Abstract
OBJECTIVE
To examine the associations of prediabetes, diabetes, and diabetes severity (as assessed by HbA1c and diabetes duration) with brain volumes and vascular pathology on brain MRI and to assess whether the associations of diabetes with brain volumes are mediated by brain vascular pathology.
RESEARCH DESIGN AND METHODS
Cross-sectional study of 1,713 participants in the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) (mean age 75 years, 60% female, 27% black, 30% prediabetes, and 35% diabetes) who underwent 3T brain MRI scans in 2011–2013. Participants were categorized by diabetes-HbA1c status as without diabetes (<5.7% [reference]), with prediabetes (5.7 to <6.5%), and with diabetes ([defined as prior diagnosis or HbA1c ≥6.5%] <7.0% vs. ≥7.0%), with further stratification by diabetes duration (<10 vs. ≥10 years).
RESULTS
In adjusted analyses, compared with participants without diabetes and HbA1c <5.7%, participants with prediabetes and those with diabetes and HbA1c <7.0% did not have significantly different brain volumes or vascular pathology (all P > 0.05), but those with diabetes and HbA1c ≥7.0% had smaller total brain volume (β −0.20 SDs, 95% CI −0.31, −0.09), smaller regional brain volumes (including frontal, temporal, occipital, and parietal lobes; deep gray matter; Alzheimer disease signature region; and hippocampus [all P < 0.05]), and increased burden of white matter hyperintensities (WMH) (P = 0.016). Among participants with diabetes, those with HbA1c ≥7.0% had smaller total and regional brain volumes and an increased burden of WMH (all P < 0.05) compared with those with HbA1c <7.0%. Similarly, participants with longer duration of diabetes (≥10 years) had smaller brain volumes and higher burden of lacunes (all P < 0.05) than those with a diabetes duration <10 years. We found no evidence for mediation by WMH in associations of diabetes with smaller brain volumes by structural equation models (all P > 0.05).
CONCLUSIONS
More-severe diabetes (defined by higher HbA1c and longer disease duration) but not prediabetes or less-severe diabetes was associated with smaller brain volumes and an increased burden of brain vascular pathology. No evidence was found that associations of diabetes with smaller brain volumes are mediated by brain vascular pathology, suggesting that other mechanisms may be responsible for these associations.
Introduction
Diabetes and prediabetes are associated with accelerated cognitive decline (1), and diabetes is associated with an approximately twofold increased risk of dementia (2). Subclinical brain pathology, as defined by small vessel disease (lacunar infarcts, white matter hyperintensities [WMH], and microhemorrhages), large vessel disease (cortical infarcts), and smaller brain volumes also are associated with an increased risk of cognitive decline and dementia (3–7). The mechanisms by which diabetes contributes to accelerated cognitive decline and dementia are not fully understood, but contributions of hyperglycemia to both cerebrovascular disease and primary neurodegenerative disease have been suggested in the literature, although results are inconsistent (2,8). Given that diabetes is a vascular risk factor, brain atrophy among individuals with diabetes may be driven by increased cerebrovascular disease. Brain magnetic resonance imaging (MRI) provides a noninvasive opportunity to study associations of hyperglycemia with small vessel disease (lacunar infarcts, WMH, microhemorrhages), large vessel disease (cortical infarcts), and brain volumes (9). The Standards for Reporting Vascular Changes on Neuroimaging statement supports brain MRI as a good surrogate marker of large and small vessel disease (9); the goal of this statement is to provide standardized definitions for vascular lesions and brain volumes. The existing body of literature on the topic of hyperglycemia with MRI findings has important limitations, including a lack of rigorous assessments of both diabetes status and brain MRI markers of subclinical cerebrovascular disease and total and regional brain volumes within the same study population. Furthermore, few prior studies evaluated the associations of prediabetes (10) and indices of diabetes severity (e.g., glycated hemoglobin [HbA1c], disease duration) (11,12) with brain MRI measures.
The community-based Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) of older individuals (mean age 75 years, range 67–90 years) provides a unique opportunity to examine the associations of prediabetes and diabetes with total and regional brain volumes and cerebrovascular pathology, including cortical infarcts, lacunar infarcts, lobar microhemorrhages, subcortical microhemorrhages, and WMH volume, on brain MRI. We hypothesized that older adults with prediabetes and diabetes have smaller total and regional brain volumes and increased burden of subclinical cerebrovascular disease compared with older individuals with normoglycemia. We also hypothesized that among individuals with diabetes, those with more-severe disease (as measured by higher HbA1c and longer disease duration) have smaller brain volumes and an increased burden of subclinical cerebrovascular disease than those with better glycemic control and shorter disease duration. Furthermore, we hypothesized that the associations of prediabetes and diabetes with smaller brain volumes is mediated by cerebrovascular disease.
Research Design and Methods
Study Population
The ARIC Study is an ongoing, community-based prospective cohort study of 15,792 middle-aged adults (age 45–64 years at baseline) recruited from four U.S. communities: Washington County, Maryland; Forsyth County, North Carolina; the suburbs of Minneapolis, Minnesota; and Jackson, Mississippi (13). Participants were initially seen at four in-person visits that occurred ∼3 years apart from 1987 to 1989 for visit 1 through 1996 to 1998 for visit 4. A fifth visit was conducted from 2011 to 2013 and was attended by 6,538 participants. Supplementary Table 1 shows the baseline (1987–1989) characteristics of ARIC participants, comparing those who attended versus those who did not attend ARIC visit 5. A subsample of participants who attended visit 5 was selected for brain MRI scans (14). Briefly, selection criteria for a visit 5 brain MRI scan were absence of MRI contraindications and any one of the following: 1) prior participation in the ARIC brain MRI ancillary study (15), 2) evidence of cognitive impairment at visit 5 (low Mini-Mental State Examination score [<21 for whites and <19 for blacks] or both low visit 5 domain z scores on two or more cognitive domains [< −1.5 SD; domains of memory, executive function, language] and cognitive decline on the Delayed Word Recall Test, the Digit Symbol Substitution Test, or the Word Fluency Test [defined as visit 5 score − highest previous score <20th percentile on one or more tests or <10th percentile on two or more tests]), or 3) a random sample of participants without evidence of cognitive impairment at visit 5. In total, 1,978 participants underwent visit 5 brain MRI scans. Of these, we excluded 80 participants with clinical neurologic disease (multiple sclerosis, stroke, surgery/radiation to skull/brain, brain tumor), 53 with incomplete MRI data or poor image quality, 32 with missing data on diabetes status or HbA1c, and 100 with missing covariates included in our statistical models, leaving a total of 1,713 participants included in the current analysis. Supplementary Table 2 shows characteristics of ARIC visit 5 participants compared with those included and excluded from the current analysis.
The ARIC Study has been approved by the institutional review boards at all participating institutions. All participants gave written informed consent at each study visit.
Diabetes Definition and Measurement of HbA1c and Fasting Glucose
Diabetes was defined by a self-reported physician diagnosis, diabetes medication use assessed at study visits and on annual follow-up telephone calls, or an HbA1c ≥6.5% measured at ARIC visit 5 (2011–2013). Diabetes duration was dichotomized as <10 years versus ≥10 years. In sensitivity analyses, we created a separate category for undiagnosed diabetes (defined as no physician diagnosis and no diabetes medication use with an HbA1c ≥6.5%).
HbA1c was measured at visit 5 (2011–2013). Whole-blood samples were assayed for HbA1c measurement by using high-performance liquid chromatography (G7 HPLC Analyzer; Tosoh Bioscience, South San Francisco, CA). HbA1c was categorized by using cut points defined in the American Diabetes Association clinical practice recommendations (16,17): <5.7% (without diabetes), 5.7 to <6.5% (prediabetes), and <7.0% and ≥7.0% (diabetes).
In sensitivity analyses, we also defined prediabetes/diabetes categories by using fasting glucose: <100 mg/dL (without diabetes), 100 to <126 mg/dL (prediabetes), and <150 and ≥150 mg/dL (with diabetes) (16). Fasting glucose was measured by hexokinase method in a subset of participants who fasted at least 8 h (n = 1,639).
Brain MRI Protocol and Image Analysis
The ARIC visit 5 (2011–2013) brain MRI scans were performed by using four 3T scanners (Maryland: Siemens Verio; North Carolina: Siemens Skyra; Minnesota: Siemens Trio; Mississippi: Siemens Skyra). The following sequences were obtained: localizer, magnetization-prepared rapid gradient-echo MP-RAGE (1.2-mm slices), axial gradient recalled echo T2-weighted imaging (T2*GRE) (4-mm slices), axial T2 fluid-attenuated inversion recovery (FLAIR) (5-mm slices), field mapping (3-mm slices), and axial diffusion tenor images (2.7-mm slices for Skyra and Verio scanners and 3-mm slices for Trio scanner). Brain volumes were measured on MP-RAGE sequences using image analysis software (FreeSurfer; http://surfer.nmr.mgh.harvard.edu) (18), WMH volume and infarcts were assessed on T2 FLAIR sequences, and microhemorrhages were assessed on T2*GRE sequences. WMH burden was measured quantitatively by using an algorithm developed at the Mayo Clinic in Rochester, Minnesota (19). Lacunar infarcts were defined as subcortical T2 FLAIR lesions with central hypointensity >3 mm and hyperintensity ≤20 mm in maximum dimension located in the caudate, lenticular nucleus, internal capsule, thalamus, brainstem, deep cerebellar white matter, centrum semiovale, or corona radiata (9,20). Cortical infarcts were defined as T2 FLAIR lesions of >20 mm in minimum diameter (21). Microhemorrhages were lesions on T2*GRE sequences of ≤5 mm in maximum diameter and were divided into lobar and subcortical microhemorrhages (9).
The following brain volumes were used as outcomes in our analyses: total brain volume, lobar volumes (frontal, parietal, temporal, and occipital), deep gray subcortical structure volume (defined as the total volume of the thalamus, caudate, putamen, and globus pallidum), total volume of an Alzheimer disease signature region (defined as the total volume of parahippocampal, entorhinal, and inferior parietal lobules; hippocampus; precuneus; and cuneus) (22), and hippocampal volume.
Statistical Analysis
Characteristics of the study population at visit 5 (2011–2013) are shown by diabetes-HbA1c category. To compare across diabetes-HbA1c categories, t tests or P values for linear trend were used to compare means for continuous variables, and χ2 tests were used to compare proportions for categorical variables. Adjusted linear and logistic regression models were used to assess the associations of diabetes-HbA1c categories with brain volumes and subclinical cerebrovascular disease. All analyses incorporated sampling weights to account for the ARIC brain MRI sampling strategy; therefore, all analyses represent an estimation of associations in the entire ARIC visit 5 (2011–2013) population. In linear regression analyses for brain volumes, each brain volume was scaled on the basis of its SD to facilitate comparison of the magnitude of association across brain regions. WMH volume was log base 2 (log2) transformed for normality. In analyses stratified by diagnosed diabetes status (defined by self-reported physician diagnosis or medication use), we also modeled the association of continuous HbA1c with total brain volume by using a restricted cubic spline with four knots placed at the 5th, 35th, 65th, and 95th percentiles (23). Secondary analyses were done to investigate associations of HbA1c and diabetes duration categories with brain volumes and markers of subclinical cerebrovascular disease among participants with diabetes (n = 602).
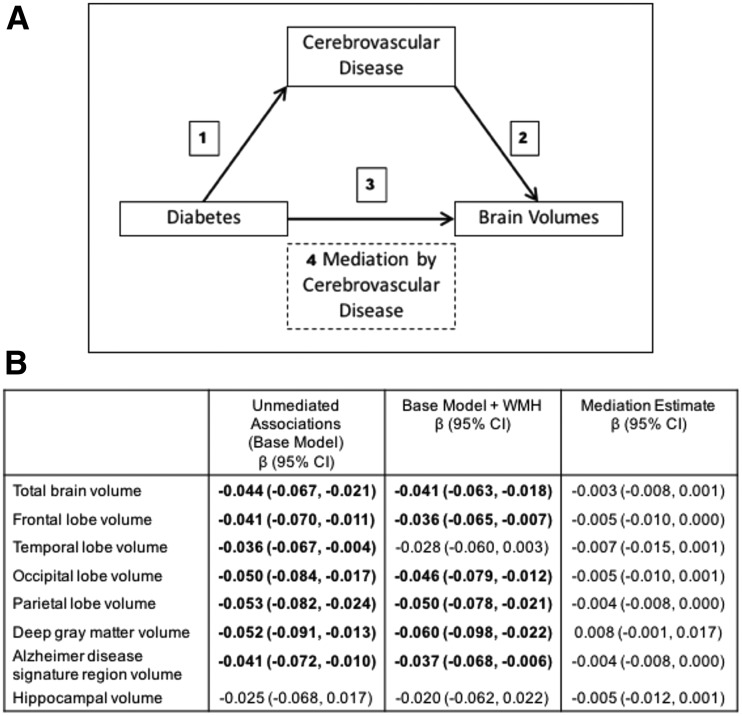
To assess whether associations of diabetes with smaller brain volumes are mediated by cerebrovascular disease, we used mediation pathway methods (24). In this analysis, we examined relationships between diabetes and MRI markers of cerebrovascular disease (path 1 in Fig. 1A), between MRI markers of cerebrovascular disease and brain volumes (path 2 in Fig. 1A), and between diabetes and brain volumes (path 3 in Fig. 1A). Finally, we assessed whether relationships between diabetes and brain volumes were attenuated when also adjusted for MRI markers of cerebrovascular disease. We formally calculated mediation estimates from indirect effects (the difference between estimates obtained when looking at the associations of diabetes with brain volumes [total effect; path 3] and estimates obtained when further adjusted for cerebrovascular disease [direct effects, path 4] by using structural equation models when significant associations were seen for paths 1, 2, and 3 [Fig. 1]) (24).
Figure 1.
Boldface data represent significance at the P < 0.05 level. Mediation model to assess whether the associations of diabetes with brain volumes are mediated by cerebrovascular disease. A: If mediation by brain vascular pathology is present, paths 1–3 should be significantly associated and path 3 should be attenuated when also adjusted for the potential mediation (path 4). B: Weighted models adjusted for age, sex, race/field center, education, smoking status, hypertension, cardiovascular disease, APOE ε4 genotype, and total intracranial volume.
All covariates used in the regression models were assessed at visit 5 (2011–2013) unless otherwise specified. Covariates were age (years), sex (male, female), race/field center (Maryland whites; Minnesota whites; North Carolina whites; North Carolina blacks; Mississippi blacks), education (less than high school, high school or equivalent, college or graduate or professional school as assessed at visit 1 [1987–1989]), cigarette smoking status (current, former, never, not reported), hypertension (systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg by self-report of physician diagnosis or hypertension medication use), cardiovascular disease (history of coronary artery disease, myocardial infarction, coronary artery bypass surgery, and/or angioplasty, including previous reviewer-adjudicated events during ARIC follow-up), and total intracranial volume (in cubic centimeters; included in models where outcome is volume).
All reported P values were based on two-sided tests, and P < 0.05 was considered statistically significant. Analyses were performed with Stata/SE 13 software (StataCorp, College Station, TX).
Results
Study Population Characteristics
Overall, the mean age of participants was 75 years, 60% were women, 27% were black, 30% had prediabetes (HbA1c 5.7 to <6.5%), and 35% had diabetes. Compared with participants without diabetes and HbA1c <5.7%, those with prediabetes (HbA1c 5.7 to <6.5%) were of similar age (75.2 vs. 75.0 years; P = 0.551), were more likely to be black (24% vs. 11%; P < 0.001), have less than a high school education (11% vs. 7%; P = 0.017), and have hypertension (71% vs. 63%; P = 0.012) (Table 1). Among participants with diabetes, those with HbA1c <7.0% versus ≥7.0% were of similar age (75.4 vs. 75.1 years; P = 0.481), but those with diabetes and HbA1c ≥7.0% were more likely to be black (39% vs. 28%; P = 0.020) and to have less than a high school education (23% vs. 16%; P = 0.031) and were more likely to have a longer duration of diabetes (12 vs. 8 years; P < 0.001).
Table 1.
Weighted participant characteristics by diabetes and HbA1c category, ARIC visit 5 (2011–2013) (n = 1,713)
| Diabetes |
|||||
|---|---|---|---|---|---|
| No diabetes (HbA1c <5.7%) | Prediabetes (HbA1c 5.7 to <6.5%) | HbA1c <7.0% | HbA1c ≥7.0% | P value‖ | |
| Patients (n) | 597 | 514 | 448 | 154 | |
| Mean age (years) | 75.0 | 75.2 | 75.4 | 75.1 | 0.654 |
| Female sex (%) | 59.7 | 61.7 | 64.4 | 60.4 | 0.595 |
| Race/field center (%) | <0.001 | ||||
| White/Minneapolis, MN | 35.1 | 29.4 | 25.4 | 19.6 | |
| White/Washington County, MD | 29.1 | 21.4 | 31.1 | 29.6 | |
| White/Forsyth County, NC | 25.1 | 25.1 | 15.6 | 12.0 | |
| Black/Forsyth County, NC | 0.3 | 1.8 | 3.3 | 1.0 | |
| Black/Jackson, MS | 10.5 | 22.3 | 24.5 | 37.9 | |
| Education* (%) | <0.001 | ||||
| Less than high school | 6.6 | 10.6 | 15.8 | 23.0 | |
| High school, GED, or vocational school | 40.0 | 40.3 | 40.6 | 44.8 | |
| College or graduate or professional school | 53.4 | 49.1 | 43.6 | 32.2 | |
| Smoking status (%) | 0.417 | ||||
| Never | 44.6 | 42.8 | 36.7 | 39.5 | |
| Former | 47.2 | 46.2 | 52.1 | 50.1 | |
| Current | 3.3 | 5.9 | 5.3 | 4.1 | |
| Not reported | 4.9 | 5.1 | 5.9 | 6.3 | |
| Hypertension (%) | 62.6 | 71.4 | 84.2 | 83.3 | <0.001 |
| Hyperlipidemia (%) | 51.8 | 60.5 | 68.8 | 73.0 | <0.001 |
| History of cardiovascular disease (%) | 5.2 | 8.4 | 8.8 | 8.4 | 0.127 |
| Atrial fibrillation (%) | 4.0 | 2.8 | 6.3 | 4.3 | 0.124 |
| Mean diabetes duration (years) | – | – | 8.2 | 12.1 | <0.001 |
| APOE ε4 genotype (%) | 0.763 | ||||
| 0 alleles | 71.2 | 73.8 | 73.7 | 71.1 | |
| 1 or 2 alleles | 28.8 | 26.2 | 26.3 | 28.9 | |
| Mean volume† (cm3) | |||||
| Total brain | 1,030.1 | 1,027.8 | 1,021.4 | 1,003.4 | <0.001 |
| Frontal lobe | 152.3 | 152.2 | 150.8 | 148.4 | <0.001 |
| Temporal lobe | 103.8 | 103.6 | 102.8 | 101.1 | <0.001 |
| Occipital lobe | 41.7 | 41.0 | 40.8 | 39.6 | <0.001 |
| Parietal lobe | 108.4 | 107.7 | 106.6 | 104.4 | <0.001 |
| Deep gray matter‡ | 30.1 | 30.3 | 30.2 | 29.3 | <0.001 |
| Alzheimer disease signature region§ | 60.5 | 60.1 | 59.6 | 58.5 | <0.001 |
| Hippocampus | 7.0 | 7.1 | 7.0 | 6.8 | <0.001 |
| Markers of subclinical cerebrovascular disease (%) | |||||
| Lobar microhemorrhages | 6.2 | 9.9 | 5.6 | 8.3 | 0.089 |
| Subcortical microhemorrhages | 17.8 | 17.7 | 16.1 | 21.7 | 0.621 |
| Cortical infarcts | 7.2 | 10.8 | 8.2 | 9.4 | 0.260 |
| Lacunar infarcts | 14.4 | 16.8 | 13.3 | 23.4 | 0.067 |
| Median WMH volume† (cm3) | 9.7 | 10.2 | 10.4 | 12.9 | 0.026 |
GED, general education development.
‖P value represents P linear trend across the median of HbA1c levels for each diabetes and HbA1c category for continuous variables and represents the χ2 P value for categorical variables.
*Assessed at ARIC visit 1 (1987–1989).
†Adjusted for total intracranial volume.
‡Defined as thalamus + putamen + caudate + globus pallidus.
§Defined as hippocampus + parahippocampal + entorhinal + inferior parietal lobule + precuneus + cuneus.
Associations of Prediabetes/Diabetes-HbA1c Categories With Brain Volumes and Markers of Subclinical Cerebrovascular Disease
Compared with participants without diabetes and HbA1c <5.7%, those with diabetes and HbA1c ≥7.0% had smaller total brain volume (β −0.20 SDs; 95% CI −0.31, −0.09) and smaller regional brain volumes, including frontal, temporal, occipital, and parietal lobes; deep gray matter; Alzheimer disease signature region; and hippocampus (all P < 0.05) (Table 2). Compared with participants with diabetes and HbA1c <7.0%, those with diabetes and HbA1c ≥7.0% had smaller total brain volume (P < 0.001), frontal lobe volume (P = 0.012), temporal lobe volume (P = 0.012), occipital lobe volume (P = 0.008), parietal lobe volume (P = 0.015), deep gray matter volume (P < 0.001), Alzheimer disease signature region volume (0.031), and hippocampal volume (P = 0.016). Both participants with diabetes and HbA1c <7.0% and those with prediabetes (HbA1c 5.7 to <6.5%) had similar total and regional brain volumes compared with participants without diabetes and HbA1c <5.7% (all P > 0.05). The continuous association of HbA1c (stratified by self-reported diabetes status) with total brain volume is shown in Supplementary Fig. 1. In sensitivity analyses, adding a separate category for individuals with undiagnosed diabetes (Supplementary Table 3), compared with participants without diabetes and HbA1c <5.7%, those with undiagnosed diabetes (n = 37) had similar brain volumes (all P > 0.05).
Table 2.
Weighted adjusted* cross-sectional associations of diabetes and HbA1c categories with brain MRI parameters, ARIC visit 5 (2011–2013) (n = 1,713)
| Diabetes |
||||
|---|---|---|---|---|
| No diabetes (HbA1c <5.7%) | Prediabetes (HbA1c 5.7 to <6.5%) | HbA1c <7.0% | HbA1c ≥7.0% | |
| Patients (n) | 597 | 514 | 448 | 154 |
| Volumes†, β (95% CI) | ||||
| Total brain | 0 (Reference) | 0.01 (−0.05, 0.08) | −0.02 (−0.08, 0.04) | −0.20 (−0.31, −0.09)‡ |
| Frontal lobe | 0 (Reference) | 0.03 (−0.05, 0.11) | −0.01 (−0.09, 0.07) | −0.15 (−0.26, −0.04)‡ |
| Temporal lobe | 0 (Reference) | 0.02 (−0.06, 0.10) | 0.01 (−0.07, 0.09) | −0.16 (−0.29, −0.02)‡ |
| Occipital lobe | 0 (Reference) | −0.04 (−0.14, 0.06) | −0.03 (−0.14, 0.07) | −0.22 (−0.36, −0.08)‡ |
| Parietal lobe | 0 (Reference) | 0.02 (−0.06, 0.10) | −0.02 (−0.10, 0.06) | −0.15 (−0.26, −0.04)‡ |
| Deep gray matter | 0 (Reference) | 0.05 (−0.06, 0.16) | 0.02 (−0.08, 0.13) | −0.28 (−0.42, −0.14)‡ |
| Alzheimer disease signature region | 0 (Reference) | 0.01 (−0.07, 0.09) | −0.02 (−0.11, 0.06) | −0.16 (−0.28, −0.03)‡ |
| Hippocampus | 0 (Reference) | 0.04 (−0.07, 0.15) | 0.00 (−0.11, 0.12) | −0.21 (−0.38, −0.03)‡ |
| Markers of subclinical cerebrovascular disease, OR (95% CI) | ||||
| Lobar microhemorrhages | 1 (Reference) | 1.62 (0.93, 2.80) | 0.91 (0.49, 1.68) | 1.33 (0.61, 2.91) |
| Subcortical microhemorrhages | 1 (Reference) | 0.88 (0.60, 1.29) | 0.78 (0.52, 1.18) | 1.07 (0.65, 1.76) |
| Cortical infarcts | 1 (Reference) | 1.44 (0.86, 2.41) | 1.02 (0.60, 1.75) | 1.13 (0.57, 2.25) |
| Lacunar infarcts | 1 (Reference) | 1.10 (0.74, 1.62) | 0.80 (0.53, 1.21) | 1.61 (0.91, 2.83) |
| Log2 WMH volume†, β (95% CI) | 0 (Reference) | 0.11 (−0.06, 0.29) | 0.01 (−0.15, 0.17) | 0.29 (0.05, 0.52)‡ |
Boldface data represent P < 0.05 compared with no diabetes and HbA1c <5.7%.
*Model adjusted for age, sex, race/field center, education, smoking status, hypertension, cardiovascular disease, APOE ε4 genotype, and total intracranial volume (when outcome is volume).
†SD units. Definition of 1 SD: total brain volume 108.1 cm3, frontal lobe volume 16.0 cm3, temporal lobe volume 11.7 cm3, occipital lobe volume 5.5 cm3, parietal lobe volume 12.6 cm3, deep gray matter volume 4.3 cm3, Alzheimer disease signature region volume 7.0 cm3, hippocampal volume 1.0 cm3.
‡P < 0.05 for comparison of diabetes and HbA1c ≥7.0% vs. diabetes and HbA1c <7.0%.
No differences in the presence of lobar microhemorrhages, subcortical microhemorrhages, cortical infarcts, and lacunar infarcts were observed among the diabetes-HbA1c categories (all P > 0.05) (Table 2). Compared with participants without diabetes and HbA1c <5.7%, those with diabetes and HbA1c ≥7.0% had increased WMH volume (P = 0.016). The WMH volume among participants with diabetes and HbA1c ≥7.0% was also significantly greater than among those with diabetes and HbA1c <7.0% (P = 0.017).
In sensitivity analyses wherein prediabetes/diabetes categories were defined by fasting glucose among 1,639 participants who fasted at least 8 h, results overall were similar in pattern to the main analyses that used HbA1c but were attenuated (Supplementary Table 4). Compared with participants without diabetes and fasting glucose <100 mg/dL, those with prediabetes (fasting glucose 100 to <126 mg/dL) had similar brain volumes and markers of cerebrovascular disease (all P > 0.05), whereas those with diabetes and fasting glucose <150 mg/dL had smaller total brain volume (β −0.09 SDs; 95% CI −0.16, −0.02), and those with diabetes and fasting glucose ≥150 mg/dL had smaller total brain volume (β −0.15 SDs; 95% CI −0.25, −0.04), deep gray matter volume (β −0.32 SDs; 95% CI −0.49, −0.15), and hippocampal volume (β −0.27 SDs; 95% CI −0.45, −0.10).
Associations of Diabetes Duration Categories With Brain Volumes and Markers of Subclinical Cerebrovascular Disease Among Participants With Diabetes
The characteristics of the 602 participants with diabetes are shown stratified by diabetes duration category in Supplementary Table 5. Those with diabetes duration ≥10 years were older than those with diabetes duration <10 years (75.9 vs. 75.0 years; P = 0.041) but were similar in terms of race and sex (all P > 0.05). Compared with participants with diabetes duration <10 years, those with diabetes duration ≥10 years has smaller adjusted total brain volume (β −0.13 SDs; 95% CI −0.20, −0.05) and smaller temporal lobe (β −0.14 SDs; 95% CI −0.24, −0.03), parietal lobe (β − 0.11 SDs; 95% CI −0.21, −0.01), and hippocampal (β −0.16 SDs; 95% CI −0.30, −0.02) volumes (Table 3). Participants with diabetes duration ≥10 years also had a 2.44 times increased odds (95% CI 1.46, 4.05) of lacunar infarcts compared with those with diabetes duration <10 years (Table 3). In sensitivity analyses, adding a separate category for individuals with undiagnosed diabetes (n = 37) (Supplementary Table 6), compared with participants with diagnosed diabetes, those with diagnosed diabetes and duration <10 years had smaller frontal lobe volumes (β −0.23 SDs; 95% CI −0.43, −0.03) and a 3.13 times increased odds (95% CI 1.11, 8.88) of subcortical microhemorrhages. Those with diagnosed diabetes and duration ≥10 years had smaller total brain; frontal, temporal, and parietal lobe; and Alzheimer disease signature region volumes and an increased odds of subcortical microhemorrhages and lacunes compared with the individuals with undiagnosed diabetes (all P < 0.05).
Table 3.
Weighted adjusted* cross-sectional associations of diabetes duration categories with brain MRI parameters among participants with diabetes, ARIC visit 5 (2011–2013) (n = 602)
| Diabetes duration <10 years (n = 342) | Diabetes duration ≥10 years (n = 260) | |
|---|---|---|
| Volumes†, β (95% CI) | ||
| Total brain | 0 (Reference) | −0.13 (−0.20, −0.05) |
| Frontal lobe | 0 (Reference) | −0.04 (−0.15, 0.06) |
| Temporal lobe | 0 (Reference) | −0.14 (−0.24, −0.03) |
| Occipital lobe | 0 (Reference) | −0.08 (−0.20, 0.04) |
| Parietal lobe | 0 (Reference) | −0.11 (−0.21, −0.01) |
| Deep gray matter | 0 (Reference) | −0.12 (−0.25, 0.02) |
| Alzheimer disease signature region | 0 (Reference) | −0.10 (−0.20, 0.01) |
| Hippocampus | 0 (Reference) | −0.16 (−0.30, −0.02) |
| Markers of subclinical cerebrovascular disease, OR (95% CI) | ||
| Lobar microhemorrhages | 1 (Reference) | 1.03 (0.50, 2.12) |
| Subcortical microhemorrhages | 1 (Reference) | 1.13 (0.69, 1.85) |
| Cortical infarcts | 1 (Reference) | 1.47 (0.76, 2.85) |
| Lacunar infarcts | 1 (Reference) | 2.44 (1.46, 4.05) |
| Log2 WMH volume†, β (95% CI) | 0 (Reference) | 0.18 (−0.03, 0.39) |
Boldface data represent P < 0.05.
*Model adjusted for age, sex, race/field center, education, smoking status, hypertension, cardiovascular disease, APOE ε4 genotype, and total intracranial volume (when outcome is volume).
†SD units. Definition of 1 SD: total brain volume 108.1 cm3, frontal lobe volume 16.0 cm3, temporal lobe volume 11.7 cm3, occipital lobe volume 5.5 cm3, parietal lobe volume 12.6 cm3, deep gray matter volume 4.3 cm3, Alzheimer disease signature region volume 7.0 cm3, hippocampal volume 1.0 cm3.
Mediation Analyses
As shown in Table 2, the diabetes and HbA1c ≥7.0% category was associated with smaller total and regional brain volumes (path 3 in Fig. 1A) and with increased WMH burden but not with any other marker of subclinical cerebrovascular disease (path 1 in Fig. 1A). Additional analyses showed that WMH burden was significantly associated with all total and regional brain volumes (all P < 0.05) (path 2 in Fig. 1A). Therefore, we assessed by using structural equation models whether associations of diabetes with smaller brain volumes were mediated by WMH burden (Fig. 1B). We did not find any evidence at the P < 0.05 level that associations of diabetes and HbA1c ≥7.0% with smaller brain volumes were mediated by WMH burden (all P values for indirect effects 0.072–0.081) (Fig. 1B).
Conclusions
In this community-based population, we found that ARIC-NCS participants with diabetes with HbA1c ≥7.0% have smaller total and regional brain volumes and an increased burden of WMH, but those with prediabetes (HbA1c 5.7 to <6.5%) and diabetes with HbA1c <7.0% have brain volumes and markers of subclinical cerebrovascular disease similar to those without diabetes. Furthermore, among participants with diabetes, those with more-severe disease (as measured by higher HbA1c and longer disease duration) had smaller total and regional brain volumes and an increased burden of cerebrovascular disease compared with those with lower HbA1c and shorter disease duration. However, we found no evidence that associations of diabetes with smaller brain volumes are mediated by cerebrovascular disease.
The findings of this study extend the current literature that suggests that diabetes is strongly associated with brain volume loss (11,25–27). Global brain volume loss (11,25–27) has been consistently reported, but associations of diabetes with smaller specific brain regions have been less robust (27,28). Similar to prior studies, the current results show that compared with individuals without diabetes, those with diabetes have smaller total brain volume (11,25–27) and regional brain volumes, including frontal and occipital lobes, deep gray matter, and the hippocampus (25,27). Furthermore, the current study suggests that greater severity of disease (as measured by HbA1c and diabetes duration) is associated with smaller total and regional brain volumes. These results are consistent with some prior studies that have included measures of disease severity (11,12) but not others (28). The current study found that participants with prediabetes and those with diabetes and HbA1c <7.0% had total and regional brain volumes similar to those without diabetes, suggesting that the effects of diabetes on brain volumes are primarily driven by disease severity (higher HbA1c and longer duration). Mechanisms whereby diabetes may contribute to brain volume loss include accelerated amyloid-β and hyperphosphorylated tau deposition as a result of hyperglycemia (29). Another possible mechanism involves pancreatic amyloid (amylin) infiltration of the brain, which then promotes amyloid-β deposition (29). Few studies have specifically looked at associations of prediabetes (defined by HbA1c and/or fasting glucose) with brain volumes. The Second Manifestations of Arterial Disease-Magnetic Resonance study reported associations of metabolic syndrome with smaller total brain volume (10); however, similar to the current study, the individual component of impaired glucose metabolism (prediabetes) was not associated with smaller total brain volume compared with normoglycemia.
Studies of diabetes with small vessel ischemic disease have shown inconsistent results, particularly for WMH (30,31), whereas associations of diabetes with lacunes have been slightly more consistent (32–35). One review on the topic of the association of diabetes with WMH (30) suggested that the inconsistencies across studies may be partially due to the advent of higher-resolution imaging and improved methodology to measure volumes of markers of cerebrovascular disease because the authors observed more significant associations between diabetes and WMH in studies that used quantitative volumetric MRI techniques versus those that used dichotomous or ordinal rating scales for WMH burden. Indeed, we used 3T MRI scanners with volumetric assessment of WMH burden and found significantly increased WMH burden among participants with diabetes, especially among those with more-severe disease as assessed by higher HbA1c, compared with those without diabetes. The introduction of higher-resolution MRI has also allowed for the assessment of microhemorrhages. Similar to prior studies (36–38), we did not find significant associations of diabetes with either lobar or subcortical microhemorrhages.
Taken together, in contrast to prior work suggesting little or no association of diabetes with small vessel disease (11,31), the current results suggest that diabetes is associated with both lower brain volumes and increased cerebrovascular pathology (WMH and lacunes). However, we did not see evidence for mediation by markers of subclinical cerebrovascular disease in the associations between diabetes and smaller brain volumes. These findings have important implications for mechanisms underlying the observed associations of diabetes with both accelerated cognitive decline (1) and increased risk of dementia (2) and suggest that diabetes may lead to a higher prevalence of subclinical cerebrovascular disease and smaller brain volumes through independent mechanisms. In contrast to the current findings in diabetes, prior work in the entire ARIC-NCS cohort (39) found that relationships between markers of cerebrovascular disease on MRI and cognitive function are mediated by a process that affects brain volumes (perhaps microinfarcts not consistently visible by MRI), suggesting that cerebrovascular disease and brain volumes may have shared underlying pathophysiological mechanisms. Although our mediation analyses were not significant at the P < 0.05 level, the P values for mediation ranged from 0.072 to 0.081, suggesting that this study was underpowered to assess mediation effects. These mediation analyses should be interpreted with caution, and future studies should also investigate mediation by cerebrovascular disease in the associations of diabetes with brain volumes in larger populations.
Strengths of this study include a detailed assessment of covariates, diabetes, and indices of diabetes severity, including HbA1c and diabetes duration, and >1,700 participants with brain MRI data. The current brain MRI data were obtained from 3T scanners and included total and regional brain volumes as well as microhemorrhages, cortical and lacunar infarcts, and WMH volumes. The study was limited by the inability to evaluate within-person changes in brain volumes and markers of subclinical cerebrovascular disease over time. Another important limitation of this cross-sectional study is the possibility of reverse causation, with participants with cognitive impairment and presumably smaller brain volumes and increased cerebrovascular disease being less compliant with medications and diet, which could then lead to poorer glycemic control. However, reverse causation is an unlikely explanation for the results given supporting evidence from prospective studies of diabetes and brain volumes and cerebrovascular disease showing associations with increased brain atrophy and increased burden of cerebrovascular lesions over time (14).
In conclusion, in this community-based population of ARIC-NCS participants, diabetes, but not prediabetes, was associated with smaller brain volumes. Furthermore, participants with more-severe diabetes (as assessed by higher HbA1c and longer disease duration) had smaller brain volumes and an increased burden of WMH and lacunes than those with less-severe disease. We found no evidence that associations of diabetes with smaller brain volumes were mediated by cerebrovascular disease, suggesting that other mechanisms may be responsible for these associations. These findings have implications for mechanisms underlying the observed associations of diabetes with both accelerated cognitive decline and increased risk of dementia, suggesting that both brain atrophy and small vessel ischemic disease may independently contribute, but additional prospective studies are needed to look directly at both cognitive function and brain imaging findings among individuals with and without diabetes over time.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff and participants of the ARIC Study for important contributions.
Funding. The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HH-SN-268201100005C, HH-SN-268201100006C, HH-SN-268201100007C, HH-SN-268201100008C, HH-SN-268201100009C, HH-SN-268201100010C, HH-SN-268201100011C, and HH-SN-268201100012C). The ARIC-NCS was supported by NHLBI grants U01-HL-096812, U01-HL-096814, U01-HL-096899, U01-HL-096902, and U01-HL-096917. A.L.C.S. is supported by the National Institute of Neurological Disorders and Stroke through an administrative supplement to award R25-NS-065729. E.S. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants K24-DK-106414 and R01-DK-089174.
Duality of Interest. D.K. serves on a data safety monitoring board for Lundbeck and for the Dominantly Inherited Alzheimer Network (DIAN) study and is an investigator in clinical trials sponsored by Biogen, TauRX Therapeutics, and Eli Lilly. R.F.G. is an associate editor for the journal Neurology. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L.C.S. performed the data analysis, contributed to the discussion, and wrote the manuscript. E.S., A.R.S., M.G., J.C., C.R.J., D.K., and T.M. contributed to the discussion and reviewed and edited the manuscript. R.F.G. performed the data analysis, contributed to the discussion, and reviewed and edited the manuscript. A.L.C.S. and R.F.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 141st Annual Meeting of the American Neurological Association, Baltimore, MD, 16–18 October 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1185/-/DC1.
References
- 1.Rawlings AM, Sharrett AR, Schneider AL, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014;161:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol 2008;7:184–190 [DOI] [PubMed] [Google Scholar]
- 3.Zahodne LB, Wall MM, Schupf N, et al. Late-life memory trajectories in relation to incident dementia and regional brain atrophy. J Neurol 2015;262:2484–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol 2012;69:1621–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 2016;73:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitagawa K, Miwa K, Yagita Y, Okazaki S, Sakaguchi M, Mochizuki H Association between carotid stenosis or lacunar infarction and incident dementia in patients with vascular risk factors. Eur J Neurol 2015;22:187–192 [DOI] [PubMed] [Google Scholar]
- 7.Ganguli M, Lee CW, Snitz BE, Hughes TF, McDade E, Chang CC. Rates and risk factors for progression to incident dementia vary by age in a population cohort. Neurology 2015;84:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015;3:75–89 [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Smith EE, Biessels GJ, et al.; Standards for Reporting Vascular Changes on Neuroimaging (STRIVE v1) . Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiehuis AM, van der Graaf Y, Mali WP, Vincken K, Muller M, Geerlings MI; SMART Study Group . Metabolic syndrome, prediabetes, and brain abnormalities on MRI in patients with manifest arterial disease: the SMART-MR study. Diabetes Care 2014;37:2515–2521 [DOI] [PubMed] [Google Scholar]
- 11.Bryan RN, Bilello M, Davatzikos C, et al. Effect of diabetes on brain structure: the Action to Control Cardiovascular Risk in Diabetes MR imaging baseline data. Radiology 2014;272:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saczynski JS, Siggurdsson S, Jonsson PV, et al. Glycemic status and brain injury in older individuals: the Age Gene/Environment Susceptibility-Reykjavik Study. Diabetes Care 2009;32:1608–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ARIC Study Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 14.Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology 2011;76:1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosley TH Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities Study. Neurology 2005;64:2056–2062 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 18.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355 [DOI] [PubMed] [Google Scholar]
- 19.Jack CR Jr, O’Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging 2001;14:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dearborn JL, Schneider AL, Sharrett AR, et al. Obesity, insulin resistance, and incident small vessel disease on magnetic resonance imaging: Atherosclerosis Risk in Communities Study. Stroke 2015;46:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology 2009;72:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerson BC, Stoub TR, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011;76:1395–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, Springer, 2001 [Google Scholar]
- 24.Rabe-Hesketh S, Skrondal A, Pickles A. Generalized multilevel structural equation modeling. Psychometrika 2004;69:167–190 [Google Scholar]
- 25.Moulton CD, Costafreda SG, Horton P, Ismail K, Fu CH. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav 2015;9:651–662 [DOI] [PubMed] [Google Scholar]
- 26.Li W, Risacher SL, Huang E, Saykin AJ; Alzheimer’s Disease Neuroimaging Initiative. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology 2016;87:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falvey CM, Rosano C, Simonsick EM, et al.; Health ABC Study . Macro- and microstructural magnetic resonance imaging indices associated with diabetes among community-dwelling older adults. Diabetes Care 2013;36:677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wisse LE, de Bresser J, Geerlings MI, et al.; Utrecht Diabetic Encephalopathy Study Group; SMART-MR Study Group . Global brain atrophy but not hippocampal atrophy is related to type 2 diabetes. J Neurol Sci 2014;344:32–36 [DOI] [PubMed] [Google Scholar]
- 29.Bharadwaj P, Wijesekara N, Liyanapathirana M, et al. The link between type 2 diabetes and neurodegeneration: roles for amyloid-β, amylin, and tau proteins. J Alzheimers Dis 2017;59:421–432 [DOI] [PubMed] [Google Scholar]
- 30.Del Bene A, Ciolli L, Borgheresi L, Poggesi A, Inzitari D, Pantoni L. Is type 2 diabetes related to leukoaraiosis? An updated review. Acta Neurol Scand 2015;132:147–155 [DOI] [PubMed] [Google Scholar]
- 31.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care 2006;29:2539–2548 [DOI] [PubMed] [Google Scholar]
- 32.Korf ES, White LR, Scheltens P, Launer LJ. Brain aging in very old men with type 2 diabetes: the Honolulu-Asia Aging Study. Diabetes Care 2006;29:2268–2274 [DOI] [PubMed] [Google Scholar]
- 33.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2002;33:21–25 [DOI] [PubMed] [Google Scholar]
- 34.Roberts RO, Knopman DS, Przybelski SA, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014;82:1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bezerra DC, Sharrett AR, Matsushita K, et al. Risk factors for lacune subtypes in the Atherosclerosis Risk in Communities (ARIC) Study. Neurology 2012;78:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 2013;36:4036–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu C, Sigurdsson S, Zhang Q, et al. Diabetes, markers of brain pathology and cognitive function: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol 2014;75:138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brundel M, Reijmer YD, van Veluw SJ, et al.; Utrecht Vascular Cognitive Impairment Study Group . Cerebral microvascular lesions on high-resolution 7-Tesla MRI in patients with type 2 diabetes. Diabetes 2014;63:3523–3529 [DOI] [PubMed] [Google Scholar]
- 39.Knopman DS, Griswold ME, Lirette ST, et al.; ARIC Neurocognitive Investigators . Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities-Neurocognitive Study. Stroke 2015;46:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.