Abstract
OBJECTIVE
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are new medications that improve cardiovascular and renal outcomes in patients with type 2 diabetes (T2D). However, the Food and Drug Administration has issued alerts regarding increased acute kidney injury (AKI) risk with canagliflozin and dapagliflozin. We aimed to assess the real-world risk of AKI in new SGLT2 inhibitor users in two large health care utilization cohorts of patients with T2D.
RESEARCH DESIGN AND METHODS
We used longitudinal data from the Mount Sinai chronic kidney disease registry and the Geisinger Health System cohort. We selected SGLT inhibitor users and nonusers (patients with T2D without SGLT2 inhibitor prescription). We determined AKI by the KDIGO (Kidney Disease: Improving Global Outcomes) definition (AKIKDIGO). We performed 1:1 nearest-neighbor propensity matching and calculated unadjusted hazard ratios (HRs) and adjusted HRs (aHRs; accounting for covariates poorly balanced) for AKI in primary and sensitivity analyses.
RESULTS
We identified 377 SGLT2 inhibitor users and 377 nonusers in the Mount Sinai cohort, of whom 3.8 and 9.7%, respectively, had an AKIKDIGO event over a median follow-up time of 14 months. The unadjusted hazards of AKIKDIGO were 60% lower in users (HR 0.4 [95% CI 0.2–0.7]; P = 0.01), which was unchanged (aHR 0.4 [95% CI 0.2–0.7]; P = 0.004) postadjustment. Similarly, we identified 1,207 SGLT2 inhibitor users and 1,207 nonusers in the Geisinger cohort, of whom 2.2 and 4.6% had an AKIKDIGO event. AKIKDIGO unadjusted hazards were lower in users (HR 0.5 [95% CI 0.3–0.8]; P < 0.01) with modest attenuation postadjustment for covariates (aHR 0.6 [95% CI 0.4–1.1]; P = 0.09). These estimates did not qualitatively change across several sensitivity analyses.
CONCLUSIONS
Our findings do not suggest an increased risk of AKI associated with SGLT2 inhibitor use in patients with T2D in two large health systems.
Type 2 diabetes (T2D) is a major public health problem. Although the incidence rate of T2D has plateaued in recent years, it still affects 29 million adults in the U.S. (1). T2D is associated with a greatly increased risk for many complications (including cardiovascular and kidney disease) and is responsible for ∼80,000 deaths/year (2). There are currently limited therapeutic options for improving cardiovascular and kidney outcomes in patients with T2D (3).
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are new medications for the treatment of patients with T2D. SGLT2 inhibitors block the reabsorption of glucose in the kidney, increase glucose excretion, and lower blood glucose levels. There are three SGLT2 inhibitors that are currently Food and Drug Administration (FDA) approved: empagliflozin, canagliflozin, and dapagliflozin. The multicenter Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG OUTCOME) trial and the CANagliflozin cardioVascular Assessment Study (CANVAS) both demonstrated lower rates of cardiovascular events and mortality with empagliflozin and canagliflozin, respectively (4,5). In addition, prespecified analyses of the trials also demonstrated a significant reduction in incidence and worsening kidney disease and the need for renal replacement therapy (6).
There have been some concerns raised regarding the risk for acute kidney injury (AKI) with two of the three approved SGLT2 inhibitors (canagliflozin and dapagliflozin) by the FDA. The FDA issued an initial warning in December 2015 and then strengthened the warning in June 2016 about the use of these two inhibitors. These warnings were prompted by 101 confirmed cases of AKI with canagliflozin or dapagliflozin reported to the FDA adverse effect reporting system from 2013 onwards (7). It certainly is possible that SGLT2 inhibitors may predispose to AKI by contributing to volume depletion because of their natriuretic properties, effects on tubuloglomerular feedback, and various other mechanisms. In addition, the volume and intrarenal hemodynamic effects of SGLT2 inhibitors may be synergistic when combined with frequently prescribed renin-angiotensin-aldosterone system antagonists and traditional diuretics in this population of patients with T2D.
However, it is unlikely that SGLT2 inhibitors increase the risk for clinically significant AKI, yet decrease the risk for chronic kidney disease (CKD), as witnessed in the EMPA-REG OUTCOME trial and CANVAS (4–6). In addition, the risk for AKI reported to the FDA must be interpreted in the context that patients with T2D are also at higher baseline risk of AKI, and, in the absence of a control group, it is unclear how much of the risk attributed to SGLT2 inhibitors could be because of baseline diabetes and related comorbidities (8).
We sought to determine the real-world risk for AKI associated with initiating SGLT2 inhibitors in two large health care utilization cohorts of patients with T2D, with and without baseline reduced estimated glomerular filtration rate (eGFR), using propensity-score matching.
Research Design and Methods
Study Setting and Design
Study Cohorts
The Mount Sinai Chronic Kidney Disease Registry is a system-wide registry of patients with eGFR <60 mL/min, ICD-CM 9/10 codes for CKD, or urinary albumin-to-creatinine ratio (ACR) >30 mg/mg receiving care at the Mount Sinai Hospital in New York, NY. For the purposes of this study, only those patients with a diagnosis of T2D (determined by a validated phenotyping algorithm) and available serum creatinine measurements between 1 January 2014 and 30 December 2016 were included (N = 12,704) (9). The registry contains deidentified electronic health record data for these patients, including physician notes, diagnoses, procedures, laboratory values, and imaging results occurring within the Mount Sinai health system. The Mount Sinai Institutional Review Board approved this study.
The Geisinger cohort represents a retrospective, community-based cohort of patients who received primary care within the Geisinger Health System. For the purposes of this study, only those patients with a diagnosis of T2D and available serum creatinine measurements between 1 January 2013 and 10 February 2017 were included (N = 56,163). T2D diagnosis was determined by ICD diagnosis codes or the use of T2D medications and qualifying laboratories. The cohort contains information on all inpatient and outpatient encounters, prescriptions, problem lists, diagnostic codes, and laboratory measurements that occur within the Geisinger Health System.
Definition of Exposure
The exposure of interest was a new prescription of an SGLT2 inhibitor, including canagliflozin, empagliflozin, or dapagliflozin. In both cohorts, this was determined by provider prescription in the electronic medical record. For SGLT2 inhibitor users, index date was defined as the first date on which an SLGT2 prescription was ordered. For nonusers, index date was defined using the creatinine measurement date after 2013.
Definition of Outcome
The primary outcome was the first AKI event after the index date detected in the inpatient setting. We identified AKI events using a laboratory-based algorithm, hereafter referred to as AKIKDIGO, which identifies events based on KDIGO (Kidney Disease: Improving Global Outcomes) serum creatinine criteria (increase in serum creatinine by ≥0.3 mg/dL within 48 h or increase in serum creatinine by ≥1.5 times baseline value in the prior 7 days) (10). Baseline creatinine was defined as outpatient creatinine before the AKI episode (if a single value was present) or the average of creatinine measurements over the past year before the AKI episode (if several values were present). For patients who experienced AKI, we also evaluated severity of AKI using the peak serum creatinine and the change in serum creatinine during an AKI event. Peak serum creatinine was defined as the maximum creatinine value measured within 10 days of an AKI event. We defined change in serum creatinine as the difference between the peak serum creatinine and the baseline value. As a sensitivity analysis, we also ascertained inpatient episodes of AKI along with the dates using ICD-9 with Clinical Modification diagnosis codes 584.xx and ICD-10 code N17.9 (hereafter referred to as AKIICD) along with the dates. For Geisinger, AKI was defined using the KDIGO creatinine-based criteria and either an increase in serum creatinine by 50% from outpatient baseline in the year prior to admission or an increase in serum creatinine by 50% over the previous 7 days using inpatient values.
Propensity Matching and Statistical Analysis
All new users of SGLT2 inhibitors were identified from the electronic medical record. Users were excluded if they were missing data on eGFR prior to prescription (N = 466 in Mount Sinai and N = 560 in Geisinger). SGLT2 user follow-up period started on the date of first prescription and ended on the date of last outpatient encounter. Nonuser follow-up period started with a first outpatient visit occurring between 2013 and 2015 and ended with an outpatient visit in December 2016 in Mount Sinai and February 2017 in Geisinger. We defined AKI episodes for users and nonusers as any AKI event occurring within the respective follow-up periods.
Control subjects were selected among patients who never received a prescription for SLGT2 inhibitors and who had a diagnosis of diabetes after 1 January 2013. Propensity scores were calculated using logistic regression of SLGT2 inhibitor use on age, race, sex, year of prescription/creatinine measurement, prevalent congestive heart failure, prevalent coronary artery disease, hypertension status, insulin use, antihypertensive medication use, nonsteroidal anti-inflammatory drug use, diuretic use, eGFR, and HbA1c, with matching on previous pharmacist visit, diabetes duration, previous AKI episode, previous endocrinologist visit, insurance status, and ACE inhibitor/angiotensin receptor blocker use (only in the Geisinger cohort), generating a separate score at each outpatient visit for control subjects. For participants with missing values in any of these covariates, exact matches were required on missing status. Propensity matching was performed with a 1:1 match for case and control subjects in which the nearest neighbor was selected without replacement.
We compared differences in demographics, comorbidities, physiologic variables, laboratory values, and medication regimens using χ2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Survival analysis was performed among case and control subjects using Cox proportional hazards regression from index date to the minimum of event date, death date, or end of follow-up (30 December 2016 in Mount Sinai and 10 February 2017 in Geisinger). Adjusted analysis was performed with additional adjustment for propensity score as well as covariates with significant differences among case and control subjects (race, HbA1c, smoking, thiazide diuretics, and metformin usage [in the Mount Sinai cohort] and diastolic blood pressure, total cholesterol, HbA1c, hemoglobin, albuminuria, antihypertensives, loop diuretics, thiazide diuretics, and metformin use [in the Geisinger cohort]).
In sensitivity analyses, we analyzed users of each type of SGLT2 inhibitor (canagliflozin, dapagliflozin, and empaglifozin) and then individuals not missing any covariate data. Finally, severity of AKI using peak serum creatinine, change in serum creatinine, and need for acute dialysis during AKI episode were compared for the SGLT2 inhibitor user group and nonuser nearest-neighbor matched group. All analyses were performed using SAS 9.2 (SAS Institute Inc.), R Version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria), and Stata version 14.2 (StataCorp, College Station, TX) for the Geisinger analysis. We considered two-tailed P values ≤0.05 as statistically significant.
Results
Study Populations
We identified a total of 388 SGLT2 inhibitor users and 12,316 patients with T2D not on SGLT2 inhibitors with eGFR data in the Mount Sinai Registry prior to matching. Compared with nonusers, SGLT2 inhibitor users tended to be younger and comprised of a larger proportion of females and smaller proportion of African Americans and with significantly lower comorbidities compared with SGLT2 inhibitor nonusers. They also had higher BMI, worse HbA1c levels, and higher eGFRs compared with nonusers. These results are shown in Supplementary Table 1.
After propensity matching, we identified 377 SGLT2 inhibitor users and 377 nonusers in the Mount Sinai Chronic Kidney Disease Registry. The majority of users were on canagliflozin (71.8%), followed by dapagliflozin (19.4%), and then empagliflozin (8.9%). Users and nonusers were well matched except for race (African American race, 18.3% in users vs. 29.6% in nonusers), hemoglobin levels (13.2 vs. 12.2 g/dL in users vs. nonusers), HbA1c (8.0 vs. 7.5% in users vs. nonusers), thiazide diuretics (42.2 vs. 31.2% in users vs. nonusers), and metformin usage (89.3 vs. 83.3% in users vs. nonusers) in the Mount Sinai cohort (Table 1). Users also had longer follow-up time compared with nonusers (435 vs. 351 days). The median number of creatinine measurements over follow-up was four (interquartile range [IQR] 2–7) in users and three (IQR 2–9) in nonusers (P = 0.05).
Table 1.
Baseline characteristics stratified by SGLT2 inhibitor user and nonuser status in the Mount Sinai and Geisinger propensity-matched cohorts
| Mount Sinai cohort |
Geisinger cohort |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| User (n = 372) | Nonuser (n = 372) | P1 | User (n = 1,207) | Nonuser (n = 1,207) | P2 | |||||||||||
| Demographics | ||||||||||||||||
| Age, years | 63.0 (56–70) | 63.0 (54–72) | 0.85 | 58.0 (51–66) | 58.2 (51–66) | 0.71 | ||||||||||
| Male | 205 (55.1) | 194 (52.2) | 0.42 | 641 (53.1) | 641 (53.1) | 1.00 | ||||||||||
| Race | <0.01 | 0.34 | ||||||||||||||
| White | 152 (40.9) | 124 (33.3) | 1,172 (97.1) | 1,182 (97.9) | ||||||||||||
| Black | 68 (18.3) | 110 (29.6) | 15 (1.2) | 13 (1.1) | ||||||||||||
| Other | 152 (40.9) | 138 (37.1) | 20 (1.7) | 12 (1.0) | ||||||||||||
| Comorbidities | ||||||||||||||||
| Smoker1 | 36 (9.7) | 93 (25.0) | <0.01 | 666 (55.2) | 666 (55.2) | 1.00 | ||||||||||
| Heart failure | 61 (16.4) | 63 (16.9) | 0.84 | 25 (2.1) | 30 (2.5) | 0.50 | ||||||||||
| Coronary artery disease | 130 (35.0) | 116 (31.2) | 0.28 | 148 (12.3) | 148 (12.3) | 1.00 | ||||||||||
| Hypertension | 335 (90.1) | 339 (91.1) | 0.62 | 788 (65.3) | 788 (65.3) | 1.00 | ||||||||||
| Stroke | 19 (5.1) | 28 (7.5) | 0.18 | 28 (2.3) | 42 (3.5) | 0.09 | ||||||||||
| Physiologic variables | ||||||||||||||||
| BMI, kg/m2 | 31.6 (28.1–36.7) | 30.8 (26.7–36.0) | 0.11 | 34.6 (31–39) | 34.3 (30–38) | 0.19 | ||||||||||
| Systolic blood pressure, mmHg | 131.2 (123.4–140.6) | 130.8 (121.9–141.0) | 0.66 | 128.0 (118–136) | 127.7 (118–138) | 0.43 | ||||||||||
| Diastolic blood pressure, mmHg | 74.8 (70.0–80.0) | 73.4 (67.6–79.0) | 0.08 | 74.7 (68–80) | 74.0 (68–80) | 0.04 | ||||||||||
| Laboratory variables | ||||||||||||||||
| HbA1c, % | 8.0 (7.3–9.0) | 7.5 (6.7–8.8) | <0.01 | 8.2 (7.4–8.8) | 7.7 (6.7–8.3) | <0.01 | ||||||||||
| Total cholesterol, mg/dL | 160.3 (141.0–188.0) | 163.0 (136.5–196.7) | 0.40 | 172.1 (147–186) | 168.6 (144–185) | 0.03 | ||||||||||
| Hemoglobin, g/dL | 13.2 (12.1–14.2) | 12.2 (10.8–13.4) | <0.01 | 14.1 (13.8–14.4) | 13.9 (13.3–14.6) | <0.01 | ||||||||||
| eGFR,2 mL/min/1.73 m2 | 63.7 (52.3–78.2) | 60.6 (46.9–81.5) | 0.08 | 87.4 (76.8–100.1) | 87.2 (78.1–98.4) | 0.66 | ||||||||||
| UACR3 | 27.5 (12.0–64.8) | 16.0 (6.9–70.0) | 0.40 | 15.0 (8.0–29.5) | 13.0 (7.0–29.0) | 0.51 | ||||||||||
| Medications | ||||||||||||||||
| Metformin | 332 (89.3) | 310 (83.3) | 0.02 | 1,028 (85.2) | 705 (58.4) | <0.01 | ||||||||||
| Insulin | 252 (67.7) | 252 (67.7) | 0.99 | 197 (16.3) | 197 (16.3) | 1.00 | ||||||||||
| ARB | 148 (39.8) | 154 (41.1) | 0.65 | 111 (9.2) | 111 (9.2) | 1.00 | ||||||||||
| ACE inhibitors | 158 (42.5) | 164 (44.1) | 0.66 | 536 (44.4) | 536 (44.4) | 1.00 | ||||||||||
| Other antihypertensive | 326 (87.6) | 328 (88.2) | 0.82 | 811 (67.2) | 635 (52.6) | <0.01 | ||||||||||
| Loop diuretics | 101 (27.2) | 92 (24.7) | 0.45 | 134 (11.1) | 90 (7.5) | <0.01 | ||||||||||
| Thiazide diuretics | 157 (42.2) | 116 (31.2) | <0.01 | 161 (13.3) | 131 (10.9) | 0.06 | ||||||||||
| NSAIDs | 3 (0.8) | 1 (0.3) | 0.32 | 284 (23.5) | 284 (23.5) | 1.00 | ||||||||||
| Follow-up in days4 | 436 (262–686) | 351 (219–654) | <0.01 | 458 (240–688) | 439 (214–686) | 0.84 | ||||||||||
| SGLT2 inhibitor type | ||||||||||||||||
| Canagliflozin | 267 (71.8) | NA | NA | 753 (60.6) | NA | NA | ||||||||||
| Dapagliflozin | 72 (19.4) | NA | NA | 134 (10.8) | NA | NA | ||||||||||
| Empagliflozin | 33 (8.9) | NA | NA | 355 (28.6) | NA | NA | ||||||||||
Continuous variables are presented as median (IQR), whereas categorical variables are presented as n (%). P1 and P2 are P values for primary and secondary analyses, respectively. ARB, angiotensin receptor blocker; NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug.
1Smoking status was considered positive if ever smoker.
2Calculated by the Chronic Kidney Disease Epidemiology Collaboration equation.
3Geisinger cohort was missing 23.4% of urine ACR (UACR). Mount Sinai cohort was missing 85% of UACR.
4In Mount Sinai, follow-up in days defined as time from start of SGLT2 inhibitor prescription to last outpatient encounter in users and time from first outpatient visit occurring between 2013 and 2015 to last outpatient visit in 2016 in nonusers. In Geisinger, follow-up time was defined from first SGLT2 inhibitor prescription in users and creatinine assessment in matched index year in nonusers until event or 10 February 2017.
We then identified 2,560 SGLT2 inhibitor users and 53,603 patients with T2D not on SGLT2 inhibitors in the Geisinger cohort. SLGT2 users were younger and more often male than nonusers. They also had more coronary artery disease, higher BMI, higher HbA1c levels, and higher eGFRs compared with nonusers.
After propensity matching, we identified 1,207 SGLT2 inhibitor users and 1,207 nonusers who were well matched except for HbA1c (8.2% in users vs. 7.7% in nonusers), metformin usage (85.2% in users vs. 58.4% in nonusers), antihypertensive usage (67.2% in users vs. 52.6% in nonusers), loop diuretics (11.1% in users vs. 7.5% in nonusers), and thiazide diuretics (13.3% in users vs. 10.9% in nonusers) in the Geisinger cohort (Table 1). Follow-up time was similar in users and nonusers (458 vs. 439 days). The median number of creatinine measurements was five (IQR 3–9) in the user and six (IQR 3–12) in the nonuser groups (P < 0.001).
AKI Events and Severity in the Mount Sinai Cohort
The proportion of patients with at least one AKIKDIGO event was 5.2% (20 out of 388) in the SGLT2 inhibitor user cohort and 1,304 out of 12,316 (10.6%) in the whole nonuser cohort with a rate of 0.03/patient-year in users and 0.06/patient-year in nonusers. After propensity matching, the proportion of SGLT2 inhibitor users and nonusers in the Mount Sinai cohort with an AKIKDIGO event was 3.8 and 9.7%, respectively (P = 0.002) (Table 2), with an incidence rate of 3/100 patient-years in users and 8/100 patient-years in nonusers. We also compared the severity of AKI events as defined by changes in creatinine from baseline and peak creatinine measures during an AKI event. Median changes in serum creatinine from baseline for the user and nonuser groups were 0.5 mg/dL (IQR 0.4–0.7) and 0.9 mg/dL (IQR 0.8–1.3; P = 0.004), respectively (Table 2). Accordingly, median peak creatinine measures in the user group were lower (1.6 mg/dL; IQR 1.4–1.8) compared with the nonuser group (1.9 mg/dL; IQR 1.6–2.4; P = 0.02) (Table 2). Sensitivity analyses using AKIICD for all 372 patients and AKIKDIGO/AKIICD for those with nonmissing data (n = 292) yielded similar results. Acute dialysis for AKI occurred in only one patient in both user and nonuser groups in the Mount Sinai cohort. Of note, 7 out of the 14 SGLT2 users had their medication discontinued and were not restarted.
Table 2.
AKI outcomes in the SGLT2 inhibitor user and nonuser groups in the Mount Sinai and Geisinger propensity-matched cohorts
| Mount Sinai cohort |
Geisinger cohort |
|||||
|---|---|---|---|---|---|---|
| User (n = 372) | Nonuser (n = 372) | P1 | User (n = 1,207) | Nonuser (n = 1,207) | P2 | |
| AKIKDIGO–inpatient | 14 (3.8) | 36 (9.7) | 0.002 | 26 (2.2) | 55 (4.6) | 0.001 |
| AKIICD | 22 (5.9) | 40 (10.8) | 0.02 | 15 (1.2) | 36 (3.0) | 0.003 |
| Peak creatinine in AKIKDIGO events | 1.6 (1.4–1.8) | 1.9 (1.6–2.4) | 0.02 | 1.7 (1.4–2.6) | 1.6 (1.3–2.4) | 0.91 |
| Change in serum creatinine during AKIKDIGO events | 0.5 (0.4–0.7) | 0.9 (0.8–1.3) | 0.004 | 0.6 (0.5–1.0) | 0.6 (0.4–1.2) | 0.80 |
| Need for acute dialysis | 1 (0.3) | 1 (0.3) | 1.00 | 0 (0.0) | 1 (0.1) | 0.317 |
P1 and P2 are P values for primary and secondary analyses, respectively.
AKI Events and Severity in the Geisinger Replication Cohort
As in the Sinai cohort, the majority of SGLT2 inhibitor users were on canagliflozin. In the Geisinger replication cohort, cumulative incidence of AKIKDIGO events was lower for SGLT2 inhibitor users compared with nonusers, with rates of 2.2 and 4.6%, respectively (P < 0.01), with an incidence rate of 1.7/100 patient-years in users and 3.8/100 patient-years in nonusers. Sensitivity analysis using an AKIICD event to define an AKI event also yielded lower rates of AKI in the SGLT2 inhibitor user group (1.2 vs. 3.0%; P < 0.01). Concerning the severity of AKI events, there was no difference in the peak serum creatinine (1.7 vs. 1.6 mg/dL; P = 0.9) or change in creatinine (0.6 vs. 0.6 mg/dL; P = 0.8) for users versus nonusers. Only one nonuser required acute dialysis during the follow-up period. Only 3 of the 26 SGLT2 inhibitor users had their medication continued; 23 were discontinued without restarting.
Association Between SGLT2 Inhibitor and AKI
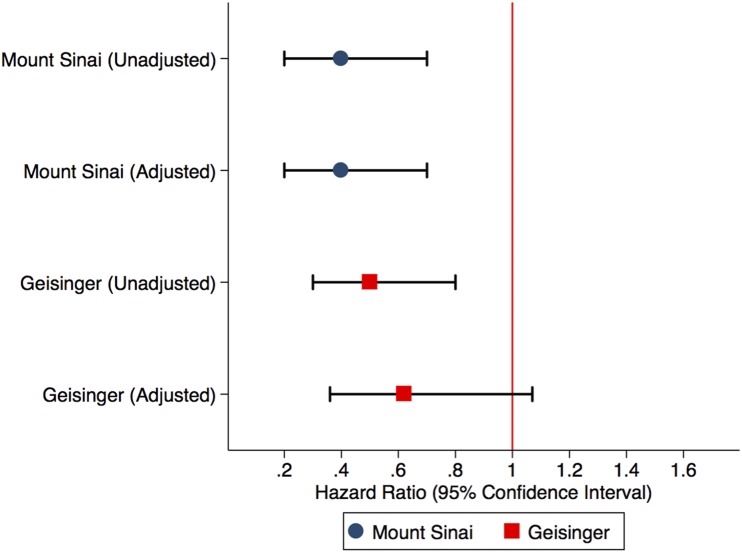
In the Mount Sinai cohort, the unadjusted hazard ratios (HRs) of AKIKDIGO were 60% lower in the user group (0.4 [95% CI 0.2–0.7]; P = 0.01) compared with the nonuser group. After adjusting for metformin use, HbA1c, smoking, thiazide use, and race, the adjusted HR (aHR) of AKIKDIGO remained unchanged (0.4 [95% CI 0.2–0.7]; P = 0.004). Similarly, in the Geisinger cohort, AKIKDIGO unadjusted hazards were lower in the user group (HR 0.5 [95% CI 0.3–0.8]; P < 0.01). After adjusting for diastolic blood pressure, total cholesterol, HbA1c, hemoglobin, albuminuria, antihypertensive use, loop diuretic use, thiazide diuretic use, and metformin use, the Geisinger cohort still had lower, albeit nonsignificant, adjusted hazards of AKIKDIGO in SGLT2 inhibitor users compared with their nonuser counterparts (aHR 0.6 [95% CI 0.4–1.1]; P = 0.09) (Fig. 1). Using the AKIICD definition had similar results in the Mount Sinai and Geisinger cohorts. Using user/nonusers not missing any covariate data (n = 584 in Mount Sinai and n = 1,160 in Geisinger) showed no increased hazard of AKIKDIGO in the user group (Table 3).
Figure 1.
Unadjusted HRs and aHRs of AKI with 95% CIs in the Mount Sinai and Geisinger propensity-matched cohorts. The HRs are generated after adjustment for covariates poorly matched for, which include metformin use, thiazides, smoking, HbA1c, and race (Mount Sinai cohort) and diastolic blood pressure, total cholesterol, HbA1c, hemoglobin, albuminuria, antihypertensive use, loop diuretic use, thiazide diuretic use, and metformin use (Geisinger cohort).
Table 3.
Sensitivity analyses of AKI in Mount Sinai and Geisinger propensity-matched cohort
| Mount Sinai cohort |
Geisinger cohort |
|||
|---|---|---|---|---|
| Unadjusted (95% CI) | Adjusted (95% CI) | Unadjusted (95% CI) | Adjusted (95% CI) | |
| Using AKIKDIGO definition | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 0.5 (0.3–0.8) | 0.6 (0.4–1.1) |
| Using AKI ICD codes | 0.5 (0.3–0.8) | 0.5 (0.3–0.9) | 0.43 (0.23–0.79) | 0.56 (0.27–1.16) |
| User/nonusers not missing any covariate data | 0.7 (0.3–1.3) | 0.7 (0.3–1.4) | 0.70 (0.37–1.33) | 0.81 (0.40–1.66) |
| Canagliflozin | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.54 (0.32–0.93) | 0.61 (0.33–1.12) |
| Dapagliflozin | 1.0 (0.5–2.4) | 1.1 (0.5–2.7) | 0.16 (0.02–1.33) | 0.32 (0.02–5.18) |
| Empagliflozin | NA | NA | 0.50 (0.15–1.66) | 0.96 (0.21–4.35) |
Results for empagliflozin in Mount Sinai cohort not available (NA) because of small sample size and lack of model convergence.
We also investigated whether or not there was differential risk of AKIKDIGO associated specifically with canagliflozin or dapagliflozin. In both the Mount Sinai and Geisinger cohorts, the unadjusted and adjusted point estimates were qualitatively similar to the overall results (Table 3). Sufficient data on empaglifozin were only available in the Geisinger cohort; results were similar as well (aHR for AKIKDIGO 0.96 [0.2–4.4]).
Conclusions
In two large contemporary cohorts from two major health care systems with different ethnic mixes, comorbidity burden, and differing levels of renal function, we did not observe any increased risk of AKI with SGLT2 inhibitor usage over nearly a year and a half of follow-up. In contrast, we observed trends toward decreased risk of AKI with SGLT2 inhibitor use before and after propensity matching, the estimates of which were qualitatively similar across several sensitivity analyses. When AKI occurred, the severity was not worse in those on SGLT2 inhibitors, as estimated by peak serum creatinine or change in creatinine. Finally, there was no increased risk of AKI specifically for canagliflozin and dapagliflozin, for which there is concern of AKI, and alerts have been issued.
Considering the mechanism of action of SGLT2 inhibitors, it is not inconceivable that they might cause acute perturbations in renal function. SGLT2 inhibitors inhibit the cotransporter in the proximal tubule, leading to increased natriuresis and glycosuria (11). The additional natriuretic effect of SGLT2 inhibition may predispose patients, particularly those on diuretics and renin-angiotensin-aldosterone system antagonists, to experience abrupt reductions in GFR. The FDA reports, as well as commentaries from various authors, have warned and speculated about AKI associated with SGLT2 inhibitors (12,13). As is the norm for postmarketing surveillance, reports of AKI to the FDA are vitally necessary for vigilance for adverse effects of newly approved medications, but the lack of a comparison or control group makes it impossible to know the relative risk, especially in a group at high baseline risk of AKI, and thus the potential public health impact.
However, there are now several lines of evidence that SGLT2 inhibitors do not increase the risk for AKI. In the EMPA-REG OUTCOME trial, empagliflozin decreased the risk for AKI (HR 0.75 [95% CI 0.57–0.98] with baseline eGFR <60; and HR 0.77 [95% CI 0.57–1.04] with baseline eGFR >60). In pooled analyses of 5,598 participants enrolled in seven placebo-controlled and randomized controlled trials of canagliflozin (14), and in the recently published CANVAS, there were no statistically significant differences in the number of renal-related adverse events versus placebo (19.7 vs. 17.4/1,000 patient-years) (5). Although the trial data can be reassuring, AKI events in these trials were assessed via Medical Dictionary for Regulatory Activities definitions (including acute prerenal failure, azotemia, blood creatinine increased, GFR decreased, renal failure, renal failure acute, and renal impairment) and thus lack specificity. Another argument against SGLT2 inhibitors truly causing AKI is that it would be theoretically difficult to harmonize increased AKI risk with the observed long-term efficacy for reduction of several CKD end points by ∼40% for empagliflozin and canagliflozin in the two large trials, given the widely acknowledged association between AKI and CKD (15). The concern for AKI among clinicians is real, as evidenced by the fact that 50–75% of patients who experienced AKI in the two cohorts did not have the SGLT2 inhibitors restarted after the AKI episode, potentially depriving them of the long-term cardio- and renoprotective benefits because of the episode of creatinine elevation.
In our study, the point estimates for risk of AKI were qualitatively similar for all three types of SGLT2 inhibitors, suggesting a class effect. We believe our data complement and build upon the clinical trial data by the implementation of both laboratory-based data and diagnostic codes to ascertain AKI events, before and after propensity matching and additional adjustment, in two large health care systems that are free from the selection bias and Hawthorne effect of clinical trial enrollment. Ongoing clinical trials, including the Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE) trial of canagliflozin and Renoprotective Effects of Dapagliflozin in Type 2 Diabetes (RED) trial, Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58), and A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (Dapa-CKD) of dapagliflozin, should provide final definitive evidence in regards to AKI risk with these agents.
Our findings need to be interpreted with consideration of some limitations. First, ascertainment bias because of the retrospective nature of data is a possibility. However, frequency of creatinine measurements between users and nonusers was very similar; thus, ascertainment bias was likely minimal. We could not account for socioeconomic status of the patients from the Mount Sinai cohort because data pertaining to these domains are poorly collated and described in the electronic medical record. However, we included insurance status for propensity matching in the Geisinger cohort and found similar results. In addition, urine ACR measurements were missing in 85% of the Mount Sinai cohort. However, we used albuminuria measurements from the Geisinger cohort in the propensity match and found similar results. Finally, residual confounding and confounding by indication cannot be ruled out, although these results are remarkably similar to the rate ratios for AKI in the EMPA-REG OUTCOME trial and CANVAS.
In conclusion, our findings suggest that there is no evidence for an increased risk of AKI in real-life SGLT2 inhibitor users compared with matched nonusers with over 1 year of follow-up in two large health systems, encompassing a multiethnic, urban population and a rural, predominantly white population. These data suggest that the potential risk of AKI with SGLT2 inhibitors, including canagliflozin and dapagliflozin, is likely attributable to the high-risk population and not related to any inherent nephrotoxicity of these agents. Our analyses also demonstrate that advanced pharmacoepidemiologic methods can be leveraged to evaluate potential risks of new and emerging drug classes. In the meantime, we believe the fear of AKI associated with SGLT2 inhibitor use can be tempered to avoid inappropriate discouragement of use of this novel class of agents that otherwise appear to afford significant long-term cardiovascular and renal protection in patients with T2D.
Supplementary Material
Article Information
Funding. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under awards K23-DK-107908 (to G.N.N.), K23-DK-1065501 (to A.C.), and R01-DK-096549/U01-DK-106962 (to S.G.C.).
Duality of Interest. S.G.C. serves as a consultant in regards to SGLT2 inhibitors for Janssen Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions. G.N.N. contributed data and administrative support and wrote the manuscript. R.F. contributed to statistical analyses and wrote the manuscript. A.C. contributed data and contributed to discussion. A.Su., K.C., P.P., and A.Sa. contributed to statistical analyses. B.F. contributed to methodology. M.E.G. contributed data and administrative support. S.G.C. contributed data and administrative support and wrote the manuscript. All authors reviewed and edited the manuscript and contributed to discussion. G.N.N. and S.G.C. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1011/-/DC1.
References
- 1.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Golden SH, Anderson C, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research; American Diabetes Association . Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2015;38:1777–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triggle CR, Ding H. Cardiovascular impact of drugs used in the treatment of diabetes. Ther Adv Chronic Dis 2014;5:245–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 5.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 12 June 2017 [Epub ahead of print]. DOI:10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 6.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. FDA drug safety communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) [article online], 2016. Available from https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm. Accessed 21 April 2017
- 8.Girman CJ, Kou TD, Brodovicz K, et al. Risk of acute renal failure in patients with type 2 diabetes mellitus. Diabet Med 2012;29:614–621 [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni GN, Gottesman O, Linneman JG, et al. Development and validation of an electronic phenotyping algorithm for chronic kidney disease. AMIA Annu Symp Proc. 2014;2014:907–916 [PMC free article] [PubMed] [Google Scholar]
- 10.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group . Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther 2014;5:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol 2016;12:711–712 [DOI] [PubMed] [Google Scholar]
- 13.Heyman SN, Khamaisi M, Rosen S, Rosenberger C, Abassi Z. Potential hypoxic renal injury in patients with diabetes on SGLT2 inhibitors: caution regarding concomitant use of NSAIDs and iodinated contrast media. Diabetes Care 2017;40:e40–e41 [DOI] [PubMed] [Google Scholar]
- 14.Qiu R, Balis D, Xie J, Davies MJ, Desai M, Meininger G. Longer-term safety and tolerability of canagliflozin in patients with type 2 diabetes: a pooled analysis. Curr Med Res Opin 2017;33:553–562 [DOI] [PubMed] [Google Scholar]
- 15.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.