Abstract
Adult pleomorphic rhabdomyosarcoma (RMS) is a rare and recalcitrant, highly-malignant mesenchymal tumor in need of improved therapeutic strategies. Our laboratory pioneered the patient-derived orthotopic xenograft (PDOX) nude mouse model with the technique of surgical orthotopic implantation (SOI). We previously described the development of a PDOX model of adult pleomorphic RMS where the tumor behaved similar to the patient donor. A high-grade pleomorphic rhabdomyosarcoma from a striated muscle was previously grown orthotopically in the right biceps-femoris muscle of nude mice to establish the PDOX model. In the present study, the PDOX models were randomized into the following treatment groups when tumor volume reached 100 mm3: G1, control without treatment; G2, cyclophosphamide (CPA) 140 mg/kg, intraperitoneal (i.p.) injection, weekly, for 3 weeks; G3, temozolomide (TEM), 25 mg/kg, per oral (p.o.), daily, for 21 days; G4, temozolomide (TEM) 25 mg/kg, p.o., daily, for 21 days combined with irinotecan (IRN), 4 mg/kg, i.p., daily for 21 days. After 3 weeks, treatment of PDOX with TEM combined with IRN was so powerful that it resulted in tumor regression and the smallest tumor volume compared to other groups. The RMS PDOX model should be of use to design the treatment program for the patient and for drug discovery and evaluation for this recalcitrant tumor type.
Keywords: rhabdomosarcoma, nude mice, patient-derived orthotopic xenograft (PDOX), temozolomide, irinotecan, combination
INTRODUCTION
Rhabdomyosarcoma (RMS) originates in striated muscle cells. The majority of RMS cases occur below the age of 18. Approximately 40% of soft tissue sarcomas (STS) are RMS. RMS can occur in any site on the body but is primarily found in the head, neck, orbit, genitourinary tract, genitals, and extremities [1–3]. RMS is divided into three histological subsets:
Embryonal rhabdomyosarcoma (ERMS) is the most common with approximately 60-70% of childhood cases. ERMS usually occurs in patients 4 years old or younger with 4 cases per 1 million children. Head and neck as well as the genitourinary track are most common sites. ERMS usually has morphology similar to developing muscle cells of a 6-8 week-old embryo, hence the name. ERMS also has two subtypes, botryoid and spindle cell [4, 5].
Alveolar rhabdomyosarcoma (ARMS) has an incidence of 1 case per 1 million in patients aged 0 to 19. ARMS occurs most commonly in extremities, trunk, and peritoneum and is more aggressive than ERMS. ARMS is the most common form of RMS observed in young adults and teenagers. ARMS has densely-packed, round cells that are similar to pulmonary alveoli, hence the name [5, 6].
Pleiomorphic rhabdomyhosarcoma (PRMS) comprises poorly differentiated anaplastic cells. PRMS is the most aggressive type of RMS [7]. PRMS occurs most often in adults and rarely in children. RMS is usually in the extremities [8, 9].
We previously developed a patient-derived orthotopic xenograft (PDOX) mouse model of PRMS. We compared the PDOX model to a subcutaneous (s.c.)-transplant model. An RMS from a striated muscle of a male patient was grown orthotopically in the right biceps- femoris muscle or right quadriceps muscle of nude mice to establish a PDOX model. The RMS was also grown subcutaneously in nude mice. PDOX tumors grew at a statistically-significant faster rate compared to the s.c. tumors. Recurrence after surgical resection occurred only in PDOX tumors, not in the s.c. model. Histologically, only the PDOX model was shown to be invasive. These results indicated that the PDOX model of adult RMS is malignant and the subcutaneous model is benign [10].
In the present study, we used the adult PRMS PDOX model to identify an effective drug or combination for this recalcitrant disease.
RESULTS AND DISCUSSION
Efficacy of cyclophosphamide (CPA), temozolomide (TEM) and TEM combined with irinotecan (IRN) on the PRMS PDOX
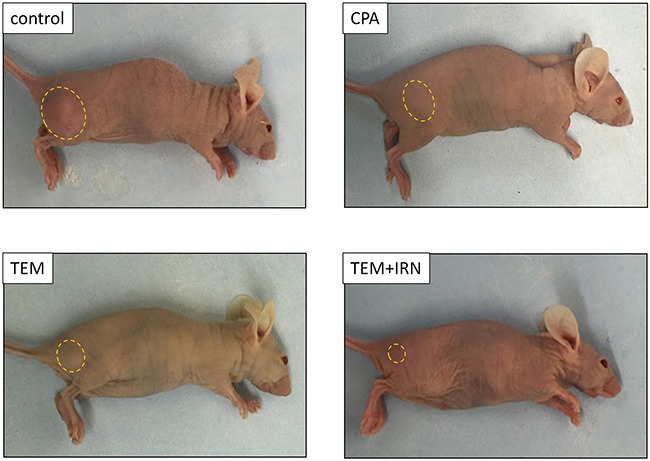
Please see Figure 1 for treatment schema. All treatments significantly inhibited the PRMS PDOX growth compared to untreated controls on day 21 after initiation. Tumor volumes at day 21 were: control (G1): 640.6 ± 225.5 mm3; CPA (G2): 301.1 ± 43.6 mm3, p=0.002; TEM (G3): 215.2 ± 40.6 mm3, p=0.0004; TEM combined with IRN (G4): 93.6 ± 8.7 mm3, p<0.0001. TEM combined with IRN showed significantly more efficacy compared to other therapies evaluated: CPA (p<0.0001), TEM (p=0.0002) (Figures 2 and 3). There were no animal deaths in any group. The body weight of treated mice was not significantly different in any group (Figure 4). No significant other side effects were observed either.
Figure 1. Treatment schema.
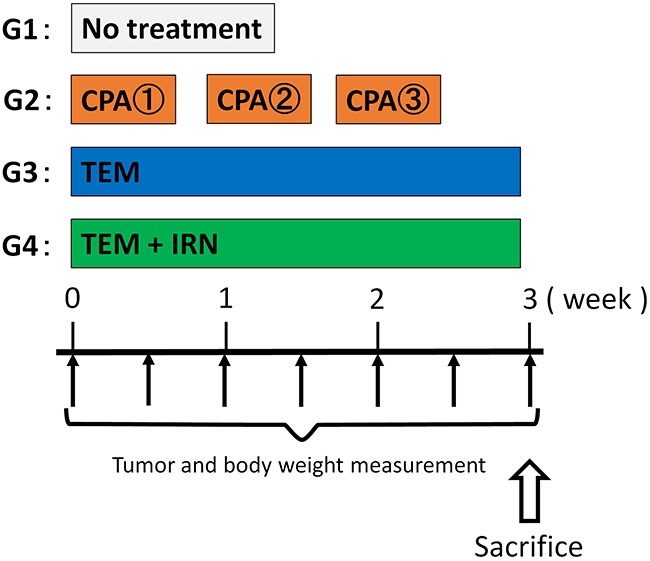
Figure 2. Efficacy of cyclophosphamide (CPA), temozolomide (TEM) and TEM combined with irinotecan (IRN).
CPA (140 mg/kg, i.p., qw×3); TEM (25 mg/kg, p.o., qd×21); TEM (25 mg/kg, p.o., qd×21) combined with IRN (4 mg/kg, i.p., qd×21). Tumor volume was measured at the indicated time points after the onset of treatment. Please see the Materials and Methods for details. N = 8 mice/group. *P=0.0002; **P<0.0001.
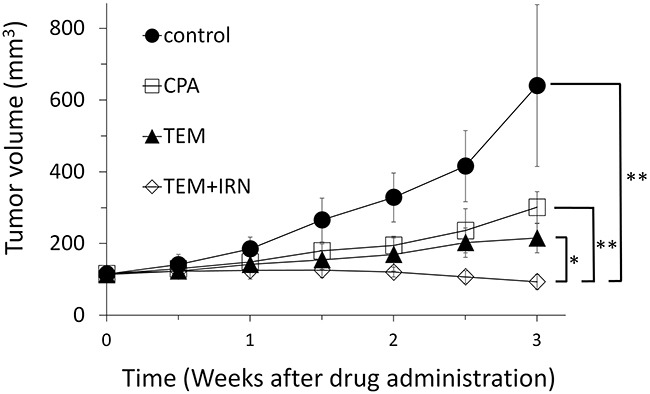
Figure 3. Efficacy of CPA, TEM and TEM combined with IRN.
Photographs of representative PDOX mouse models from each treatment group at day 21.
Figure 4. Effect of treatment on body weight.
Bar graph shows body weight in each group at pre-treatment and 3 weeks after drug administration.
Histology of the PRMS PDOX and patient tumor
A high-power photomicrograph of the original patient tumor displayed solid sheets of tumor cells characterized by pleomorphic, hyperchromatic, enlarged nuclei with coarse chromatin and moderate amounts of lightly eosinophilic cytoplasm. Numerous mitotic figures, including atypical forms are present. A high-power image of the orthotopically-implanted tumors had very similar features, including pleomorphic, hyperchromatic, enlarged nuclei with coarse chromatin and moderate amounts of lightly eosinophilic cytoplasm. Numerous mitotic figures, including atypical forms are also present (Figure 5), demonstrating the fidelity of the PDOX tumor.
Figure 5. Histology of the original patient tumor and the untreated control PDOX tumor.
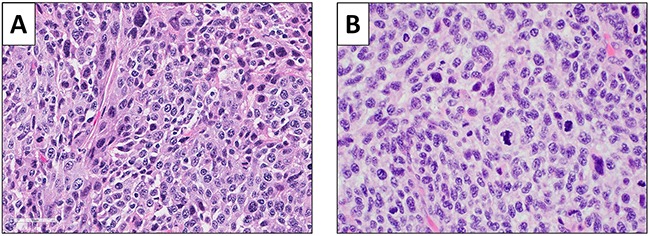
(A) Original patient tumor. (B) Untreated control PDOX tumor. See Materials and Methods for details.
The donor patient was newly diagnosed with PRMS which is considered a high-grade sarcoma. The patient had not received any prior systemic treatment. There is no consensus as to what the standard of care therapy is for this disease [11–13]. Therefore, it was decided to choose some systemic therapies that are used for RMS such as IRN and CPA. It was decided to try IRN + TMZ since this is a combination regimen that has been used for various malignancies and has shown efficacy against a wide range of tumors and several forms of sarcoma. Although this combination is not commonly used for PRMS, our unexpected results show tumor regression with this combination. This promising result makes the combination of TEM and IRN a candidate therapy for the donor patient of the PRMS PDOX, since tumor regression in the model suggested efficacy in the clinic [14]. A future study will determine if the combination of TEM and IRN is synergistic or additive in this model of PRMS.
Previously-developed concepts and strategies of highly selective tumor targeting can take advantage of molecular targeting of tumors, including tissue-selective therapy which focuses on unique differences between normal and tumor tissues [15–20].
CONCLUSIONS
RMS is a recalcitrant disease. It usually occurs in children and young adults. In the present case, it occurred in 68-year-old man. We previously established a PDOX model of pleomorphic RMS (PRMS) [10] that is highly malignant compared to the same tumor grown subcutaneously. Therefore, we assume the PDOX model represents the patient since PRMS is a highly-malignant tumor. The present results show that the combination of TEM and IRN was so powerful that it was able to regress the RMS PDOX, indicating potential of a similar response in the patient [14].
The combination of vincristine, actinomycin D, cyclophosphamide (VAC) is sometimes used to treat RMS and will be used for future experiments comparing VAC with TEM+IRN [21]. The results of the present study suggests that potential powerful therapy can be identified for many adult PRMS patients. The PRMS PDOX can be used for discovery and evaluation of novel therapeutics for this recalcitrant disease as well.
MATERIALS AND METHODS
Animal care
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 4–6 weeks old, were used in this study. Animals were housed in a barrier facility on a high efficiency particulate arrestance (HEPA)-filtered rack under standard conditions of 12-hour light/dark cycles. The animals were fed an autoclaved laboratory rodent diet. All animal studies were conducted with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873-1. In order to minimize any suffering of the animals, anesthesia and analgesics were used for all surgical experiments. Animals were anesthetized by subcutaneous injection of a 0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate.
Patient-derived tumor
A 68-year-old male diagnosed with pleomorphic RMS had a large primary tumor in the right high thigh previously underwent surgical resection at Department of Surgery, University of California, Los Angeles (UCLA). The patient did not receive any chemotherapy or radiotherapy prior to surgery. Written informed consent was obtained from the patient as part of a UCLA Institutional Review Board (IRB #10-001857)-approved protocol [10].
Surgical orthotopic implantation (SOI) to establish an RMS PDOX model
Our laboratory pioneered the PDOX nude mouse model with the technique of surgical orthotopic implantation (SOI), including pancreatic [22–25], breast [26], ovarian [27], lung [28], cervical [29], colon [30–32], stomach [33], sarcoma [34–38], and melanoma [39–41].
The PRMS tumor was previously established at AntiCancer, Inc. [10]. Tumors were initially grown subcutaneously after transplantation of 5 mm fragments. After 3 weeks growth, tumors were harvested and cut into small fragments (3-4 mm). After nude mice were anesthetized, a 5 mm skin incision was made on the right high thigh, then the biceps femoris or quadriceps was split to make space for the tumor. A single tumor fragment was implanted orthotopically into the space to establish a PDOX model [10]. The wound was closed with 6-0 nylon suture (Ethilon, Ethicon, Inc., NJ, USA).
Treatment design
PRMS PDOX mouse models were randomized into 4 groups of 8 mice each: G1, control without treatment; G2, CPA 140 mg/kg, i.p., qw×3; G3, TEM 25 mg/kg, p.o., qd×21; G4, TEM 25 mg/kg, p.o., qd×21 combined with IRN 4 mg/kg, i.p., qd×21. Tumor length, width and mouse body weight were measured twice in a week. Tumor volume was calculated by following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± SD.
Histological analysis
Fresh tumor specimens were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections (3 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H&E) staining was performed according to standard protocol. Histological examination was performed with a BHS system microscope. Images were acquired with INFINITY ANALYZE software (Lumenera Corporation, Ottawa, Canada).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–352. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 2.Maurer HM, Beltangady M, Gehan EA, Crist W, Hammond D, Hays DM, Heyn R, Lawrence W, Newton W, Ortega J, Ruymann FB, Soule E, Tefft M. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Maurer HM, Gehan EA, Beltangady M, Crist W, Dickman PS, Donaldson SS, Fryer C, Hammond D, Hays DM, Herrmann J, Heyn R, Jones PM, Lawrence W, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Newton WA, Jr, Gehan EA, Webber BL, Marsden HB, van Unnik AJ, Hamoudi AB, Tsokos MG, Shimada H, Harms D, Schmidt D, Ninfo V, Cavazzana AO, Gonzales-Crussi F, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer. 1995;76:1073–1085. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Qualman SJ, Coffin CM, Newton WA, Hojo H, Triche TJ, Parham DM, Crist WM. Intergroup Rhabdomyosarcoma Study: update for pathologists. Pediatr Dev Pathol. 1998 Nov-Dec;1:550–561. doi: 10.1007/s100249900076. [DOI] [PubMed] [Google Scholar]
- 6.Meyer WH. Holland-Frei Cancer Medicine. 6th ed. Hamilton (ON): BC Decker; 2003. “Rhabdomyosarcoma”. [Google Scholar]
- 7.Meza JL, Anderson J, Pappo AS, Meyer WH, Children’s Oncology Group Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children’s Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 8.Raney RB, Jr, Tefft M, Maurer HM, Ragab AH, Hays DM, Soule EH, Foulkes MA, Gehan EA. Disease patterns and survival rate in children with metastatic soft-tissue sarcoma. A report from the Intergroup Rhabdomyosarcoma Study (IRS)-I. Cancer. 1988;62:1257–1266. doi: 10.1002/1097-0142(19881001)62:7<1257::aid-cncr2820620703>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Hays DM, Lawrence W, Jr, Wharam M, Newton W, Jr, Ruymann FB, Beltangady M, Maurer HM. Primary reexcision for patients with ‘microscopic residual’ tumor following initial excision of sarcomas of trunk and extremity sites. J Pediatr Surg. 1989;24:5–10. doi: 10.1016/s0022-3468(89)80290-8. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi K, Kawaguchi K, Kiyuna T, Murakami T, Miwa S, Nelson SD, Dry SM, Li Y, Singh A, Kimura H, Hayashi K, Yamamoto N, Tsuchiya H, et al. Patient-derived orthotopic xenograft (PDOX) mouse model of adult rhabdomyosarcoma invades and recurs after resection in contrast to the subcutaneous ectopic model. Cell Cycle. 2017;16:91–94. doi: 10.1080/15384101.2016.1252885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNall-Knapp RY, Williams CN, Reeves EN, Heideman RL, Meyer WH. Extended phase I evaluation of vincristine, irinotecan, temozolomide, and antibiotic in children with refractory solid tumors. Pediatr. Blood Cancer. 2010;54:909–915. doi: 10.1002/pbc.22460. [DOI] [PubMed] [Google Scholar]
- 12.Wagner LM, Crews KR, Iacono LC, Houghton PJ, Fuller CE, McCarville MB, Goldsby RE, Albritton K, Stewart CF, Santana VM. Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin Cancer Res. 2004;10:840–848. doi: 10.1158/1078-0432.ccr-03-0175. [DOI] [PubMed] [Google Scholar]
- 13.Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, Meyers PA. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029–1034. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 14.Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, Clarke RB, Clevers H, Coukos G, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017 doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 15.Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today. 2003;8:1104–1107. doi: 10.1016/s1359-6446(03)02806-x. [DOI] [PubMed] [Google Scholar]
- 16.Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle. 2005;4:1518–1521. doi: 10.4161/cc.4.11.2208. [DOI] [PubMed] [Google Scholar]
- 17.Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug ransporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia. 2001;15:936–941. doi: 10.1038/sj.leu.2402127. [DOI] [PubMed] [Google Scholar]
- 18.Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–391. doi: 10.1038/sj.leu.2404075. [DOI] [PubMed] [Google Scholar]
- 19.Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget. 2011;2:222–233. doi: 10.18632/oncotarget.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer. 2003;89:1147–1151. doi: 10.1038/sj.bjc.6601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollár A, Langer R, Ionescu C, Cullmann JL, Klenke FM. Pleomorphic rhabdomyosarcoma with an impressive response to chemotherapy: case report and review of the literature. Tumori. 2016:102. doi: 10.5301/tj.5000476. [DOI] [PubMed] [Google Scholar]
- 22.Hiroshima Y, Zhang Y, Murakami T, Maawy AA, Miwa S, Yamamoto M, Yano S, Sato S, Momiyama M, Mori R, Matsuyama R, Chishima T, Tanaka K, et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograph (PDOX) and cell line mouse models. Oncotarget. 2014;5:12346–12357. doi: 10.18632/oncotarget.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiroshima Y, Maawy A, Zhang Y, Murakami T, Momiyama M, Mori R, Matsuyama R, Katz MH, Fleming JB, Chishima T, Tanaka K, Ichikawa Y, Endo I, et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19–9-conjugated fluorophore. PLOS ONE. 2014;9:e114310. doi: 10.1371/journal.pone.0114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiroshima Y, Maawy AA, Katz MH, Fleming JB, Bouvet M, Endo I, Hoffman RM. Selective efficacy of zoledronic acid on metastasis in a patient-derived orthotopic xenograph (PDOX) nude-mouse model of human pancreatic cancer. J Surg Oncol. 2015;111:311–315. doi: 10.1002/jso.23816. [DOI] [PubMed] [Google Scholar]
- 26.Fu X, Le P Hoffman RM. A metastatic-orthotopic transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13:901–904. [PubMed] [Google Scholar]
- 27.Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993;13:283–286. [PubMed] [Google Scholar]
- 28.Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51:992–995. doi: 10.1002/ijc.2910510626. [DOI] [PubMed] [Google Scholar]
- 29.Hiroshima Y, Zhang Y, Zhang M, Maawy A, Mii S, Yamamoto M, Uehara F, Miwa S, Yano S, Murakami T, Momiyama M, Chishima T, Tanaka K, et al. Establishment of a patient-derived orthotopic xenograph (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLOS ONE. 2015;10:e0117417. doi: 10.1371/journal.pone.0117417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu X, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM, Bouvet M. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi: 10.1002/jso.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiroshima Y, Maawy A, Metildi CA, Zhang Y, Uehara F, Miwa S, Yano S, Sato S, Murakami T, Momiyama M, Chishima T, Tanaka K, Bouvet M, et al. Successful fluorescence-guided surgery on human colon cancer patient-derived orthotopic xenograft mouse models using a fluorophore-conjugated anti-CEA antibody and a portable imaging system. J Laparoendosc Adv Surg Tech A. 2014;24:241–247. doi: 10.1089/lap.2013.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa T, Kubota T, Watanabe M, Kitajima M, Fu X, Hoffman RM. Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 1993;53:608–612. doi: 10.1002/ijc.2910530414. [DOI] [PubMed] [Google Scholar]
- 34.Murakami T, DeLong J, Eilber FC, Zhao M, Zhang Y, Zhang N, Singh A, Russell T, Deng S, Reynoso J, Quan C, Hiroshima Y, Matsuyama R, et al. Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft PDOX model. Oncotarget. 2016;7:12783–12790. doi: 10.18632/oncotarget.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiroshima Y, Zhao M, Zhang Y, Zhang N, Maawy A, Murakami T, Mii S, Uehara F, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, et al. Tumor-targeting Salmonella typhimurium A1-R arrests a chemo-resistant patient soft-tissue sarcoma in nude mice. PLoS One. 2015;10:e0134324. doi: 10.1371/journal.pone.0134324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiyuna T, Murakami T, Tome Y, Kawaguchi K, Igarashi K, Zhang Y, Zhao M, Li Y, Bouvet M, Kanaya F, Singh A, Dry S, Eilber FC, et al. High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget. 2016;7:33046–33054. doi: 10.18632/oncotarget.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami T, Singh AS, Kiyuna T, Dry SM, Li Y, James AW, Igarashi K, Kawaguchi K, DeLong JC, Zhang Y, Hiroshima Y, Russell T, Eckardt MA, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2016;7:47556–47564. doi: 10.18632/oncotarget.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiroshima Y, Zhang Y, Zhang N, Uehara F, Maawy A, Murakami T, Mii S, Yamamoto M, Miwa S, Yano S, Momiyama M, Mori R, Matsuyama R, et al. Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res. 2015;35:697–701. [PubMed] [Google Scholar]
- 39.Yamamoto M, Zhao M, Hiroshima Y, Zhang Y, Shurell E, Eilber FC, Bouvet M, Noda M, Hoffman RM. Efficacy of tumor-targeting Salmonella typhimurium A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One. 2016;11:e0160882. doi: 10.1371/journal.pone.0160882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawaguchi K, Murakami T, Chmielowski B, Igarashi K, Kiyuna T, Unno M, Nelson SD, Russell TA, Dry SM, Li Y, Eilber FC, Hoffman RM. Vemurafenib-resistant BRAF-V600E mutated melanoma is regressed by MEK targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget. 2016;7:71737–71743. doi: 10.18632/oncotarget.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi K, Igarashi K, Murakami T, Chmiewloski B, Kiyuna T, Zhao M, Zhang Y, Singh A, Unno M, Nelson SD, Russell T, Dry SM, Li Y, et al. Tumor-targeting Salmonella typhimurium A1-R combined with Temozolomide regresses malignant melanoma with a BRAF-V600 mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget. 2016;7:85929–85936. doi: 10.18632/oncotarget.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]


