Abstract
To report a single-institution experience of gamma evaluations with 2%/1 mm for stereotactic ablative radiotherapy (SABR) delivered with volumetric modulated arc therapy (VMAT) technique, from January 2014 to January 2016. A total of 168 SABR VMAT plans were analyzed with a gamma criterion of 2%/1 mm, a threshold value of 10%, and a tolerance level of 90%. Of the 168 cases, four cases failed with 2%/1 mm. The average passing rate was 97.0% ± 2.5%. Three of the four failed cases showed passing rates higher than 90%, which was achieved by shifting the measuring device by 1 mm in the left-to-right or anterior-to-posterior directions. One failed case showed a passing rate higher than 90%, which was achieved by changing the threshold value from 10% to 5%, leading to an increase in the number of tested points from 26 to 51. Concerns regarding the high susceptibility of the gamma criterion of 2%/1 mm to setup errors of the measuring device are unnecessary based on our two-year experience, since only four cases failed with the 2%/1 mm from a total of 168 clinical cases. Therefore, the gamma criterion of 2%/1 mm could be successfully applied in the clinic with its high sensitivity to detect errors in VMAT plans.
Keywords: 2D gamma evaluation, pre-treatment patient-specific quality assurance, volumetric modulated arc therapy, stereotactic ablative radiotherapy, gamma criterion
INTRODUCTION
Intensity modulated radiation therapy (IMRT) as well as volumetric modulated arc therapy (VMAT) can deliver highly conformal prescription doses to target volumes while minimizing doses to organs at risk (OARs) in proximity to the target volumes, which enables high local control as well as reduction of complications related to radiotherapy [1–5]. This could be achieved by using the inverse planning algorithm and modulations of photon beam intensities [6, 7]. In the case of IMRT, the photon beam intensities are modulated with superpositions of photon beams with various beam apertures defined by the multi-leaf collimators (MLCs), i.e., static IMRT, or movements of each MLC with various speeds during beam-on time, i.e., dynamic IMRT [8, 9]. In rare instances, IMRT could be delivered with compensators manufactured for each field at the institutions in which an MLC system was not available [10, 11]. On the contrary, for VMAT, the photon beam intensities are modulated with simultaneous modulations of three parameters: the gantry rotation speeds, dose-rates, and MLC positions [7, 12]. For both the IMRT and VMAT, high modulation of photon beam intensities may generate a clinically better quality treatment plan; however, it could cause discordance in dose distributions between the calculated dose in the treatment planning system (TPS) and the actual dose delivered to a patient during treatment [13–15]. Since IMRT and VMAT use larger amount of monitor units than a conventional radiotherapy technique such as 3D conformal radiation therapy, in addition to generating a steep dose fall-off between the target volume and nearby OARs, the discordance in dose distributions between the calculation and delivery of IMRT or VMAT could result in critical medical malpractice [16]. Therefore, pre-treatment patient-specific quality assurance (QA) for IMRT and VMAT plans is highly recommended in the clinic as a verification procedure of the treatment plan before patient treatment [16].
As a pre-treatment QA for IMRT and VMAT, 2D gamma evaluation, which is generally performed in the clinic, compares the calculated planar dose distribution in the TPS with the planar dose distribution measured with 2D array dosimeters [17]. For IMRT, 2D gamma evaluation with a gamma criterion of 3%/3 mm has been generally recommended and is routinely applied in the clinic [16]. However, for VMAT, 2D gamma evaluation with a gamma criterion of 2%/2 mm was recommended by Heilemann et al. and Fredh et al. [18, 19]. They investigated the sensitivity of the 2D global gamma evaluation with 2%/2 mm comparing that with 3%/3 mm to detect errors that were artificially introduced into the VMAT plans. They showed higher sensitivity of the gamma criterion of 2%/2 mm to detect errors in the VMAT plans and recommended its use in the clinic for pre-treatment VMAT QA. For stereotactic ablative radiotherapy (SABR), which should be performed with care owing to its large fraction sizes as well as small fraction numbers, we previously recommended 2D global gamma evaluation with a gamma criterion of 2%/1 mm because of its higher sensitivity in detecting delivery errors in the SABR VMAT plans than those with 2%/2 mm, 1.5%/1.5 mm, and 1%/2 mm [20]. In our previous study, a tolerance level of 90% with a MapCHECK2 (Sun Nuclear Corporation, Melbourne, FL, USA) dosimeter and a tolerance level of 80% with an EBT2 film (Ashland Inc., Covington, KY, USA) was found to be appropriate with the gamma criterion of 2%/1 mm for pre-treatment VMAT QA for SABR.
Although 2D global gamma evaluation with a strict gamma criterion of 2%/1 mm could detect the discordance between the calculated and the measured planar dose distributions better than that with the conventional 2%/2 mm, the setup error of the measurement device could be a detrimental factor in the pre-treatment VMAT QA for SABR [21]. In other words, the gamma-passing rate could be lower than the tolerance level owing to the setup errors of the measurement device, although there is no problem in the SABR VMAT plans. Since the distance to agreement (DTA) of the conventional 2 mm was reduced to 1 mm, 2D gamma evaluation with 2%/1 mm could be more susceptible to setup errors of the measurement device. In the clinic, this could be problematic as it consumes a significant amount of time and human resources. Therefore, we report the results of 2D global gamma evaluation with a gamma criterion of 2%/1 mm for SABR VMAT in this study, which was performed in our institution for a two-year period, from January 2014 to January 2016. Although this report is from a single institution experience, considerable numbers of SABR for a variety of treatment sites with various modulation degrees were performed in our institution; therefore, it could be informative for other clinics to perform VMAT QA for SABR with a gamma criterion of 2%/1 mm.
RESULTS
Gamma passing rates and modulation degree of VMAT plans
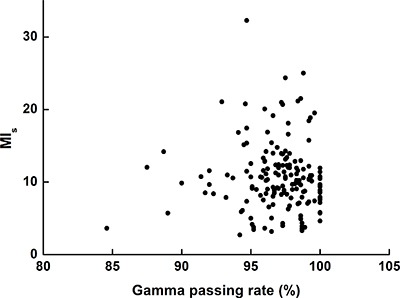
The distributions of the 2D global gamma-passing rates with 2%/1 mm for SABR VMAT for two years in our institution are shown in Figure 1. The number of cases with a gamma passing rate of 100% with 2%/1 mm was 20 (11.9% of the total examined cases). The number of cases with gamma passing rates greater than or equal to 98%, 96%, 94%, 92%, and 90% was 66 (39.3%), 128 (76.2%), 154 (91.7%), 161 (95.8%), and 164 (97.6%), respectively. From a total of 168 cases, 4 cases (2.4%) failed, with a gamma criterion of 2%/1 mm and a tolerance level of 90%. The gamma passing rates of each treatment site are summarized in Table 1. The average gamma passing rate of lung SABR was the highest among all (97.7% ± 2.4%) while that of C-spine SABR was the lowest among all (94.1% ± 3.2%). No noticeable differences were observed among the gamma passing rates of SABR VMAT plans for each treatment site.
Figure 1. 2D global gamma passing rates with a gamma criterion of 2%/1 mm for stereotactic ablative radiotherapy delivered with volumetric modulated arc therapy technique.

Table 1. Details of the analyzed VMAT plan cases for two years.
| Treatment sites | Total number of cases | Prescription (Gy) | Fraction number (Gy) | MIs | %GP with 2%/1 mm (%) |
|---|---|---|---|---|---|
| Head & Neck | 1 | 24 | 3 | 18.44 | 99.2 |
| C-spine | 6 | 16 ± 5.4 | 1 ± 0.0 | 13.3 ± 3.9 | 94.1 ± 3.2 |
| T-spine | 12 | 19 ± 3.6 | 1.3 ± 1.2 | 10.3 ± 3.6 | 95.3 ± 3.7 |
| Lung | 82 | 57.0 ± 5.7 | 4.0 ± 0.3 | 9.8 ± 2.6 | 97.7 ± 2.4 |
| Liver | 28 | 39.9 ± 9.6 | 3.3 ± 0.4 | 10.5 ± 4.9 | 97.1 ± 1.8 |
| Abdomen | 16 | 34.4 ± 7.0 | 3.3 ± 1.0 | 11.3 ± 8.0 | 97.1 ± 1.6 |
| Pelvis | 9 | 28.3 ± 5.7 | 3.3 ± 2.8 | 11.2 ± 7.5 | 96.2 ± 1.7 |
| L-spine | 12 | 19.3 ± 3.7 | 1.2 ± 0.6 | 12.2 ± 3.5 | 96.0 ± 2.6 |
| Thorax | 2 | 29.5 ± 13.4 | 4.0 ± 1.4 | 22.8 ± 13.4 | 95.8 ± 3.1 |
| Total | 168 | 43.1 ± 16.4 | 3.2 ± 1.3 | 10.7 ± 4.7 | 97.0 ± 2.5 |
Abbreviations: VMAT, volumetric modulated arc therapy; MIs, modulation index evaluating the speed of multi-leaf collimators; %GP with 2%/1 mm, global gamma passing rate with a gamma criterion of 2%/1 mm
The values of the modulation index (MIs) in terms of MLC speed are summarized in Table 1 [14]. The MIs values have been plotted as a function of the gamma passing rates with 2%/1 mm in Figure 2. Per the previous study, as the modulation degree increases, the MIs values increase and the gamma passing rates decrease; therefore, the MIs values are inversely proportional to the gamma passing rates [14]. However, this tendency is not observed in Figure 2. Consequently, the Pearson correlation coefficient between the gamma passing rates and the MIs values was 0.014 with a p value of 0.861, showing no correlation between the gamma passing rates for SABR VMAT and the MIs values.
Figure 2. Values of the modulation index evaluating multi-leaf collimator speed (MIs) plotted as a function of the gamma passing rates.
Analysis of the failed cases with a gamma criterion of 2%/1 mm
The details of the four SABR VMAT plans, which failed with a gamma criterion of 2%/1 mm and a tolerance level of 90%, are summarized in Table 2. One lung case and three spine cases failed, showing gamma passing rates less than 90% (89.0% for the lung case, 88.7% for the C-spine case, 87.5% for the L-spine case, and 84.6% for the T-spine case). When the conventional gamma criterion of 2%/2 mm was applied for those four cases, all the gamma passing rates were higher than 95%, as shown in Figure 3.
Table 2. Details of the failed cases of the VMAT plans with a gamma criterion of 2%/1 mm and a tolerance level of the global gamma passing rate of 90% for two years.
| Failed case | Treatment sites | Prescription (Gy) | Fraction number | MIs | %GP with 2%/1 mm | %GP with 2%/2 mm |
|---|---|---|---|---|---|---|
| 1 | T-spine | 13 | 1 | 3.63 | 84.6 | 100 |
| 2 | Lung | 60 | 4 | 5.73 | 89.0 | 100 |
| 3 | L-spine | 20 | 1 | 12.03 | 87.5 | 97.1 |
| 4 | C-spine | 16 | 1 | 14.18 | 88.7 | 95.6 |
Abbreviations: VMAT, volumetric modulated arc therapy; MIs, modulation index evaluating the speed of multi-leaf collimator; %GP with 2%/1 mm, global gamma passing rate with a gamma criterion of 2%/1 mm; %GP with 2%/2 mm, global gamma passing rate with a gamma criterion of 2%/2 mm.
Figure 3. 2D global gamma analyses for the four failed stereotactic ablative radiotherapy plans delivered with volumetric modulated arc therapy technioque.
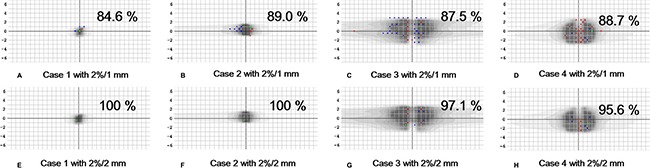
Points with measured values higher than the calculated values are shown in red dots while points with measured values lower than the calculated values are shin in blue dots.
The MIs values of the four failed cases ranged from 3.63 to 14.18. The MIs values of the lung and T-spine cases were 3.63 and 5.73, respectively, which were much lower than the average MIs value (10.7) calculated from the wholly examined cases (168 cases). The MIs values of the L-spine and C-spine cases were 12.03 and 14.18, respectively, which were slightly higher than the average MIs value. However, the MIs values of the L-spine and C-spine cases constituted the top 26.8% and the top 15.8% of the all the examined cases, respectively.
The changes in the gamma passing rates of the failed cases after simulating setup errors, which included yaw rotational setup errors and translational setup errors, in the left-to-right (LR), superior-to-inferior (SI), and anterior-to-posterior (AP) directions are summarized in Table 3. For the T-spine SABR VMAT plan, no improvement in the gamma-passing rate was observed by simulating setup errors. However, for the rest of the failed cases, gamma-passing rates were greater than 90% with a shift of 1 mm. For the lung and L-spine SABR VMAT plan, shifting the measurement device by 1 mm in the LR direction increased the gamma passing rates to 100% and 91.6%, respectively. For the C-spine SABR VMAT plan, by shifting the measurement device by 1 mm in the AP direction, the passing rate increased to 94.2%.
Table 3. Changes in gamma passing rates of the failed cases by simulating setup errors.
| Gamma passing rate with 2%/1 mm (%) | ||||
|---|---|---|---|---|
| Couch rotation | –0.2° | –0.1° | 0.1° | 0.2° |
| T-spine | 84.6 | 84.6 | 84.6 | 84.6 |
| Lung | 89.4 | 89.5 | 87.2 | 87.2 |
| L-spine | 87.3 | 87.9 | 87.8 | 87.5 |
| C-spine | 88.6 | 88.6 | 88.4 | 88.9 |
| SI shift | –0.2 cm | –0.1 cm | 0.1 cm | 0.2 cm |
| T-spine | 83.0 | 84.6 | 84.6 | 84.6 |
| Lung | 82.2 | 89.0 | 89.0 | 85.5 |
| L-spine | 88.4 | 87.5 | 87.5 | 86.0 |
| C-spine | 88.0 | 88.7 | 88.7 | 86.2 |
| LR shift | –0.2 cm | –0.1 cm | 0.1 cm | 0.2 cm |
| T-spine | 82.7 | 84.6 | 84.6 | 84.1 |
| Lung | 97.5 | 100 | 87.6 | 85.5 |
| L-spine | 90.4 | 91.6 | 87.5 | 85.5 |
| C-spine | 85.5 | 88.7 | 88.7 | 86.0 |
| AP shift | –0.2 cm | –0.1 cm | 0.1 cm | 0.2 cm |
| T-spine | 73.1 | 79.2 | 57.6 | 24.3 |
| Lung | 64.0 | 73.3 | 55.6 | 54.5 |
| L-spine | 82.6 | 86.2 | 85.6 | 81.4 |
| C-spine | 81.7 | 84.7 | 94.2 | 93.2 |
Abbreviations: SI, superior to inferior direction; LR, left to right direction; AP, anterior to posterior direction
Note: The gamma passing rates higher than 90% are shown in italic.
The changes in the gamma passing rates of the failed cases and the number of tested points during gamma evaluation in terms of the threshold values are shown in Table 4. The number of tested points increased as the threshold values decreased. The T-spine, lung, and C-spine SABR VMAT plans showed higher gamma passing rates than the tolerance level of 90% with a threshold value of 5%. In the case of the T-spine plan, the gamma-passing rate did not change with the setup error simulations; however, it changed to 92.2% upon lowering the threshold value to 5%. In this case, the number of tested points increased from 26 to 51 by changing the threshold value from 10% to 5%, which was a drastic increase (96.2%) in the number of the tested points.
Table 4. Changes in gamma passing rates and number of tested points of the failed cases with increasing threshold values.
| Target volume size (cc) | Gamma passing rate with 2%/1 mm (%) | ||||||
|---|---|---|---|---|---|---|---|
| 5% | 10% | 15% | 20% | 25% | 30% | ||
| T-spine | 0.5 | 92.2 | 84.6 | 77.8 | 73.3 | 80.0 | 80.0 |
| Lung | 3.2 | 92.0 | 89.0 | 85.1 | 76.2 | 71.4 | 71.0 |
| L-spine | 153.7 | 89.2 | 87.5 | 87.1 | 87.5 | 87.0 | 85.4 |
| C-spine | 75.7 | 91.0 | 88.7 | 84.2 | 79.9 | 76.4 | 73.6 |
| No. of tested points during gamma evaluation | |||||||
|---|---|---|---|---|---|---|---|
| 5% | 10% | 15% | 20% | 25% | 30% | ||
| T-spine | 0.5 | 51 | 26 | 18 | 15 | 10 | 10 |
| Lung | 3.2 | 125 | 91 | 67 | 42 | 35 | 31 |
| L-spine | 153.7 | 351 | 305 | 278 | 256 | 246 | 206 |
| C-spine | 75.7 | 310 | 247 | 184 | 139 | 123 | 106 |
Note: The gamma passing rates higher than 90% are shown in italic.
DISCUSSION
In this study, we demonstrated the gamma evaluation results for SABR VMAT with a gamma criterion of 2%/1 mm from January 2014 to January 2016. For two years, a total of 168 cases of gamma evaluations for SABR VMAT plans were carried out and four of them failed with 2%/1 mm and 90% tolerance level (failure rate of 2.4%) [20]. The decision to use the gamma criterion of 2%/1 mm rather than 2%/2 mm was meant to reduce the DTA from 2 mm to 1 mm. This could increase the sensitivity of the gamma evaluation in detecting errors in the treatment plans; however, it makes the gamma evaluation more susceptible to setup errors of the measurement device, which is impractical in the clinic. The results of this study revealed that no concerns are necessary in the use of 2%/1 mm with a 90% tolerance level since only four of the 168 cases failed with gamma evaluation using the 2%/1 mm gamma criterion for SABR VMAT. The gamma evaluation with 2%/1 mm was successfully applied in the clinic for the pre-treatment patient-specific VMAT QA for SABR in our institution for two years.
The calculation resolution of the reference 2D dose distribution was 1 mm while the detector resolution of the MapCHECK2 dosimeter was 7.07 mm in the diagonal direction. Some previous studies showed that the grid size of the detector array could affect the gamma passing rates, therefore, the results in this study is only valid for the MapCHECK2 dosimeter or detector arrays with similar detector resolutions to that of the MapCHECK2 dosimeter [22, 23]. Since those previous studies recommended the calculation grid size should be smaller than the mearement grid size, we used calculation grid size of 1 mm in this study [22, 23].
Previously, Park et al. showed considerable correlations with statistical significance between the MIs values and gamma passing rates [14]. In that study, as the modulation degree of the VMAT plans increased, the gamma passing rates decreased and the values of MIs increased. Upon reviewing the four failed cases of our study, we found that the MIs values of these cases were not particularly high; therefore, the failures with the gamma evaluation were apparently not caused by the high modulation of the VMAT plans. The gamma passing rates of the other SABR VMAT plans, which were acquired in the same week in which the failed cases were acquired, showed higher gamma passing rates than the tolerance level. Therefore, no systemic errors existed in the linac delivery system or in the TPS. When we applied gamma evaluation with 2%/2 mm, i.e., increased the DTA, the passing rates of those failed cases became greater than 95%. In this respect, we assumed that the setup errors of the measurement device may have caused the low passing rates [21]. By simulating setup errors, i.e., shifting the measuring device by 1 mm in the LR direction, the VMAT plans for the lung SABR and the L-spine SABR showed gamma passing rates greater than 90% by. In the case of the VMAT plan for C-spine SABR, by shifting the measuring device by 1 mm in the AP direction, a passing rate of 94.2% was achieved. However, there was no improvement in the gamma-passing rate of the VMAT plan for T-spine SABR with the setup error simulation. We observed that the number of the tested points during gamma evaluation for the T-spine SABR VMAT was extremely small, i.e., only 26 points. Therefore, we decreased the threshold value to 5%, and the number of the tested points increased to 51 (a 96% increase). Subsequently, we achieved 92.2% passing rate with the 2%/1 mm for the T-spine SABR VMAT. Besides the T-spine VMAT, the VMAT plans for lung SABR and C-spine SABR showed gamma passing rates greater than 90% when the threshold value was changed to 5%. The number of tested points of the VMAT plans for the lung SABR and C-spine SABR increased by 37% and 26%, respectively, when the threshold value was changed to 5%. We observed a decreasing tendency in the gamma passing rates with increasing threshold values, which was in agreement with the results in the study by Steers et al., which showed increased sensitivity of the gamma evaluation with increasing threshold values [24]. Therefore, the low gamma-passing rate of the VMAT plan for T-spine SABR may have been caused by the small number of tested points, since the gamma-passing rate is a percent value. After searching the error source for each of the four failed cases, we verified the four VMAT plans with regard to their deliverability for patient treatment.
Several studies recently showed irrelevance of the 2D gamma passing rates toward the changes in the clinically relevant dose-volumetric parameters between the treatment plan and actual delivery [22, 25]. Information of the 2D gamma evaluation is not enough to detect errors affecting delivered doses which are three-dimensional inside a patient's body [26]. Moreover, since gamma evaluation is a comprehensive evaluation tool for a plan as a whole and not an evaluation tool for the delivered doses to each organ individually, the dose delivery accuracy to each structure including the target volumes and OARs, could not be verified individually with gamma evaluation [13]. Despite these limitations, 2D gamma evaluation is still performed in the clinic widely because no better alternative exists. To overcome the limited information of the 2D gamma evaluation, 3D gel dosimeters were proposed for the pre-treatment patient-specific QA for IMRT or VMAT [27]. However, the accuracy of the various 3D gel dosimeters is not adequate to be used in the clinic yet, owing to the high uncertainty. Some dosimeters recently introduced in the clinic are capable of measuring quasi-3D dose distributions, such as COMPASS™ system (IBA Dosimetry GmbH, Schwarzenbruck, Germany), ArcCHECK™ (Sun Nuclear Corporation, Melbourne, FL, USA), and OCTAVIUS 4D™ system (PTW, Freiburg, Germany) [22, 26, 28]. Although these dosimetry systems can provide more information than the 2D dosimeters, they have their own limitations. Although the COMPASS system can reconstruct dose distributions with actually measured fluences during beam delivery, the dose distribution is reconstructed with its own dose calculation algorithm. Therefore, this is not fully based on the measurement [26]. For the ArcCHECK system, the measured fluence could be used for the reconstruction of the dose distribution in the patient CT image with the 3DVH™ software (Sun Nuclear Corporation, Melbourne, FL, USA). However, this system also has the same limitation as that of the COMPASS system [28]. In the case of OCTAVIUS 4D system, the delivered-dose discrepancy in individual organs cannot be evaluated since that system does not utilize patient CT images [22]. Moreover, these quasi-3D dosimetry systems are not available to all radiotherapy institutions. On the other hand, some suggested the utilization of linac log files for the pre-treatment QA, which are recorded in the linac operating system during the actual delivery of a plan [29]. This method has an intrinsic disadvantage as a verification method for IMRT or VMAT plans because it is not an independent verification method, i.e., the accuracy of the delivered doses accomplished with the radiotherapy system including the linac and the TPS is verified with the same system. In this respect, 2D gamma evaluation is still used generally in the clinic. Therefore, enhancing the performance of 2D gamma evaluation has some merits [18–20, 24].
We previously suggested a gamma criterion of 2%/1 mm for pre-treatment SABR VMAT QA rather than 2%/2 mm to enhance the sensitivity of gamma evaluation in detecting errors in the SABR VMAT plans [20]. There might be some concern regarding the use of the gamma criterion of 2%/1 mm in the clinic because it could make the gamma evaluation susceptible to setup errors during measurements. However, as shown in the results of this study, the gamma evaluation with 2%/1 mm could be successfully applied in the clinic. Based on our two-year experience, use of the gamma criterion of 2%/1 mm for SABR VMAT can be easily adopted in the clinic while enhancing the sensitivity of the gamma evaluation to detect errors in a treatment plan.
MATERIALS AND METHODS
VMAT plan information
168 VMAT plans for SABR were generated and delivered to patients from January 2014 to January 2016 in our institution. The treatment plan details are summarized in Table 1. The target volumes of SABR were located in the lungs (82 cases), spine (30 cases), liver (28 cases), abdomen (16 cases), pelvis (9 cases), thorax (2 cases), and the head and neck (1 case). The prescription doses ranged from 6 Gy to 60 Gy and the fraction sizes ranged from 3 Gy to 24 Gy. The fraction number ranged from 1 to 10. Every patient underwent CT scans with a Brillance CT Big Bore™ (Philips, Amesterdam, Netherlands). During CT scans, patients were immobilized with appropriate immobilization devices compatible for each treatment site. For the treatment sites such as the lung, where respiratory motion is considerable, the motion was minimized with a Body Pro-Lok system (CIVICO, Orange City, IA, USA) and internal target volumes (ITVs) were defined with the 4D CT. For SABR, the ITV, rather than the respiratory gating technique, was used. All the VMAT plans were generated with an Eclipse™ system using the TrueBeam STx™ with a high-definition MLC (Varian Medical Systems, Palo Alto, CA, USA). According to the treatment site, a 6 MV flattening filter free (FFF) photon beam or 10 MV FFF photon beam was used. For optimization of the VMAT plans, a progressive resolution optimizer (PRO3, version 10, Varian Medical Systems, Palo Alto, CA, USA) was used. For the calculation of dose distributions, the anisotropic analytic algorithm (AAA, version 10, Varian Medical Systems, Palo Alto, CA, USA) was used. Except for lung SABR, a dose calculation grid of 2 mm was used for all the other cases. In the case of lung SABR, a dose calculation grid of 1 mm was used [30].
2D global gamma evaluation with a gamma criterion of 2%/1 mm
Before treatment, every SABR plan was verified using the 2D global gamma-index method with absolute doses by measuring planar dose distributions with the MapCHECK2 dosimeter. The MapCHECK2 dosimeter is a detector array with a total of 1527 solid state diodes. The diagonal detector spacing was 7.07 mm and the detector spacing parallel to X and Y axes were both 10 mm. Unlike the MapCHECK dosimeter, the MapCHECK2 dosimeter is compatible with VMAT using the Isocentric Mounting Fixture™ or MapPHAN™ (Sun Nuclear Corporation, Melbourne, FL, USA). In this study, the MapCHECK2 dosimeter was inserted into the MapPHAN which is a solid water phantom with a hole for insertion of the MapCHECK2. For the calculation of reference dose distributions, CT images of the MapCHECK2 inserted into the MapPHAN were acquired with a slice thickness of 1 mm. With these CT images, the reference planar dose distributions were calculated with the Eclipse system with a calculation grid of 1 mm. The MapCHECK2 dosimeter was calibrated every month. When measuring 2D dose distributions, the MapCHECK2 inserted into the MapPHAN was setup with a light field and room laser system in the treatment room. After measuring dose distributions for each SABR VMAT plan, 2D gamma evaluations were performed with the SNC patient software (version 6.1.2, Sun Nuclear Corporation, Melbourne, FL, USA). The global gamma evaluation with a gamma criterion of 2%/1 mm was performed with absolute doses. The points with doses less than 10% of the maximum measured dose were ignored for the gamma evaluation, i.e., the threshold value was set as 10%.
Evaluation of modulation degree
To examine the correlation of the gamma passing rate to the modulation degree, we calculated the MIs [14]. The MIs values were calculated with the DICOM-RT formatted treatment plan files exported from the Eclipse system. For correlation analysis, the Pearson correlation coefficient was calculated between gamma passing rates and the MIs values.
Analysis of the failed cases
Among 168 tested cases with the 2D global gamma evaluation, the failed cases with a tolerance level of 90% were analyzed. We traced the reason of the failure with gamma evaluation with 2%/1 mm for the failed cases by simulating setup errors and changing the threshold values. We simulated setup errors by rotating the couch from −0.2° to 0.2° (yaw rotational error) at intervals of 0.1°, translating the couch from −2 mm to 2 mm at intervals of 1 mm in the LR, SI, and AP directions. We examined the changes in the gamma passing rates by changing the threshold values from 5% to 50% at intervals of 5%.
Abbreviations
- IMRT
intensity modulated radiation therapy
- VMAT
volumetric modulated arc therapy
- OAR
organ at risk
- MLC
multi-leaf collimator
- TPS
treatment planning system
- MU
monitor unit
- 3D CRT
3D conformal radiation therapy
- QA
quality assurance
- SABR
stereotactic ablative radiotherapy
- DTA
distance to agreement
- MI
modulation index
- LR
left-to-right
- SI
superior-to-inferior
- AP
anterior-to-posterior
- ITV
internal target volume
- FFF
flattening filter free
- PRO
progressive resolution optimizer
- AAA
anisotropic analytic algorithm
Footnotes
Author contributions
JIK and JMP conceived of the study concept, analysed data, obtained funding, and drafted the manuscript. MSC participated in data collection and data checking. HGW, EKC, HJK, and JHK analyzed the data from a clinical perspective and discussed to improve the significance of this study. All authors read and approved the final manuscript. All authors had full access to all the data and had final responsibility for the decision to submit for publication.
CONFLICTS OF INTEREST
The authors indicated no conflicts of interest.
FUNDING
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (No. 1631200).
REFERENCES
- 1.Kim YS, Lee J, Park JI, Sung W, Lee SM, Kim GE. Volumetric modulated arc therapy for carotid sparing in the management of early glottic cancer. Radiat Oncol J. 2016;34:18–25. doi: 10.3857/roj.2016.34.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostheimer C, Hubsch P, Janich M, Gerlach R, Vordermark D. Dosimetric comparison of intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) in total scalp irradiation: a single institutional experience. Radiat Oncol J. 2016;34:313–321. doi: 10.3857/roj.2016.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JM, Kim K, Chie EK, Choi CH, Ye SJ, Ha SW. RapidArc vs intensity-modulated radiation therapy for hepatocellular carcinoma: a comparative planning study. Br J Radiol. 2012;85:e323–329. doi: 10.1259/bjr/19088580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu T, Shen C, Ou X, He X, Ying H, Hu C. The role of adjuvant chemotherapy in nasopharyngeal carcinoma with bulky neck lymph nodes in the era of IMRT. Oncotarget. 2016;7:21013–21022. doi: 10.18632/oncotarget.7849. https://doi.org/10.18632/oncotarget.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J, Hu W, Cai G, Wang J, Xie J, Peng J, Zhang Z. Dosimetric comparisons of VMAT, IMRT and 3DCRT for locally advanced rectal cancer with simultaneous integrated boost. Oncotarget. 2016;7:6345–6351. doi: 10.18632/oncotarget.6401. https://doi.org/10.18632/oncotarget.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahme A. Optimization of stationary and moving beam radiation therapy techniques. Radiother Oncol. 1988;12:129–140. doi: 10.1016/0167-8140(88)90167-3. [DOI] [PubMed] [Google Scholar]
- 7.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 8.Nicolini G, Fogliata A, Cozzi L. IMRT with the sliding window: comparison of the static and dynamic methods. Dosimetric and spectral analysis. Radiother Oncol. 2005;75:112–119. doi: 10.1016/j.radonc.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Seco J, Evans PM, Webb S. Analysis of the effects of the delivery technique on an IMRT plan: comparison for multiple static field, dynamic and NOMOS MIMiC collimation. Phys Med Biol. 2001;46:3073–3087. doi: 10.1088/0031-9155/46/12/301. [DOI] [PubMed] [Google Scholar]
- 10.Waghorn BJ, Staton RJ, Rineer JM, Meeks SL, Langen K. A comparison of the dosimetric effects of intrafraction motion on step-and-shoot, compensator, and helical tomotherapy-based IMRT. J Appl Clin Med Phys. 2013;14:4210. doi: 10.1120/jacmp.v14i3.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartrum T, Bailey M, Nelson V, Grace M. Linear attenuation coefficients for compensator based IMRT. Australas Phys Eng Sci Med. 2007;30:281–287. doi: 10.1007/BF03178438. [DOI] [PubMed] [Google Scholar]
- 12.Park JM, Wu HG, Kim JH, Carlson JN, Kim K. The effect of MLC speed and acceleration on the plan delivery accuracy of VMAT. Br J Radiol. 2015:20140698. doi: 10.1259/bjr.20140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JM, Park SY, Kim H. Modulation index for VMAT considering both mechanical and dose calculation uncertainties. Phys Med Biol. 2015;60:7101–7125. doi: 10.1088/0031-9155/60/18/7101. [DOI] [PubMed] [Google Scholar]
- 14.Park JM, Park SY, Kim H, Kim JH, Carlson J, Ye SJ. Modulation indices for volumetric modulated arc therapy. Phys Med Biol. 2014;59:7315–7340. doi: 10.1088/0031-9155/59/23/7315. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Park JM, Sung W, Kim IH, Ye SJ. Texture analysis on the edge-enhanced fluence of VMAT. Radiat Oncol. 2015;10:74. doi: 10.1186/s13014-015-0382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzell GA, Burmeister JW, Dogan N, LoSasso TJ, Mechalakos JG, Mihailidis D, Molineu A, Palta JR, Ramsey CR, Salter BJ, Shi J, Xia P, Yue NJ, et al. IMRT commissioning: multiple institution planning and dosimetry comparisons, a report from AAPM Task Group 119. Med Phys. 2009;36:5359–5373. doi: 10.1118/1.3238104. [DOI] [PubMed] [Google Scholar]
- 17.Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25:656–661. doi: 10.1118/1.598248. [DOI] [PubMed] [Google Scholar]
- 18.Fredh A, Scherman JB, Fog LS, Munck af Rosenschold P. Patient QA systems for rotational radiation therapy: a comparative experimental study with intentional errors. Med Phys. 2013;40:031716. doi: 10.1118/1.4788645. [DOI] [PubMed] [Google Scholar]
- 19.Heilemann G, Poppe B, Laub W. On the sensitivity of common gamma-index evaluation methods to MLC misalignments in Rapidarc quality assurance. Med Phys. 2013;40:031702. doi: 10.1118/1.4789580. [DOI] [PubMed] [Google Scholar]
- 20.Kim JI, Park SY, Kim HJ, Kim JH, Ye SJ, Park JM. The sensitivity of gamma-index method to the positioning errors of high-definition MLC in patient-specific VMAT QA for SBRT. Radiat Oncol. 2014;9:167. doi: 10.1186/1748-717X-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low DA, Morele D, Chow P, Dou TH, Ju T. Does the gamma dose distribution comparison technique default to the distance to agreement test in clinical dose distributions? Med Phys. 2013;40:071722. doi: 10.1118/1.4811141. [DOI] [PubMed] [Google Scholar]
- 22.Jin X, Yan H, Han C, Zhou Y, Yi J, Xie C. Correlation between gamma index passing rate and clinical dosimetric difference for pre-treatment 2D and 3D volumetric modulated arc therapy dosimetric verification. Br J Radiol. 2015;88:20140577. doi: 10.1259/bjr.20140577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bresciani S, Di Dia A, Maggio A, Cutaia C, Miranti A, Infusino E, Stasi M. Tomotherapy treatment plan quality assurance: the impact of applied criteria on passing rate in gamma index method. Med Phys. 2013;40:121711. doi: 10.1118/1.4829515. [DOI] [PubMed] [Google Scholar]
- 24.Steers JM, Fraass BA. IMRT QA: Selecting gamma criteria based on error detection sensitivity. Med Phys. 2016;43:1982. doi: 10.1118/1.4943953. [DOI] [PubMed] [Google Scholar]
- 25.Nelms BE, Zhen H, Tome WA. Per-beam, planar IMRT QA passing rates do not predict clinically relevant patient dose errors. Med Phys. 2011;38:1037–1044. doi: 10.1118/1.3544657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JI, Choi CH, Wu HG, Kim JH, Kim K, Park JM. Correlation analysis between 2D and quasi-3D gamma evaluations for both intensity-modulated radiation therapy and volumetric modulated arc therapy. Oncotarget. 2017;8:5449–5459. doi: 10.18632/oncotarget.12279. https://doi.org/10.18632/oncotarget.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldock C, De Deene Y, Doran S, Ibbott G, Jirasek A, Lepage M, McAuley KB, Oldham M, Schreiner LJ. Polymer gel dosimetry. Phys Med Biol. 2010;55:R1–63. doi: 10.1088/0031-9155/55/5/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajasekaran D, Jeevanandam P, Sukumar P, Ranganathan A, Johnjothi S, Nagarajan V. A study on correlation between 2D and 3D gamma evaluation metrics in patient-specific quality assurance for VMAT. Med Dosim. 2014;39:300–308. doi: 10.1016/j.meddos.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Agnew CE, King RB, Hounsell AR, McGarry CK. Implementation of phantom-less IMRT delivery verification using Varian DynaLog files and R/V output. Phys Med Biol. 2012;57:6761–6777. doi: 10.1088/0031-9155/57/21/6761. [DOI] [PubMed] [Google Scholar]
- 30.Ong CL, Cuijpers JP, Senan S, Slotman BJ, Verbakel WF. Impact of the calculation resolution of AAA for small fields and RapidArc treatment plans. Med Phys. 2011;38:4471–4479. doi: 10.1118/1.3605468. [DOI] [PubMed] [Google Scholar]


