Abstract
Pancreatic islets produce and secrete cytokines and chemokines in response to inflammatory and metabolic stress. The physiological role of these “isletokines” in health and disease is largely unknown. We observed that islets release multiple inflammatory mediators in patients undergoing islet transplants within hours of infusion. The proinflammatory cytokine interferon-γ–induced protein 10 (IP-10/CXCL10) was among the highest released, and high levels correlated with poor islet transplant outcomes. Transgenic mouse studies confirmed that donor islet–specific expression of IP-10 contributed to islet inflammation and loss of β-cell function in islet grafts. The effects of islet-derived IP-10 could be blocked by treatment of donor islets and recipient mice with anti–IP-10 neutralizing monoclonal antibody. In vitro studies showed induction of the IP-10 gene was mediated by calcineurin-dependent NFAT signaling in pancreatic β-cells in response to oxidative or inflammatory stress. Sustained association of NFAT and p300 histone acetyltransferase with the IP-10 gene required p38 and c-Jun N-terminal kinase mitogen-activated protein kinase (MAPK) activity, which differentially regulated IP-10 expression and subsequent protein release. Overall, these findings elucidate an NFAT-MAPK signaling paradigm for induction of isletokine expression in β-cells and reveal IP-10 as a primary therapeutic target to prevent β-cell–induced inflammatory loss of graft function after islet cell transplantation.
Introduction
Islet endocrine cells have been shown to produce cytokines under conditions of physical, inflammatory, and metabolic stress (1–6). Expression of interleukin (IL)-1β has been observed in β-cells exposed to hyperglycemic conditions and in patients with type 2 diabetes (7). IL-1β signaling further promotes cytokine expression in β-cells (3,5,6,8–10). Acute effects of IL-1β to enhance insulin expression and induce proliferation in β-cells indicate a physiological role of cytokines to enable islets to adapt to inflammatory stress and metabolic demand (11–16). However, inappropriate expression of cytokines by islets is associated with endoplasmic reticulum and oxidative stress responses that promote β-cell death and dysfunction (17–20). Overexposure of islets to osmotic, metabolic, oxidative, or inflammatory stress induces p38 and c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) signaling (21–24). This leads to downstream effects of nuclear factor-κB to upregulate genes that promote apoptosis (17,25).
During islet transplantation, islets are exposed to multiple physical and chemical stresses throughout the islet cell isolation and infusion procedures that induce expression of cytokines. These inflammatory mediators are secreted by islets and persist for days within culture. Upon transplantation of islets, an innate immune response is observed that is largely responsible for a loss of up to 50% or more of the initial islet graft mass during the immediate posttransplant period. This phenomenon was first described as an instant blood-mediated inflammatory reaction that is characterized by a heparin-sensitive, platelet-mediated activation of the complement cascade (26,27). We hypothesized that release of islet-derived cytokines by stressed islets contributes to the early inflammatory loss of islet cell tissue upon transplantation.
In this study, we monitored circulating inflammatory mediators in patients immediately after islet transplantation and identified interferon-γ–induced protein 10 (IP-10/CXCL10) as a major chemokine released immediately upon islet infusion that adversely affects transplant outcomes. Protection of islet grafts with β-cell–specific deletion of IP-10 in an islet transplant model confirmed a role of donor islet–specific IP-10 to contribute to early inflammatory loss of islet graft function. Further analysis of IP-10 in β-cells indicated that NFAT and stress-activated MAPK signaling directly induced expression of the IP-10 gene in response to oxidative or inflammatory stress. Moreover, high glucose stimulated release of IP-10 protein from stressed β-cells. Finally, a monoclonal antibody (mAb) directed toward IP-10 could prevent early loss of islet grafts transplanted in mice. These findings highlight IP-10 as a key isletokine that can be targeted to prevent islet inflammation and improve islet cell transplant outcomes.
Research Design and Methods
Cell and Tissue Samples
Blood samples were collected from 34 patients undergoing islet transplants at Baylor University Medical Center at time intervals after islet infusion in accordance with Institutional Review Board–approved protocols. Isolated human islets from multiple donors (>90% purity) were provided by the Integrated Islet Distribution Program at City of Hope and from the cGMP Islet Cell Processing Laboratory at Baylor University Medical Center. C57BL/6 mouse pancreatic islets were isolated as described below. Isolated islets were cultured 1–2 days before use. Isolated islets, FACS-purified β-cells, and the MIN6 β-cell line were cultured in RPMI 1640 or Krebs-Ringer bicarbonate HEPES buffer media at 37°C in 5% CO2 humidified air.
Mouse Islet Isolation
The use and care of laboratory animals for all studies were performed in accordance with guidelines and protocols approved by the Institutional Animal Care and Use Committee. Islets were isolated from wild-type and IP-10 knockout (IP-10−/−) C57BL/6 9- to 12-week-old male mice (The Jackson Laboratory). Pancreata were removed from mice after type V collagenase (C9263; Sigma-Aldrich) perfusion, digested for 18 min at 37°C, and washed with Hanks’ balanced salt solution. Islets were separated from digested pancreas preparations by centrifugation on a 1.077 and 1.100 g/mL Ficoll gradient solution. The separated islet tissue layer was collected and washed with Hanks’ balanced salt solution. Intact islets were hand selected from the purified preparation by pipette and cultured overnight at 37°C.
Reagents and Recombinant DNA Constructs
Antibodies were used as follows: NFATc2, p300, and HDAC3 (Santa Cruz Biotechnology, Inc.), and extracellular signal–regulated kinase (ERK)1/2, p38, and JNK (Cell Signaling Technology). The mouse IP-10 promoter-reporter vector containing Gaussia luciferase reporter was obtained from GeneCopoeia. Anti–IP-10 mAb was obtained from the Baylor Research Institute Biotechnology Core. Vectors expressing green fluorescent protein (GFP) and mouse NFATc2 or sequence-scrambled short hairpin (sh)RNA were obtained from OriGene. Expression vectors containing dominant negative NFAT PxIxIT motif (dnNFAT) and mutated dnNFAT AxAxAA motif (dnNFATm) were previously described (5,28).
DNA Transfection and Promoter Assays
MIN6 cells were transfected with plasmids and shRNA constructs by Lipofectamine 2000 24 h before purification and cell treatments. Cells were harvested with 1× Reporter Lysis buffer, and luciferase/Renilla enzyme activity was detected by the Dual-Luciferase Reporter Assay System (Promega) using a TD20/20 Luminometer.
Flow Cytometry
Human islets and MIN6 cells underwent FACS on a MoFlo high-speed cell sorter. β-Cells were purified based on zinc-sensitive fluorescence of Newport Green 2′,7′-dichlorofluorescein diacetate, as previously described (5). Newport Green–stained β-cell fractions were screened for exclusive expression of insulin by immunoassay. MIN6 cells transfected with shNFAT-GFP and sh-scrambled-GFP were trypsinized and purified based on relative GFP fluorescence.
Immunoassays
Proteins from human serum collected from patients undergoing islet transplants and supernatant samples from isolated human islets (300 islet equivalent [IEQ] in 1 mL medium) were measured by multiplex assays with magnetic beads using the Luminex platform system (EMD Millipore). ELISAs were performed to measure IP-10 and insulin using a Cytation 5 microplate reader (BioTek).
Immunofluorescent Staining
Formalin-fixed and paraffin-embedded sections of isolated human islets were incubated with primary antibody (1:250) in PBS containing 0.1% Triton X-100 and 4% BSA overnight at 4°C. Samples were washed, underwent fluorescein isothiocyanate and Cy3 conjugated secondary antibody (1:500), and imaged by an Olympus BX61 TRF Fluorescent Microscope.
Islet Transplantation
Wild-type C57BL/6, IP-10−/− C57BL/6, and nude mice (Envigo) (9- to 12-week old males) were injected with streptozotocin (STZ; 200 mg/kg body weight). Mice with blood glucose >400 mg/dL for ≥2 consecutive days were selected as diabetic recipients for islet transplantation. For mouse islet recipients, 200 islets were infused to C57BL/6 recipients via the portal vein. For human islet recipients, 2,000 IEQ were infused to subcapsular kidney sites in nude mice, and kidneys were removed after 6 h for analysis of islet graft cytokine gene expression.
Glucose Measurements
Blood glucose and body weight were monitored twice weekly for up to 30 days. After 30 days, mice were fasted for 8 h before an intraperitoneal administration of 20% glucose solution at 2 g glucose/kg body weight. Blood glucose was measured at 30-min intervals for intraperitoneal glucose tolerance test (IPGTT) analyses.
Nuclear Run-on Assay
MIN6 cells were treated and lysed in Tris buffer containing 0.5% Triton X-100. Nuclei were washed and isolated by centrifugation on a 0.6 mol/L sucrose cushion at 600g in a table top centrifuge. Intact fractionated nuclei were incubated at 30°C for 30 min in Tris buffer containing 2 units RNasin recombinant ribonuclease inhibitor (Promega) and 30% glycerol in the presence of 200 μmol/L ribonucleoside triphosphate. RNA was isolated using TRI Reagent and transcribed to cDNA. Relative changes in gene targets and 18S mRNA were quantified by quantitative (q)PCR.
Chromatin Immunoprecipitation Assays
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (6). Briefly, MIN6 cells were fixed, and chromatin DNA-protein was cross-linked with 1% formaldehyde for 10 min, quenched with 125 mmol/L glycine for 5 min, and washed with PBS. Fixed cells were sonicated with a Bioruptor 200 (Diagenode) and lysed in radioimmunoprecipitation assay buffer containing protease inhibitor cocktail. DNA-protein complexes were immunoprecipitated with the indicated antibodies or IgG isotype controls and extensively washed. Cross-links were reversed at 65°C for 4 h in Tris buffer containing 5 mol/L NaCl and 0.5 mol/L EDTA. DNA was extracted by phenol/CHCl3 and precipitated with ethanol, followed by proteinase K and ribonuclease A treatment. Precipitated DNA and 1% control inputs were analyzed by qPCR.
Real-time qPCR
qPCR was performed using the Bio-Rad CFX Connect system with RT2 SYBR Green qPCR Mastermix. RT2 qPCR Primer Assays were used to detect specific cDNA-converted mRNA transcripts for 18S and target genes (Qiagen). Primer sequences used to detect input, and immunoprecipitated DNA sequences from −266 to −122 of the 5′-flank mouse IP-10 promoter region were 5′-TCTGCAAGGCACTCATCTGAT and 5′-CGAGGGCATTGCTTGTGTTT.
Results
High IP-10 Released From Islets Upon Transplantation Is Associated With Poor Clinical Transplant Outcomes
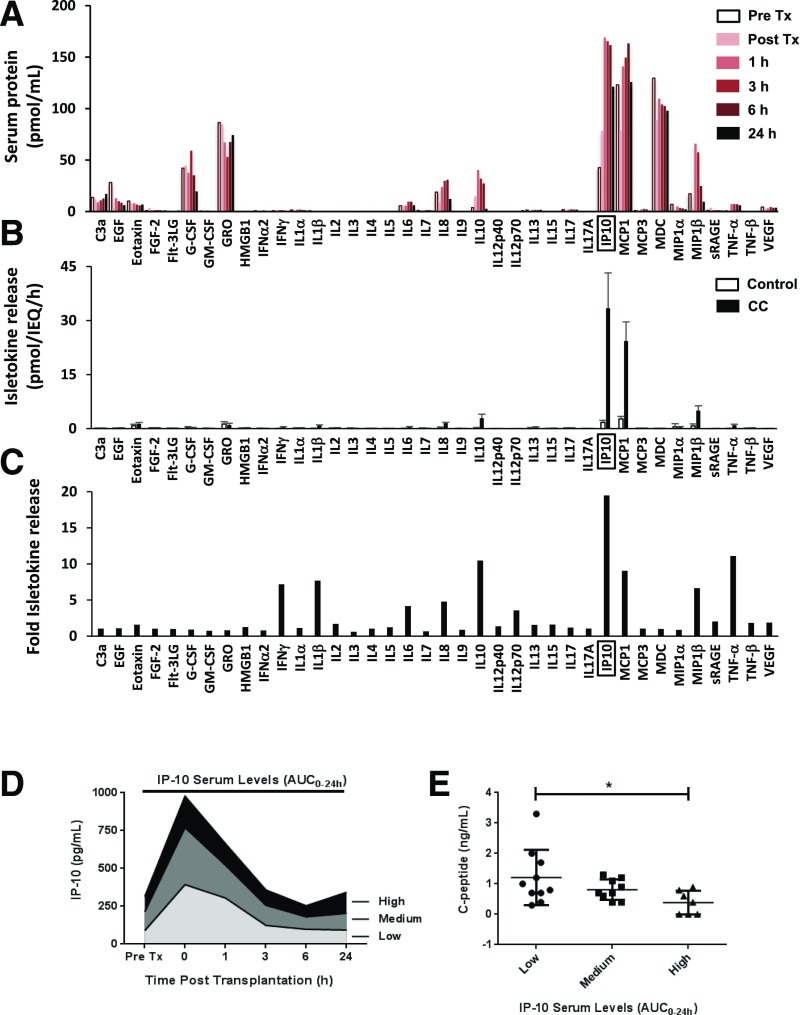
We analyzed clinical samples for inflammatory mediators present in the blood plasma circulation immediately after islet infusion. Samples from islet transplant patients before and after islet infusion were analyzed by Luminex multiplex protein screening to identify early inflammatory mediators present upon infusion into the portal vein. Several isletokines were present in the blood circulation within hours of islet transplantation (Fig. 1A), with particularly high levels of IP-10, MCP-1, and MDC (>100 pmol/mL). Notably, IP-10 showed the largest increase in expression (approximately fourfold) in the circulation within 1 h of islet infusion relative to pretransplant control samples.
Figure 1.
Analysis of inflammatory mediators released from human islets. A: Screening of inflammatory mediators present in patient blood serum samples (n = 34) after islet transplantation (Tx). B: Release of inflammatory mediators from isolated human islets after 2-h exposure to CC. C: Fold release of inflammatory mediators from isolated human islets exposed to CC. D: IP-10 serum levels detected in patients undergoing autologous islet transplantation. Data are represented by AUC0–24h in groups of low (<2 pg ⋅ h/IEQ/kg, n = 10), medium (2–4 pg ⋅ h/IEQ/kg, n = 9), and high (>4 pg ⋅ h/IEQ/kg, n = 7) IP-10. E: Correlation of IP-10 levels released in blood circulation within 24 h of islet transplantation (AUC0–24h) to C-peptide output (pg/mL) as a measure of graft function. Error bars represent SD. Asterisk denotes statistical significance (*P < 0.05) in mean values based on a two-way ANOVA and the Tukey multiple comparison test. Data shown are representative results from three independent experiments using three replicate assays for human islet and patient samples.
To identify isletokines directly produced by islets under inflammatory conditions, we exposed isolated human islets (3,000 IEQ) to a proinflammatory cytokine cocktail (CC) containing 5 ng/mL IL-1β, 10 ng/mL tumor necrosis factor-α (TNF-α), and 50 ng/mL interferon-γ (IFN-γ) for 2 h. Islets were washed extensively, and we analyzed proteins released into the medium 6 h after treatment. The analysis showed that chemokines IP-10, MCP-1, and MIP-1β had the highest rates of release (>5 pmol/IEQ/h) (Fig. 1B). Large inductions (more than fivefold) were observed for IFN- γ, IL-1β, IL-10, IP-10, MCP-1, MIP-1β, and TNF-α (Fig. 1C). Among all cytokines tested, IP-10 had the highest increase in induction and amount of protein released into the circulation after islet infusion or into the medium in vitro after islet exposure to inflammatory stress.
To investigate the role of IP-10 in innate inflammatory reactions immediately after islet transplantation in isolation from adaptive immune responses associated with allotransplantation, we analyzed serum samples from autologous islet transplant patients (n = 26) collected at various intervals immediately after infusion and correlated them to transplant outcomes assessed more than 6 months after transplantation. Transplant patients were grouped by IP-10 area under the curve (AUC) plasma levels over 24 h (AUC0–24h) of low (<2 pg ⋅ h/IEQ/kg, n = 10), medium (2–4 pg ⋅ h/IEQ/kg, n = 9), and high (>4 pg ⋅ h/IEQ/kg, n = 7) (Fig. 1D). The analyses showed that patients in the group with higher levels of IP-10 had significantly reduced islet graft function as assessed by C-peptide (Fig. 1E). Collectively, these data indicate that IP-10 plays a role in the inflammatory response and loss of islet function observed during the immediate peritransplant period. The data also suggest that that lowering IP-10 released by islets could improve transplant outcomes.
Effects of IP-10 on Islet Transplant Outcomes Is Donor-Islet Specific and Can Be Reversed by Anti–IP-10 mAb
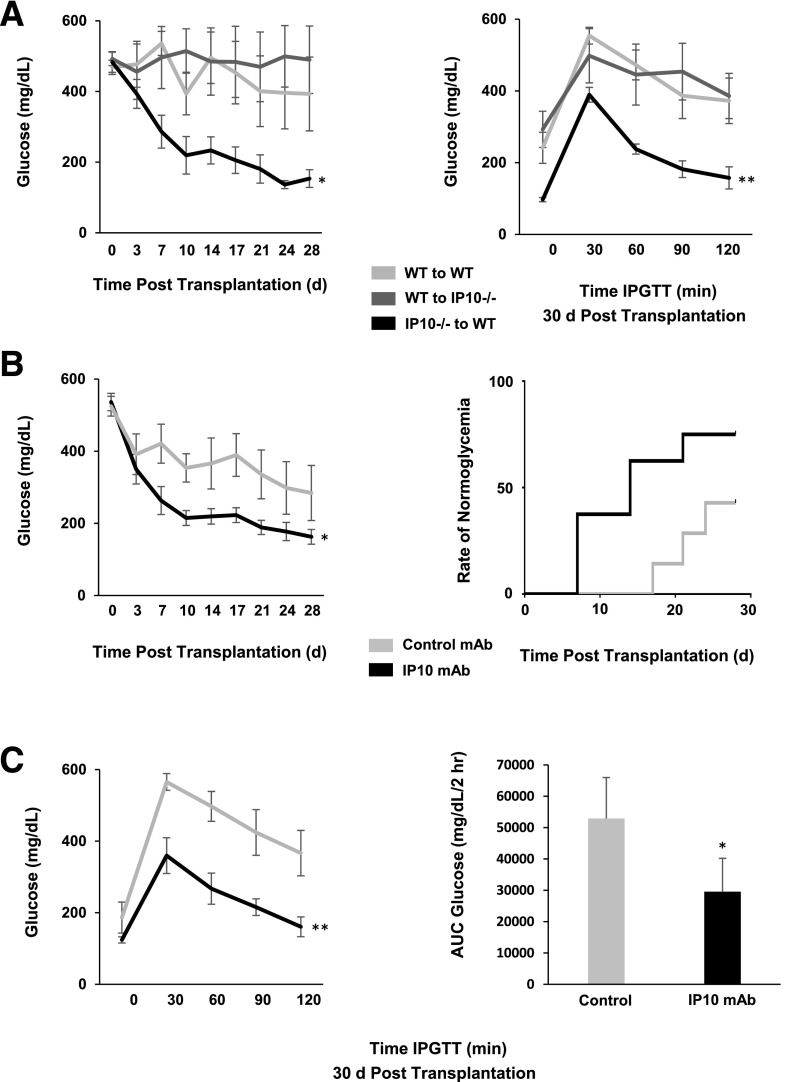
To determine the direct contributions of islet-derived IP-10 on transplant outcomes, we used transgenic IP-10−/− mice to compare effects of IP-10 gene deletion in islet donor tissue and in islet recipients on graft function. STZ-induced diabetic mice were recipients of marginal doses of 200 isolated mouse donor islets intraportally. Blood glucose was monitored in recipients up to 30 days after transplantation, and glucose profiles were assessed by IPGTT (Fig. 2A). Hyperglycemia was reversed in those receiving IP-10−/− donor islets up to 30 days after transplantation. IPGTTs showed reversal of blood glucose to normal concentrations within 2 h of the glucose challenge. In contrast, recipients of wild-type donor islets remained diabetic and exhibited poor glucose tolerance 30 days after transplantation. The results indicate that IP-10 specifically derived from β-cells of donor islets contributes to loss of islet graft endocrine function in mouse islet transplants.
Figure 2.
Effect of IP-10 gene deletion and antibody neutralization on islet transplant outcomes. A: Blood glucose profiles (left) and IPGTT (right) comparing STZ-diabetic recipients of wild-type (WT) and IP-10−/− donor islets up to 30 days after transplantation. B: Transplant outcomes comparing blood glucose profiles (left) and rate of achieving normal glucose (<200 mg/dL) (right) between STZ-diabetic recipients of WT donor islet preparations with IP-10 mAb or IgG1K isotype mAb control. C: IPGTT time course (left) and AUC0–2h (right) comparing IP-10 mAb–treated mice and controls 30 days after transplantation. Error bars represent SD. Asterisks denote statistical significance (*P < 0.05, **P < 0.01) in mean values compared with controls based on the two-tailed Student t test. Data shown are representative of results from at least three independent experiments using three replicate assays with four to seven mice per treatment group.
The clinical and animal transplant data from these studies suggested that lowering or ablating IP-10 could improve islet transplant outcomes. We therefore sought to neutralize the effects of IP-10 with an anti–IP-10 mAb in a syngeneic mouse islet transplant model. Donor islet preparations were pretreated for 10 min with 100 μg anti–IP-10 or IgG1K isotype control mAb and then transplanted intraportally to STZ-diabetic mouse recipients. Diabetes was reversed in the IP-10 mAb–treated mice that received marginal doses of islets, and the rate of normoglycemia was significantly improved compared with the mAb isotype control group (Fig. 2B). IP-10 mAb–treated mice also displayed improved blood glucose profiles, with AUC glucose concentrations significantly lower than those of controls (Fig. 2C). These data indicate that IP-10 production by donor islets is detrimental to islet graft function and that ablation or neutralization of donor islet IP-10 can improve islet transplantation.
IP-10 Is Expressed in Islet β-Cells in Response to Stress-Induced Calcineurin and MAPK Signaling
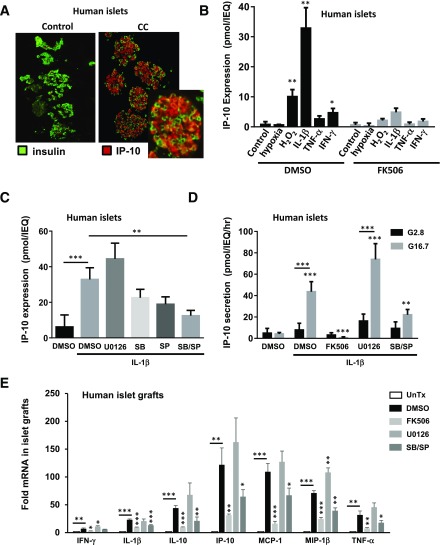
The results from mouse islet transplant experiments suggested that targeting IP-10 expression by stressed islets may also provide a point of intervention to preserve islet graft function. To identify stress-mediated signaling responses that may induce expression of IP-10 in islets during transplantation procedures, we treated human islets in vitro with the proinflammatory CC as well as hypoxia and H2O2 to generate oxidative stress. Exposure to cytokines resulted in a large induction of IP-10 primarily in insulin-producing pancreatic β-cells within isolated islets treated for 24 h before fixation and immunofluorescent antibody staining (Fig. 3A). Further analysis by ELISA indicated that IL-1β produced the largest effect on IP-10 protein expression in human islets compared with TNF-α, IFN-γ, hypoxia, and H2O2 within 6 h of exposure (Fig. 3B). In each case, induction of IP-10 protein was suppressed by the selective protein phosphatase 2B calcineurin inhibitor FK506 (1 μmol/L). This suggests that inflammatory and oxidative stress induces IP-10 expression in islets by calcineurin-dependent signaling pathways.
Figure 3.
Expression and release of IP-10 by human islets in response to stress signaling. A: Immunofluorescent costaining of IP-10 and insulin in isolated human islets exposed to CC for 24 h, with inset at original magnification ×5. B: Induction of IP-10 protein in response to 6-h treatment with hypoxia, H2O2, IL-1β, TNF-α, and IFN-γ in the presence of DMSO control (left) and FK506 (right). C: Effect of MAPK inhibitors U0126, SB203580, and SP600125 on IL-1β–induced IP-10 protein expression. D: Glucose-stimulated release of IP-10 protein from islets pretreated with IL-1β and inhibitors or DMSO control. E: Induction of isletokine mRNA in human islet grafts transplanted into the mice treated with inhibitors or DMSO vehicle control relative to untransplanted (UnTx) islets. Error bars represent SD. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) in mean values for treatments compared with untreated control (*above bar) or condition indicated by lines (*above line) as determined by the Student t test (B and E) and by two-way ANOVA with the Dunnett (C) and Šidák multiple comparison test (D). Data shown are representative of results from at least three independent experiments using three replicate assays.
We further investigated requirements of MAPKs ERK1/2, p38, and JNK to induce IP-10 by the use of selective MAPK signaling inhibitors U0126 (10 μmol/L), SB203580 (10 μmol/L), and SP600125 (10 μmol/L), respectively. Whereas p38 and JNK inhibitors prevented induction of IP-10 protein expression, inhibiting ERK1/2 signaling sustained, and in some cases, enhanced, the response with 6 h of IL-1β treatment (Fig. 3C). These results indicate that stress-activated MAPKs p38 and JNK have selective if not reciprocal roles in the induction of intracellular IP-10 protein expression in human islets in response to IL-1β.
We also examined conditions that could influence IP-10 release from stressed islets. We observed that human islets treated with IL-1β for 6 h had significantly increased rates of IP-10 release (>40 pmol/IEQ/h) when exposed to high glucose (Fig. 3D). The effect of glucose on the release of IP-10 from islets was likely independent of insulin action, because exogenous insulin up to 100 nmol/L could not directly stimulate IP-10 secretion alone or in combination with glucose (Supplementary Fig. 1). Glucose alone was not sufficient to induce IP-10 expression but was required for IP-10 release after 6 h induction with IL-1β. Moreover, glucose-stimulated IP-10 protein release was significantly reduced in human islets exposed to IL-1β in the presence of p38 and JNK but not ERK1/2 inhibitor. Notably, glucose-stimulated insulin secretion was not affected by MAPK inhibitors (Supplementary Fig. 2). Taken together, these data indicate that calcineurin signaling and MAPKs p38 and JNK are required for IP-10 expression and that glucose can stimulate its subsequent release from human islets. Importantly, the results demonstrate that IP-10 signaling pathways can be targeted for suppression without affecting β-cell function.
Calcineurin and MAPKs Regulate NFAT-Mediated Isletokine Gene Transcription in Pancreatic β-Cells
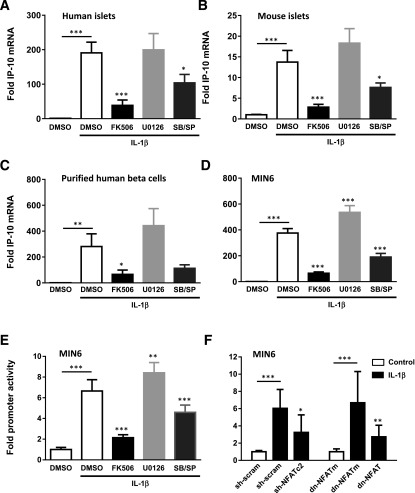
The previous experiments demonstrated that human islets could express IP-10 in response to stress-activated signaling pathways. To determine whether islets expressed isletokines during islet transplantation, we transplanted isolated human islets into the kidney capsules of diabetic nude mice and removed the islet grafts by scalpel dissection after 6 h to evaluate the expression of isletokine genes. The gene expression assays showed a significant induction of mRNA in human islet xenografts for all isletokines tested (Fig. 3E). In each case, isletokine mRNA was blocked or largely reduced by pretreatment of islets with calcineurin and MAPK p38 and JNK inhibitors. These results indicate that IP-10 and other isletokines are induced in human islets upon transplantation at the transcriptional level via a common mechanism involving calcineurin and MAPK signaling pathways. Similarly, IP-10 mRNA was induced in isolated human and mouse islets in vitro within 2 h of exposure to IL-1β and suppressed by both calcineurin and MAPK p38 and JNK inhibitors (Fig. 4A and B). These responses were also observed in FACS-purified human β-cells and MIN6 β-cell lines (Fig. 4C and D). The results were further corroborated using a different set of highly selective MAPK inhibitors in MIN6 cells (Supplementary Fig. 3). Moreover, calcineurin and MAPKs p38 and JNK were required to induce IP-10 gene promoter activity in MIN6 cells as detected by luciferase promoter-reporter assays (Fig. 4E). These experiments collectively demonstrate that IP-10 induction is regulated by calcineurin and MAPKs in pancreatic islet β-cells during islet transplantation and under conditions of inflammatory stress.
Figure 4.
Induction of the IP-10 gene by MAPK and NFAT signaling in pancreatic β-cells. Fold induction of IP-10 mRNA expression after 2-h exposure to IL-1β in human islets (A), mouse islets (B), purified human β-cells (C), and the MIN6 β-cell line (D). Induction of the mouse IP-10 gene promoter in MIN6 cells and blockade by calcineurin and MAPK pharmacological inhibitors (E) and NFAT shRNA interference knockdown and dnNFAT (F). Error bars represent SD. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) in mean values for treatments compared with untreated control (*above bar) or condition indicated by lines (*above line) as determined by two-way ANOVA and the Dunnett multiple comparison test. Data shown are representative of results from at least three independent experiments using three replicate assays.
That calcineurin activates downstream target NFAT-family transcription factors to directly regulate cytokine genes in immune cells has been well established (29–39). Interfering with the ability of calcineurin to bind to and dephosphorylate NFAT-family member substrates prevents NFAT from regulating cytokine gene transcription. Thus, we used an RNA interference knockdown approach to test the requirement of NFAT in the induction of IP-10 promoter activity in MIN6 cells. Overexpression of NFATc2 shRNA reduced induction of IP-10 promoter activity in MIN6 cells treated for 6 h with IL-1β compared with scrambled shRNA controls. In addition, overexpression of a dnNFAT PxIxIT motif to interfere with NFAT interaction with calcineurin suppressed IL-1β–induced IP-10 promoter activity compared with an alanine-substituted AxAxAA dnNFATm control (Fig. 5F). These results indicated that NFAT is an essential component for the activation of the IP-10 gene promoter by calcineurin in pancreatic β-cells.
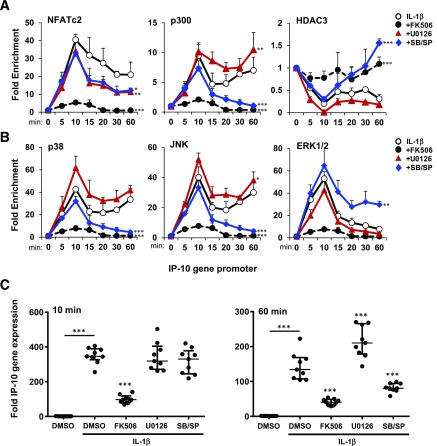
Figure 5.
Signaling requirements for interaction of NFAT and associated factors with the IP-10 gene promoter. ChIP time course analysis of promoter association of NFAT, p300, and HDAC3 (A) and MAPKs p38, JNK, and ERK1/2 (B) with the IP-10 promoter in MIN6 cells exposed to IL-1β in the presence of calcineurin and MAPK pharmacological inhibitors. C: Nuclear run-on assay of IP-10 mRNA released from nuclei isolated from MIN6 exposed to IL-1β for 10 min (left) and 60 min (right) in the presence of calcineurin and MAPK pharmacological inhibitors. Error bars represent SD. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) in mean values for treatments compared with untreated control (*above bar) or condition indicated by lines (*above line) as determined by two-way ANOVA with the Dunnett (A and B) and Tukey multiple comparison test (C). Data shown are representative of results from three independent experiments using two (A and B) or three (C) replicate assays.
To determine whether NFAT directly interacts with the IP-10 gene in β-cells, we performed ChIP assays to examine the association of NFATc2 with the 5′ flanking IP-10 gene promoter in MIN6 cells. Time course analyses indicated that the IP-10 promoter was enriched with NFATc2 up to 40-fold within 10 min of IL-1β exposure (Fig. 5A). The association of NFATc2 with the IP-10 promoter was largely sustained for up to 60 min. In contrast, FK506 abolished NFATc2 binding. MAPK inhibitors had little effect on the acute (10 min) association of NFATc2 with the IP-10 gene but showed a significant reduction in a sustained (60 min) response. Histone acetylase p300 showed a biphasic association with the IP-10 promoter. However, whereas ERK1/2 inhibition had no effect on p300 association, inhibitors of p38 and JNK prevented a sustained response. The biphasic kinetics of p300 were inverse to what was observed with the HDAC3 association where p38 and JNK inhibition enhanced the response to IL-1β. This inverse relationship indicates competitive MAPK signaling requirements for p300 and HDAC3 to regulate the IP-10 promoter. The results also suggest that calcineurin signaling is required for relative changes of association of p300 and HDAC3 with the IP-10 promoter to occur.
MAPKs have previously been shown to translocate to the nucleus and associate with DNA-protein complexes within gene promoters (13,40,41). Thus, we also examined effects of IL-1β to induce MAPK association with the IP-10 promoter. p38 and JNK both associated with the IP-10 promoter with similar kinetics as observed with p300 (Fig. 5B). This contrasted with ERK1/2, which was transiently enriched within 10 min but sharply subsided by 60 min. In each case, FK506 prevented MAPKs from associating with the IP-10 promoter. MAPK inhibitors had little effect on the acute accumulation of MAPKs on the promoter but selectively inhibited late-phase responses. Together, these results suggest that calcineurin signaling is required for NFAT, p300, HDAC3, and MAPK association with the IP-10 promoter and that MAPK activity is required for sustained formation of the protein-DNA chromatin complex.
The transient association of transcription factors versus sustained complex formation on the DNA promoter may impart differences in transcriptional activity. We therefore performed nuclear run-on assays to measure the transcriptional output of nuclei from MIN6 cells exposed to conditions to simulate the transcriptional complexes described above. The results showed a calcineurin-dependent induction of IP-10 mRNA output for both the 10-min and 60-min stimulations (Fig. 5C). MAPK inhibitors had no effect on output at 10 min when all factors were observed on the gene promoter. However, inhibition of p38 and JNK resulted in diminished IP-10 mRNA output by 60 min. These conditions correlated with the loss of p300, p38, and JNK on the IP-10 promoter (Fig. 5A and B). U0126 had no effect on promoter output at 60 min when ERK1/2 was absent, confirming that ERK1/2 is not required for IP-10 sustained p300 binding and IP-10 transcription (Fig. 5B and C). Overall, these data suggest that calcineurin and NFATc2 signaling are required for recruitment of enzymes p300 and MAPKs to regulate the IP-10 promoter and that p38 and JNK MAPK-selective activity is required for DNA-protein complex stability to promote a sustained transcriptional response.
Discussion
The current study reveals a role of islet-derived cytokines in mediating acute inflammatory damage to islet grafts during the early stages of islet transplantation. High levels of circulating IP-10 observed in transplant patients within hours of islet infusion correlate with poor long-term outcomes in graft function. The source of IP-10 released from islets was confirmed in a mouse transplant model, where β-cell–specific ablation of IP-10 in donor islets could significantly improve glucose tolerance in STZ-induced diabetic recipients compared with wild-type donor islets. This was further highlighted by improvements in islet transplant outcomes by the use of anti–IP-10 mAb to neutralize IP-10 secreted from islets transplanted into diabetic mouse recipients. The acute detrimental effects of locally released IP-10 on transplanted islets is likely indirect because direct treatment of isolated islets with concentrations of up to 100 ng/mL recombinant IP-10 did not affect β-cell function or apoptosis within 24 h of exposure (Supplementary Fig. 4A and B).
These results provide proof of principle for the potential use of anticytokine therapy to protect islets during clinical islet transplantation procedures. Indeed, promising results have been observed with the use of anti-inflammatory recombinant protein antagonists to neutralize cytokines during islet transplantation. Our center has successfully used etanercept and anakinra to block TNF-α and IL-1β, respectively, to improve islet transplant outcomes (42). These observations were corroborated in a controlled mouse transplant study that demonstrated combined effects of etanercept and anakinra to improve islet cell function and engraftment in immunodeficient mice (43). These results show a combinatorial approach to targeting circulating inflammatory mediators, such as IP-10, TNF-α, and IL-1β, could conceivably have synergistic effects to improve islet transplant outcomes or reduce IEQ tissue required. The use of neutralizing protein antagonists to selectively target key isletokines released upon islet infusion to improve transplant outcomes warrants further exploration for clinical use.
The study also identifies upstream molecular targets to block isletokine production by islet β-cells. In vitro and in vivo results both indicated that a set of common signaling components is activated by oxidative or inflammatory stress, which induces expression of isletokine genes in β-cells. One common signaling component is the calcium-dependent calcineurin/NFAT pathway. The selective calcineurin inhibitor FK506 suppressed multiple cytokines in human islet grafts transplanted in mice. Further analysis showed that the downstream transcriptional target of calcineurin, NFATc2, and histone acetylase p300 were associated with the IP-10 promoter in response to IL-1β.
The other identified common signaling component involves stress-activated MAPKs. The activity of chromatin-bound p38 and JNK promoted the association of both NFATc2 and p300 while opposing the accumulation of HDAC3. Transcriptional activity of IP-10 in β-cells correlated closely with kinetics of promoter-bound NFATc2 and p300, indicating that p38 and JNK were required for gene expression. ERK1/2, however, had little effect on the IP-10 gene promoter, which highlights a kinase-selective role of stress-activated p38 and JNK in islet inflammation. Together, these results indicate that multiple isletokines expressed during islet transplantation can be blocked by perturbing calcineurin/NFATc2 and p38/JNK signaling.
NFAT interacts with basic helix-loop-helix (bZIP) family proteins to regulate cytokine gene promoters (44–50). These bZIP proteins can enhance or repress promoter activity, depending on the cell signaling and gene promoter context. We previously showed that NFAT requires bZIPs c-Jun and ATF2 to activate the TNF-α promoter in β-cells when exposed to high glucose and IL-1β (5). However, under normal conditions, β-cell–specific bZIP MafA interacts with NFAT to both regulate the insulin gene in response to glucose conditions and maintain the TNF-α gene in a silenced state (5,6). Further studies are required to determine specific NFATc2-interacting cofactors that bind to and regulate IP-10 and other isletokine promoters in β-cells during islet cell transplantation.
Lastly, proinflammatory cytokines are systemically elevated during the progression of type 1 and type 2 diabetes. Although inflammatory cells largely contribute to this inflammatory state, the role of isletokines in the context of diabetes has not been fully explored. Considering that islets can evoke an inflammatory response when stressed and transplanted, β-cells could conceivably contribute to inflammatory responses during a metabolic or inflammatory disease state. Thus, β-cells could play a role in the initiation or exacerbation of their own immune destruction by release of isletokines when damaged or distressed. In this broader context, targeting isletokines may have the potential to prevent or delay progression of metabolic diseases propagated by islet inflammation and β-cell–targeted immune destruction.
Supplementary Material
Article Information
Acknowledgments. The authors thank Nofit Borenstein-Auerbach and Rituparna Bhattacharjee for technical assistance and Monila Khadka for database analysis assistance, all from Baylor Scott & White Research Institute. The authors acknowledge the editorial assistance of Cindy Orticio, Baylor Scott & White Health.
Funding. This work was supported by Roche Diagnostic Corporation, Baylor Scott & White Health, and the Tubitak Graduate Scholarship to G.B. The National Institutes of Health grants U19-AI-057234 funded mAb development and 1UC4-DK-098085 funded the Integrated Islet Distribution Program.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. G.Y., F.K., P.B.S., R.S., C.C., C.M.D., S.Z., G.B., and M.A.K. researched data. M.F.L. contributed to discussion. B.N. contributed to discussion and reviewed and edited the manuscript. M.C.L. researched data and wrote the manuscript. M.C.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 2015 International Pancreas and Islet Transplant Association, Melbourne, Australia, 15–19 November 2015; the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016; the 2015 American Transplant Congress, Philadelphia, PA, 2–6 May 2015; and at the 2016 American Transplant Congress, Boston, MA, 11–15 June 2016.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db17-0578/-/DC1.
References
- 1.Chen MC, Schuit F, Eizirik DL. Identification of IL-1beta-induced messenger RNAs in rat pancreatic beta cells by differential display of messenger RNA. Diabetologia 1999;42:1199–1203 [DOI] [PubMed] [Google Scholar]
- 2.Cardozo AK, Kruhøffer M, Leeman R, Orntoft T, Eizirik DL. Identification of novel cytokine-induced genes in pancreatic beta-cells by high-density oligonucleotide arrays. Diabetes 2001;50:909–920 [DOI] [PubMed] [Google Scholar]
- 3.Kutlu B, Cardozo AK, Darville MI, et al. . Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes 2003;52:2701–2719 [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen ML, Rønn SG, Bruun C, et al. . IL-1beta-induced chemokine and Fas expression are inhibited by suppressor of cytokine signalling-3 in insulin-producing cells. Diabetologia 2009;52:281–288 [DOI] [PubMed] [Google Scholar]
- 5.Lawrence MC, Naziruddin B, Levy MF, Jackson A, McGlynn K. Calcineurin/nuclear factor of activated T cells and MAPK signaling induce TNF-alpha gene expression in pancreatic islet endocrine cells. J Biol Chem 2011;286:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence MC, Borenstein-Auerbach N, McGlynn K, et al. . NFAT targets signaling molecules to gene promoters in pancreatic β-cells. Mol Endocrinol 2015;29:274–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maedler K, Sergeev P, Ris F, et al. . Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia 2003;46:255–266 [DOI] [PubMed] [Google Scholar]
- 9.Taylor-Fishwick DA, Weaver JR, Grzesik W, et al. . Production and function of IL-12 in islets and beta cells. Diabetologia 2013;56:126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes M, Kutlu B, Miani M, et al. . Temporal profiling of cytokine-induced genes in pancreatic β-cells by meta-analysis and network inference. Genomics 2014;103:264–275 [DOI] [PubMed] [Google Scholar]
- 11.Comens PG, Wolf BA, Unanue ER, Lacy PE, McDaniel ML. Interleukin 1 is potent modulator of insulin secretion from isolated rat islets of Langerhans. Diabetes 1987;36:963–970 [DOI] [PubMed] [Google Scholar]
- 12.Maedler K, Schumann DM, Sauter N, et al. . Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes 2006;55:2713–2722 [DOI] [PubMed] [Google Scholar]
- 13.Lawrence MC, Shao C, McGlynn K, Naziruddin B, Levy MF, Cobb MH. Multiple chromatin-bound protein kinases assemble factors that regulate insulin gene transcription. Proc Natl Acad Sci U S A 2009;106:22181–22186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roep BO, Kleijwegt FS, van Halteren AG, et al. . Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol 2010;159:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arous C, Ferreira PG, Dermitzakis ET, Halban PA. Short term exposure of beta cells to low concentrations of interleukin-1β improves insulin secretion through focal adhesion and actin remodeling and regulation of gene expression. J Biol Chem 2015;290:6653–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajmrle C, Smith N, Spigelman AF, et al. Interleukin-1 signaling contributes to acute islet compensation. JCI Insight 2016;1:e86055 [DOI] [PMC free article] [PubMed]
- 17.Ortis F, Pirot P, Naamane N, et al. . Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia 2008;51:1213–1225 [DOI] [PubMed] [Google Scholar]
- 18.Gurzov EN, Ortis F, Cunha DA, et al. . Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ 2009;16:1539–1550 [DOI] [PubMed] [Google Scholar]
- 19.Hasnain SZ, Borg DJ, Harcourt BE, et al. . Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med 2014;20:1417–1426 [DOI] [PubMed] [Google Scholar]
- 20.Gorasia DG, Dudek NL, Veith PD, et al. . Pancreatic beta cells are highly susceptible to oxidative and ER stresses during the development of diabetes. J Proteome Res 2015;14:688–699 [DOI] [PubMed] [Google Scholar]
- 21.Paraskevas S, Aikin R, Maysinger D, et al. . Activation and expression of ERK, JNK, and p38 MAP-kinases in isolated islets of Langerhans: implications for cultured islet survival. FEBS Lett 1999;455:203–208 [DOI] [PubMed] [Google Scholar]
- 22.Nishiki Y, Adewola A, Hatanaka M, Templin AT, Maier B, Mirmira RG. Translational control of inducible nitric oxide synthase by p38 MAPK in islet β-cells. Mol Endocrinol 2013;27:336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguchi H, Nakai Y, Ueda M, et al. . Activation of c-Jun NH2-terminal kinase (JNK) pathway during islet transplantation and prevention of islet graft loss by intraportal injection of JNK inhibitor. Diabetologia 2007;50:612–619 [DOI] [PubMed] [Google Scholar]
- 24.Fukuda K, Tesch GH, Yap FY, et al. . MKK3 signalling plays an essential role in leukocyte-mediated pancreatic injury in the multiple low-dose streptozotocin model. Lab Invest 2008;88:398–407 [DOI] [PubMed] [Google Scholar]
- 25.Abdelli S, Ansite J, Roduit R, et al. . Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 2004;53:2815–2823 [DOI] [PubMed] [Google Scholar]
- 26.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Ups J Med Sci 2000;105:125–133 [DOI] [PubMed] [Google Scholar]
- 27.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 2002;51:1779–1784 [DOI] [PubMed] [Google Scholar]
- 28.Chow CW, Rincón M, Davis RJ. Requirement for transcription factor NFAT in interleukin-2 expression. Mol Cell Biol 1999;19:2300–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science 1988;241:202–205 [PubMed] [Google Scholar]
- 30.Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science 1989;246:1617–1620 [DOI] [PubMed] [Google Scholar]
- 31.Bierer BE, Mattila PS, Standaert RF, et al. . Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A 1990;87:9231–9235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flanagan WM, Corthésy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 1991;352:803–807 [DOI] [PubMed] [Google Scholar]
- 33.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992;357:695–697 [DOI] [PubMed] [Google Scholar]
- 34.Cockerill PN, Shannon MF, Bert AG, Ryan GR, Vadas MA. The granulocyte-macrophage colony-stimulating factor/interleukin 3 locus is regulated by an inducible cyclosporin A-sensitive enhancer. Proc Natl Acad Sci U S A 1993;90:2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J, Yaseen NR, Hogan PG, Rao A, Sharma S. Phosphorylation of the transcription factor NFATp inhibits its DNA binding activity in cyclosporin A-treated human B and T cells. J Biol Chem 1995;270:20653–20659 [DOI] [PubMed] [Google Scholar]
- 36.Wesselborg S, Fruman DA, Sagoo JK, Bierer BE, Burakoff SJ. Identification of a physical interaction between calcineurin and nuclear factor of activated T cells (NFATp). J Biol Chem 1996;271:1274–1277 [DOI] [PubMed] [Google Scholar]
- 37.Loh C, Shaw KT, Carew J, et al. . Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J Biol Chem 1996;271:10884–10891 [DOI] [PubMed] [Google Scholar]
- 38.Luo C, Burgeon E, Carew JA, et al. . Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol Cell Biol 1996;16:3955–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamura H, Aramburu J, García-Rodríguez C, et al. . Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell 2000;6:539–550 [DOI] [PubMed] [Google Scholar]
- 40.Lawrence MC, McGlynn K, Shao C, et al. . Chromatin-bound mitogen-activated protein kinases transmit dynamic signals in transcription complexes in beta-cells. Proc Natl Acad Sci U S A 2008;105:13315–13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson JD, LeBoeuf RC, Bomsztyk K. Direct recruitment of insulin receptor and ERK signaling cascade to insulin-inducible gene loci. Diabetes 2011;60:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto S, Takita M, Chaussabel D, et al. . Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant 2011;20:1641–1647 [DOI] [PubMed] [Google Scholar]
- 43.McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant 2012;12:322–329 [DOI] [PubMed] [Google Scholar]
- 44.Jain J, McCaffrey PG, Miner Z, et al. . The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 1993;365:352–355 [DOI] [PubMed] [Google Scholar]
- 45.Northrop JP, Ullman KS, Crabtree GR. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem 1993;268:2917–2923 [PubMed] [Google Scholar]
- 46.Tsai EY, Jain J, Pesavento PA, Rao A, Goldfeld AE. Tumor necrosis factor alpha gene regulation in activated T cells involves ATF-2/Jun and NFATp. Mol Cell Biol 1996;16:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun LJ, Peterson BR, Verdine GL. Dual role of the nuclear factor of activated T cells insert region in DNA recognition and cooperative contacts to activator protein 1. Proc Natl Acad Sci U S A 1997;94:4919–4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang TT, Chow CW. Transcription cooperation by NFAT.C/EBP composite enhancer complex. J Biol Chem 2003;278:15874–15885 [DOI] [PubMed] [Google Scholar]
- 49.Falvo JV, Lin CH, Tsytsykova AV, et al. . A dimer-specific function of the transcription factor NFATp. Proc Natl Acad Sci U S A 2008;105:19637–19642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters RD, Drullinger LF, Kugel JF, Goodrich JA. NFATc2 recruits cJun homodimers to an NFAT site to synergistically activate interleukin-2 transcription. Mol Immunol 2013;56:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.