Abstract
Objective:
The purpose of this study was to identify the expression characteristics of circular RNAs in the peripheral blood of coronary artery disease patients and type 2 diabetes mellitus patients.
Methods:
Circular RNA in the peripheral blood from 6 control individuals, 6 coronary artery disease patients, 6 type 2 diabetes mellitus patients and 6 coronary artery disease combined with type 2 diabetes mellitus patients was collected for microarray analysis, and a further independent cohort consisting of 20 normal individuals, 20 type 2 diabetes mellitus subjects and 20 coronary artery disease subjects was used to verify the expression of five circular RNAs chosen for further analysis. The findings were then tested in a third cohort using quantitative real-time polymerase chain reaction.
Results:
In total, 40 circular RNAs differentially expressed between the three experimental groups and the control group were identified by microarray analysis: 13 were upregulated in the experimental groups, while 27 were downregulated. Of the five circular RNAs chosen for further analysis, three were significantly downregulated in the experimental groups. The crude odds ratios and adjusted odds ratios of hsa-circRNA11783-2 showed significant differences in both the coronary artery disease group and type 2 diabetes mellitus group. We then verified hsa-circRNA11783-2 in the third cohort, and it remained closely related to both coronary artery disease and type 2 diabetes mellitus.
Conclusion:
Hsa-circRNA11783-2 is closely related to both coronary artery disease and type 2 diabetes mellitus.
Keywords: Coronary artery disease, type 2 diabetes mellitus, circular RNA, circulating circular RNA, microarray analysis
Introduction
In 2015, the mortality associated with coronary artery disease (CAD) was 2.6% higher than in 2013, and with an ageing population in China, the number of deaths from cardiovascular disease (CVD) is still increasing rapidly.1 According to the 7th edition of the Diabetes Atlas by the International Diabetes Federation (IDF), there were nearly 410 million diabetes mellitus (DM) patients around the world in 2015, of which approximately 46.5% have not yet been diagnosed. The China Cardiac Survey collected clinical data from 3513 patients in seven cities across the country, which revealed a high percentage (up to 77%) of hyperglycaemia in patients with CAD.2
Over the past few years, non-coding RNAs have been considered the ‘dark matter’ of the genome because of their indefinite types and functions. However, studies have shown that non-coding RNAs are able to regulate gene expression even though they cannot code for proteins directly.3 Circular RNAs (circRNAs) are a class of closed circular non-coding RNAs derived from reverse splicing of exons, introns or both.4,5 With a ring structure lacking the 5′-end cap and the 3′-end poly(A) tail, circRNAs cannot be recognized or hydrolysed by RNA exonuclease, and therefore, circRNAs possess relatively higher biological stability than linear RNA.6 The regulatory gene expression mechanisms of circRNAs vary. As an ‘miRNA sponge’, circRNAs play an important regulatory role at the post-transcriptional level via competitive binding to miRNAs.7 The circRNAs can also regulate transcription by interacting with small nuclear RNA (snRNA) or RNA polymerase II in the nucleus8 and competitively regulate RNA splicing by binding to transcription factors.9 In addition, the expression of circRNAs is tissue specific and time specific; for example, ciRS-7 is highly expressed in brain tissue.10 Moreover, there are significant differences in the type and expression of circRNAs at different stages of biological development.11,12
Previous research has shown that circRNAs are closely related to certain human diseases. Burd et al.13 found that cANRIL, the circular isoform of long non-coding RNA (lncRNA)-ANRIL, may influence the development of CAD by affecting the expression of the INK4/ARF gene. Wang et al.14 discovered that miR-7 might be involved in the occurrence of DM by reducing regeneration of pancreatic β cells. Qin et al.15 demonstrated that hsa_circ_0005075 is a potential biomarker for liver cancer. In this study, we investigated the relationship between circRNAs with CAD and type 2 diabetes mellitus (T2DM). Microarray analysis was conducted to compare the expression profiles of circRNAs in the peripheral blood of normal individuals, CAD patients, T2DM patients and CAD combined with T2DM patients, and the results were verified in larger cohorts.
Materials and methods
Patient population
The 289 participants in the study were divided into three cohorts (the clinical and demographic features of each cohort are shown in Supplementary Tables 1 to 3). Participants were enrolled from the Departments of Cardiology and Endocrinology of the People’s Hospital of Zhengzhou University from July 2015 to June 2016. All participants received coronary angiography (CAG) and an oral glucose tolerance test (OGTT) to identify the presence of CAD and/or T2DM. Clinically matched participants (24 in total; control group, n = 6; T2DM group, n = 6; CAD group, n = 6; CAD combined with T2DM group, n = 6) were enrolled for microarray analysis. CircRNA expression was verified in the second cohort (control group, n = 20; T2DM group, n = 20; CAD group, n = 20), and the circRNA with the best performance was selected and tested in the third cohort (control group, n = 60; T2DM group, n = 64; CAD group, n = 81). The exclusion criteria were as follows: (1) liver or kidney dysfunction, (2) autoimmune disease, (3) malignancy, (4) any other clinically acute or chronic inflammatory systemic disease, (5) uncontrolled hypertension, (6) endocrine disease aside from T2DM or (7) previous history of acute myocardial infarction (AMI), percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).
Definition of CAD and T2DM
In this study, CAD was diagnosed according to the American College of Cardiology/American Heart Association guidelines (at least one coronary artery stenosis: ⩾50%).16 The extent of coronary artery stenosis was measured by two independent cardiologists by visual observation.
T2DM was diagnosed according to the 1998 Standards of the World Health Organization (WHO);17 T2DM was diagnosed when one of the following criteria were met: (1) fasting blood glucose (FPG) ⩾ 126 mg/dL (7.0 mmol/L), where fasting was defined in the study as abstinence from any calories for at least 8 h or (2) 2-h post-load plasma glucose ⩾ 200 mg/dL (11.1 mmol/L) during the OGTT.
In this study, control individuals showed no sign of coronary atherosclerosis or microvascular disease investigated by negative treadmill exercise testing (TET) and emission computed tomography (ECT), and their 2 h post-load plasma glucose levels in the OGTT and FPG test were less than 140 mg/dL (7.8 mmol/L) and 110 mg/dL (6.1 mmol/L), respectively.
Collection of blood samples, RNA extraction and quantitative real-time polymerase chain reaction
After an overnight fast, 2 mL of blood was collected from the median cubital vein in the early morning before breakfast and stored in a vacuum blood collection tube containing ethylenediaminetetraacetic acid (EDTA) anticoagulant. Within 20 min (during which time the blood samples were stored in an ice box at 4°C), total RNA was extracted from the whole blood (1 mL) with a total RNA rapid extraction kit (CapitalBio Corp., Beijing, China) following the manufacturer’s instructions. RNA was dissolved in ribonuclease (RNase)-free water, and the concentration and purity of total RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The integrity of RNA was assessed with 1% formaldehyde denaturing gel electrophoresis. Subsequently, reverse transcription was performed by applying a PrimeScript RT reagent kit (Takara Bio, Inc., Shiga, Japan) to obtain the complementary DNA (cDNA). Quantitative Real-Time Polymerase Chain Reaction (Q-PCR) was performed using SYBR-Green Premix Ex Taq (Takara Bio, Inc.) and monitored with an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Life Technologies, Foster City, CA, USA). The relative expression levels of circRNAs were determined by Q-PCR. Primers used for Q-PCR are listed in Supplementary Table 4.
Microarray analysis of circRNA
Participants (normal individuals, CAD subjects, T2DM subjects and subjects with both CAD and T2DM; six cases in each group) were enrolled to conduct the microarray analysis. Digestion, dephosphorylation, degeneration and amplification of RNA were performed in accordance with the instructions of the manufacturer. RNA was labelled with Cy3-dCTP, and purified RNA was hybridized to a microarray (human circRNA Array V2.0, CapitalBio., Beijing, China) containing 170,340 human circRNA probes. GeneSpring Software V13.0 (Agilent Technologies, Santa Clara, CA, USA) was employed to analyse microarray data. To select the differentially expressed circRNAs, a threshold value of fold change ⩾2 or ⩽−2 with a p < 0.05 determined by a t test was used.
Statistical analysis
Variables of different distributions are expressed as the mean ± standard deviation or percentages. In the scatterplot showing circRNA expression, the horizontal lines represent the median values. A chi-square test was conducted for categorical variables; Kolmogorov–Smirnov and Shapiro–Wilk tests were performed to check data normality for continuous variables, followed by testing for homogeneity of variance. Significant differences in clinical and demographic indicators were tested using one-way analysis of variance (ANOVA) or a Kruskal–Wallis H test. Logistic regression analysis was applied to obtain odds ratios (ORs) after the relative expression of circRNAs expanded to 10 times, and adjusted ORs were also estimated after shared risk factors for CAD and T2DM were introduced: smoking, hypertension, total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL). For all tests, a p value under 0.05 was considered statistically significant. All analyses were performed using SPSS Statistics 22.0 software (SPSS Inc., Chicago, IL, USA).
Results and discussion
Characteristics of circRNA expression profiles
The results showed significant differences in circRNA expression between the control group and the three experimental groups. Microarray analysis identified 2036 circRNAs differentially expressed between the control group and the CAD group; 376 were upregulated and 1660 were downregulated in the CAD group (Supplementary Table 5), while 489 were differentially expressed between the control group and the T2DM group. In addition, 78 were upregulated in the T2DM group, whereas 411 were downregulated (Supplementary Table 6). Similarly, 220 circRNAs were also differentially expressed in the control group and the CAD and T2DM group, of which 147 were upregulated, and the other 73 were downregulated (Supplementary Table 7). Further analysis indicated that 40 circRNAs were differentially expressed in all three experimental groups, of which 13 circRNAs were upregulated and 27 were downregulated in the experimental groups (Supplementary Table 8). Then, seven circRNAs were screened under improved screening criteria, with a p value less than 0.005 and fold change >2.1; five circRNAs showing relatively larger fold changes were then selected for the following validation, namely, hsa_circ_0009036, hsa_circ_0054129, hsa-circRNA11806-28, hsa-circRNA6510-1 and hsa-circRNA11783-2 (highlighted in Supplementary Table 8).
The circRNA expression profile verified by Q-PCR
We used the second cohort (control group, n = 20; T2DM group, n = 20; and CAD group, n = 20) to validate the five circRNAs selected in the previous step and the results are shown in Figure 1. The expression levels of hsa_circ_0009036 and hsa_circ_0054129 were not significantly different between groups, while the levels of hsa-circRNA11806-28, hsa-circRNA6510-1 and hsa-circRNA11783-2 were significantly lower in both the T2DM group and CAD group compared with the control group (Table 1).
Figure 1.
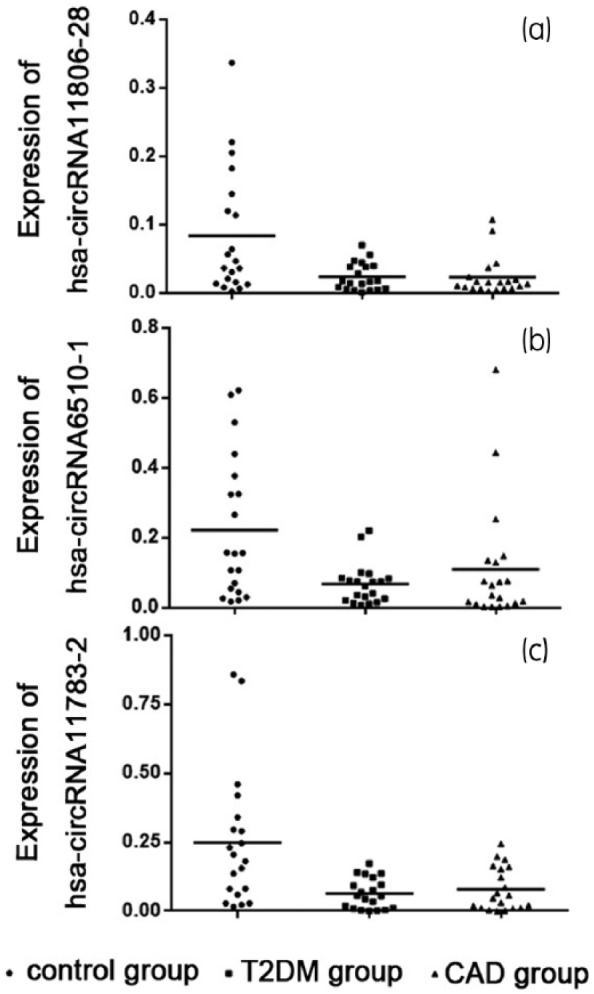
Expression levels of downregulated circRNAs quantified by Q-PCR. The cohort included 20 control individuals, 20 CAD patients and 20 T2DM patients. The relative circRNA levels were normalized to the level of the control (β-actin): expression levels of (a) hsa-circRNA11806-28, (b) hsa-circRNA6510-1 and (c) hsa-circRNA11783-2.
Table 1.
Validation of downregulated circRNAs by Q-PCR.
| CAD group vs control group |
T2DM group vs control group | |||
|---|---|---|---|---|
| Fold change | p value | Fold change | p value | |
| hsa-circRNA11806-28 | 0.3 | 0.007 | 0.3 | 0.027 |
| hsa-circRNA6510-1 | 0.5 | 0.016 | 0.3 | 0.007 |
| hsa-circRNA11783-2 | 0.3 | 0.003 | 0.3 | 0.001 |
circRNAs: circular RNAs; Q-PCR: quantitative real-time polymerase chain reaction; CAD: coronary artery disease; T2DM: type 2 diabetes mellitus.
Logistic regression analysis of the differentially expressed circRNAs
Logistic regression analysis was further performed to compare correlations between the three circRNAs and CAD and T2DM, and the analysis was adjusted by introducing risk factors: smoking, hypertension, TC, TG, HDL and LDL (Table 2). The results indicated that hsa-circRNA11783-2 had a stronger correlation with CAD and T2DM than hsa-circRNA11806-28 and hsa-circRNA6510-1. We then verified hsa-circRNA11783-2 in the third cohort.
Table 2.
Logistic regression analysis of downregulated circRNAs in the peripheral blood of CAD and T2DM patients.
| hsa-circRNA11806-28 |
hsa-circRNA6510-1 |
hsa-circRNA11783-2 | ||||
|---|---|---|---|---|---|---|
| CAD | T2DM | CAD | T2DM | CAD | T2DM | |
| Crude OR | 0.127 | 0.091 | 0.711 | 0.369 | 0.414 | 0.275 |
| 95% CI | 0.019–0.830 | 0.009–0.865 | 0.484–1.044 | 0.160–0.851 | 0.203–0.842 | 0.105–0.719 |
| p value | 0.031 | 0.037 | 0.082 | 0.019 | 0.015 | 0.008 |
| Adjusted OR | 0.145 | 0.205 | 0.757 | 0.272 | 0.394 | 0.048 |
| 95% CI | 0.020–1.038 | 0.030–1.389 | 0.478–1.201 | 0.073–1.018 | 0.185–0.838 | 0.003–0.829 |
| p value | 0.055 | 0.105 | 0.237 | 0.053 | 0.016 | 0.037 |
circRNAs: circular RNAs; CAD: coronary artery disease; T2DM: type 2 diabetes mellitus; OR: odds ratio; CI: confidence interval.
Independent cohort validation with larger sample size
The significance of differential hsa-circRNA11783-2 expression was validated in the third cohort (control group, n = 60; T2DM group, n = 64; and CAD group, n = 81) (Figure 2). Hsa-circRNA11783-2 was downregulated in both the CAD and T2DM groups compared with the control group, and the corresponding fold changes were 0.4 (p = 0.014) and 0.5 (p < 0.001) in the CAD and T2DM groups, respectively. Logistic regression analysis indicated that the crude OR of hsa-circRNA11783-2 was 0.740 [95% confidence interval (CI) = (0.630–0.869), p < 0.001] in the CAD group and 0.778 [(0.659–0.919), p = 0.003] in the T2DM group. After introducing the shared risk factors of CAD and T2DM (TC, TG, HDL, LDL, smoking and hypertension), the adjusted OR was 0.688 [(0.571–0.829), p < 0.001] in the CAD group and 0.723 [(0.591–0.883), p = 0.002] in the T2DM group. The results suggested that hsa-circRNA11783-2 is closely related to both CAD and T2DM.
Figure 2.
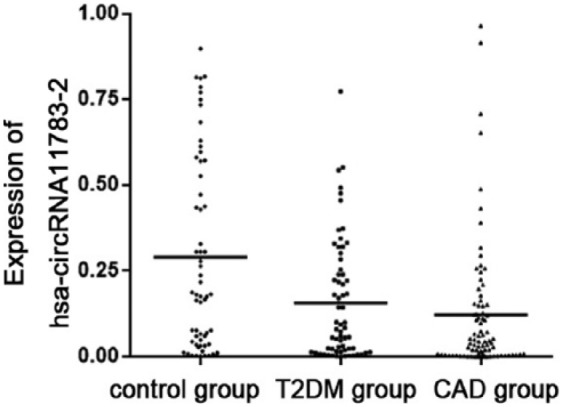
Expression levels of hsa-circRNA11783-2 quantified by Q-PCR. The cohort included 60 control individuals, 81 CAD patients and 64 T2DM patients.
Discussion
The high morbidity and mortality of CAD and T2DM impose a huge social and economic burden worldwide, which severely reduces quality of life. The mortality of patients with CAD complicated by T2DM is significantly higher than that of patients with normal glucose metabolism.18,19 The single-nucleotide polymorphism rs10911021 is closely related to the morbidity and mortality of CAD patients in the setting of T2DM.20 Thus, it is important to find targeted circRNAs associated with both CAD and T2DM that could further our understanding of the associations between CAD and T2DM at the genetic level.
In this study, we first explored differentially expressed circRNAs in control individuals and patients with CAD or T2DM and in patients with both CAD and T2DM. A total of 40 circRNAs were differentially expressed between the control group and the three experimental groups. The gene ontology and pathway enrichment analysis results suggested that these circRNAs are correlated with biological adhesion, cell adhesion and mitotic cell cycle. Then, five selected circRNAs were verified in a second cohort with a larger sample size. The results suggested that hsa-circRNA11783-2 is more closely related to CAD and T2DM than the other selected circRNAs. Subsequently, the above results were verified in a third cohort, and the significant role of hsa-circRNA11783-2 in CAD and T2DM was confirmed by the corresponding results.
There is currently no definitive evidence demonstrating the biological function of hsa-circRNA11783-2. Hsa-circRNA11783-2 is located at chr18:28650691–28672263, and its source gene name is ENST00000251081. This gene encodes a member of the desmocollin protein subfamily. Mutations in this gene are associated with CVDs,21 but there is no evidence showing that this gene is related to diabetes. CircRNAs can regulate gene expression by acting as ‘miRNA sponges’. There are some binding sites for miR-608 and miR-3907 in hsa-circRNA11783-2, and these miRNAs are associated with tumours and the female reproductive tract.22,23
This study is the first to explore the expression of circRNAs in subjects with CAD and T2DM. Our results indicated that hsa-circRNA11783-2 is closely related to both conditions. However, this study is single centred, and the geographical distribution of participants was relatively concentrated. Therefore, it remains uncertain whether these results are relevant to subjects from other regions and countries. To study a homogeneous population, we excluded patients with kidney disease and prior CVD, and our conclusions need to be validated in cohorts with a larger sample size and in patients with these conditions. Finally, the specific roles of hsa-circRNA11783-2 in CAD and T2DM require more attention and further exploration in future studies.
Conclusion
In conclusion, this study is the first to verify that certain circRNAs are correlated with CAD and T2DM. Hsa-circRNA11783-2 exhibited the strongest correlation with both CAD and T2DM.
Supplementary Material
Acknowledgments
The authors thank the volunteers who participated in this study and the doctors who helped with patient recruitment. X.L. and Z.Z. contributed equally to this work.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval and consent to participate: This study received approval from The Ethics Committee of People’s Hospital of Zhengzhou University. Written informed consent was obtained from all participants prior to the initiation of protocol-specified procedures. The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Consent for publication was obtained from all participants in a written form.
Funding: This work was supported by the Science and Technology Department of Henan Province (grant number: 122102310620).
References
- 1. Naghavi M, Wang H, Lozano R, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385: 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu D-Y, Pan C-Y, Yu J-M. The relationship between coronary artery disease and abnormal glucose regulation in China: the China Heart Survey. Eur Heart J 2006; 27: 2573–2579. [DOI] [PubMed] [Google Scholar]
- 3. Cech TR, Steitz JA. The noncoding RNA revolution – trashing old rules to forge new ones. Cell 2014; 157: 77–94. [DOI] [PubMed] [Google Scholar]
- 4. Barrett SP, Wang PL, Salzman J. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife 2015; 4: e07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 2015; 22: 256–264. [DOI] [PubMed] [Google Scholar]
- 6. Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013; 19: 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495: 333–338. [DOI] [PubMed] [Google Scholar]
- 8. You X, Vlatkovic I, Babic A, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 2015; 18: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salzman J, Chen RE, Olsen MN, et al. Cell-type specific features of circular RNA expression. PLoS Genet 2013; 9: e1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Zheng Q, Bao C, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 2015; 25: 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin X, Lo H-C, Wong DT, et al. Noncoding RNAs in human saliva as potential disease biomarkers. Front Genet 2015; 6: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495: 384–388. [DOI] [PubMed] [Google Scholar]
- 13. Burd CE, Jeck WR, Liu Y, et al. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 2010; 6: e1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Liu J, Liu C, et al. MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic β-cells. Diabetes 2013; 62: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin M, Liu G, Huo X, et al. Hsa_circ_0001649: a circular RNA and potential novel biomarker for hepatocellular carcinoma. Cancer Biomark 2016; 16: 161–169. [DOI] [PubMed] [Google Scholar]
- 16. Scanlon PJ, Faxon DP, Audet A-M, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation 1999; 99: 2345–2357. [DOI] [PubMed] [Google Scholar]
- 17. Alberti KG, Zimmet PF. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 18. Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med 2001; 161: 1717–1723. [DOI] [PubMed] [Google Scholar]
- 19. Dale AC, Vatten LJ, Nilsen TI, et al. Secular decline in mortality from coronary heart disease in adults with diabetes mellitus: cohort study. BMJ 2008; 337: a236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Look AHEAD Research Group. Prospective association of GLUL rs10911021 with cardiovascular morbidity and mortality among individuals with type 2 diabetes: the Look AHEAD study. Diabetes 2016; 65: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alsabeq B, Krahn AD, Conacher S, et al. Arrhythmogenic right ventricular cardiomyopathy with recessive inheritance related to a new homozygous desmocollin-2 mutation. Can J Cardiol 2014; 30: 696.e1–696.e3. [DOI] [PubMed] [Google Scholar]
- 22. Cummins JM, He Y, Leary RJ, et al. The colorectal microRNAome. Proc Natl Acad Sci USA 2006; 103: 3687–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Creighton CJ, Benham AL, Zhu H, et al. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PLoS One 2010; 5: e9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


