1.00
For the Index60 <1.00 cohort (n = 1,124, age 16.2 ± 11.8 years, BMI z score 0.43 ± 1.0):
IND/DYS− = incident Index60 ≥1.00 OGTT without concomitant dysglycemia
IND/DYS+ = incident Index60 ≥1.00 OGTT with concomitant dysglycemia
Note that in the above nomenclature, the incident dysglycemic OGTT always starts with DYS, whereas the incident Index60 ≥1.00 always starts with IND.
Risk of Type 1 Diabetes After Incident Dysglycemic or Incident Index60 ≥1.00 OGTT
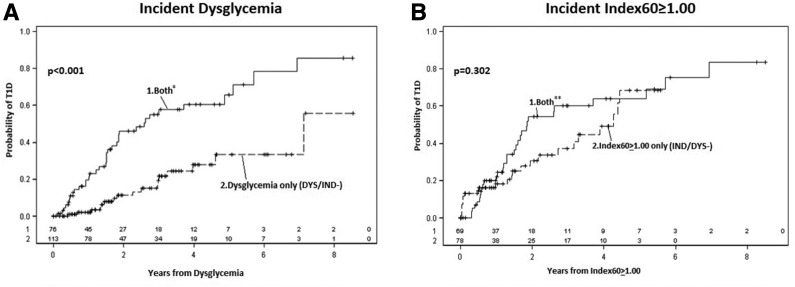
Figure 1A compares the cumulative incidence for type 1 diabetes after the first dysglycemic OGTT between DYS/IND− and DYS/IND+. The cumulative incidence for type 1 diabetes after DYS/IND+ was significantly higher (P < 0.001) than the cumulative incidence after DYS/IND−.
Figure 1.
A: Comparisons of cumulative incidence curves for type 1 diabetes (T1D) after incident dysglycemia according to concomitant Index60 ≥1.00. The cumulative incidence for T1D is substantially higher if there is concomitant Index60 ≥1.00. B: Comparisons of cumulative incidence curves for T1D after incident Index60 ≥1.00 according to concomitant dysglycemia. The difference between the cumulative incidence curves is much smaller after incident Index60 ≥1.00 than after incident dysglycemia. *Both indicates incident dysglycemia with concomitant Index60 ≥1.00 (DYS/IND+). **Both indicates incident Index60 ≥1.00 with concomitant dysglycemia (IND/DYS+).
Figure 1B compares the cumulative incidence for type 1 diabetes after the first Index60 ≥1.00 OGTT between IND/DYS− and IND/DYS+. The cumulative incidence of type 1 diabetes after IND/DYS+ was somewhat higher than the cumulative incidence for IND/DYS− but not significantly (P = 0.30).
The cumulative incidence of type 1 diabetes after IND/DYS− was significantly higher than that after DYS/IND− (3-year risk 0.34 [n = 65] vs. 0.20 [n = 100]; P < 0.01). The IND/DYS− OGTTs identified a much younger population than that identified by DYS/IND− OGTTs (mean age 13.9 ± 11.1 vs. 19.9 ± 12.9 years; P = 0.002). After adjustment for age, the difference in cumulative incidence was smaller but remained significant (P = 0.029).
In summary, the cumulative incidence of type 1 diabetes was similar between IND/DYS− and IND/DYS+, but the cumulative incidence of type 1 diabetes was greater for DYS/IND+ than for DYS/IND−. Moreover, the cumulative incidence of type 1 diabetes was greater for IND/DYS− than for DYS/IND−.
Risk of Type 1 Diabetes at Last OGTT (Among Participants Not Diagnosed) After Having Incident Dysglycemia or Incident Index60 ≥1.00
Table 1 shows DPTRS values at the last visit among those who did not progress to type 1 diabetes (nonprogressors) according to whether a prior incident dysglycemic OGTT or a prior incident Index60 ≥1.00 OGTT had occurred. DPTRS values at the last OGTT were higher (P < 0.001) for the nonprogressors who previously had DYS/IND+ than for nonprogressors who previously had DYS/IND− (5-year risk estimates from last OGTT 0.73 and 0.37, respectively). In contrast, DPTRS values at the last visit did not differ significantly (P = 0.42) between the nonprogressors who previously had IND/DYS+ and the nonprogressors who previously had IND/DYS− (5-year risk estimates from last OGTT 0.75 and 0.65, respectively). In a comparison between nonprogressors who previously had DYS/IND− and nonprogressors who previously had IND/DYS−, DPTRS values at the last visit were significantly higher (P < 0.001) in the latter (5-year risk estimates from last OGTT 0.49 [n = 36] vs. 0.30 [n = 80]).
Table 1.
DPTRS values and 5-year risk estimates at last visit among individuals with incident dysglycemia or incident Index60 ≥1.00 OGTTs who did not progress to type 1 diabetes
| Incident dysglycemia |
Incident Index60 ≥1.00 |
||
|---|---|---|---|
| Index60 <1.00 (n = 92) | Index60 ≥1.00 (n = 40) | No dysglycemia (n = 50) | Dysglycemia (n = 38) |
| 5.93 ± 1.56 (0.37) | 7.64 ± 1.32 (0.73)† | 7.27 ± 1.44 (0.65) | 7.52 ± 1.33 (0.75) |
Data are mean ± SD (5-year estimate from the last visit derived from DPTRS). Higher DPTRS values signify a greater risk for type 1 diabetes.
†P < 0.001 for comparison of incident dysglycemia and Index60 ≥1.00 with incident dysglycemia and Index60 <1.00.
In summary, the risk for type 1 diabetes among nonprogressors at the last OGTT was similar between those who previously had IND/DYS− and those who had IND/DYS+, but the risk was greater for those who previously had DYS/IND+ than for those who had DYS/IND−. Moreover, the risk for type 1 diabetes at the last visit was greater for IND/DYS− than for DYS/IND−.
Prediction Accuracy
An analysis was performed of the AUCROC integrated over the time of follow-up after the incident Index60 ≥1.00 and incident dysglycemic OGTTs. Among participants with an incident Index60 ≥1.00 OGTT, the presence or absence of concomitant dysglycemia contributed little to the prediction of type 1 diabetes beyond the contribution of incident Index60 ≥1.00; the integrated AUCROC of dysglycemia for predicting type 1 diabetes was only 0.48, which is close to the null value for no contribution (0.50). The AUCROC P values at each year of follow-up were 0.97 at 1 year, 0.05 at 2 years, 0.66 at 3 years, 0.51 at 4 years, and 0.20 at 5 years. In contrast, among those with an incident dysglycemic OGTT, the presence or absence of concomitant Index60 ≥1.00 contributed appreciably to the prediction of type 1 diabetes beyond the contribution of incident dysglycemia; the integrated AUCROC of Index60 ≥1.00 for predicting type 1 diabetes was 0.68. The AUCROC P values at each year of follow-up were <0.001 at 1 year, <0.001 at 2 years, <0.01 at 3 years, <0.05 at 4 years, and 0.13 at 5 years.
In summary, when incident Index60 ≥1.00 occurred, the presence or absence of concomitant dysglycemia did not add to the prediction of type 1 diabetes. On the other hand, when incident dysglycemia occurred, the presence or absence of Index60 ≥1.00 added to the prediction of type 1 diabetes.
Comparisons of DYS/IND− and IND/DYS− With Type 1 Diabetes for Associations With Baseline Variables
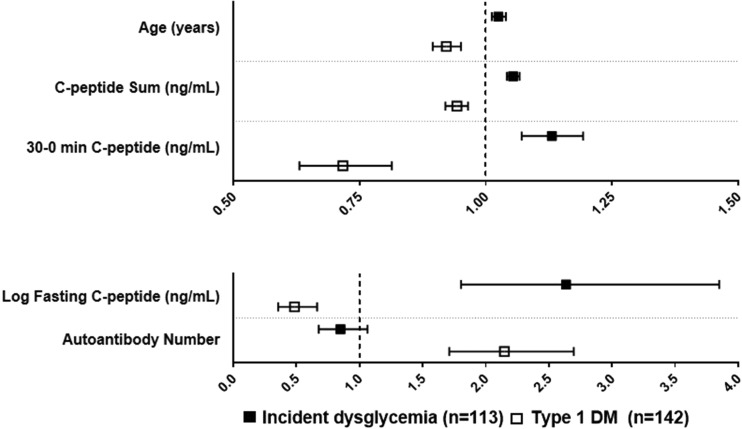
We examined the consistency of HRs between DYS/IND− and type 1 diabetes (as end points) for associations with baseline variables in the same cohort (normoglycemic OGTTs at baseline). The data showed a discordance between DYS/IND− and type 1 diabetes for HRs with several baseline variables (Fig. 2 and Supplementary Table 2). Specifically, the HRs of DYS/IND− with age, log fasting C-peptide, C-peptide sum, and 30- to 0-min C-peptide difference were in opposite directions to the HRs of type 1 diabetes with those variables (positive for DYS/IND− and inverse for type 1 diabetes). Moreover, all the HRs were statistically significant (P < 0.001) in the opposite directions. The HRs of DYS/IND− and type 1 diabetes with autoantibody number (any combination of the four antibodies tested) were also in opposite directions, inverse for DYS/IND− and positive for type 1 diabetes; the HR was significant for type 1 diabetes (P < 0.001) but not for DYS/IND− (P = 0.16). The HRs of DYS/IND− and type 1 diabetes were only concordant for the glucose sum (P < 0.001 for both).
Figure 2.
HRs with 95% CIs of DYS/IND− and of type 1 diabetes for associations with baseline variables. The HRs of DYS/IND− are discordant from the HRs of type 1 diabetes. Specifically, for age and each C-peptide variable, the HR and 95% CI (all positive) of DYS/IND− is on the opposite side of the null value (i.e., 1.0) from the HR and 95% CI (all inverse) of type 1 diabetes. The HR of DYS/IND− and autoantibody number is inverse and on the opposite side of the null value from the HR of type 1 diabetes and autoantibody number; however, the 95% CI for the HR of type 1 diabetes overlaps the null value. The two panels are shown with different scales to encompass all the variables. DM, diabetes.
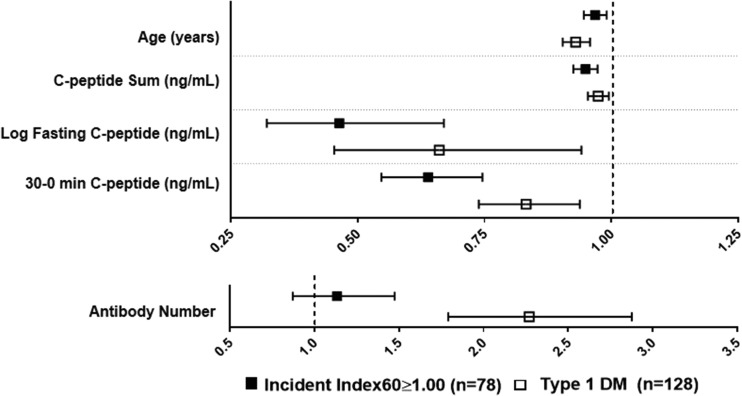
In contrast, among the cohort with Index60 <1.00 at baseline, the HRs of IND/DYS− with the aforementioned baseline variables were similar to the HRs of type 1 diabetes with those variables (Fig. 3 and Supplementary Table 3), with all being in the same direction (inverse) and significant. The HRs of IND/DYS− and type 1 diabetes were also concordant for the glucose sum (both positive and significant). The directionality of the HRs of IND/DYS− and of type 1 diabetes with autoantibody number was also the same (positive for both); the HR was significant for type 1 diabetes (P < 0.001) but not for IND/DYS− (P = 0.14).
Figure 3.
HRs with 95% CIs of IND/DYS− and of type 1 diabetes for associations with baseline variables. The HRs of IND/DYS− are concordant with the HRs of type 1 diabetes. Specifically, for age and each C-peptide variable, the HR and 95% CI of IND/DYS− is on the same side of the null value (i.e., 1.0) as the HR and 95% CI (all inverse) of type 1 diabetes. The HR of IND/DYS− and autoantibody number is also positive and on the same side of the null value as the HR of type 1 diabetes and autoantibody number; however, the 95% CI for the HR of type 1 diabetes overlaps the null value. The two panels are shown with different scales to encompass all the variables. DM, diabetes.
Diabetes autoantibody profiles were evaluated between DYS/IND− and IND/DYS− (Supplementary Table 4). No significant differences in the frequencies of autoantibodies for GAD and insulin or for islet cell cytoplasmic autoantibodies were found between the cohorts. A difference of borderline significance (P = 0.045) was found in the frequency of IA2 autoantibodies between the cohorts.
In summary, associations with age and C-peptide variables were more consistent between IND/DYS− and type 1 diabetes than between DYS/IND− and type 1 diabetes. DYS/IND− and type 1 diabetes differed in the directionality of their associations with age and C-peptide variables.
Conclusions
The findings suggest that incident dysglycemia in the absence of Index60 ≥1.00 (i.e., DYS/IND−) has shortcomings as a prediagnostic end point for type 1 diabetes. On the basis of their level of risk, a number of individuals who have incident dysglycemia without Index60 ≥1.00 might not ultimately progress to type 1 diabetes. The uncertain progression to type 1 diabetes is further supported by evidence that the associations of DYS/IND− with several baseline variables (including age, C-peptide measures, and autoantibody number) differ in directionality from the associations of type 1 diabetes with those same baseline variables.
As a measure that includes both glucose and C-peptide information, Index60 ≥1.00 has promise as a prediagnostic end point. In direct statistical comparisons between participants with DYS/IND− and those with IND/DYS−, the cumulative incidence for type 1 diabetes was appreciably higher after IND/DYS−. Moreover, among those who did not progress to type 1 diabetes, DPTRS values at the last visit were higher for those who had IND/DYS−. In addition, IND/DYS− outperformed DYS/IND− with regard to prediction accuracy and consistency with type 1 diabetes for associations with the baseline variables.
The lower risk of type 1 diabetes for DYS/IND− and its opposite directionality of associations with baseline variables to that of type 1 diabetes have implications beyond identifying optimal prediagnostic end points. These findings suggest that even among individuals with two or more autoantibodies who develop dysglycemia, outcomes besides type 1 diabetes are not uncommon. Some individuals could revert to an ongoing normoglycemic state, some could develop type 2 diabetes or other forms of diabetes, or some could have either intermittent dysglycemia or continuing dysglycemia without progression to type 1 diabetes. Thus, those who develop dysglycemia, even in the presence of autoantibodies, could be heterogeneous with varying outcomes. IND/DYS− appears to be more centered on the pathway to a type 1 diabetes outcome than DYS/IND−. The much older age at the time of the DYS/IND− OGTTs supports this view.
The number of individuals who developed incident dysglycemia was greater than the number who developed incident Index60 ≥1.00. This consideration is important because the number who reach an end point could affect the length and cost of a prevention trial. However, if DYS/IND− were excluded because of its questionable performance as a prediagnostic end point, the number of individuals with incident dysglycemia would be less than the number with incident Index60 ≥1.00.
Few studies have specifically examined potential prediagnostic end points for type 1 diabetes. In one study, changes in HbA1c and area under the curve C-peptide over a 2-year period suggested the possibility that metabolic markers can be used as intermediate end points for type 1 diabetes (16).
A limitation of the current study was the lack of confirmation of the prediagnostic end points because the PTP study was not designed to assess these end points. Protocols that use prediagnostic end points would likely require confirmation. Another limitation is the possibility that slow progression to type 1 diabetes was not captured during the follow-up period.
The PTP represents a much older population than at-risk cohorts followed from birth or a young age (17) for the development of autoantibodies and ultimately type 1 diabetes. Children from those cohorts who develop multiple autoantibodies are at a very high lifetime risk for type 1 diabetes. The uncertainty of the ultimate development of type 1 diabetes for those with multiple autoantibodies and DYS/IND− in the PTP highlights the importance of age as a modifier of risk for type 1 diabetes. Thus, the possible heterogeneity of outcomes in older, autoantibody-positive populations with dysglycemia deserves further exploration. A better understanding of the diverse outcomes that could occur would help to refine the design of type 1 diabetes prevention trials.
A major difference between dysglycemia and Index60 is that the latter combines both glucose and C-peptide information, which could be the main explanation for the superior performance of Index60 ≥1.00. Although the choice of a prediagnostic end point partially depends on the nature of the prevention trial, consideration should be given for developing prediagnostic end points for type 1 diabetes that combine glucose and C-peptide information, such as Index60 ≥1.00.
Supplementary Material
Article Information
Funding. The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the cooperative agreements U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085465, U01-DK-085453, U01-DK-085461, U01-DK-085466, U01-DK-085499, U01-DK-085504, U01-DK-085509, U01-DK-103180, U01-DK-103153, U01-DK-085476, U01-DK-103266, U01-DK-103282, U01-DK-106984, U01-DK-106994, U01-DK-107013, U01-DK-107014, and UC4-DK-106993; JDRF; the Victorian State Government Operational Infrastructure Support Program; and the National Health and Medical Research Council Research Institute Infrastructure Support Scheme.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or JDRF.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.M.N. analyzed data and wrote the manuscript. D.B. and S.G. provided statistical guidance and reviewed the manuscript. M.A.A., P.C., R.G., W.R., J.M.W., D.M.W., and C.E.-M. assisted in the analysis and reviewed the manuscript. D.W. reviewed the manuscript. J.S.S. assisted in the analysis and reviewed the manuscript. A.M. and J.M.S. assisted in the analysis and in writing the manuscript. J.M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0916/-/DC1.
References
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986;314:1360–1368 [DOI] [PubMed] [Google Scholar]
- 2.Gorsuch AN, Spencer KM, Lister J, et al. Evidence for a long prediabetic period in type I (insulin-dependent) diabetes mellitus. Lancet 1981;2:1363–1365 [DOI] [PubMed] [Google Scholar]
- 3.Baekkeskov S, Landin M, Kristensen JK, et al. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest 1987;79:926–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarn AC, Smith CP, Spencer KM, Bottazzo GF, Gale EAM. Type I (insulin dependent) diabetes: a disease of slow clinical onset? Br Med J (Clin Res Ed) 1987;294:342–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sosenko JM, Palmer JP, Greenbaum CJ, et al. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial–Type 1. Diabetes Care 2006;29:643–649 [DOI] [PubMed] [Google Scholar]
- 6.Sosenko JM, Skyler JS, Beam CA, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in Diabetes Prevention Trial–Type 1 participants. Diabetes 2013;62:4179–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Trial—Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 [DOI] [PubMed] [Google Scholar]
- 8.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 9.Gale EA, Bingley PJ, Emmett CL, Collier T; European Nicotinamide Diabetes Intervention Trial (ENDIT) Group . European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004;363:925–931 [DOI] [PubMed] [Google Scholar]
- 10.Sosenko JM, Palmer JP, Rafkin-Mervis L, et al.; Diabetes Prevention Trial–Type 1 Study Group . Incident dysglycemia and progression to type 1 diabetes among participants in the Diabetes Prevention Trial–Type 1. Diabetes Care 2009;32:1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sosenko JM, Skyler JS, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial–Type 1 Study Group . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosenko JM, Mahon J, Rafkin L, et al.; Diabetes Prevention Trial-Type 1 and TrialNet Study Groups . A comparison of the baseline metabolic profiles between Diabetes Prevention Trial-Type 1 and TrialNet Natural History Study participants. Pediatr Diabetes 2011;12:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sosenko JM, Skyler JS, Palmer JP, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial–Type 1 Study Group . The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 2013;36:2615–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosenko JM, Krischer JP, Palmer JP, et al.; Diabetes Prevention Trial–Type 1 Study Group . A risk score for type 1 diabetes derived from autoantibody-positive participants in the Diabetes Prevention Trial–Type 1. Diabetes Care 2008;31:528–533 [DOI] [PubMed] [Google Scholar]
- 15.Uno H, Cai T, Tian L, Wei LJ. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc 2007;102:527–537 [Google Scholar]
- 16.Krischer JP; Type 1 Diabetes TrialNet Study Group . The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 2013;56:1919–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.